Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Procedures
2.2. Statistical Analysis
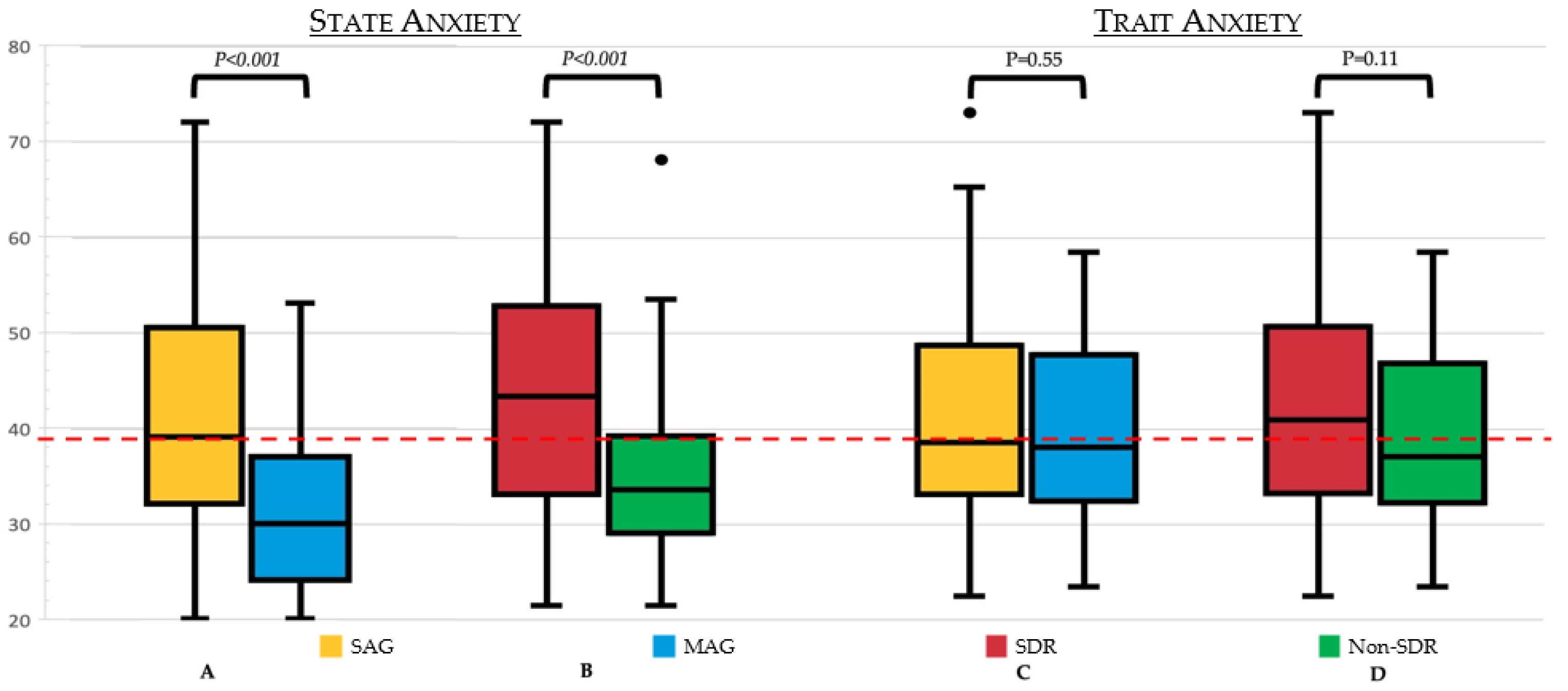
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.K.; Vadas, P. Anaphylaxis: Mechanisms and management. Clin. Exp. Allergy 2011, 41, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Montoro, J.; Mullol, J.; Jáuregui, I.; Dávila, I.; Ferrer, M.; Bartra, J.; del Cuvillo, A.; Sastre, J.; Valero, A. Stress and Allergy. J. Investig. Allergol. Clin. Immunol. 2009, 19 (Suppl. S1), 40–47. [Google Scholar] [CrossRef] [PubMed]
- Baiardini, I.; Gaeta, F.; Molinengo, G.W.; Braido, F.; Canonica, G.; Romano, A. Quality-of-life issues in survivors to anaphylactic reactions to drugs. Allergy 2015, 70, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Boyton, R.J. COVID-19 vaccination: The road ahead. Science 2022, 375, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; Del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021, 76, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA-J. Am. Med. Assoc. 2021, 325, 780. [Google Scholar] [CrossRef] [PubMed]
- Klimek, L.; Jutel, M.; Akdis, C.A.; Bousquet, J.; Akdis, M.; Torres-Jaen, M.; Agache, I.; Canonica, G.W.; Del Giacco, S.; O’Mahony, L.; et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—An EAACI-ARIA Position Paper. Allergy 2020, 76, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.-R.; Cucca, V.; Asperti, C.; Ramirez, G.A.; Della-Torre, E.; Moro, M.; Zandalasini, C.; Di Napoli, D.; Ambrosio, A.; Signorelli, C.; et al. Efficacy of a rational algorithm to assess allergy risk in patients receiving the BNT162b2 vaccine. Vaccine 2021, 39, 6464–6469. [Google Scholar] [CrossRef] [PubMed]
- Chiang, V.; Saha, C.; Yim, J.; Au, E.Y.; Kan, A.K.; Hui, K.S.H.; Li, T.S.; Lo, W.L.W.; Hong, Y.D.; Ye, J.; et al. The Role of the Allergist in Coronavirus Disease 2019 Vaccine Allergy Safety: A Pilot Study on a “Hub-and-Spoke” Model for Population-Wide Allergy Service. Ann. Allergy Asthma Immunol. 2022, 129, 308–312.e1. [Google Scholar] [CrossRef] [PubMed]
- Decreto Legge 12 Marzo 2021, Gazzetta Ufficiale n.72 del 24-03-2021. Available online: https://www.gazzettaufficiale.it/gazzetta/serie_generale/caricaDettaglio?dataPubblicazioneGazzetta=2021-03-24&numeroGazzetta=72 (accessed on 3 July 2022).
