Study of Excipients in Delayed Skin Reactions to mRNA Vaccines: Positive Delayed Intradermal Reactions to Polyethylene Glycol Provide New Insights for COVID-19 Arm
Abstract
:1. Background
2. Methods
2.1. Patients
2.2. Clinical Protocol
2.3. Skin Testing Protocol
3. Results
3.1. Demographic Baseline
3.2. Clinical Characterization of Skin Reactions Due to mRNA COVID-19 Vaccines
3.3. Patch Testing Results
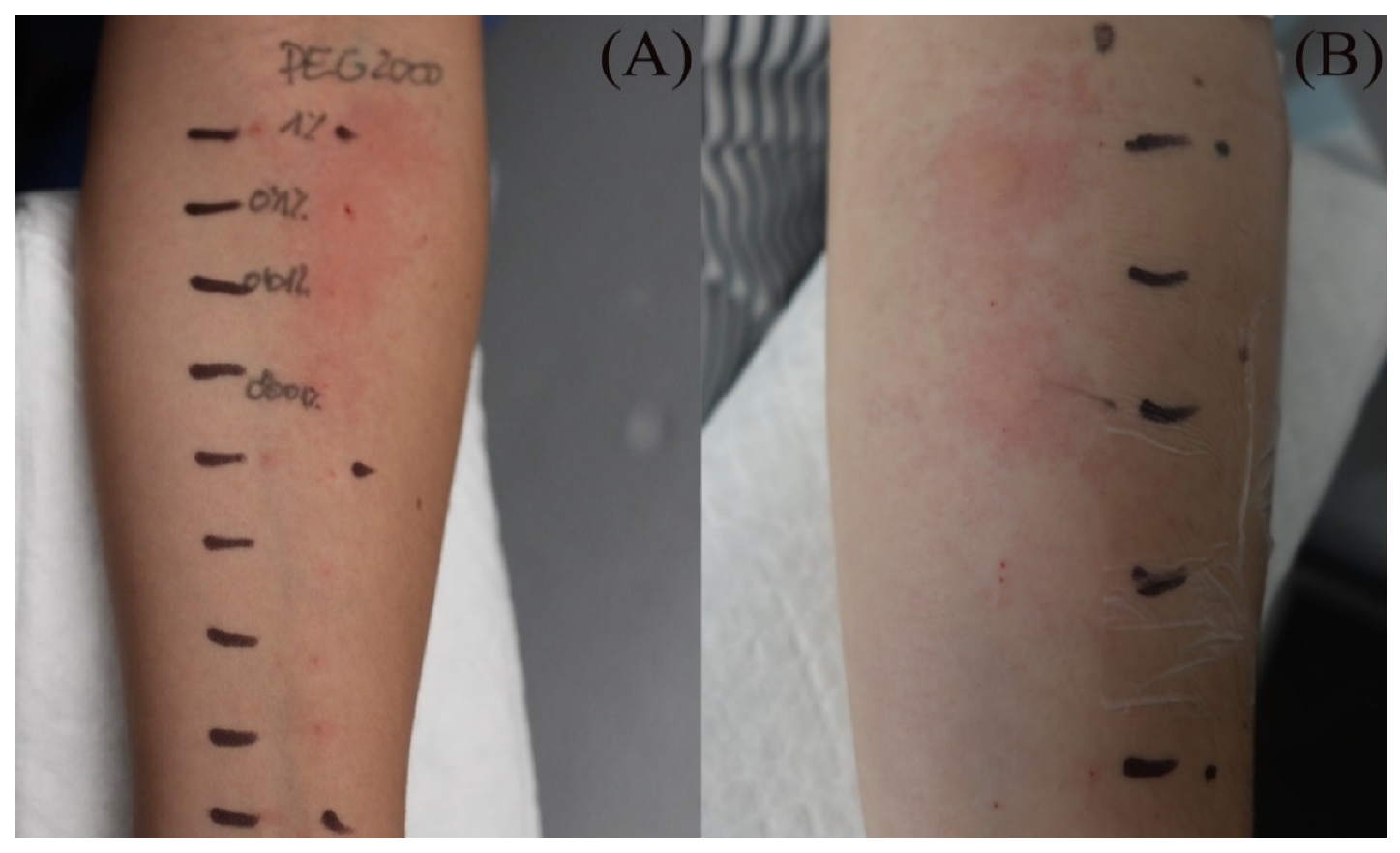
3.4. Intradermal Testing Results in Patients with Delayed Reactions
3.5. Skin Testing Health Care Workers without Skin Reactions
3.6. Skin Biopsy of PEG Reactions
4. Discussion
4.1. Skin Reactions Features
4.2. Skin Testing Results for PEG
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Riesco, M.R.; Nebreda, D.R.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-COV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2022, 186, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kempf, W.; Kettelhack, N.; Kind, F.; Courvoisier, S.; Galambos, J.; Pfaltz, K. ‘COVID arm’—Histological features of a delayed-type hypersensitivity reaction to Moderna mRNA-1273 SARS-CoV2 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e730–e732. [Google Scholar] [CrossRef]
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Hammerle, J.; Fernandez-Escribano, M.; Moreno-Del Real, C.M.; Garcia-Abellas, P.; Carretero-Barrio, I.; Solano-Solares, E.; de-la-Hoz-Caballer, B.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: A clinical and histological characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Allergy to COVID-19 vaccines: A current update. Allergol. Int. 2021, 70, 313–318. [Google Scholar] [CrossRef]
- Nilsson, L.; Csuth, Á.; Storsaeter, J.; Garvey, L.H.; Jenmalm, M.C. Vaccine allergy: Evidence to consider for COVID-19 vaccines. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 401–409. [Google Scholar] [CrossRef]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19, Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Restivo, V.; Candore, G.; Barrale, M.; Caravello, E.; Graziano, G.; Onida, R.; Raineri, M.; Tiralongo, S.; Brusca, I. Allergy to Polyethilenglicole of Anti-SARS CoV2 Vaccine Recipient: A Case Report of Young Adult Recipient and the Management of Future Exposure to SARS-CoV2. Vaccines 2021, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard-Mouritsen, M.A.; Jensen, B.M.; Poulsen, L.K.; Duus Johansen, J.; Garvey, L.H. Optimizing investigation of suspected allergy to polyethylene glycols. J. Allergy Clin. Immunol. 2022, 149, 168–175. [Google Scholar] [CrossRef]
