The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Laboratory Analysis
2.4. Statistical Analysis
2.5. Ethics Approval
3. Results
3.1. Characteristics of the Study Participants
3.2. Prevalence of SARS-CoV-2 Infection among HCWs
3.3. The SARS-CoV-2 Infection Risk Factors among HCWs
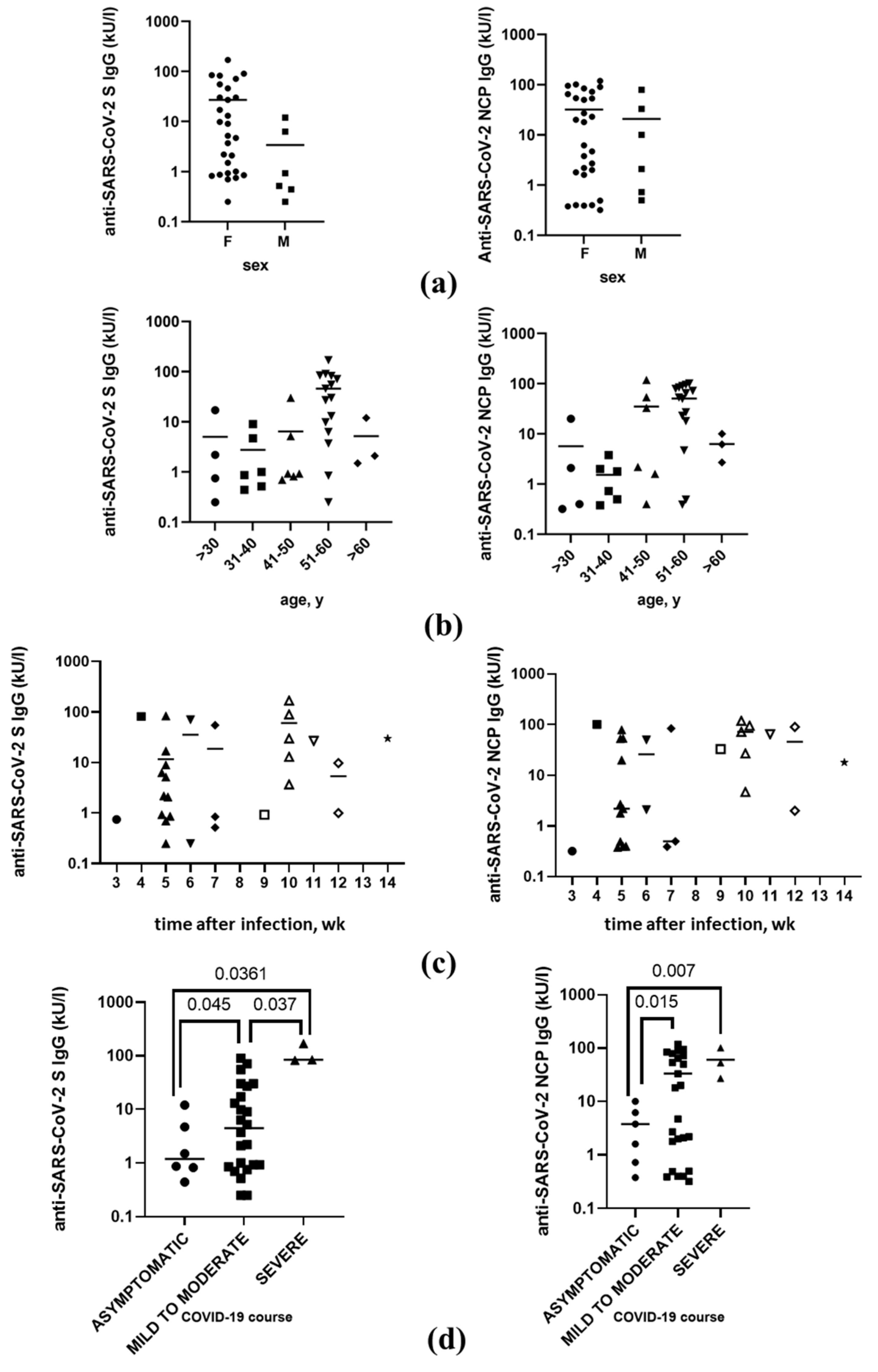
