Differential Homing Receptor Profiles of Lymphocytes Induced by Attenuated versus Live Plasmodium falciparum Sporozoites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Flow Cytometric Analysis
2.3. Statistical Analysis
3. Results
3.1. Immunization and Challenge with Live Sporozoites Induce Significant Changes in the Frequency of SPZ-Specific B Cells with a Unique Homing Receptor Profile
3.2. Challenge with Live Sporozoites, but Not Immunization, Induces Significant Changes in the Homing Receptor Profiles of SPZ-Specific CD4+ T Cells
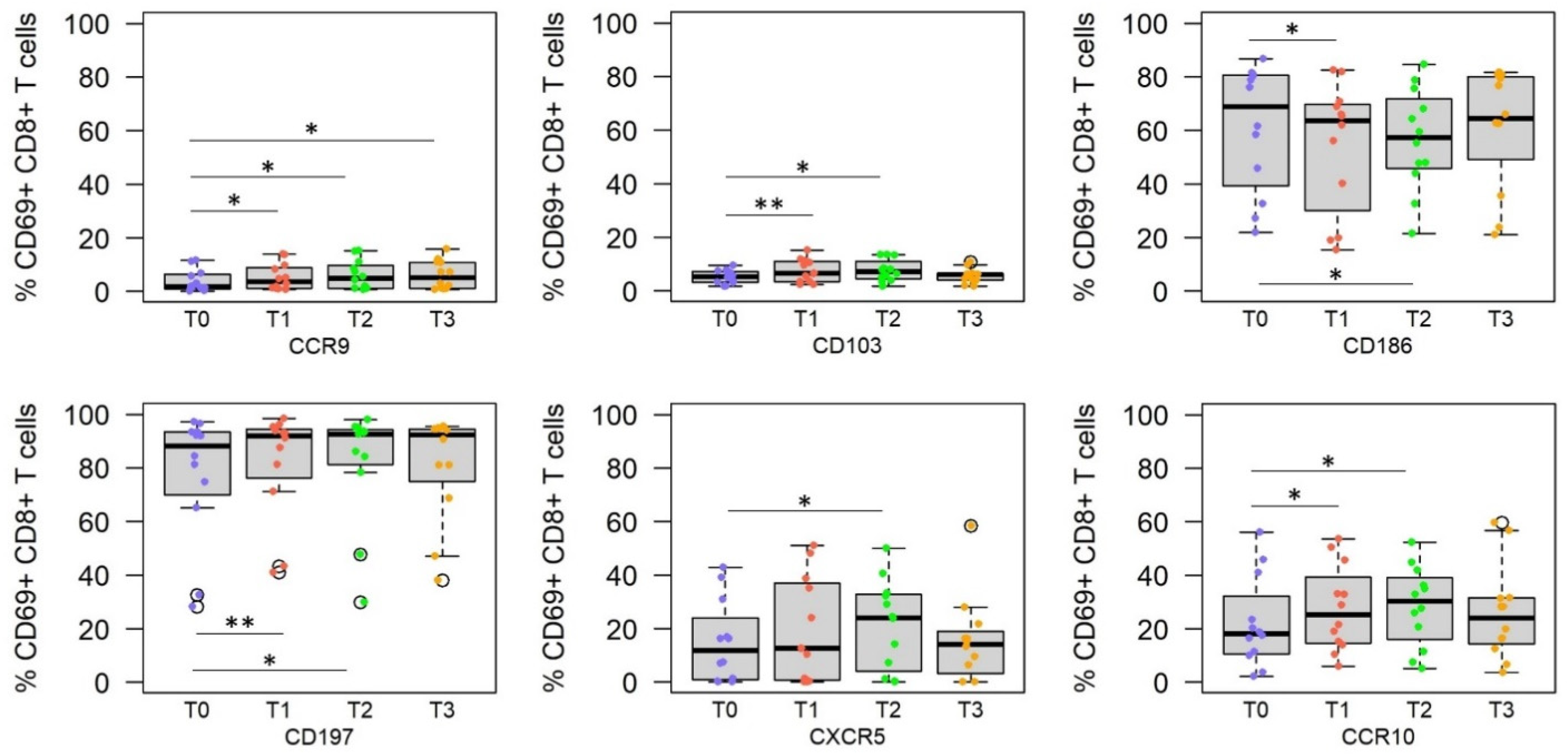
3.3. RAS Vaccine and Live SPZ Challenge Induce a Complex Profile of Homing Receptors on SPZ-Specific CD8+ T Cells
3.4. Homing Receptor Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, Y.; Kobayashi, M. Lymphocyte ‘Homing’ and Chronic Inflammation. Pathol. Int. 2015, 65, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, C.C.; Peske, J.D.; Engelhard, V.H. Peripheral Tissue Homing Receptor Control of Naïve, Effector, and Memory CD8 T Cell Localization in Lymphoid and Non-Lymphoid Tissues. Front. Immunol. 2013, 4, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.; Iwasaki, A. Tissue-Resident Memory T Cells. Immunol. Rev. 2013, 255, 165–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thome, J.J.; Yudanin, N.; Ohmura, Y.; Kubota, M.; Grinshpun, B.; Sathaliyawala, T.; Kato, T.; Lerner, H.; Shen, Y.; Farber, D.L. Spatial Map of Human T Cell Compartmentalization and Mainte-nance over Decades of Life. Cell 2014, 159, 814–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, D.J.; Butcher, E.C. Rapid Acquisition of Tissue-specific Homing Phenotypes by CD4+ T Cells Activated in Cutaneous or Mucosal Lymphoid Tissues. J. Exp. Med. 2002, 195, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Crowl, J.T.; Heeg, M.; Ferry, A.; Milner, J.J.; Omilusik, K.D.; Toma, C.; He, Z.; Chang, J.T.; Goldrath, A.W. Tissue-Resident Memory Cd8(+) T Cells Possess Unique Transcriptional, Epigenetic and Functional Adaptations to Different Tissue Environ-ments. Nat. Immunol. 2022, 23, 1121–1131. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, D.; de Menezes, M.N.; Holz, L.E.; Ghilas, S.; Heath, W.R.; Beattie, L. Heath, and Lynette Beattie. Har-nessing Liver-Resident Memory T Cells for Protection against Malaria. Expert Rev. Vaccines 2021, 20, 127–141. [Google Scholar] [CrossRef]
- Lefebvre, M.N.; Harty, J.T. You Shall Not Pass: Memory CD8 T Cells in Liver-Stage Malaria. Trends Parasitol. 2020, 36, 147–157. [Google Scholar] [CrossRef]
- Slütter, B.; Van Braeckel-Budimir, N.; Abboud, G.; Varga, S.M.; Salek-Ardakani, S.; Harty, J.T. Dynamics of Influen-za-Induced Lung-Resident Memory T Cells Underlie Waning Heterosubtypic Immunity. Sci. Immunol. 2017, 2, eaag2031. [Google Scholar] [CrossRef] [Green Version]
