Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
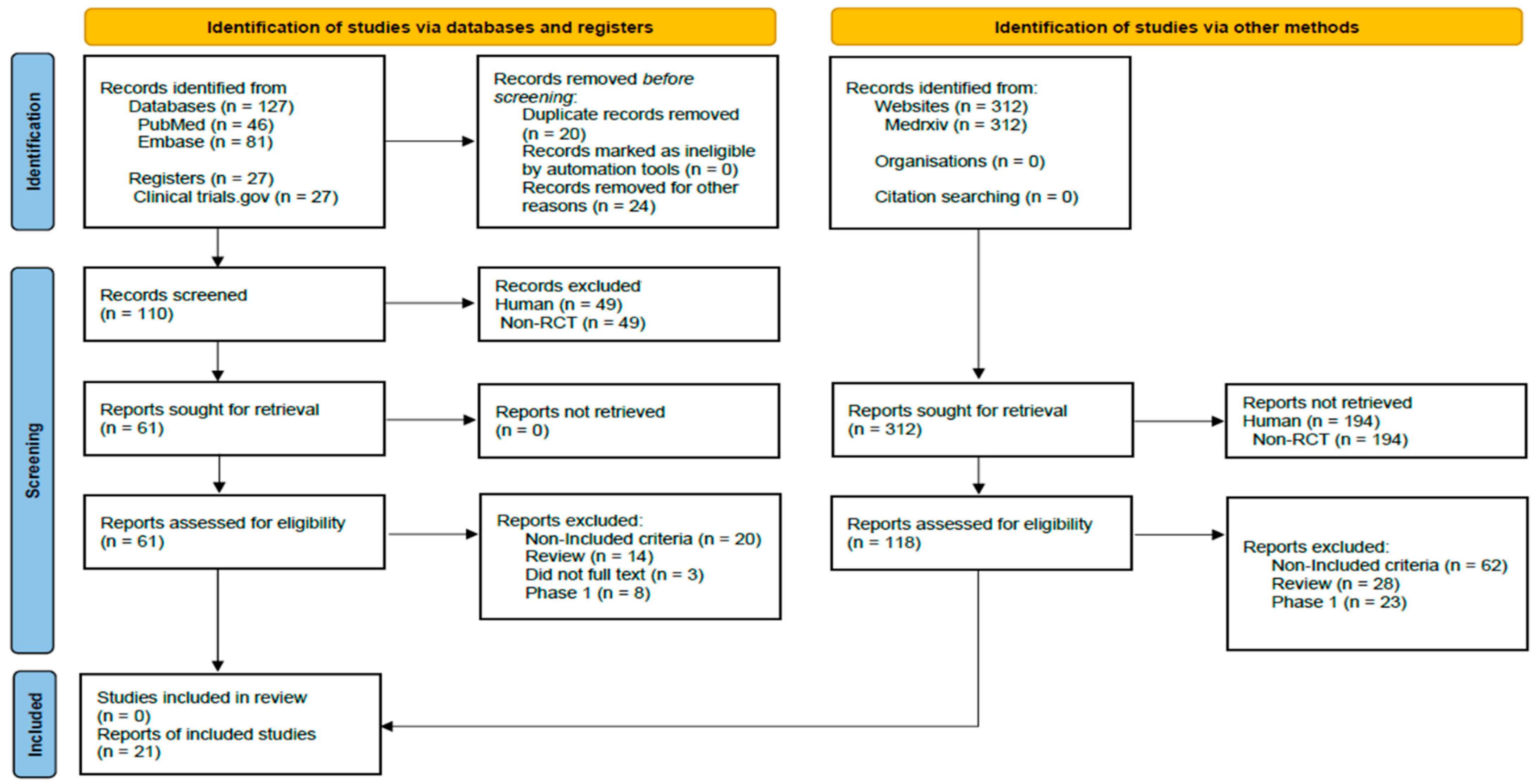
2.1. Search Strategy and Selection Criteria
2.2. Outcomes and Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purja, S.; Shin, H.; Lee, J.-Y.; Kim, E. Is loss of smell an early predictor of COVID-19 severity: A systematic review and meta-analysis. Arch. Pharmacal Res. 2021, 44, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Majumdar, P.; Niyogi, S. SARS-CoV-2 mutations: The biological trackway towards viral fitness. Epidemiol. Infect. 2021, 149, 1–19. [Google Scholar] [CrossRef]
- Liu, C.; Shi, W.; Becker, S.T.; Schatz, D.G.; Liu, B.; Yang, Y. Structural basis of mismatch recognition by a SARS-CoV-2 proofreading enzyme. Science 2021, 373, 1142–1146. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Richterman, A. SARS-CoV-2 Transmission and Prevention in the Era of the Delta Variant. Infect. Dis. Clin. N. Am. 2022, 36, 267–293. [Google Scholar] [CrossRef]
- McLean, G.; Kamil, J.; Lee, B.; Moore, P.; Schulz, T.F.; Muik, A.; Sahin, U.; Türeci, O.; Pather, S. The Impact of Evolving SARS-CoV-2 Mutations and Variants on COVID-19 Vaccines. mBio 2022, 3, e02979-21. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A. Evidence Synthesis for Decision Making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef]
- Verardi, S.; Casciani, C.U.; Nicora, E.; Forzano, F.; Origone, A.; Valle, I.; Catania, G.; Salanitri, G.; Salcuni, P.; Azzarone, M.; et al. A multicentre study on LMW-heparin effectiveness in preventing postsurgical thrombosis. Int. Angiol. 1998, 7 (Suppl. 3), 19–24. [Google Scholar]
- Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 2002, 21, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.P.; Fleurence, R.; Devine, B.; Itzler, R.; Barrett, A.; Hawkins, N.; Lee, K.; Boersma, C.; Annemans, L.; Cappelleri, J.C. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: Report of the ISPRR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Value Health 2011, 14, 417–428. [Google Scholar] [CrossRef]