3.4. Anti-SARS-CoV-2 Antibodies Level after the Infection
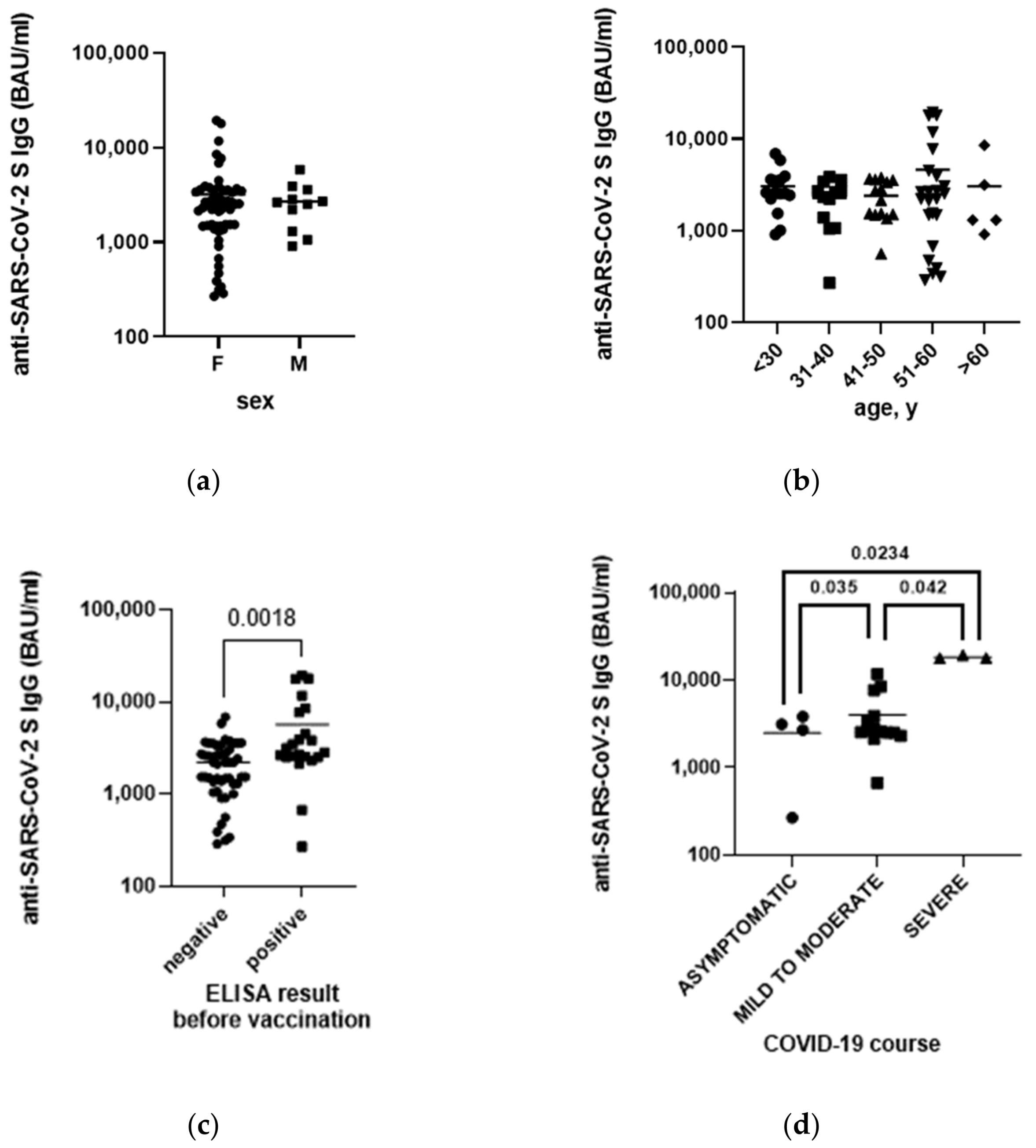
3.5. Anti-SARS-CoV-2 Antibodies Level after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Coronavirus. Available online: https://www.who.int/health-topics/coronavirus (accessed on 25 July 2022).
