Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine
Abstract
:1. Introduction
2. Experimental Section
2.1. Samples, Receptors, and Biosensors
2.2. Sample and Biosensor Preparation
2.3. Biolayer Interferometry Quantification Assay
2.3.1. Biosensors, Receptors, and VP7 Antibodies
2.3.2. Quantification Assay Analysis
2.4. Statistical Analysis
3. Results and Discussion
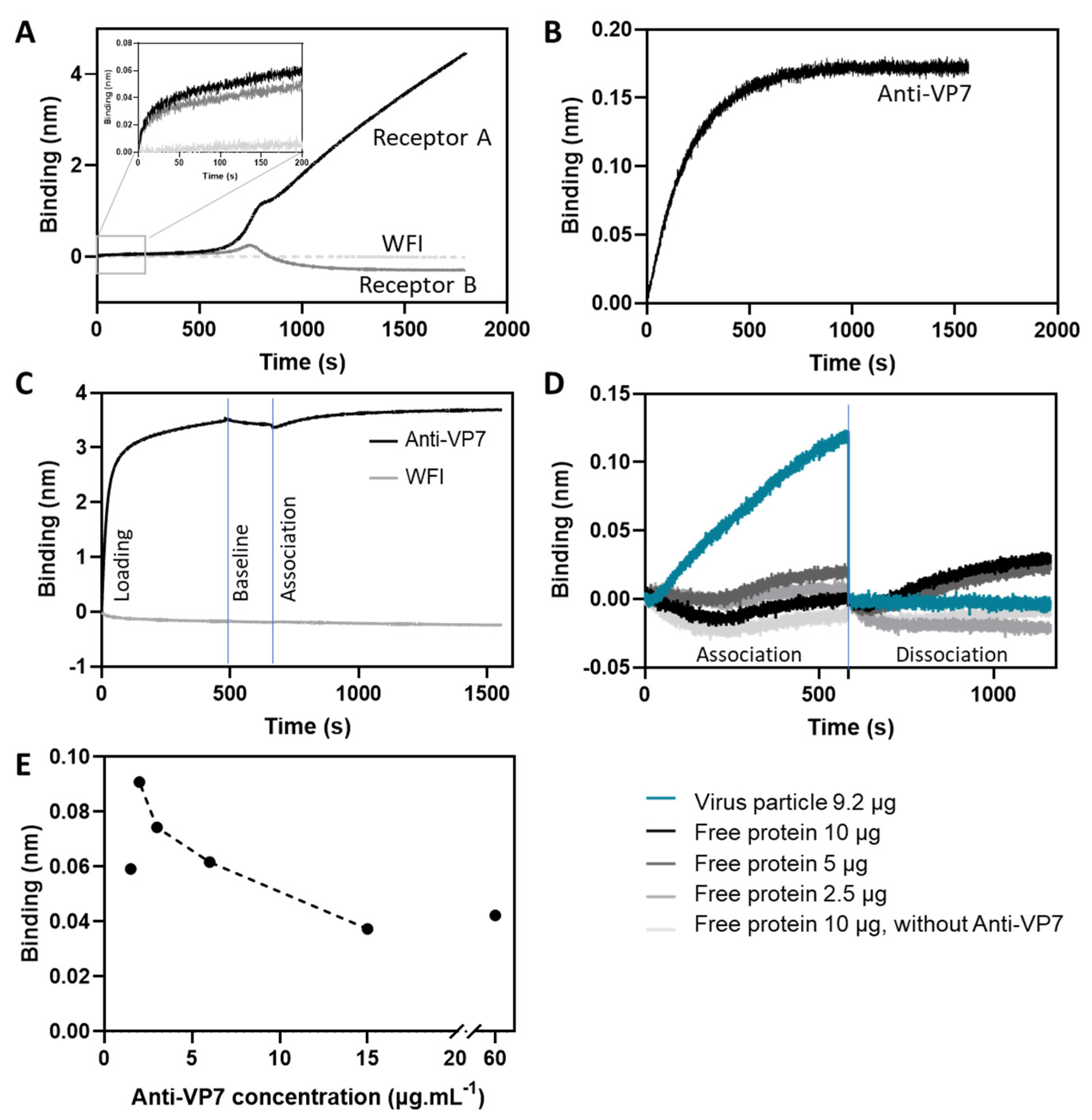
3.1. Criteria Definition and Optimization for Assay Implementation
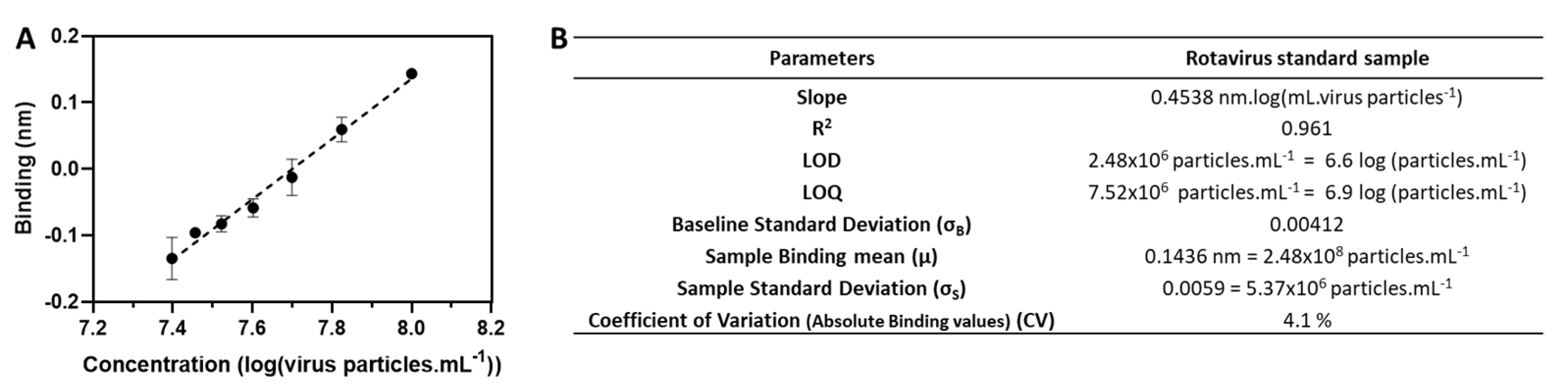
3.2. Assay Performance
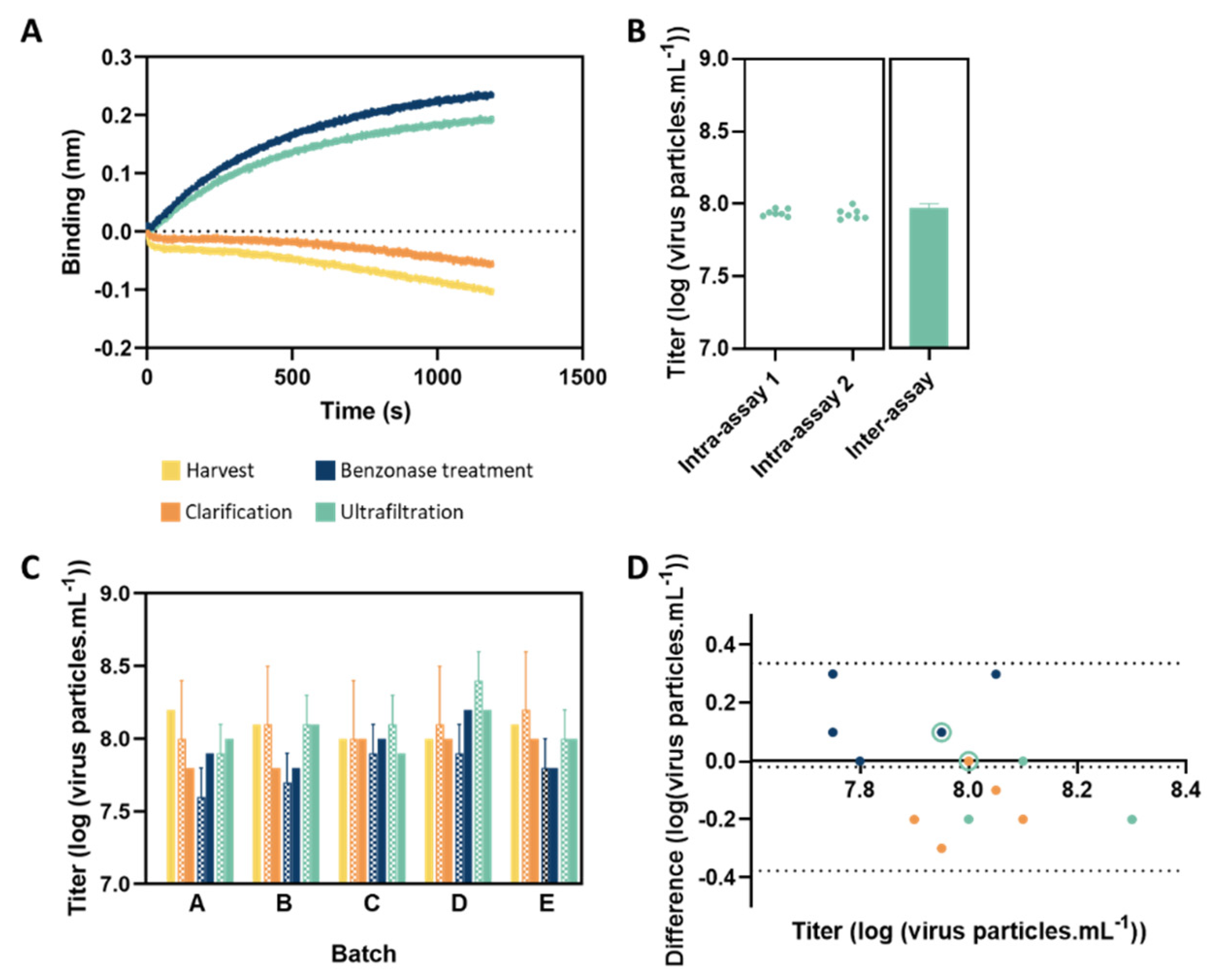
3.3. Analysis of In-Process Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958. [Google Scholar] [CrossRef] [PubMed]
- Hallowell, B.D.; Chavers, T.; Parashar, U.; Tate, J.E. Global Estimates of Rotavirus Hospitalizations Among Children Below 5 Years in 2019 and Current and Projected Impacts of Rotavirus Vaccination. J. Pediatric. Infect Dis. Soc. 2022, 11, 149–158. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Vaccine in National Immunization Programme Update. Available online: Www.Who.Int/Immunization/Monitoring_surveillance/VaccineIntroStatus.Pptx?Ua=1 (accessed on 20 January 2022).
- Burke, R.M.; Tate, J.E.; Kirkwood, C.D.; Steele, A.D.; Parashar, U.D. Current and New Rotavirus Vaccines. Curr. Opin. Infect. Dis. 2019, 32, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Buttery, J.P.; Kirkwood, C. Rotavirus Vaccine Implementation: Evidence to Fill the Gap? Lancet Glob. Health 2021, 9, e885–e886. [Google Scholar] [CrossRef]
- Newall, A.T.; Leong, R.N.; Reyes, J.F.; Curns, A.T.; Rudd, J.; Tate, J.; Macartney, K.; Parashar, U. Rotavirus Vaccination Likely to Be Cost Saving to Society in the United States. Clin. Infect. Dis. 2021, 73, 1424–1430. [Google Scholar] [CrossRef]