- Booth, J.S.; Goldberg, E.; Patil, S.A.; Barnes, R.S.; Greenwald, B.D.; Sztein, M.B. Age-Dependency of Terminal Ileum Tissue Resident Memory T Cell Responsiveness Profiles to S. Typhi Following Oral Ty21a Immunization in Humans. Immune Ageing 2021, 18, 19. [Google Scholar] [CrossRef]
- McCarthy, K.R.; Von Holle, T.A.; Sutherland, L.L.; Oguin, I.I.I.T.H.; Sempowski, G.D.; Harrison, S.C.; Moody, M.A. Differential Immune Imprinting by Influenza Virus Vaccination and Infection in Nonhuman Pri-mates. Proc. Natl. Acad. Sci. USA 2021, 118, e2026752118. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Changrob, S.; Li, L.; Wilson, P.C. Imprinting, immunodominance, and other impediments to generating broad influenza immunity. Immunol. Rev. 2020, 296, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.M.; Palkola, N.V.; Arvilommi, H.S.; Kantele, J.M. Distinctive homing profile of pathogen-specific activated lymphocytes in human urinary tract infection. Clin. Immunol. 2008, 128, 427–434. [Google Scholar] [CrossRef]
- Kantele, A.; Kantele, J.M.; Savilahti, E.; Westerholm, M.; Arvilommi, H.; Lazarovits, A.; Butcher, E.C.; Mäkelä, P.H. Homing Potentials of Circulating Lymphocytes in Humans Depend on the Site of Activation: Oral, but Not Parenteral, Typhoid Vac-cination Induces Circulating Antibody-Secreting Cells That All Bear Homing Receptors Directing Them to the Gut. J. Immunol. 1997, 158, 574. [Google Scholar]
- Grover, A.; Kim, G.J.; Lizée, G.; Tschoi, M.; Wang, G.; Wunderlich, J.R.; Rosenberg, S.A.; Hwang, S.T.; Hwu, P. Intralymphatic Dendritic Cell Vaccination Induces Tumor Antigen-Specific, Skin-Homing T Lymphocytes. Clin. Cancer Res. 2006, 12, 5801–5808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palkola, N.V.; Pakkanen, S.H.; Kantele, J.M.; Pakarinen, L.; Puohiniemi, R.; Kantele, A. Differences in Homing Potentials of Streptococcus Pneumoniae–Specific Plasmablasts in Pneumococcal Pneumonia and after Pneumo-coccal Polysaccharide and Pneumococcal Conjugate Vaccinations. J. Infect. Dis. 2015, 212, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Toapanta, F.R.; Simon, J.K.; Barry, E.M.; Pasetti, M.F.; Levine, M.M.; Kotloff, K.L.; Sztein, M.B. Gut-homing conventional plasmablasts and CD27− plasmablasts elicited after a short time of exposure to an oral live-attenuated Shigella vaccine candidate in humans. Front. Immunol. 2014, 5, 374. [Google Scholar] [CrossRef]
- Benlahrech, A.; Harris, J.; Meiser, A.; Papagatsias, T.; Hornig, J.; Hayes, P.; Lieber, A.; Athanasopoulos, T.; Bachy, V.; Csomor, E.; et al. Adenovirus vector vaccination induces expansion of memory CD4 T cells with a mucosal homing phenotype that are readily susceptible to HIV-1. Proc. Natl. Acad. Sci. USA 2009, 106, 19940–19945. [Google Scholar] [CrossRef] [Green Version]
- Neidleman, J.; Luo, X.; McGregor, M.; Xie, G.; Murray, V.; Greene, W.C.; Sulggi, A.L.; Roan, N.R. Mrna Vaccine-Induced T Cells Respond Identically to Sars-Cov-2 Variants of Concern but Differ in Longevity and Homing Properties Depending on Prior Infection Status. eLife 2021, 10, e72619. [Google Scholar] [CrossRef]