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 Antibodies and Associated Factors in Healthcare Workers: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef]
- Lorent, D.; Nowak, R.; Roxo, C.; Lenartowicz, E.; Makarewicz, A.; Zaremba, B.; Nowak, S.; Kuszel, L.; Stefaniak, J.; Kierzek, R.; et al. Prevalence of Anti-SARS-CoV-2 Antibodies in Poznań, Poland, after the First Wave of the COVID-19 Pandemic. Vaccines 2021, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 Vaccine: Where Are We Now and Where Should We Go? Expert Rev. Vaccines 2021, 20, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A Comprehensive Status Report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, I.-T.; Chao, C.-M.; Lee, P.-I.; Ko, W.-C.; Hsueh, P.-R. COVID-19 Vaccines: Concerns beyond Protective Efficacy and Safety. Expert Rev. Vaccines 2021, 20, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Peng, Y.; Shen, E.; Huang, Q.; Chen, Y.; Liu, P.; Guo, C.; Feng, Z.; Gao, L.; Zhang, X.; et al. A Comprehensive Analysis of the Efficacy and Safety of COVID-19 Vaccines. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2794–2805. [Google Scholar] [CrossRef] [PubMed]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- MRNA-1273 Vaccine (Moderna) against COVID-19 Background Document (Draft). Available online: https://www.who.int/publications-detail-redirect/mrna-1273-vaccine-(moderna)-against-covid-19-background-document-(draft) (accessed on 25 July 2022).
- Background Document on MRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available online: https://www.who.int/publications-detail-redirect/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 25 July 2022).
- The Role of Community Health Workers in COVID-19 Vaccination: Implementation Support Guide. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-NDVP-CHWs-role-2021.1 (accessed on 25 July 2022).
- Narodowy Program Szczepień Przeciw COVID-19—Szczepienie Przeciwko COVID-19—Portal Gov.pl. Available online: https://www.gov.pl/web/szczepimysie/narodowy-program-szczepien-przeciw-covid-19 (accessed on 22 August 2022).
- Mapa Zarażeń—COVID-HUB. Available online: https://covidhub.psnc.pl/mapa/ (accessed on 25 July 2022).
- Zaszczepionych 93,24% Lekarzy. Available online: http://www.mp.pl/social/article/277067 (accessed on 22 August 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of MRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Kasztelewicz, B.; Janiszewska, K.; Burzyńska, J.; Szydłowska, E.; Migdał, M.; Dzierżanowska-Fangrat, K. Prevalence of IgG Antibodies against SARS-CoV-2 among Healthcare Workers in a Tertiary Pediatric Hospital in Poland. PLoS ONE 2021, 16, e0249550. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, R.J.; Woźniak-Grygiel, E.; Wąsik, M.; Kasperczyk, J.; Gawrylak-Dryja, E.; Mond-Paszek, R.; Konka, A.; Badura-Brzoza, K.; Fronczek, M.; Golec, M.; et al. SARS-CoV-2 Antibody Screening in Healthcare Workers in Non-Infectious Hospitals in Two Different Regions of Southern Poland (Upper Silesia and Opole Voivodeships): A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4376. [Google Scholar] [CrossRef] [PubMed]
