Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review
Abstract
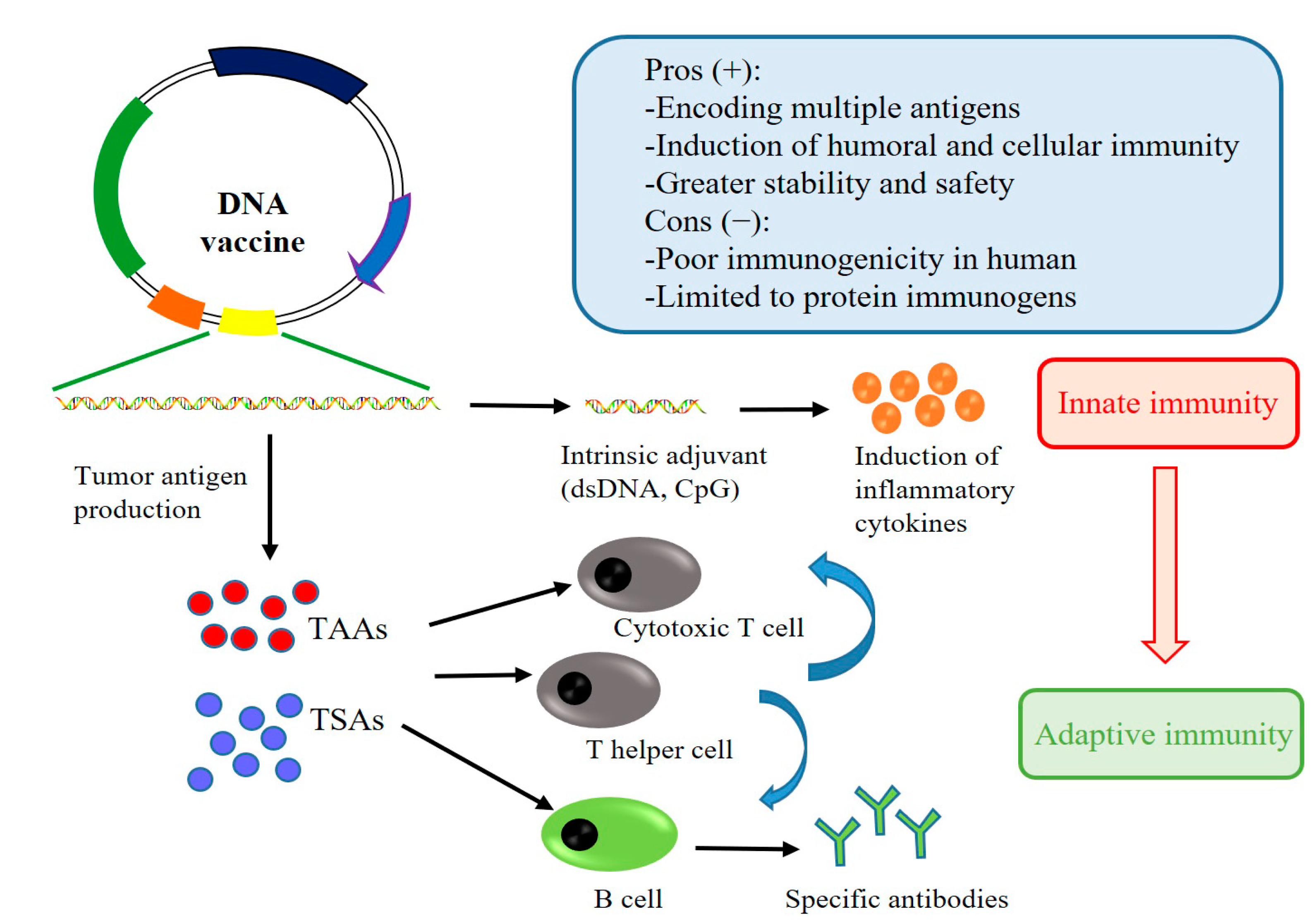
:1. Introduction
2. DNA Vaccines of Lung Cancer: Mechanisms of Immune Activation
3. Potent Antigens Selection for Lung Cancer
4. DNA Vaccine Delivery Platforms
5. Adjuvants
6. Recent Clinical Trials Based on DNA Vaccines against Lung Cancer
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Aldea, M.; Besse, B.; Planchard, D.; Reck, M.; Giaccone, G.; Soria, J.C. Small cell lung cancer: A slightly less orphan disease after immunotherapy. Ann. Oncol. 2021, 32, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Saltos, A.; Shafique, M.; Chiappori, A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front. Oncol. 2020, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Antonarelli, G.; Corti, C.; Tarantino, P.; Ascione, L.; Cortes, J.; Romero, P.; Mittendorf, E.A.; Disis, M.L.; Curigliano, G. Therapeutic cancer vaccines revamping: Technology advancements and pitfalls. Ann. Oncol. 2021, 32, 1537–1551. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Faghfuri, E.; Pourfarzi, F.; Faghfouri, A.H.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Baradaran, B. Recent developments of RNA-based vaccines in cancer immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhao, K.; Song, X.; Song, T.; Wang, X.; Zhang, X.; Yue, B.; Chu, Y. Heterologous Prime-Boost Immunization with DNA Vaccine and Modified Recombinant Proteins Enhances Immune Response against Trueperella pyogenes in Mice. Vaccines 2022, 10, 839. [Google Scholar] [CrossRef]
- Rezaei, T.; Davoudian, E.; Khalili, S.; Amini, M.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Strategies in DNA vaccine for melanoma cancer. Pigment. Cell Melanoma Res. 2021, 34, 869–891. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Pappa, A.; Chlichlia, K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol. Ther. 2016, 165, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Porter, K.R.; Raviprakash, K. DNA Vaccine Delivery and Improved Immunogenicity. Curr. Issues Mol. Biol. 2017, 22, 129–138. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Leitner, W.W. Vaccination Using Gene-Gun Technology. Methods Mol. Biol. 2015, 1325, 289–302. [Google Scholar]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Santangelo, P.J.; Prausnitz, M.R. DNA uptake, intracellular trafficking and gene transfection after ultrasound exposure. J. Control. Release 2016, 234, 1–9. [Google Scholar] [CrossRef]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grodeland, G.; Fredriksen, A.B.; Løset, G.; Vikse, E.; Fugger, L.; Bogen, B. Antigen Targeting to Human HLA Class II Molecules Increases Efficacy of DNA Vaccination. J. Immunol. 2016, 197, 3575–3585. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, M.; Hirohashi, Y.; Nakatsugawa, M.; Murai, A.; Kubo, T.; Hashimoto, S.; Tokita, S.; Murata, K.; Kanaseki, T.; Tsukahara, T.; et al. Tumor-infiltrating CD8(+) T cells recognize a heterogeneously expressed functional neoantigen in clear cell renal cell carcinoma. Cancer Immunol. Immunother. 2022, 71, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2018, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Redman, J.M.; Collins, J.M.; Bilusic, M. Cancer vaccines: Enhanced immunogenic modulation through therapeutic combinations. Hum. Vaccin. Immunother. 2017, 13, 2561–2574. [Google Scholar] [CrossRef]