- Burke, R.M.; Tate, J.E.; Parashar, U.D. Global Experience With Rotavirus Vaccines. J. Infect Dis. 2021, 224, S792–S800. [Google Scholar] [CrossRef]
- Karafillakis, E.; Hassounah, S.; Atchison, C. Effectiveness and Impact of Rotavirus Vaccines in Europe, 2006–2014. Vaccine 2015, 33, 2097–2107. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Ma, L.-F.; Carey, M.E.; Steele, A.D. The Rotavirus Vaccine Development Pipeline. Vaccine 2019, 37, 7328–7335. [Google Scholar] [CrossRef]
- Kollaritsch, H.; Kundi, M.; Giaquinto, C.; Paulke-Korinek, M. Rotavirus Vaccines: A Story of Success. Clin. Microbiol. Infect. 2015, 21, 735–743. [Google Scholar] [CrossRef]
- Snelling, T.L.; Andrews, R.M.; Kirkwood, C.D.; Culvenor, S.; Carapetis, J.R. Case-Control Evaluation of the Effectiveness of the G1P [8] Human Rotavirus Vaccine during an Outbreak of Rotavirus G2P [4] Infection in Central Australia. Clin. Infect. Dis. 2011, 52, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.E.; Ngabo, F.; Donnen, P.; Gatera, M.; Uwimana, J.; Rugambwa, C.; Mwenda, J.M.; Parashar, U.D. Effectiveness of Pentavalent Rotavirus Vaccine Under Conditions of Routine Use in Rwanda. Clin. Infect. Dis. 2016, 62, S208–S212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Liu, P.; Zhu, F. The Performance of Licensed Rotavirus Vaccines and the Development of a New Generation of Rotavirus Vaccines: A Review. Hum. Vaccines Immunother. 2021, 17, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, G.; Särnefält, A.; Kumar, A. Considerations for Bioanalytical Characterization and Batch Release of COVID-19 Vaccines. NPJ Vaccines 2021, 6, 53. [Google Scholar] [CrossRef]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Ranheim, T.; Mathis, P.K.; Joelsson, D.B.; Smith, M.E.; Campbell, K.M.; Lucas, G.; Barmat, S.; Melissen, E.; Benz, R.; Lewis, J.A.; et al. Development and Application of a Quantitative RT-PCR Potency Assay for a Pentavalent Rotavirus Vaccine (RotaTeq®). J. Virol. Methods 2006, 131, 193–201. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Moleirinho, M.G.; Wheatley, D.; Welsh, J.; Gantier, R.; Alves, P.M.; Peixoto, C.; Carrondo, M.J.T. Universal Label-Free in-Process Quantification of Influenza Virus-like Particles. Biotechnol. J. 2017, 12, 1700031. [Google Scholar] [CrossRef]
- Durous, L.; Julien, T.; Padey, B.; Traversier, A.; Rosa-Calatrava, M.; Blum, L.J.; Marquette, C.A.; Petiot, E. SPRi-Based Hemagglutinin Quantitative Assay for Influenza Vaccine Production Monitoring. Vaccine 2019, 37, 1614–1621. [Google Scholar] [CrossRef]
- Nilsson, C.E.; Abbas, S.; Bennemo, M.; Larsson, A.; Hämäläinen, M.D.; Frostell-Karlsson, Å. A Novel Assay for Influenza Virus Quantification Using Surface Plasmon Resonance. Vaccine 2010, 28, 759–766. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology (CPMP/ICH/381/95); European Medicines Agency: Amsterdam, The Netherlands, 1995.
- Food and Drug Administration. ICH Guidance for Industry, Q2B Validation of Analytical Procedures: Methodology (FDA-1996-D-0169); Food and Drug Administration: Silver Spring, ML, USA, 1996.
- Bland, J.; Altman, D. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The Sweet Spot: Defining Virus–Sialic Acid Interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Bodle, J.; Verity, E.E.; Ong, C.; Vandenberg, K.; Shaw, R.; Barr, I.G.; Rockman, S. Development of an Enzyme-Linked Immunoassay for the Quantitation of Influenza Haemagglutinin: An Alternative Method to Single Radial Immunodiffusion. Influenza Other Respir. Viruses 