- Korona-Głowniak, I.; Mielnik, M.; Podgajna, M.; Grywalska, E.; Hus, M.; Matuska, K.; Wojtysiak-Duma, B.; Duma, D.; Glowniak, A.; Malm, A. SARS-CoV-2 Seroprevalence in Healthcare Workers before the Vaccination in Poland: Evolution from the First to the Second Pandemic Outbreak. Int. J. Environ. Res. Public Health 2022, 19, 2319. [Google Scholar] [CrossRef]
- Piccoli, L.; Ferrari, P.; Piumatti, G.; Jovic, S.; Rodriguez, B.F.; Mele, F.; Giacchetto-Sasselli, I.; Terrot, T.; Silacci-Fregni, C.; Cameroni, E.; et al. Risk Assessment and Seroprevalence of SARS-CoV-2 Infection in Healthcare Workers of COVID-19 and Non-COVID-19 Hospitals in Southern Switzerland. Lancet Reg. Health—Eur. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M.; et al. SARS-CoV-2-Specific Antibody Detection in Healthcare Workers in Germany with Direct Contact to COVID-19 Patients. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 128, 104437. [Google Scholar] [CrossRef]
- Psichogiou, M.; Karabinis, A.; Pavlopoulou, I.D.; Basoulis, D.; Petsios, K.; Roussos, S.; Pratikaki, M.; Jahaj, E.; Protopapas, K.; Leontis, K.; et al. Antibodies against SARS-CoV-2 among Health Care Workers in a Country with Low Burden of COVID-19. PLoS ONE 2020, 15, e0243025. [Google Scholar] [CrossRef]
- Brant-Zawadzki, M.; Fridman, D.; Robinson, P.A.; Zahn, M.; Chau, C.; German, R.; Breit, M.; Bock, J.R.; Hara, J. SARS-CoV-2 Antibody Prevalence in Health Care Workers: Preliminary Report of a Single Center Study. PLoS ONE 2020, 15, e0240006. [Google Scholar] [CrossRef]
- Brant-Zawadzki, M.; Fridman, D.; Robinson, P.A.; Zahn, M.; Chau, C.; German, R.; Breit, M.; Burke, E.; Bock, J.R.; Hara, J. Prevalence and Longevity of SARS-CoV-2 Antibodies Among Health Care Workers. Open Forum Infect. Dis. 2021, 8, ofab015. [Google Scholar] [CrossRef]
- Bogogiannidou, Z.; Vontas, A.; Dadouli, K.; Kyritsi, M.A.; Soteriades, S.; Nikoulis, D.J.; Mouchtouri, V.A.; Koureas, M.; Kazakos, E.I.; Spanos, E.G.; et al. Repeated Leftover Serosurvey of SARS-CoV-2 IgG Antibodies, Greece, March and April 2020. Eurosurveillance 2020, 25, 2001369. [Google Scholar] [CrossRef] [PubMed]
- Menachemi, N. Population Point Prevalence of SARS-CoV-2 Infection Based on a Statewide Random Sample—Indiana, April 25–29, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.; Cieslak, P.; Linder, M. Notes from the Field: Seroprevalence Estimates of SARS-CoV-2 Infection in Convenience Sample—Oregon, May 11–June 15, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1100–1101. [Google Scholar] [CrossRef] [PubMed]
- Zejda, J.E.; Brożek, G.M.; Kowalska, M.; Barański, K.; Kaleta-Pilarska, A.; Nowakowski, A.; Xia, Y.; Buszman, P. Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Random Sample of Inhabitants of the Katowice Region, Poland. Int. J. Environ. Res. Public Health 2021, 18, 3188. [Google Scholar] [CrossRef]