- Hoaglin, D.C.; Hawkins, N.; Jansen, J.P.; Scott, D.A.; Itzler, R.; Cappelleri, J.C.; Boersma, C.; Thompson, D.; Larholt, K.M.; Diaz, M.; et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: Report of the ISPRR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health 2011, 14, 429–437. [Google Scholar] [CrossRef]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Mak. 2013, 33, 618–640. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Network Meta-Analysis. Meta-Analysis with R.; Springer International Publishing: Cham, Switzerland, 2015; pp. 187–216. [Google Scholar]
- Dobler, C.C.; Wilson, M.E.; Murad, M.H. A pulmonologist’s guide to understanding network meta-analysis. Eur. Respir. J. 2018, 52, 1800525. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H.; for the GRADE Working Group. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Morton, S.C.; Murad, M.H.; O’Connor, E.; Lee, C.S.; Booth, M.; Vandermeer, B.W.; Snowden, J.M.; D’Anci, K.E.; Fu, R.; Gartlehner, G.; et al. AHRQ Methods for Effective Health Care. Quantitative Synthesis—An Update. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Zeng, B.; Le Gao, L.; Zhou, Q.; Yu, K.; Sun, F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: A systematic review and meta-analysis. BMC Med. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, Z.; Luo, W.; Si, S.; Mo, M.; Zhou, H.; Xin, X.; Liu, H.; Yu, Y. Efficacy and Safety of COVID-19 Vaccines in Phase III Trials: A Meta-Analysis. Vaccines 2021, 9, 582. [Google Scholar] [CrossRef]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Daussy, C.; Pied, N.; Wodrich, H. Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties. Viruses 2021, 13, 1221. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Toubasi, A.A.; Al-Sayegh, T.N.; Obaid, Y.Y.; Al-Harasis, S.M.; AlRyalat, S.A.S. Efficacy and safety of COVID-19 vaccines: A network meta-analysis. J. Evidence-Based Med. 2022; Online version. [Google Scholar] [CrossRef]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 vaccines: Neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef] [PubMed]
- REUTERS. Sinopharm, Sinovac COVID-19 Vaccine Data Show Efficacy: WHO. Available online: https://www.reuters.com/article/us-health-coronavirus-who-china-vaccines-idUSKBN2BN1K8 (accessed on 21 March 2021).