- Seong, Y.; Lazarus, N.H.; Sutherland, L.; Habtezion, A.; Abramson, T.; He, X.-S.; Greenberg, H.; Butcher, E.C. Trafficking receptor signatures define blood plasmablasts responding to tissue-specific immune challenge. JCI Insight 2017, 2, e90233. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.E.; Tewari, K.; Lyke, K.E.; Sim BK, L.; Billingsley, P.F.; Laurens, M.B.; Gunasekera, A.; Chakravarty, S.; James, E.R.; Sedegah, M.; et al. Hoffman. Live Attenuated Malaria Vaccine Designed to Protect through Hepatic Cd8+ T Cell Immunity. Science 2011, 334, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Li, Q.; Caridha, D.; O’Neil, M.T.; Ockenhouse, C.F.; Hickman, M.; Angov, E. Protective Immune Mechanisms against Pre-Erythrocytic Forms of Plasmodium Berghei Depend on the Target Anti-gen. Trials Vaccinol. 2014, 3, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Mac-Daniel, L.; Buckwalter, M.R.; Gueirard, P.; Ménard, R. Myeloid Cell Isolation from Mouse Skin and Draining Lymph Node Following Intradermal Immunization with Live Attenuated Plasmodium Sporozoites. J. Vis. Exp. 2016, e53796. [Google Scholar] [CrossRef] [Green Version]
- Ménard, R.; Tavares, J.; Cockburn, I.; Markus, M.; Zavala, F.; Amino, R. Looking under the Skin: The First Steps in Malarial Infection and Immunity. Nat. Rev. Microbiol. 2013, 11, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.J.; Tse, S.-W.; Zavala, F. From the draining lymph node to the liver: The induction and effector mechanisms of malaria-specific CD8+ T cells. Semin. Immunopathol. 2015, 37, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Cockburn, I.; Kuk, S.; Overstreet, M.; Sacci, J.B.; Zavala, F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 2007, 13, 1035–1041. [Google Scholar] [CrossRef]
- Ishizuka, A.S.; Lyke, E.K.; DeZure, A.; Berry, A.; Richie, T.L.; Mendoza, F.H.; Enama, E.M.; Gordon, I.J.; Chang, L.-J.; Sarwar, U.N.; et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 2016, 22, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Simms, E.P.; Ellis, T.M. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin. Diagn. Lab. Immunol. 1996, 3, 301–304. [Google Scholar] [CrossRef] [Green Version]
- Hickey, B.; Teneza-Mora, N.; Lumsden, J.; Reyes, S.; Sedegah, M.; Garver, L.; Hollingdale, M.R.; Banania, J.G.; Ganeshan, H.; Dowler, M.; et al. IMRAS—A clinical trial of mosquito-bite immunization with live, radiation-attenuated P. falciparum sporozoites: Impact of immunization parameters on protective efficacy and generation of a repository of immunologic reagents. PLoS ONE 2020, 15, e0233840. [Google Scholar] [CrossRef]