- Benenson, S.; Oster, Y.; Cohen, M.J.; Nir-Paz, R. BNT162b2 MRNA Covid-19 Vaccine Effectiveness among Health Care Workers. N. Engl. J. Med. 2021, 384, 1775–1777. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S.; Migliore, G.; Vimercati, L.; Martinelli, A.; Lobifaro, A.; Diella, G.; Stefanizzi, P.; on behalf of the Control Room Working Group. BNT162b2 MRNA COVID-19 Vaccine Effectiveness in the Prevention of SARS-CoV-2 Infection and Symptomatic Disease in Five-Month Follow-Up: A Retrospective Cohort Study. Vaccines 2021, 9, 1143. [Google Scholar] [CrossRef]
- Bedston, S.; Akbari, A.; Jarvis, C.I.; Lowthian, E.; Torabi, F.; North, L.; Lyons, J.; Perry, M.; Griffiths, L.J.; Owen, R.K.; et al. COVID-19 Vaccine Uptake, Effectiveness, and Waning in 82,959 Health Care Workers: A National Prospective Cohort Study in Wales. Vaccine 2022, 40, 1180–1189. [Google Scholar] [CrossRef]
- Kayı, İ.; Madran, B.; Keske, Ş.; Karanfil, Ö.; Arribas, J.R.; Psheniсhnaya, N.; Petrosillo, N.; Gönen, M.; Ergönül, Ö. The Seroprevalence of SARS-CoV-2 Antibodies among Health Care Workers before the Era of Vaccination: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1242–1249. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Joung, S.; Liu, Y.; Wu, M.; Weber, B.; Claggett, B.; Botting, P.G.; Sun, N.; Driver, M.; Kao, Y.H.; et al. Demographic and Clinical Characteristics Associated with Variations in Antibody Response to BNT162b2 COVID-19 Vaccination among Healthcare Workers at an Academic Medical Centre: A Longitudinal Cohort Analysis. BMJ Open 2022, 12, e059994. [Google Scholar] [CrossRef]
- Alishaq, M.; Nafady-Hego, H.; Jeremijenko, A.; Ajmi, J.A.A.; Elgendy, M.; Vinoy, S.; Fareh, S.B.; Plaatjies, J.V.; Nooh, M.; Alanzi, N.; et al. Risk Factors for Breakthrough SARS-CoV-2 Infection in Vaccinated Healthcare Workers. PLoS ONE 2021, 16, e0258820. [Google Scholar] [CrossRef]
- Bampoe, S.; Lucas, D.N.; Neall, G.; Sceales, P.; Aggarwal, R.; Caulfield, K.; Siassakos, D.; Odor, P.M. A Cross-Sectional Study of Immune Seroconversion to SARS-CoV-2 in Frontline Maternity Health Professionals. Anaesthesia 2020, 75, 1614–1619. [Google Scholar] [CrossRef]
- Coxon, K.; Turienzo, C.F.; Kweekel, L.; Goodarzi, B.; Brigante, L.; Simon, A.; Lanau, M.M. The Impact of the Coronavirus (COVID-19) Pandemic on Maternity Care in Europe. Midwifery 2020, 88, 102779. [Google Scholar] [CrossRef]
- Schmitt, N.; Mattern, E.; Cignacco, E.; Seliger, G.; König-Bachmann, M.; Striebich, S.; Ayerle, G.M. Effects of the Covid-19 Pandemic on Maternity Staff in 2020—A Scoping Review. BMC Health Serv. Res. 2021, 21, 1364. [Google Scholar] [CrossRef]
- Russell, A.; Hsu, E.B.; Fenstermacher, K.Z.J.; Ricketts, E.P.; Dashler, G.; Chen, A.; Shaw-Saliba, K.; Caturegli, P.P.; Pekosz, A.; Rothman, R.E. Characteristics of SARS-CoV-2 Seropositivity among Emergency Department Healthcare Workers at a Tertiary Care Center in Baltimore. Healthcare 2022, 10, 576. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P. Immune Determinants of COVID-19 Disease Presentation and Severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef]