- Bianchi, A.; Bottau, P.; Calamelli, E.; Caimmi, S.; Crisafulli, G.; Franceschini, F.; Liotti, L.; Mori, F.; Paglialunga, C.; Saretta, F.; et al. Hypersensitivity to polyethylene glycol in adults and children: An emerging challenge. Acta Biomed. 2021, 92, e2021519. [Google Scholar]
- Wenande, E.; Garvey, L.H. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin. Exp. Allergy 2016, 46, 907–922. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef]
- Barbaud, A.; Gonçalo, M.; Bruynzeel, D.; Bircher, A.J. European Society of Contact Dermatitis. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermat. 2001, 45, 321–328. [Google Scholar] [CrossRef]
- Scala, E.; Giani, M.; Pirrotta, L.; Guerra, E.; Locanto, M.; De Pità, O.; Puddu, P. Selective severe anaphylactic reaction due to ketorolac tromethamine without nonsteroidal anti-inflammatory drug intolerance. J. Allergy Clin. Immunol. 2001, 107, 557. [Google Scholar] [CrossRef]
- de Groot, A.C. Patch Testing. In Test Concentrations and Vehicles for 4900 Chemicals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Fisher, A.A. Contact Dermatitis, 3rd ed.; Lea & Febiger: Philadelphia, PA, USA, 1986. [Google Scholar]
- Bohn, S.; Hurni, M.; Bircher, A.J. Contact allergy to trometamol. Contact Dermat. 2001, 44, 319. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010, 10, 338–349, Erratum in Lancet Infect Dis. 2010, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A. Skin tests in the diagnosis of drug hypersensitivity reactions. Curr. Pharm. Des. 2008, 14, 2778–2791. [Google Scholar] [CrossRef] [PubMed]
- Tramontana, M.; Bianchi, L.; Biondi, F.; Hansel, K.; Malatesta, N.; Marietti, R.; Stingeni, L. A case of delayed allergy to polyethylene glycol 2000 and polysorbate 80 confirmed by patch test: Consequences for anti-SARS-CoV2 vaccination? Contact Dermat. 2022, 87, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Maoz-Segal, R.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz, Y.; Niznik, S.; Deutch, M.; Elbaz, E.; Genaim, H.; et al. Assessment of Immediate Allergic Reactions After Immunization with the Pfizer BNT162b2 Vaccine Using Intradermal Skin Testing With the COVID-19 Vaccines. J. Allergy Clin. Immunol. Pract. 2022, 10, 2677–2684. [Google Scholar] [CrossRef]
- Lim, X.R.; Tan, J.W.L.; Chan, G.Y.L.; Hou, J.; Xie, L.; Goh, V.H.L.; Boon, J.; Lee, S.S.M.; Teo, C.M.-L.; Tan, S.C.; et al. Evaluation of Patients with Vaccine Allergies Prior to mRNA-Based COVID-19 Vaccination. Vaccines 2022, 10, 1025. [Google Scholar] [CrossRef]
- Cox, F.; Khalib, K.; Conlon, N. PEG That Reaction: A Case Series of Allergy to Polyethylene Glycol. J. Clin. Pharmacol. 2021, 61, 832–835. [Google Scholar] [CrossRef]