- Mordmüller, B.; Supan, C.; Sim, K.L.; Gómez-Pérez, G.P.; Salazar, C.L.O.; Held, J.; Bolte, S.; Esen, M.; Tschan, S.; Joanny, F.; et al. Direct Venous Inoc-ulation of Plasmodium Falciparum Sporozoites for Controlled Human Malaria Infection: A Dose-Finding Trial in Two Centres. Malar. J. 2015, 14, 117. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.M.; Gouirand, V.; Rosenblum, M.D. T-Cell Adhesion in Healthy and Inflamed Skin. JID Innov. 2021, 1, 100014. [Google Scholar] [CrossRef]
- Billerbeck, E.; Kang, Y.-H.; Walker, L.; Lockstone, H.; Grafmueller, S.; Fleming, V.; Flint, J.; Willberg, C.B.; Bengsch, B.; Seigel, B.; et al. Analysis of Cd161 Expression on Human Cd8+ T Cells Defines a Distinct Functional Subset with Tissue-Homing Properties. Proc. Natl. Acad. Sci. USA 2010, 107, 3006–3011. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, C. The Roles of Liver-Resident Lymphocytes in Liver Diseases. Front. Immunol. 2019, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Lipp, M. Shaping up Adaptive Immunity: The Impact of Ccr7 and Cxcr5 on Lymphocyte Trafficking. Microcirculation 2003, 10, 325–334. [Google Scholar] [CrossRef]
- Tubo, N.J.; McLachlan, J.B.; Campbell, J.J. Chemokine Receptor Requirements for Epidermal T-Cell Trafficking. Am. J. Pathol. 2011, 178, 2496–2503. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.M. 10—Chemokines and Chemokine Receptors. In Clinical Immunology, 5th ed.; Robert, R.R., Thomas, A.F., William, T.S., Harry, W.S., Anthony, J.F., Cornelia, M.W., Eds.; Elsevier: London, UK, 2019; pp. 157–170.e1. [Google Scholar]
- Hart, A.L.; Ng, S.C.; Mann, E.; Al-Hassi, H.O.; Bernardo, D.; Knight, S.C. Homing of immune cells: Role in homeostasis and intestinal inflammation. Inflamm. Bowel Dis. 2010, 16, 1969–1977. [Google Scholar] [CrossRef]
- Mura, M.; Chaudhury, S.; Farooq, F.; Duncan, E.H.; Beck, K.; Bergmann-Leitner, E.S. Optimized flow cytometric protocol for the detection of functional subsets of low frequency antigen-specific CD4+ and CD8+ T cells. MethodsX 2020, 7, 101005. [Google Scholar] [CrossRef]
- Haeberlein, S.; Chevalley-Maurel, S.; Ozir-Fazalalikhan, A.; Koppejan, H.; Winkel, B.M.F.; Ramesar, J.; Khan, S.M.; Sauerwein, R.W.; Roestenberg, M.; Janse, C.J.; et al. Protective immunity differs between routes of administration of attenuated malaria parasites independent of parasite liver load. Sci. Rep. 2017, 7, 10372. [Google Scholar] [CrossRef] [Green Version]
- Nardin, E.; Zavala, F.; Nussenzweig, V.; Nussenzweig, R.S. Pre-erythrocytic malaria vaccine: Mechanisms of protective immunity and human vaccine trials. Parassitologia 1999, 41, 397–402. [Google Scholar]
- Hoffman, S.L.; Goh, L.M.L.; Luke, T.C.; Schneider, I.; Le, T.P.; Doolan, D.L.; Sacci, J.; de la Vega, P.; Dowler, M.; Paul, C.; et al. Protection of Humans against Malaria by Immunization with Radiation-Attenuated Plasmodium falciparum Sporozoites. J. Infect. Dis. 2002, 185, 1155–1164. [Google Scholar] [CrossRef] [Green Version]