- Mougel, A.; Terme, M.; Tanchot, C. Therapeutic Cancer Vaccine and Combinations With Antiangiogenic Therapies and Immune Checkpoint Blockade. Front. Immunol. 2019, 10, 467. [Google Scholar] [CrossRef]
- Weng, T.Y.; Yen, M.C.; Huang, C.T.; Hung, J.J.; Chen, Y.L.; Chen, W.C.; Wang, C.Y.; Chang, J.Y.; Lai, M.D. DNA vaccine elicits an efficient antitumor response by targeting the mutant Kras in a transgenic mouse lung cancer model. Gene Ther. 2014, 21, 888–896. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef]
- Chiu, L.C.; Lin, S.M.; Lo, Y.L.; Kuo, S.C.; Yang, C.T.; Hsu, P.C. Immunotherapy and Vaccination in Surgically Resectable Non-Small Cell Lung Cancer (NSCLC). Vaccines 2021, 9, 689. [Google Scholar] [CrossRef]
- García-Pardo, M.; Gorria, T.; Malenica, I.; Corgnac, S.; Teixidó, C.; Mezquita, L. Vaccine Therapy in Non-Small Cell Lung Cancer. Vaccines 2022, 10, 740. [Google Scholar] [CrossRef]
- Jou, J.; Harrington, K.J.; Zocca, M.B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Tagliamonte, M. Selecting Target Antigens for Cancer Vaccine Development. Vaccines 2020, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. Genomic prediction of neoantigens: Immunogenomics before NGS. Nat. Rev. Genet. 2021, 22, 550–551. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Rouleau, E.; Besse, B. Clinical utility of tumor mutational burden in patients with non-small cell lung cancer treated with immunotherapy. Transl. Lung Cancer Res. 2018, 7, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Bravaccini, S.; Bronte, G.; Ulivi, P. TMB in NSCLC: A Broken Dream? Int. J. Mol. Sci. 2021, 22, 6536. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.N.; Choi, J.; Mani, N.; Sanmamed, M.F.; Datar, I.; Sowell, R.; Du, V.Y.; Kaftan, E.; Goldberg, S.; Dong, W.; et al. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat. Commun. 2018, 9, 3196. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Creaney, J.; Redwood, A.; Robinson, B. The Current Lung Cancer Neoantigen Landscape and Implications for Therapy. J. Thorac. Oncol. 2021, 16, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, M.; Zhang, X.; Wang, Y.; Dai, L. Autoantibodies to tumor-associated antigens in lung cancer diagnosis. Adv. Clin. Chem. 2021, 103, 1–45. [Google Scholar]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Bentzen, A.K.; Marquard, A.M.; Lyngaa, R.; Saini, S.K.; Ramskov, S.; Donia, M.; Such, L.; Furness, A.J.; McGranahan, N.; Rosenthal, R.; et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 2016, 34, 1037–1045. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Smith, K.N.; Forde, P.M.; Niknafs, N.; Bhattacharya, R.; White, J.; Zhang, T.; Adleff, V.; Phallen, J.; Wali, N.; et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017, 7, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Zhou, D.; Wu, W.; Tan, W.L.; Wang, J.; Zhou, C.; Lou, Y. MHC class II restricted neoantigen peptides predicted by clonal mutation analysis in lung adenocarcinoma patients: Implications on prognostic immunological biomarker and vaccine design. BMC Genom. 