- Goepfert, P.A.; Fu, B.; Chabanon, A.-L.; Bonaparte, M.I.; Davis, M.G.; Essink, B.J.; Frank, I.; Haney, O.; Janosczyk, H.; Keefer, M.C.; et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: Interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect. Dis. 2021, 21, 1257–1270. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Arora, S.; Monreal, M.; Jimenez, D.; Krumholz, H.M.; Goldhaber, S.Z.; Elkind, M.S.; Piazza, G. Cerebral Venous Sinus Thrombosis in the U.S. Population, After Adenovirus-Based SARS-CoV-2 Vaccination, and After COVID-19. J. Am. Coll. Cardiol. 2021, 78, 408–411. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.; Turner, D.; Turner, R. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Sa, S.; Lee, C.W.; Shim, S.R.; Yoo, H.; Choi, J.; Kim, J.H.; Lee, K.; Hong, M.; Han, H.W. The Safety of mRNA-1273, BNT162b2 and JNJ-78436735 COVID-19 Vaccines: Safety Monitoring for Adverse Events Using Real-World Data. Vaccines 2022, 10, 320. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv 2021. [Google Scholar] [CrossRef]
- Tang, P.; Hasan, M.R.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Khatib, H.A.A.; Al Mukdad, S.; Coyle, P.; Ayoub, H.H.; Kanaani, Z.A.; et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. medRxiv 2021. [Google Scholar] [CrossRef]
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Peng, Q.; Zhou, R.; Wang, Y.; Zhao, M.; Liu, N.; Li, S.; Huang, H.; Yang, D.; Au, K.-K.; Wang, H.; et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine 2022, 77, 103904. [Google Scholar] [CrossRef] [PubMed]
- The Economist. Which Vaccine Is the Most Effective against the Delta Variant? Available online: https://www.economist.com/graphic-detail/2021/11/17/which-vaccine-is-the-most-effective-against-the-delta-variant (accessed on 13 December 2021).
- Kim, H.-S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef] [PubMed]
- Ramagopalan, S.V.; Simpson, A.; Sammon, C. Can real-world data really replace randomised clinical trials? BMC Med. 2020, 18, 1–2. [Google Scholar] [CrossRef]
- Cines, D.B.; Bussel, J.B. SARS-CoV-2 Vaccine–Induced Immune Thrombotic Thrombocytopenia. N. Engl. J. Med. 2021, 384, 2254–2256. [Google Scholar] [CrossRef]




| Vaccine Type | Investigational Name | Company | Trade Name | Recommended | Adjuvant | Dosage |
|---|---|---|---|---|---|---|
| mRNA-based * | mRNA-1273 | Moderna | Spikevax | Adults 18 and older | None | Two doses, 28 days apart |
| BNT162b1 | Pfizer/BioNTech | Comirnaty | Adults aged 16 and older (Emergency Use Authorization for ages 12–15) | None | Two doses, 21 days apart | |
| Pro-Subunit | NVX-CoV2373 | Novavax | Covovax | Ages 12–84 | Matrix-M1 | Two doses, 21 days apart |
| ZF2001 | Anhui Zhifei Longcom | Zifivax | Adults 18 and older | Aluminum hydroxide | Three doses, over a period of 2 months | |