- Kebaier, C.; Voza, T.; Vanderberg, J. Kinetics of Mosquito-Injected Plasmodium Sporozoites in Mice: Fewer Sporozoites Are Injected into Sporozoite-Immunized Mice. PLoS Pathog. 2009, 5, e1000399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, A.; Jyh, L.H.; Susan, N.C.; Joanna, R.G.; William, R.H.; Laura, K.M.; Scott, N.M. Chemokine Receptor–Dependent Control of Skin Tissue–Resident Memory T Cell Formation. J. Immunol. 2017, 199, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.E.; Davis, M.; Boekschoten, M.; Amsen, D.; Dascher, C.C.; Ryffel, B.; Swanson, J.; Muller, M.; Blander, J.M. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature 2011, 474, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Parmar, R.; Patel, H.; Yadav, N.; Parikh, R.; Patel, K.; Mohankrishnan, A.; Bhurani, V.; Joshi, U.; Dalai, S.K. Infectious Sporozoites of Plasmodium berghei Effectively Activate Liver CD8α+ Dendritic Cells. Front. Immunol. 2018, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, F.; Cameron, O.T.; Sidobre, S.; Manlongat, N.; Kronenberg, M.; Briskin, M.J.; Dustin, M.L.; Littman, D.R. Intravascular Immune Surveillance by CXCR6+ NKT Cells Patrolling Liver Sinusoids. PLOS Biol. 2005, 3, e113. [Google Scholar] [CrossRef] [Green Version]
- Stegmann, K.A.; Robertson, F.; Hansi, N.; Gill, U.; Pallant, C.; Christophides, T.; Pallett, L.J.; Peppa, D.; Dunn, C.; Fusai, G. Cxcr6 Marks a Novel Subset of T-Betloeomeshi Natural Killer Cells Residing in Human Liver. Sci. Rep. 2016, 6, 26157. [Google Scholar] [CrossRef] [Green Version]
- Tse, S.-W.; Radtke, A.J.; Espinosa, D.A.; Cockburn, I.; Zavala, F. The Chemokine Receptor CXCR6 Is Required for the Maintenance of Liver Memory CD8+ T Cells Specific for Infectious Pathogens. J. Infect. Dis. 2014, 210, 1508–1516. [Google Scholar] [CrossRef]
- Wiggins, B.G.; Pallett, L.J.; Li, X.; Davies, S.P.; Amin, O.E.; Gill, U.S.; Kucykowicz, S.; Patel, A.M.; Aliazis, K.; Liu, Y.S.; et al. The Human Liver Microenvironment Shapes the Homing and Function of Cd4+ T-Cell Populations. Gut 2022, 71, 1399–1411. [Google Scholar] [CrossRef]
- Wong, M.T.; Ong, D.E.H.; Lim, F.S.H.; Teng, K.W.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, S.L.; Billingsley, P.F.; James, E.; Richman, A.; Loyevsky, M.; Li, T.; Chakravarty, S.; Gunasekera, A.; Chattopadhyay, R.; Li, M.; et al. Development of a Metabolically Active, Non-Replicating Sporozoite Vaccine to Prevent Plasmodium falciparum Malaria. Hum. Vaccine 2010, 6, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Sheikh-Mohamed, S.; Sanders, E.C.; Gommerman, J.L.; Tal, M.C. Guardians of the Oral and Nasopharyngeal Galaxy: Iga and Protection against Sars-Cov-2 Infection. Immunol. Rev. 2022, 309, 75–85. [Google Scholar] [CrossRef]