2018, 19, 582. [Google Scholar] [CrossRef]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.; Jhunjhunwala, S.; Kowanetz, M.; O’Gorman, W.E.; Hegde, P.S.; Sumatoh, H.; Lee, B.H.; Nardin, A.; Becht, E.; Flynn, S.; et al. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J. Immunother. Cancer 2019, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Cheng, R.; Pan, Y.; Zhang, H.; He, Y.; Su, C.; Ren, S.; Zhou, C. Heterogeneity of neoantigen landscape between primary lesions and their matched metastases in lung cancer. Transl. Lung Cancer Res. 2020, 9, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Q.; Zhang, R.; Xie, L.; Shu, Y.; Gao, S.; Wang, P.; Su, X.; Qin, Y.; Wang, Y.; et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target. Ther. 2021, 6, 26. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Li, H.M.; Guo, K.; Yu, Z.; Feng, R.; Xu, P. Diagnostic value of protein chips constructed by lung-cancer-associated markers selected by the T7 phage display library. Thorac. Cancer 2015, 6, 469–474. [Google Scholar] [CrossRef]
- Dai, L.; Tsay, J.C.; Li, J.; Yie, T.A.; Munger, J.S.; Pass, H.; Rom, W.N.; Zhang, Y.; Tan, E.M.; Zhang, J.Y. Autoantibodies against tumor-associated antigens in the early detection of lung cancer. Lung Cancer 2016, 99, 172–179. [Google Scholar] [CrossRef]
- Dai, L.; Li, J.; Tsay, J.J.; Yie, T.A.; Munger, J.S.; Pass, H.; Rom, W.N.; Tan, E.M.; Zhang, J.Y. Identification of autoantibodies to ECH1 and HNRNPA2B1 as potential biomarkers in the early detection of lung cancer. Oncoimmunology 2017, 6, e1310359. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qu, Y.; Li, J.; Wang, X.; Wang, K.; Wang, P.; Jiang, B.H.; Zhang, J. Serological proteome analysis approach-based identification of ENO1 as a tumor-associated antigen and its autoantibody could enhance the sensitivity of CEA and CYFRA 21-1 in the detection of non-small cell lung cancer. Oncotarget 2017, 8, 36664–36673. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Song, G.; Chen, D.; Li, Y.; Liu, S.; Hu, S.; Rosa, C.; Eichinger, D.; Pino, I.; Zhu, H.; et al. Identification of Serological Biomarkers for Early Diagnosis of Lung Cancer Using a Protein Array-Based Approach. Mol. Cell. Proteom. 2017, 16, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yu, R.; Wang, H.; Yan, D.; Yuan, Q.; Ma, Y.; Slamon, D.; Hou, D.; Wang, H.; Wang, Q. Significance of tumor-associated autoantibodies in the early diagnosis of lung cancer. Clin. Respir. J. 2018, 12, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Lipok, M.; Szlachcic, A.; Kindela, K.; Czyrek, A.; Otlewski, J. Identification of a peptide antagonist of the FGF1-FGFR1 signaling axis by phage display selection. FEBS Open Bio 2019, 9, 914–924. [Google Scholar] [CrossRef]
- Zang, R.; Li, Y.; Jin, R.; Wang, X.; Lei, Y.; Che, Y.; Lu, Z.; Mao, S.; Huang, J.; Liu, C.; et al. Enhancement of diagnostic performance in lung cancers by combining CEA and CA125 with autoantibodies detection. Oncoimmunology 2019, 8, e1625689. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, L.; Li, W.; Zhou, S.; Xu, S. Diagnostic value of multiple tumor-associated autoantibodies in lung cancer. OncoTargets Ther. 2019, 12, 457–469. [Google Scholar] [CrossRef]