| CoV2 preS dTM | Sanofi | - | Adults 18 and older | AF03 | Two doses, 21 days apart | |
| Adenovirus-based | Gam-COVID-Vac | Gamaleya | Sputnik V | Adults 18 and older | None | Two doses, 21 days apart |
| Ad26.COV2.S | Johnson & Johnson | COVID-19 Vaccine Janssen | Adults 18 and older | None | Single shot | |
| AZD1222 | Oxford/AstraZeneca | Covishield or Vaxzevria | Adults 18 and older | None | Two doses 28–84 days apart | |
| Ad5-nCoV | CanSino | Convidecia | Adults 18 and older | None | Single dose | |
| Inactivated virus | MINHAI | SZKT | Kconvax | Adults 18 and older | Aluminum hydroxide | Two doses, 28 days apart |
| BBIBP-CorV | Sinopharm | - | Adults 18 and older | Aluminum hydroxide | Two doses, 21 days apart | |
| WIV04 | Sinopharm | - | Adults 18 and older | Aluminum hydroxide | Two doses, 21 days apart | |
| CoronaVac | Sinovac | CoronaVac | Adults 18 and older | Aluminum hydroxide | Two doses, 14–28 days apart |
| Study | Registered Trial Number | Phase | Intervention | Control | Patients | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Treatment/Company | Dose (μg) | Total | Mean Age (years) | Female (%) | |||||
| Banden_2021 | NCT04470427 | Ⅲ | mRNA-1273/Moderna | 100 | Placebo | 30,351 | 51.4 | 56 | 5 |
| Chu_2021-1 | NCT04405076 | Ⅱ | 50 | Placebo | 200 | 36.95 | 52 | 7 | |
| Chu_2021-2 | NCT04405076 | Ⅱ | 100 | Placebo | 200 | 37.8 | 49.4 | 7 | |
| Chu_2021-3 | NCT04405076 | Ⅱ | 50 | Placebo | 200 | 64.55 | 45 | 7 | |
| Chu_2021-4 | NCT04405076 | Ⅱ | 100 | Placebo | 200 | 50.6 | 51.5 | 7 | |
| Formica_2021-1 | NCT04368988 | Ⅱ | NVX-CoV2373/Novavax | 5 | Placebo | 513 | 51.55 | 51.05 | 23 |
| Formica_2021-2 | NCT04368988 | Ⅱ | 25 | Placebo | 514 | 52.15 | 50.55 | 23 | |
| Shinde_2021 | NCT04533399 | Ⅱ | 5 | Placebo | 4382 | 32 | 42.6 | 25 | |
| Toback_2021 | NCT04583995 | Ⅲ | 5 | Placebo | 14,039 | 56 | 48.4 | 24 | |
| Logunov_2021 | NCT04530396 | Ⅲ | Gam-COVID-Vac/Gamaleya | 0.5 mL/dose | Placebo | 19,866 | 45.3 | 48.5 | 8 |
| Pan_2021-1 | ChiCTR2000038804 | Ι–Ⅱ | MINHAI/SZKT | 5 | Placebo | 150 | 35.85 | 48.5 | 21 |
| Pan_2021-2 | ChiCTR2000038804 | Ι–Ⅱ | 10 | Placebo | 150 | 45.55 | 51 | 21 | |
| Pan_2021-3 | ChiCTR2000038804 | Ι–Ⅱ | 5 | Placebo | 150 | 42.05 | 60 | 21 | |
| Pan_2021-4 | ChiCTR2000038804 | Ι–Ⅱ | 10 | Placebo | 150 | 43.1 | 54 | 21 | |
| Polack_2021 | NCT04368728 | Ⅱ–Ⅲ | BNT162b1/Pfizer/BioNTech | 30 | Placebo | 37,706 | NA | 55 | 1 |
| Sadoff_2021 | NCT04505722 | Ⅲ | Ad26.COV2.S/Johnson & Johnson | 0.5 mL/dose | Placebo | 43,783 | 52 | 57 | 12 |
| Sadoff_2021.01-1 | NCT04436276 | Ι–Ⅱ | 5 × 1010 vp/mL | Placebo | 244 | 35.75 | 51.5 | 11 | |
| Sadoff_2021.01-2 | NCT04436276 | Ι–Ⅱ | 1 × 1011 vp/mL | Placebo | 240 | 70.2 | 52.5 | 11 | |
| Sadoff_2021.01-3 | NCT04436276 | Ι–Ⅱ | 5 × 1010 vp/mL | Placebo | 242 | 69.75 | 50.5 | 11 | |
| Sadoff_2021.01-4 | NCT04436276 | Ι–Ⅱ | 1 × 1011 vp/mL | Placebo | 242 | 69.95 | 52 | 11 | |