- Talaei, M.; Faustini, S.; Holt, H.; Jolliffe, D.A.; Vivaldi, G.; Greenig, M.; Perdek, N.; Maltby, S.; Bigogno, C.M.; Symons, J.; et al. Determinants of Pre-Vaccination Antibody Responses to SARS-CoV-2: A Population-Based Longitudinal Study (COVIDENCE UK). BMC Med. 2022, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex Differences in Immune Responses That Underlie COVID-19 Disease Outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Moncunill, G.; Aguilar, R.; Ribes, M.; Ortega, N.; Rubio, R.; Salmerón, G.; Molina, M.J.; Vidal, M.; Barrios, D.; Mitchell, R.A.; et al. Determinants of Early Antibody Responses to COVID-19 MRNA Vaccines in a Cohort of Exposed and Naïve Healthcare Workers. EBioMedicine 2022, 75, 103805. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination. Cell 2022, 185, 1025–1040. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021, 385, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels After Vaccination. Front. Public Health 2021, 9, 1964. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Peng, Y.; Liang, Y.; Wei, J.; Xing, L.; Guo, L.; Li, X.; Li, J.; Wang, J.; et al. Longitudinal Analysis of Antibody Dynamics in COVID-19 Convalescents Reveals Neutralizing Responses up to 16 Months after Infection. Nat. Microbiol. 2022, 7, 423–433. [Google Scholar] [CrossRef]
- Wolszczak-Biedrzycka, B.; Bieńkowska, A.; Dorf, J. Assessment of Post-Vaccination Antibody Response Eight Months after the Administration of BNT1622b2 Vaccine to Healthcare Workers with Particular Emphasis on the Impact of Previous COVID-19 Infection. Vaccines 2021, 9, 1508. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Antoni, D.; Manoussopoulos, Y.; Stefanou, P.; Argyropoulou, S.; Vrioni, G.; Tsakris, A. Age and Sex Associations of SARS-CoV-2 Antibody Responses Post BNT162b2 Vaccination in Healthcare Workers: A Mixed Effects Model across Two Vaccination Periods. PLoS ONE 2022, 17, e0266958. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.; Whitaker, M.; Flower, B.; Tang, S.N.; Atchison, C.; Darzi, A.; Donnelly, C.A.; Cann, A.; Diggle, P.J.; Ashby, D.; et al. Population Antibody Responses Following COVID-19 Vaccination in 212,102 Individuals. Nat. Commun. 2022, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial Observations on Age, Gender, BMI and Hypertension in Antibody Responses to SARS-CoV-2 BNT162b2 Vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease Severity Dictates SARS-CoV-2-Specific Neutralizing Antibody Responses in COVID-19. Signal. Transduct. Target. Ther. 2020, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and Immunological Assessment of Asymptomatic SARS-CoV-2 Infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of a Fourth Dose of MRNA COVID-19 Vaccine against All-Cause Mortality in Long-Term Care Facility Residents and in the Oldest Old: A Nationwide, Retrospective Cohort Study in Sweden. Lancet Reg. Health—Eur. 2022, 21, 100466. [Google Scholar] [CrossRef] [PubMed]

| DIDaCN 1 | GaOW 2 | |
|---|---|---|
| HCWs | ||
| Study participants | 50 | 40 |
| Female | 40 | 35 |
| Male | 10 | 5 |
| <30 y.o. | 16 | 3 |
| 31–40 y.o. | 13 | 2 |
| 41–50 y.o. | 11 | 9 |