- Filon, F.L.; Lazzarato, I.; Patriarca, E.; Iavernig, T.; Peratoner, A.; Perri, G.; Ponis, G.; Rocco, G.; Cegolon, L. Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy). Vaccines 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Wolfson, A.R.; Wickner, P.G.; Cogan, A.S.; McMahon, A.E.; Saff, R.; Robinson, L.B.; Phillips, E.; Blumenthal, K.G. COVID-19 Vaccination in Patients with Reported Allergic Reactions: Updated Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Larson, H.; Dubé, È.; Fisher, A. Vaccine Hesitancy: Drivers and How the Allergy Community Can Help. J. Allergy Clin. Immunol. Pract. 2021, 9, 3568–3574. [Google Scholar] [CrossRef] [PubMed]
- Bagos-Estevez, A.G.; Ledford, D.K. Anaphylaxis: Definition, Epidemiology, Diagnostic Challenges, Grading System. Immunol. Allergy Clin. N. Am. 2021, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Article 29 Data Protection Working Party. 2014. Available online: https://ec.europa.eu/justice/article-29/documentation/opinion-recommendation/files/2014/wp216_en.pdf (accessed on 17 September 2022).
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. S11), S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.G.; Waal-Manning, H.J.; Spears, G.F. Some norms and reliability data for the State-Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br. J. Clin. Psychol. 1983, 22 Pt 4, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Vai, B.; Cazzetta, S.; Ghiglino, D.; Parenti, L.; Saibene, G.; Toti, M.; Verga, C.; Wykowska, A.; Benedetti, F. Risk Perception and Media in Shaping Protective Behaviors: Insights From the Early Phase of COVID-19 Italian Outbreak. Front. Psychol. 2020, 11, 563426. [Google Scholar] [CrossRef] [PubMed]
- Bendau, A.; Plag, J.; Petzold, M.B.; Ströhle, A. COVID-19 vaccine hesitancy and related fears and anxiety. Int. Immunopharmacol. 2021, 97, 107724. [Google Scholar] [CrossRef] [PubMed]
- Losappio, L.M.; Cappai, A.; Arcolaci, A.; Badiu, I.; Bonadonna, P.; Boni, E.; Bussolino, C.; Caminati, M.; Galati, P.; Heffler, E.; et al. Anxiety and Depression Effects During Drug Provocation Test. J. Allergy Clin. Immunol. Pract. 2018, 6, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Soyyiğit, Ş.; Aydın, Ö.; Yılmaz, I.; Özdemir, S.K.; Cankorur, V.; Atbaşoğlu, C.; Çelik, G.E. Evaluation of drug provocation test–related anxiety in patients with drug hypersensitivity. Ann. Allergy Asthma Immunol. 2016, 117, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.W.; Bender, F.L.; Ballou, S.; Kelley, J.M.; Wilhelm, M.; Miller, F.G.; Rief, W.; Kaptchuk, T.J. Frequency of Adverse Events in the Placebo Arms of COVID-19 Vaccine Trials: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e2143955. [Google Scholar] [CrossRef] [PubMed]
| Total Sample | Severe Allergic Group | Mild Allergic Group | p | |
|---|---|---|---|---|
| N (100%) | 116 (100) | 89 (77) | 27 (23) | NA |
| Females: n (%) | 90 (78) | 76 (86) | 15 (51) | <0.010 |