| Xia_2020.10-1 | ChiCTR2000032459 | Ι–Ⅱ | BBIBP-CorV/Sinopharm | 8 | Placebo | 112 | 60 | 45 | 20 |
| Xia_2020.10-2 | ChiCTR2000032459 | Ι–Ⅱ | 4 | Placebo | 112 | 54 | 51.5 | 20 | |
| Xia_2020.10-3 | ChiCTR2000032459 | Ι–Ⅱ | 4 | Placebo | 112 | 55 | 42.5 | 20 | |
| Xia_2020.10-4 | ChiCTR2000032459 | Ι–Ⅱ | 4 | Placebo | 112 | 57 | 50.5 | 20 | |
| Kaabi_2021-2 | NCT04510207 | Ⅲ | 4 | Placebo | 25,463 | 36.1 | 15.35 | 19 | |
| Kaabi_2021-1 | NCT04510207 | Ⅲ | WIV04/Sinopharm_Wuhan | 5 | Placebo | 25,480 | 36.15 | 15.6 | 19 |
| Xia_2020-1 | ChiCTR2000031809 | Ι–Ⅱ | 5 | Placebo | 112 | 35.1 | 52 | 18 | |
| Xia_2020-2 | ChiCTR2000031809 | Ι–Ⅱ | 5 | Placebo | 112 | 35.1 | 48.5 | 18 | |
| Yang_2021-1 | NCT04466085 | Ι–Ⅱ | ZF2001/Anhui Zhifei Longcom | 25 | Placebo | 300 | 56 | 48.5 | 26 |
| Yang_2021-2 | NCT04466085 | Ι–Ⅱ | 50 | Placebo | 300 | 58.5 | 51 | 26 | |
| Yang_2021-3 | NCT04466085 | Ι–Ⅱ | 25 | Placebo | 300 | 43.05 | 52 | 26 | |
| Yang_2021-4 | NCT04466085 | Ι–Ⅱ | 50 | Placebo | 300 | 43.3 | 54.5 | 26 | |
| Zhang_2021-1 | NCT04352608 | Ι–Ⅱ | CoronaVac/Sinovac | 3 | Placebo | 180 | 42.8 | 56.65 | 16 |
| Zhang_2021-2 | NCT04352608 | Ι–Ⅱ | 6 | Placebo | 180 | 43 | 59.15 | 16 | |
| Zhang_2021-3 | NCT04352608 | Ι–Ⅱ | 3 | Placebo | 180 | 42.9 | 48.75 | 16 | |
| Zhang_2021-4 | NCT04352608 | Ι–Ⅱ | 6 | Placebo | 180 | 45.65 | 48.75 | 16 | |
| Wu_2021-1 | NCT04383574 | Ι–Ⅱ | 1.5 | Placebo | 150 | 48.5 | 56 | 14 | |
| Wu_2021-2 | NCT04383574 | Ι–Ⅱ | 3 | Placebo | 150 | 48.5 | 52 | 14 | |
| Wu_2021-3 | NCT04383574 | Ι–Ⅱ | 6 | Placebo | 149 | 51 | 49.4 | 14 | |
| Bueno_2021 | NCT04651790 | Ⅱ | 3 | Placebo | 310 | NA | NA | 15 | |
| Palacios_2021 | NCT04456595 | Ⅲ | 3 | Placebo | 12,396 | 64.2 | 64.2 | 17 | |
| Zhu_2020-1 | NCT04341389 | Ⅱ | Ad5-nCoV/CanSino | 1 × 1011 vp/mL | Placebo | 379 | 39.6 | 49.9 | 9 |
| Zhu_2020-2 | NCT04341389 | Ⅱ | 5 × 1010 vp/mL | Placebo | 255 | 39.45 | 49.9 | 9 | |
| Madhi_2021 | NCT04444674 | Ι–Ⅱ | AZD1222/ Oxford/ AstraZeneca | 5 × 1010 vp/mL | Placebo | 2021 | NA | 43.5 | 10 |
| Goepfert_2021-1 | NCT04537208 | Ι–Ⅱ | CoV2 preS Dtm-AS03/Sanofi | 1.3 | Placebo | 57 | 33.65 | 47 | 22 |
| Goepfert_2021-2 | NCT04537208 | Ι–Ⅱ | 1.3 | Placebo | 111 | 32.85 | 45 | 22 | |
| Goepfert_2021-3 | NCT04537208 | Ι–Ⅱ | 2.6 | Placebo | 56 | 32.25 | 54.5 | 22 | |
| Goepfert_2021-4 | NCT04537208 | Ι–Ⅱ | 2.6 | Placebo | 114 | 33.45 | 66.55 | 22 | |
| Goepfert_2021-5 | NCT04537208 | Ι–Ⅱ | 1.3 | Placebo | 57 | 60.15 | 52.5 | 22 | |
| Goepfert_2021-6 | NCT04537208 | Ι–Ⅱ | 1.3 | Placebo | 111 | 60.65 | 47 | 22 | |
| Goepfert_2021-7 | NCT04537208 | Ι–Ⅱ | 2.6 | Placebo | 56 | 60.1 | 62.5 | 22 | |
| Goepfert_2021-8 | NCT04537208 | Ι–Ⅱ | 2.6 | Placebo | 114 | 61.7 | 68 | 22 | |
| Comparisons (vs. Placebo) | Study No. | Effect Size (95% CI) | Study Design | Grade | |
|---|---|---|---|---|---|