| 51–60 y.o. | 9 | 20 |
| >60 y.o. | 1 | 6 |
| Physicians | 16 | 6 |
| Nurses/Midwives | 25 | 29 |
| Others | 9 | 5 |
| HCUs | ||
| Hospital beds | 20 | 28 + 3 3 |
| Admitted patients/month in 2020, mean | 273 | 150 |
| COVID-19 patients in 2020 | 292 + 2443 4 | 3 |
| SARS-CoV-2 infection risk | HIGH | LOW |
| Seroprevalence (95% CI) | |||||
|---|---|---|---|---|---|
| September 2020 | December 2020 | February 2021 | September 2021 | ||
| N | 90 | 90 | 90 | 75 | |
| Total | anti-S | 0% a (0.00–4.09) | 37.8% b (28.46–48.10) | 100% c (95.91–100) | 89.3% d (80.34–94.50) |
| anti-NCP | nd | nd | 26.0% (18.41–35.37) | 17.1% (10.28–27.10) | |
| N | 50 | 50 | 50 | 41 | |
| DIDaCN 1 | anti-S | 0% a (0.00–7.14) | 26.0% b (15.87–36.55) | 100% c (92.90–100) | 85.40% c (71.56–93.12) |
| anti-NCP | nd | nd | 14.0% (6.95–26.19) | 5.71% (1.02–18.61) | |
| N | 40 | 40 | 40 | 34 | |
| GaOW 2 | anti-S | 0% a (0.00–8.76) | 52.5% b (37.50–67.07) | 100% c (91.24–100) | 94.12% c (80.91–98.96) |
| anti-NCP | nd | nd | 38.00% (25.86–51.85) | 26.83% (15.70–41.93) | |
| Seroprevalence (95% CI) | |||
|---|---|---|---|
| Total | DIDaCN 1 | GaOW 2 | |
| Total | 37.8% ab (28.46–48.10) | 26% a (15.87–36.55) | 52.5% b (37.50–67.07) |
| Sex | |||
| Female | 37.3% (27.26-48.65) | 22.5% (12.32–37.50) | 54.3% (38.19–69.53) |
| Male | 40.0% (19.82–64.25) | 40% (16.82–68.73) | 40% (7.11–76.93) |
| Age | |||
| <30 | 21.0% (8.51–43.33) | 25% (10.18–49.49) | 0% (0.00–56.15) |
| 31–40 | 40.0% (19.82–64.25) | 30.8% (12.68–57.63) | 100% (17.77–100) |
| 41–50 | 30.0% (14.55–51.90) | 27.2% (9.75–56.57) | 33.3% (12.06–64.58) |
| 51–60 | 51.7% (34.43–68.61) | 22.2% (3.95–54.74) | 65% (43.29–81.88) |
| >60 | 42.9% (15.82–74.95) | 0% (0.00–94.87) | 50% (18.76–81.24) |
| Occupation | |||
| Physicians | 36.4% (19.73–57.05) | 31.3% (14.17-55.60) | 50% (18.76-81.24) |
| Nurses/midwives | 44.4% ab (32.00–57.62) | 24% a (11.50–43.43) | 62.1% b (44.00–77.31) |
| Others | 14.3% (2.54–39.94) | 22.2% (3.95–54.74) | 0% (0.00–43.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorent, D.; Nowak, R.; Luwański, D.; Pisarska-Krawczyk, M.; Figlerowicz, M.; Zmora, P. The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination. Vaccines 2022, 10, 1576. https://doi.org/10.3390/vaccines10101576
Lorent D, Nowak R, Luwański D, Pisarska-Krawczyk M, Figlerowicz M, Zmora P. The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination. Vaccines. 2022; 10(10):1576. https://doi.org/10.3390/vaccines10101576
Chicago/Turabian StyleLorent, Dagny, Rafał Nowak, Dawid Luwański, Magdalena Pisarska-Krawczyk, Magdalena Figlerowicz, and Paweł Zmora. 2022. "The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination" Vaccines 10, no. 10: 1576. https://doi.org/10.3390/vaccines10101576
APA StyleLorent, D., Nowak, R., Luwański, D., Pisarska-Krawczyk, M., Figlerowicz, M., & Zmora, P. (2022). The Longitudinal Analysis on the Anti-SARS-CoV-2 Antibodies among Healthcare Workers in Poland—Before and after BNT126b2 mRNA COVID-19 Vaccination. Vaccines, 10(10), 1576. https://doi.org/10.3390/vaccines10101576