- Pei, L.; Liu, H.; Ouyang, S.; Zhao, C.; Liu, M.; Wang, T.; Wang, P.; Ye, H.; Wang, K.; Song, C.; et al. Discovering novel lung cancer associated antigens and the utilization of their autoantibodies in detection of lung cancer. Immunobiology 2020, 225, 151891. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, X.; Sun, Y. Functional characterization of SOX2 as an anticancer target. Signal Transduct. Target. Ther. 2020, 5, 135. [Google Scholar] [CrossRef]
- Luo, B.; Mao, G.; Ma, H.; Chen, S. The role of seven autoantibodies in lung cancer diagnosis. J. Thorac. Dis. 2021, 13, 3660–3668. [Google Scholar] [CrossRef]
- Kraynyak, K.A.; Bodles-Brakhop, A.; Bagarazzi, M. Tapping the Potential of DNA Delivery with Electroporation for Cancer Immunotherapy. Cancer Vaccines 2017, 405, 55–78. [Google Scholar]
- Lee, S.H.; Danishmalik, S.N.; Sin, J.I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vaccin. Immunother. 2015, 11, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Lione, L.; Salvatori, E.; Petrazzuolo, A.; Massacci, A.; Maggio, R.; Confroti, A.; Compagnone, M.; Aurisicchio, L.; Ciliberto, G.; Palombo, F. Antitumor efficacy of a neoantigen cancer vaccine delivered by electroporation is influenced by microbiota composition. Oncoimmunology 2021, 10, 1898832. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Hoai, T.; Pezzutto, A.; Westermann, J. Gene Gun Her2/neu DNA Vaccination: Evaluation of Vaccine Efficacy in a Syngeneic Her2/neu Mouse Tumor Model. Methods Mol. Biol. 2015, 1317, 17–37. [Google Scholar]
- Lai, M.D.; Yen, M.C.; Lin, C.M.; Tu, C.F.; Wang, C.C.; Lin, P.S.; Yang, H.J.; Lin, C.C. The effects of DNA formulation and administration route on cancer therapeutic efficacy with xenogenic EGFR DNA vaccine in a lung cancer animal model. Genet. Vaccines Ther. 2009, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sethi, T.K.; Taylor, D.; Kim, Y.J.; Johnson, D.B. Breakthrough concepts in immune-oncology: Cancer vaccines at the bedside. J. Leukoc. Biol. 2020, 108, 1455–1489. [Google Scholar] [CrossRef] [PubMed]
- Eby, J.M.; Barse, L.; Henning, S.W.; Rabelink, M.J.; Klarquist, J.; Gilbert, E.R.; Hammer, A.M.; Fernandez, M.F.; Yung, N.; Khan, S.; et al. Alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 1 can support immune responses toward tumors overexpressing ganglioside D3 in mice. Cancer Immunol. Immunother. 2017, 66, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Garza-Morales, R.; Perez-Trujillo, J.J.; Martinez-Jaramillo, E.; Saucedo-Cardenas, O.; Loera-Arias, M.J.; Garcia-Garcia, A.; Rodriguez-Rocha, H.; Yolcu, E.; Shirwan, H.; Gomez-Gutierrez, J.G.; et al. A DNA Vaccine Encoding SA-4-1BBL Fused to HPV-16 E7 Antigen Has Prophylactic and Therapeutic Efficacy in a Cervical Cancer Mouse Model. Cancers 2019, 11, 96. [Google Scholar] [CrossRef]