- Bianchi, L.; Biondi, F.; Hansel, K.; Murgia, N.; Tramontana, M.; Stingeni, L. Skin tests in urticaria/angioedema and flushing to Pfizer-BioNTech SARS-CoV-2 vaccine: Limits of intradermal testing. Allergy 2021, 76, 2605–2607. [Google Scholar] [CrossRef]
- Loli-Ausejo, D.; Gómez-Armayones, S.; Sáez-Peñataro, J.; González-Matamala, M.; Mascaró, B.; Muñoz-Cano, R.; Bartra, J. COVID-19 vaccine tolerabilty in a patient with a delayed allergic reaction to polyethylene glycol: A case report. J. Investig. Allergol. Clin. Immunol. 2022, 33. [Google Scholar] [CrossRef]
- Wolfson, A.R.; Robinson, L.B.; Li, L.; McMahon, A.E.; Cogan, A.S.; Fu, X.; Wickner, P.; Samarakoon, U.; Saff, R.R.; Blumenthal, K.G.; et al. First-Dose mRNA COVID-19 Vaccine Allergic Reactions: Limited Role for Excipient Skin Testing. J. Allergy Clin. Immunol. Pract. 2021, 9, 3308–3320. [Google Scholar] [CrossRef]
- Pickert, J.; Hennighausen, I.; Mühlenbein, S.; Möbs, C.; Pfützner, W. Immediate-Type Hypersensitivity to Polyethylene Glycol (PEG) Including a PEG-containing COVID-19 Vaccine Revealed by Intradermal Testing. J. Investig. Allergol. Clin. Immunol. 2021, 31, 526–527. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, M.M.; Sitek, A.N.; Kinate, S.A.; Joshi, A.Y.; Park, M.A. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: Early report. Ann. Allergy Asthma Immunol. 2021, 126, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; Marcelino, J.; Ferreira, F.; Silva, R.; Proença, M.; Tomaz, E. Skin testing with Pfizer SARS-CoV-2 vaccine and PEG 2000. Asia Pac. Allergy. 2021, 11, e18. [Google Scholar] [CrossRef]
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021, 76, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Y.; Goldberg, I.; Sprecher, E.; Slodownik, D. Patch testing versus interferon-gamma release assay in evaluation of drug eruptions. Fundam. Clin. Pharmacol. 2022, 36, 414–420. [Google Scholar] [CrossRef]
| Skin Reactions | DLLR | ILR | Morbilliform and Pityriasis Rosea-Like Rash | Urticariform Rash | Psoriasiform Rash | BMS |
|---|---|---|---|---|---|---|
| Clinical picture | 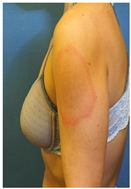 n = 18 | 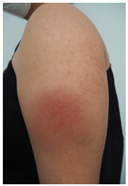 n = 5 | 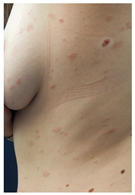 n = 4 | 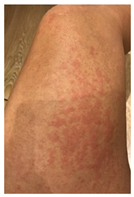 n = 2 | 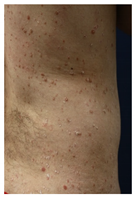 n = 1 | No image n = 1 |
| Median age (IQR) | 38.9 (47–30) | 47.6 (61.5–34) | 43.8 (59.5–28) | 35.5 | 72 | 44 |
| Sex, n (%) | ||||||
| Women | 16 (88.9) | 5 | 3 | 2 | None | 1 |