| Vaccine efficacy, RR | |||||
| mRNA-1273 | 1 | 0.05 (0.01, 0.47) | RCT | ⊕⊕⊕○ Moderate | |
| NVX-CoV2373 | 2 | 0.23 (0.05, 1.07) | RCT | ⊕⊕○○ Low | |
| BNT162b1 | 1 | 0.06 (0.01, 0.50) | RCT | ⊕⊕⊕○ Moderate | |
| Gam-COVID-Vac | 1 | 0.09 (0.01, 0.79) | RCT | ⊕⊕⊕○ Moderate | |
| Ad26.COV2.S | 1 | 0.32 (0.04, 2.61) | RCT | ⊕⊕⊕○ Moderate | |
| AZD1222 | 1 | 0.25 (0.02, 2.84) | RCT | ⊕⊕⊕○ Moderate | |
| BBIBP-CorV | 1 | 0.27 (0.03, 2.24) | RCT | ⊕⊕⊕○ Moderate | |
| WIV04 | 1 | 0.36 (0.04, 3.00) | RCT | ⊕⊕⊕○ Moderate | |
| CoronaVac | 1 | 0.50 (0.06, 4.07) | RCT | ⊕⊕⊕○ Moderate | |
| Immunogenicity of neutralizing antibodies to live SARS-CoV-2, SMD | |||||
| mRNA-1273 | 1 | 1605.34 (1534.68, 1676.00) | RCT | ⊕⊕⊕○ Moderate | |
| NVX-CoV2373 | 1 | 1360.28 (1019.39, 1701.18) | RCT | ⊕⊕○○ Low | |
| Gam-COVID-Vac | 1 | 42.91 (−14.77, 100.58) | RCT | ⊕⊕⊕○ Moderate | |
| Ad26.COV2.S | 1 | 223.28 (159.11, 287.44) | RCT | ⊕⊕⊕○ Moderate | |
| BBIBP-CorV | 2 | 142.29 (113.22, 171.36) | RCT | ⊕⊕⊕○ Moderate | |
| WIV04 | 2 | 125.45 (86.83, 164.06) | RCT | ⊕⊕⊕○ Moderate | |
| CoronaVac | 1 | 35.01 (15.19, 54.83) | RCT | ⊕⊕⊕○ Moderate | |
| ZF2001 | 1 | 13.97 (−13.92, 41.86) | RCT | ⊕⊕⊕○ Moderate | |
| CoV2 preS Dtm-AS03/ Sanofi | 1 | 22.15 (0.93, 43.36) | RCT | ⊕⊕⊕○ Moderate | |
| Ad5-nCoV | 1 | 18.90 (−20.53, 58.33) | RCT | ⊕⊕⊕○ Moderate | |
| MINHAI | 1 | 72.08 (43.25, 100.91) | RCT | ⊕⊕⊕○ Moderate | |
| Immunogenicity of specific IgG, SMD | |||||
| MINHAI | 1 | 1250.50 (942.25, 1558.74) | RCT | ⊕⊕⊕○ Moderate | |
| NVX-CoV2373 | 1 | 1421.49 (426.76, 2416.22) | RCT | ⊕⊕○○ Low | |
| Gam-COVID-Vac | 1 | 8965.45 (7361.81, 10569.09) | RCT | ⊕⊕⊕○ Moderate | |
| WIV04 | 1 | 143.96 (−234.55, 522.47) | RCT | ⊕⊕⊕○ Moderate | |
| CoronaVac | 1 | 1161.64 (907.67, 1415.62) | RCT | ⊕⊕⊕○ Moderate | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Purja, S.; Shin, H.; Kim, M.S.; Park, S.; Kronbichler, A.; Smith, L.; Eisenhut, M.; Shin, J.I.; Kim, E. Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis. Vaccines 2022, 10, 1572. https://doi.org/10.3390/vaccines10101572
Oh S, Purja S, Shin H, Kim MS, Park S, Kronbichler A, Smith L, Eisenhut M, Shin JI, Kim E. Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis. Vaccines. 2022; 10(10):1572. https://doi.org/10.3390/vaccines10101572
Chicago/Turabian StyleOh, SuA, Sujata Purja, Hocheol Shin, Min Seo Kim, Seoyeon Park, Andreas Kronbichler, Lee Smith, Michael Eisenhut, Jae Il Shin, and Eunyoung Kim. 2022. "Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis" Vaccines 10, no. 10: 1572. https://doi.org/10.3390/vaccines10101572
APA StyleOh, S., Purja, S., Shin, H., Kim, M. S., Park, S., Kronbichler, A., Smith, L., Eisenhut, M., Shin, J. I., & Kim, E. (2022). Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis. Vaccines, 10(10), 1572. https://doi.org/10.3390/vaccines10101572