- Imai, T. Single Amino Acid Deletion at N-Terminus of the Target Antigen in DNA Vaccine Induces Altered CD8(+) T Cell Responses against Tumor Antigen. Vaccines 2021, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Khan, N.; Jayandharan, G.R. Vector engineering, strategies and targets in cancer gene therapy. Cancer Gene Ther. 2022, 29, 402–417. [Google Scholar] [CrossRef]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, X.; Wu, Y.; Cao, P.; Movahedi, F.; Liu, J.; Wang, J.; Xu, Z.P.; Gu, W. Mannose-Functionalized Biodegradable Nanoparticles Efficiently Deliver DNA Vaccine and Promote Anti-tumor Immunity. ACS Appl. Mater. Interfaces 2021, 13, 14015–14027. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Banik, B.; Pathak, R.K.; Kumar, A.; Kolishetti, N.; Dhar, S. Nanotechnology inspired tools for mitochondrial dysfunction related diseases. Adv. Drug Deliv. Rev. 2016, 99, 52–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, Y.; Tokunaga, A.; Hashizume, J.; Nakagawa, H.; Harasawa, H.; Kurosaki, T.; Nakamura, T.; Nishida, K.; Nakashima, M.; Hashida, M.; et al. Evaluation of transgene expression characteristics and DNA vaccination against melanoma metastasis of an intravenously injected ternary complex with biodegradable dendrigraft poly-L-lysine in mice. Drug Deliv. 2021, 28, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.X.; Huang, L.; Gauthier, M.; Yang, G.; Wang, Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine 2016, 11, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Canão, F.; Ferreira, H.; Neves, N.M. Liposomal formulations for lung cancer treatment in the last two decades: A systematic review. J. Cancer Res. Clin. Oncol. 2022, 148, 2375–2386. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Tie, Y.; Zheng, H.; He, Z.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X.; et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020, 5, 6. [Google Scholar] [CrossRef]
- Lee, S.; Trinh, T.H.T.; Yoo, M.; Shin, J.; Lee, H.; Kim, J.; Hwang, E.; Lim, Y.B.; Ryou, C. Self-Assembling Peptides and Their Application in the Treatment of Diseases. Int. J. Mol. Sci. 2019, 20, 5850. [Google Scholar] [CrossRef]
- Zhu, L.; Shi, Y.; Xiong, Y.; Ba, L.; Li, Q.; Qiu, M.; Zou, Z.; Peng, G. Emerging self-assembling peptide nanomaterial for anti-cancer therapy. J. Biomater. Appl. 2021, 36, 882–901. [Google Scholar] [CrossRef] [PubMed]
- Osman, G.; Rodriguez, J.; Chan, S.Y.; Chisholm, J.; Duncan, G.; Kim, N.; Tatler, A.L.; Shakesheff, K.M.; Hanes, J.; Suk, J.S.; et al. PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J. Control. Release 2018, 285, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, W.; Wu, X.; Zhang, N.; Zhang, Y.; Ouyang, S.; Song, X.; Fang, X.; Seeram, R.; Xue, W.; et al. Functional Self-Assembling Peptide Nanofiber Hydrogels Designed for Nerve Degeneration. ACS Appl. Mater. Interfaces 2016, 8, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Rimner, A.; Weder, W.; Azzoli, C.G.; Kris, M.G.; Cascone, T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat. Rev. Clin. Oncol. 2021, 18, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Soh, J. Adjuvant therapy of operable nonsmall cell lung cancer: An update. Curr. Opin. Oncol. 2021, 33, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Gadgeel, S.M. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Bertaglia, V.; Reale, M.L.; Delcuratolo, M.D.; Tabbò, F.; Olmetto, E.; Capelletto, E.; Bironzo, P.; Novello, S. Major breakthroughs in lung cancer adjuvant treatment: Looking beyond the horizon. Cancer Treat. Rev. 2021, 101, 102308. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Shetab Boushehri, M.A.; Lamprecht, A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Ammi, R.; De Waele, J.; Willemen, Y.; Van Brussel, I.; Schrijvers, D.M.; Lion, E.; Smits, E.L. Poly(I:C) as cancer vaccine adjuvant: Knocking on the door of medical breakthroughs. Pharmacol. Ther. 2015, 146, 120–131. [Google Scholar] [CrossRef]
- Vacchelli, E.; Galluzzi, L.; Eggermont, A.; Fridman, W.H.; Galon, J.; Sautès-Fridman, C.; Tartour, E.; Zitvogel, L.; Kroemer, G. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 2012, 1, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D. Development of the CpG Adjuvant 1018: A Case Study. Methods Mol. Biol. 2017, 1494, 15–27. [Google Scholar] [PubMed]
- Topper, M.J.; Vaz, M.; Chiappinelli, K.B.; DeStefano Shields, C.E.; Niknafs, N.; Yen, R.C.; Wenzel, A.; Hicks, J.; Ballew, M.; Stone, M.; et al. Epigenetic Therapy Ties MYC Depletion to Reversing Immune Evasion and Treating Lung Cancer. Cell 2017, 171, 1284–1300.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e47. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, T.; Khalili, S.; Baradaran, B.; Mosafer, J.; Rezaei, S.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on HIV DNA vaccines development: Stepwise improvements to clinical trials. J. Control. Release 2019, 316, 116–137. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Li, W.A.; Mooney, D.J.; Dranoff, G. Advances in Therapeutic Cancer Vaccines. Adv. Immunol. 2016, 130, 191–249. [Google Scholar] [PubMed]
- Liu, Y.; Geng, Y.; Yue, B.; Lo, P.C.; Huang, J.; Jin, H. Injectable Hydrogel as a Unique Platform for Antitumor Therapy Targeting Immunosuppressive Tumor Microenvironment. Front. Immunol. 2021, 12, 832942. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Oh, J.E.; Hong, J.; Chung, Y.; Lee, Y.; Park, K.D.; Kim, S.; Yun, C.O. Optimized biodegradable polymeric reservoir-mediated local and sustained co-delivery of dendritic cells and oncolytic adenovirus co-expressing IL-12 and GM-CSF for cancer immunotherapy. J. Control. Release 2017, 259, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Nimanong, S.; Ostroumov, D.; Wingerath, J.; Knocke, S.; Woller, N.; Gürlevik, E.; Falk, C.S.; Manns, M.P.; Kühnel, F.; Wirth, T.C. CD40 Signaling Drives Potent Cellular Immune Responses in Heterologous Cancer Vaccinations. Cancer Res. 2017, 77, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. cGAS-STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.H.; O’Reilly, E.M.; Varadhachary, G.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: An open-label, multicentre, phase 1b study. Lancet Oncol. 2021, 22, 118–131. [Google Scholar] [CrossRef]
- Weiss, S.A.; Djureinovic, D.; Jessel, S.; Krykbaeva, I.; Zhang, L.; Jilaveanu, L.; Ralabate, A.; Johnson, B.; Levit, N.S.; Anderson, G.; et al. A Phase I Study of APX005M and Cabiralizumab with or without Nivolumab in Patients with Melanoma, Kidney Cancer, or Non-Small Cell Lung Cancer Resistant to Anti-PD-1/PD-L1. Clin. Cancer Res. 2021, 27, 4757–4767. [Google Scholar] [CrossRef] [PubMed]