| Men | 2 | None | 1 | None | 1 | None |
| Allergies, n (%) | 11 (61.1) | 1 | 2 | 1 | 1 | 1 |
| Past anaphylaxis, n | 2 | None | None | None | None | None |
| Chronic skin disorder, n | ||||||
| AD | 3 | 2 | None | 1 | None | None |
| CSU | 3 | 1 | 1 | 1 | None | None |
| Median onset (days) (IQR) | 8.1 (9–7) | 2.4 (3.5–1.5) | 6.8 (8.5–5) | 6 | 10 | 1 |
| Median duration (days) (IQR) | 4.8 (7–3) | 3 (4.5–1.5) | 4.3 (6–2.5) | 6.5 | 15 | 10 |
| Vaccine, n (%) | ||||||
| Moderna | 16 (88.9) | 5 | 1 | None | 1 | None |
| Pfizer | 2 | None | 3 | 2 | None | 1 |
| Dose, n (%) | ||||||
| 1st | 13 (72.2) | 5 | 3 | None | None | 1 |
| 2nd | 5 | None | 1 | 2 | 1 | None |
| Relapse with 2nd dose, n | 3 | 1 | None | None | None | None |
| Local symptoms, n (%) | 16 (88.9) | 3 | 4 | 2 | None | 1 |
| Response to treatment | All patients presented a good response to conventional treatments | |||||
| Vaccine discontinuation | No vaccine discontinuation was needed for any patient with the 2nd dose | |||||
| Skin Reaction | Intradermal Testing (Readings at 20 min/2 h/D2; if All Negative: Neg) | ||||||||||||||||
| PEG-400 | PEG-2000 | TR | 3-PC | ||||||||||||||
| 1.0% | 0.1% | 0.01% | 0.001% | 1.0% | 0.1% | 0.01% | 0.001% | 1.0% | 0.1% | 0.01% | 0.001% | 1.0% | 0.1% | 0.01% | 0.001% | ||
| Widespread skin reactions | 1 | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 2 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 3 | Neg | Neg | Neg | Neg | (+/+/−) | (+/+/−) | (+/−/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 4 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 5 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| “COVID ARMS” or DLLR | 6 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| 7 | (+/+/+) | (+/+/−) | Neg | Neg | (+/+/+) | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | (+/−/−) | Neg | Neg | Neg | |
| 8 | Neg | Neg | Neg | Neg | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 9 | (+/+/−) | (+/+/−) | (+/+/−) | Neg | (+/+/+) | (+/+/+) | (+/+/−) | (+/+/−) | (+/−/−) | (+/−/−) | (+/−/−) | (+/−/−) | Neg | Neg | Neg | Neg | |
| 10 | (+/+/−) | Neg | Neg | Neg | (+/+/−) | (+/+/−) | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 11 | Neg | Neg | Neg | Neg | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 12 | Neg | Neg | Neg | Neg | (+/+/−) | (+/−/−) | (+/−/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 13 | (+/+/−) | Neg | Neg | Neg | (+/+/−) | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 14 | Neg | Neg | Neg | Neg | (+/+/−) | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 15 | Neg | Neg | Neg | Neg | (+/+/−) | (+/+/−) | (+/+/−) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| 16 | Neg | Neg | Neg | Neg | (+/+/+) | (+/+/−) | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | |
| Skin Reaction | Type | Grading | Duration (days) | Vertical D (cm) | Horizontal D (cm) | |
|---|---|---|---|---|---|---|
| Widespread skin reactions | 1 | Psoriasiform eruption | 3 | 15 | NA | NA |
| 2 | Pityriasis rosea rash | 3 | 6 | NA | NA | |
| 3 | Morbilliform rash | 3 | 4 | NA | NA | |
| 4 | Urticariform rash | 3 | 7 | NA | NA | |
| 5 | Urticariform rash | 3 | 6 | NA | NA | |
| “COVID ARMS” or DLLR | 6 | NA | 1 | 6 | 6.5 | 8.0 |
| 7 | NA | 2 | 3 | 11.0 | 6.1 | |
| 8 | NA | 2 | 7 | 8.4 | 5.3 | |
| 9 | NA | 2 | 15 | 14.5 | 7.4 | |
| 10 | NA | 2 | 7 | 8.5 | 6.8 | |
| 11 | NA | 1 | 6 | 5.8 | 4.5 | |
| 12 | NA | 2 | 4 | 8.5 | 7.2 | |
| 13 | NA | 2 | 7 | 9.5 | 7.0 | |
| 14 | NA | 2 | 6 | 8.8 | 7.5 | |
| 15 | NA | 2 | 7 | 8.0 | 6.5 | |
| 16 | NA | 1 | 3 | 9.5 | 5.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesqué, D.; Pujol, R.M.; Marcantonio, O.; Vidal-Navarro, A.; Ramada, J.M.; Arderiu-Formentí, A.; Albalat-Torres, A.; Serra, C.; Giménez-Arnau, A.M. Study of Excipients in Delayed Skin Reactions to mRNA Vaccines: Positive Delayed Intradermal Reactions to Polyethylene Glycol Provide New Insights for COVID-19 Arm. Vaccines 2022, 10, 2048. https://doi.org/10.3390/vaccines10122048
Pesqué D, Pujol RM, Marcantonio O, Vidal-Navarro A, Ramada JM, Arderiu-Formentí A, Albalat-Torres A, Serra C, Giménez-Arnau AM. Study of Excipients in Delayed Skin Reactions to mRNA Vaccines: Positive Delayed Intradermal Reactions to Polyethylene Glycol Provide New Insights for COVID-19 Arm. Vaccines. 2022; 10(12):2048. https://doi.org/10.3390/vaccines10122048
Chicago/Turabian StylePesqué, David, Ramon Maria Pujol, Orianna Marcantonio, Ainhoa Vidal-Navarro, José María Ramada, Alba Arderiu-Formentí, Agustí Albalat-Torres, Consol Serra, and Ana María Giménez-Arnau. 2022. "Study of Excipients in Delayed Skin Reactions to mRNA Vaccines: Positive Delayed Intradermal Reactions to Polyethylene Glycol Provide New Insights for COVID-19 Arm" Vaccines 10, no. 12: 2048. https://doi.org/10.3390/vaccines10122048
APA StylePesqué, D., Pujol, R. M., Marcantonio, O., Vidal-Navarro, A., Ramada, J. M., Arderiu-Formentí, A., Albalat-Torres, A., Serra, C., & Giménez-Arnau, A. M. (2022). Study of Excipients in Delayed Skin Reactions to mRNA Vaccines: Positive Delayed Intradermal Reactions to Polyethylene Glycol Provide New Insights for COVID-19 Arm. Vaccines, 10(12), 2048. https://doi.org/10.3390/vaccines10122048