| Age Class: median (IQR) | 47 (37–57) | 47 (37–57) | 47 (32–57) | ns |
| Drug anaphylaxis: n (%) | 70 (60) | 70 (79) | 0 (0) | <0.001 |
| Drug HRs ≥ 2: n (%) | 49 (42) | 49 (56) | 0 (0) | <0.001 |
| Food anaphylaxis: n (%) | 43 (37) | 42 (48) | 0 (0) | <0.001 |
| Allergic comorbidities: n (%) | 75 (66) | 59 (67) | 15 (56) | ns |
| Rhinitis/conjunctivitis: n (%) | 37 (32) | 30 (34) | 7 (26) | ns |
| Asthma: n (%) | 33 (28) | 26 (30) | 7 (26) | ns |
| AD: n (%) | 33 (28) | 27 (31) | 5 (22) | ns |
| ACD: n (%) | 29 (25) | 27 (30) | 2 (7) | <0.050 |
| CSU: n (%) | 12 (10) | 9 (10) | 3 (11) | ns |
| Food allergy: n (%) | 47 (41) | 42 (48) | 5 (22) | <0.050 |
| Hymenoptera allergy: n (%) | 18 (16) | 19 (22) | 3 (11) | ns |
| Positive SPT: n (%) | 60 (52) | 51 (57) | 9 (33) | <0.050 |
| AntiH1 therapy: n (%) | 49 (42) | 39 (44) | 10 (37) | ns |
| Inhaled asthma therapy | 25 (21) | 19 (21) | 6 (22) | ns |
| Comorbidities: n (%) | 19 (16) | 16 (18) | 3 (11) | ns |
| Dose 1: n (%) | 16 (14) | 12 (14) | 4 (15) | ns |
| Dose 2: n (%) | 46 (40) | 36 (41) | 9 (33) | ns |
| Dose 3: n (%) | 52 (45) | 38 (43) | 14 (52) | ns |
| Symptoms: n (%) | 15 (13) | 13 (15) | 2 (7) | Ns |
| STAI-state: median (IQR) | 36.5 (30.0–47.2) | 39.0 (32.0–50.2) | 30. 0 (24.5–36.5) | <0.050 |
| STAI-trait: median (IQR) | 37.0 (32.0–48.0) | 37.50 (32.0–48.0) | 37.0 (31.5–47.0) | Ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asperti, C.; Benanti, G.; Ramirez, G.A.; Russo, M.; Vai, B.; Bramé, B.; Viapiana, N.; Nannipieri, S.; Cilona, M.B.; Mazzetti, M.; et al. Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees. Vaccines 2022, 10, 2047. https://doi.org/10.3390/vaccines10122047
Asperti C, Benanti G, Ramirez GA, Russo M, Vai B, Bramé B, Viapiana N, Nannipieri S, Cilona MB, Mazzetti M, et al. Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees. Vaccines. 2022; 10(12):2047. https://doi.org/10.3390/vaccines10122047
Chicago/Turabian StyleAsperti, Chiara, Giovanni Benanti, Giuseppe A. Ramirez, Marco Russo, Benedetta Vai, Barbara Bramé, Naomi Viapiana, Serena Nannipieri, Maria Bernadette Cilona, Martina Mazzetti, and et al. 2022. "Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees" Vaccines 10, no. 12: 2047. https://doi.org/10.3390/vaccines10122047
APA StyleAsperti, C., Benanti, G., Ramirez, G. A., Russo, M., Vai, B., Bramé, B., Viapiana, N., Nannipieri, S., Cilona, M. B., Mazzetti, M., Zuffada, S., Di Mattei, V. E., Benedetti, F., Dagna, L., & Yacoub, M.-R. (2022). Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees. Vaccines, 10(12), 2047. https://doi.org/10.3390/vaccines10122047