- Dubensky, T.W., Jr.; Kanne, D.B.; Leong, M.L. Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants. Ther. Adv. Vaccines 2013, 1, 131–143. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Motedayen Aval, L.; Pease, J.E.; Sharma, R.; Pinato, D.J. Challenges and Opportunities in the Clinical Development of STING Agonists for Cancer Immunotherapy. J. Clin. Med. 2020, 9, 3323. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Carboni, S.; Di Berardino-Besson, W.; Riva, E.; Santiago-Raber, M.L.; Belnoue, E.; Derouazi, M. STING Agonist Combined to a Protein-Based Cancer Vaccine Potentiates Peripheral and Intra-Tumoral T Cell Immunity. Front. Immunol. 2021, 12, 695056. [Google Scholar] [CrossRef]
- Xue, A.; Shang, Y.; Jiao, P.; Zhang, S.; Zhu, C.; He, X.; Feng, G.; Fan, S. Increased activation of cGAS-STING pathway enhances radiosensitivity of non-small cell lung cancer cells. Thorac. Cancer 2022, 13, 1361–1368. [Google Scholar] [CrossRef]
- Yin, M.; Hu, J.; Yuan, Z.; Luo, G.; Yao, J.; Wang, R.; Liu, D.; Cao, B.; Wu, W.; Hu, Z. STING agonist enhances the efficacy of programmed death-ligand 1 monoclonal antibody in breast cancer immunotherapy by activating the interferon-β signalling pathway. Cell Cycle 2022, 21, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.D.; Poh, C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019, 50, 78. [Google Scholar] [CrossRef] [PubMed]
- Redding, L.; Weiner, D.B. DNA vaccines in veterinary use. Expert Rev. Vaccines 2009, 8, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, S.; Getz, K.A.; Blazynski, C. Evaluating the Completeness of ClinicalTrials.gov. Ther. Innov. Regul. Sci. 2019, 53, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Fenerty, K.E.; Patronas, N.J.; Heery, C.R.; Gulley, J.L.; Folio, L.R. Resources Required for Semi-Automatic Volumetric Measurements in Metastatic Chordoma: Is Potentially Improved Tumor Burden Assessment Worth the Time Burden? J. Digit. Imaging 2016, 29, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heery, C.R.; Palena, C.; McMahon, S.; Donahue, R.N.; Lepone, L.M.; Grenga, I.; Dirmeier, U.; Cordes, L.; Marté, J.; Dahut, W.; et al. Phase I Study of a Poxviral TRICOM-Based Vaccine Directed Against the Transcription Factor Brachyury. Clin. Cancer Res. 2017, 23, 6833–6845. [Google Scholar] [CrossRef]
- Gamat-Huber, M.; Jeon, D.; Johnson, L.E.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; Rastogi, I.; Wargowski, E.; Zahm, C.D.; McNeel, D.G. Treatment Combinations with DNA Vaccines for the Treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC). Cancers 2020, 12, 2831. [Google Scholar] [CrossRef]
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune Microenvironment in Osteosarcoma: Components, Therapeutic Strategies and Clinical Applications. Front. Immunol. 2022, 13, 907550. [Google Scholar] [CrossRef]
- Weber, J.S.; Vogelzang, N.J.; Ernstoff, M.S.; Goodman, O.B.; Cranmer, L.D.; Marshall, J.L.; Miles, S.; Rosario, D.; Diamond, D.C.; Qiu, Z.; et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J. Immunother. 2011, 34, 556–567. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 8 August 2022).