2013, 7, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; King, L.R.; Manischewitz, J.; Coyle, E.M.; Golding, H. Novel Antibody-Independent Receptor-Binding SPR-Based Assay for Rapid Measurement of Influenza Vaccine Potency. Vaccine 2014, 32, 2188–2197. [Google Scholar] [CrossRef] [PubMed]
- Vachieri, S.G.; Xiong, X.; Collins, P.J.; Walker, P.A.; Martin, S.R.; Haire, L.F.; Zhang, Y.; McCauley, J.W.; Gamblin, S.J.; Skehel, J.J. Receptor Binding by H10 Influenza Viruses. Nature 2014, 511, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Pall Life Sciences Biomolecular Binding Kinetics Assays on the Octet Platform. Application Note 14. Available online: http://separations.co.za/wp-content/uploads/2017/05/OCTET.pdf (accessed on 31 July 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, S.B.; Dias, M.M.; Matheise, J.-P.; Knott, I.; Gomes-Alves, P.; Alves, P.M. Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine. Vaccines 2022, 10, 1585. https://doi.org/10.3390/vaccines10101585
Carvalho SB, Dias MM, Matheise J-P, Knott I, Gomes-Alves P, Alves PM. Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine. Vaccines. 2022; 10(10):1585. https://doi.org/10.3390/vaccines10101585
Chicago/Turabian StyleCarvalho, Sofia B., Mafalda M. Dias, Jean-Philippe Matheise, Isabelle Knott, Patrícia Gomes-Alves, and Paula M. Alves. 2022. "Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine" Vaccines 10, no. 10: 1585. https://doi.org/10.3390/vaccines10101585
APA StyleCarvalho, S. B., Dias, M. M., Matheise, J.-P., Knott, I., Gomes-Alves, P., & Alves, P. M. (2022). Biolayer Interferometry Analysis for a Higher Throughput Quantification of In-Process Samples of a Rotavirus Vaccine. Vaccines, 10(10), 1585. https://doi.org/10.3390/vaccines10101585