- Simon, J.K.; Ramirez, K.; Cuberos, L.; Campbell, J.D.; Viret, J.F.; Muñoz, A.; Lagos, R.; Levine, M.M.; Pasetti, M.F. Mucosal IgA Responses in Healthy Adult Volunteers following Intranasal Spray Delivery of a Live Attenuated Measles Vaccine. Clin. Vaccine Immunol. 2011, 18, 355–361. [Google Scholar] [CrossRef]
- Frederick, D.R.; Goggins, J.A.; Sabbagh, L.M.; Freytag, L.C.; Clements, J.D.; McLachlan, J.B. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4+ T cells following parenteral immunization. Mucosal Immunol. 2017, 11, 549–561. [Google Scholar] [CrossRef] [PubMed]



| Marker | Receptor Type | Ligand | Function/Target Tissue |
|---|---|---|---|
| CD103 (ITGAE) | Integrin | E-cadherin | Entry into epithelial tissues including liver, skin [31] |
| CD161 (KLRB1) | C-type lectin | LLT-1 | Entry into inflamed tissue, liver (IL-17 driven) [32] |
| CD183 (CXCR3) | Chemokine receptor | CXCL9, CXCL10, CXCL11 | Entry into inflamed tissue, liver [33] |
| CD185 (CXCR5) | Chemokine receptor | CXCL13 | Entry into lymph nodes, direction towards B cell-compartment (follicles/germinal centers) [34] |
| CD186 (CXCR6) | Chemokine receptor | CXCL16 | Entry into inflamed tissue, liver [33] |
| CD196 (CCR6) | Chemokine receptor | CCL20 | Entry into inflamed tissue, Th17, skin [35] |
| CD197(CCR7) | Chemokine receptor | CCL19, CCL21 | Entry into secondary lymphoid organs [33,36], direction towards T cell compartments [34] |
| CDw199 (CCR9) | Chemokine receptor | CCL25 | Entry into intestinal tissues [37] |
| CCR10 | Chemokine receptor | CCL27, CCL28 | Entry into Skin [35] |
| Comparison | Changes | B Cells | CD4+ T Cells | CD8+ T Cells |
|---|---|---|---|---|
| T0 vs. T1 i.e., Pre- vs. Post-Immune | Upregulation | CXCR5 CCR10 | CCR10 CCR9 CD103 CD197 CXCR5 | |
| Downregulation | CD197 | CD186 | ||
| T1 vs. T2/T3 i.e., Immune vs. post-challenge | Upregulation | CCR10 CD197 | CD186 CD196 | CD186 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mura, M.; Atre, T.; Savransky, T.; Bergmann-Leitner, E.S. Differential Homing Receptor Profiles of Lymphocytes Induced by Attenuated versus Live Plasmodium falciparum Sporozoites. Vaccines 2022, 10, 1768. https://doi.org/10.3390/vaccines10101768
Mura M, Atre T, Savransky T, Bergmann-Leitner ES. Differential Homing Receptor Profiles of Lymphocytes Induced by Attenuated versus Live Plasmodium falciparum Sporozoites. Vaccines. 2022; 10(10):1768. https://doi.org/10.3390/vaccines10101768
Chicago/Turabian StyleMura, Marie, Tanmaya Atre, Tatyana Savransky, and Elke S. Bergmann-Leitner. 2022. "Differential Homing Receptor Profiles of Lymphocytes Induced by Attenuated versus Live Plasmodium falciparum Sporozoites" Vaccines 10, no. 10: 1768. https://doi.org/10.3390/vaccines10101768
APA StyleMura, M., Atre, T., Savransky, T., & Bergmann-Leitner, E. S. (2022). Differential Homing Receptor Profiles of Lymphocytes Induced by Attenuated versus Live Plasmodium falciparum Sporozoites. Vaccines, 10(10), 1768. https://doi.org/10.3390/vaccines10101768