- PubMed.gov. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 8 September 2022).
- Otsuka, T.; Nishida, S.; Shibahara, T.; Temizoz, B.; Hamaguchi, M.; Shiroyama, T.; Kimura, K.; Miyake, K.; Hirata, H.; Mizuno, Y.; et al. CpG ODN (K3)-toll-like receptor 9 agonist-induces Th1-type immune response and enhances cytotoxic activity in advanced lung cancer patients: A phase I study. BMC Cancer 2022, 22, 744. [Google Scholar] [CrossRef]
- Waki, K.; Yokomizo, K.; Yoshiyama, K.; Takamori, S.; Komatsu, N.; Yamada, A. Integrity of circulating cell-free DNA as a prognostic biomarker for vaccine therapy in patients with nonsmall cell lung cancer. Immunopharmacol. Immunotoxicol. 2021, 43, 176–182. [Google Scholar] [CrossRef]
| No. of Potential NEOANTIGENS | Patient | Parameter (s) | Identified Year | References |
|---|---|---|---|---|
| 8–610 | 34 | MHC-I binding affinity | 2015 | [39] |
| 288–417 | 2 | MHC-I binding affinity | 2016 | [40] |
| 80–741 | 7 | MHC-I binding affinity | 2016 | [41] |
| 102–316 | 4 | MHC-I binding affinitySelf-similarity | 2017 | [42] |
| 12 | 147 | MHC-II binding affinity | 2018 | [43] |
| 1–219 | 12 | MHC-I binding affinityAntigen processing | 2018 | [44] |
| 1–139 | 14 | MHC-I binding affinity | 2019 | [45] |
| 54–2992 | 20 | MHC-I binding affinity | 2020 | [46] |
| 12–30 | 12 | MHC-I/MHC-II binding affinity | 2021 | [47] |
| 225 | 24 | MHC-I/MHC-II binding affinity | 2021 | [48] |
| TAAs | Gene Function | Histology | Identified Year | References |
|---|---|---|---|---|
| OLFM1 | Suppress cell growth and metastasis | LC | 2015 | [49] |
| SQLE | Cell proliferation and metastasis | LC | 2015 | [49] |
| c-Myc | Cell growth and metabolism | LC | 2016 | [50] |
| HNRNPA2B1 | mRNA metabolism and transport | LC | 2017 | [51] |
| ENO1 | Regulate cell proliferation and metastasis | NSCLC | 2017 | [52] |
| P53 | Inducing cell cycle arrest, and DNA repair | LC | 2017 | [53] |
| GBU4-5 | Cell growth and division | SCLC | 2018 | [54] |
| IGFBP-1 | Cell migration | LC | 2019 | [38] |
| FGFR1 | stem cell leukemia/lymphoma syndrome | LC | 2019 | [55] |
| CA125 | Cell adhesion, migration, and invasion | NSCLC | 2019 | [56] |
| GAGE7 | Influence cancer progression | NSCLC | 2019 | [57] |
| TOP2A | Metabolism for proteins and DNA damage | LC | 2020 | [58] |
| SOX2 | Cell proliferation, metastasis, and drug resistance | LC | 2020 | [59] |
| CAGE | Cell cycle, growth, and proliferation | NSCLC | 2021 | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Liu, L.; Lv, Z.; Zhao, K.; Yi, Q.; Zhang, J. Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review. Vaccines 2022, 10, 1586. https://doi.org/10.3390/vaccines10101586
Huang T, Liu L, Lv Z, Zhao K, Yi Q, Zhang J. Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review. Vaccines. 2022; 10(10):1586. https://doi.org/10.3390/vaccines10101586
Chicago/Turabian StyleHuang, Ting, Li Liu, Zheng Lv, Kelei Zhao, Qiong Yi, and Jing Zhang. 2022. "Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review" Vaccines 10, no. 10: 1586. https://doi.org/10.3390/vaccines10101586
APA StyleHuang, T., Liu, L., Lv, Z., Zhao, K., Yi, Q., & Zhang, J. (2022). Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review. Vaccines, 10(10), 1586. https://doi.org/10.3390/vaccines10101586






