Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
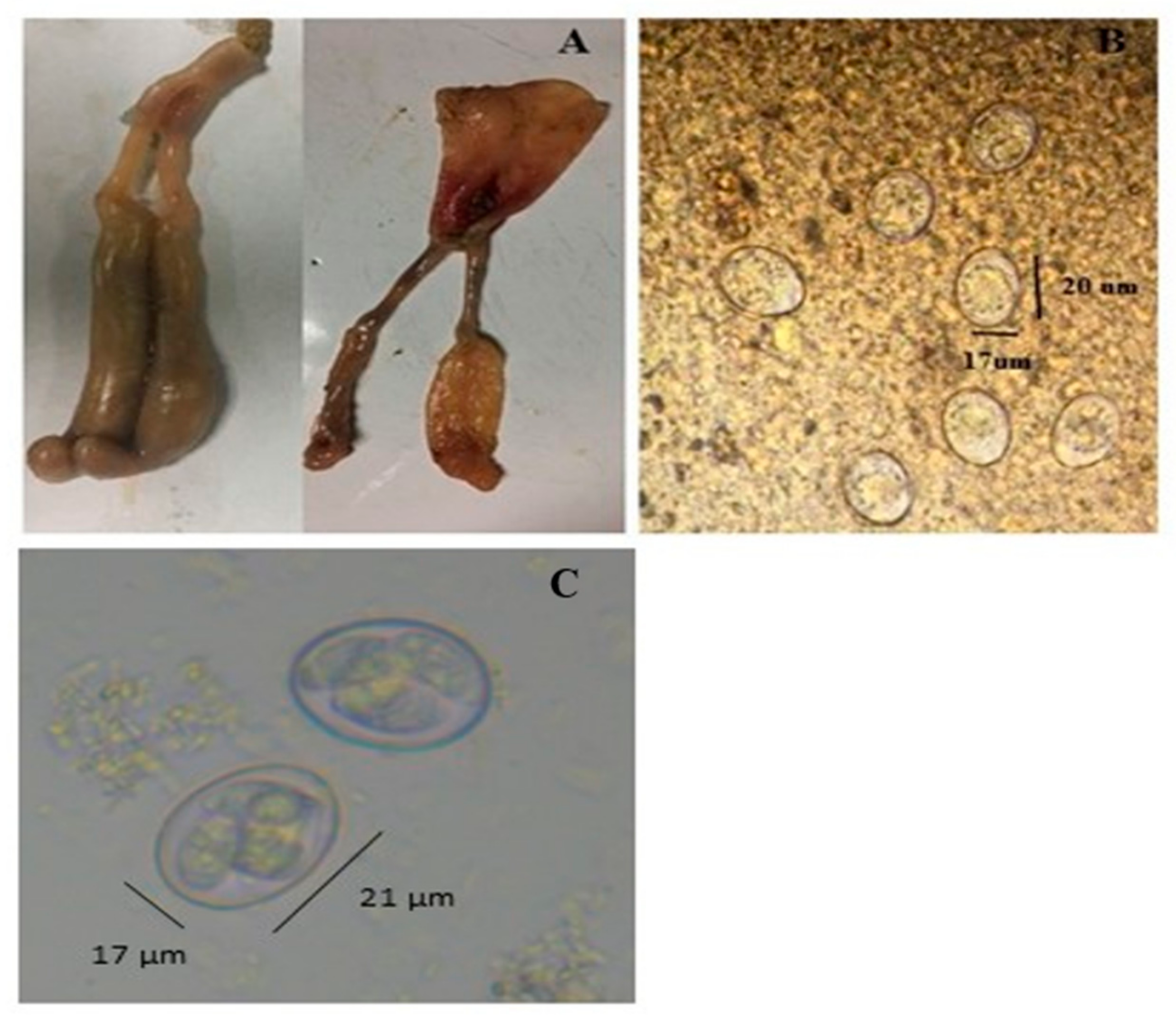
2.2. Parasite Propagation
2.3. Experimentally Treated Chicken Groups
2.4. Oocyst per Gram (OPG) and Fecal Score Assessment
2.5. Feed Conversion Ratio
2.6. Tissue and Serum Collection
2.7. Immune Response
2.7.1. Cell-mediated Response to Dinitrochlorobenzene (DNCB)
2.7.2. Humoral Immune Response to Sheep Red Blood Cells (SRBCs)
2.8. Serum Chemistry
2.9. RNA Extraction, cDNA Preparation, and Determination of mRNA Gene Expression
2.10. Statistical Analysis
3. Results
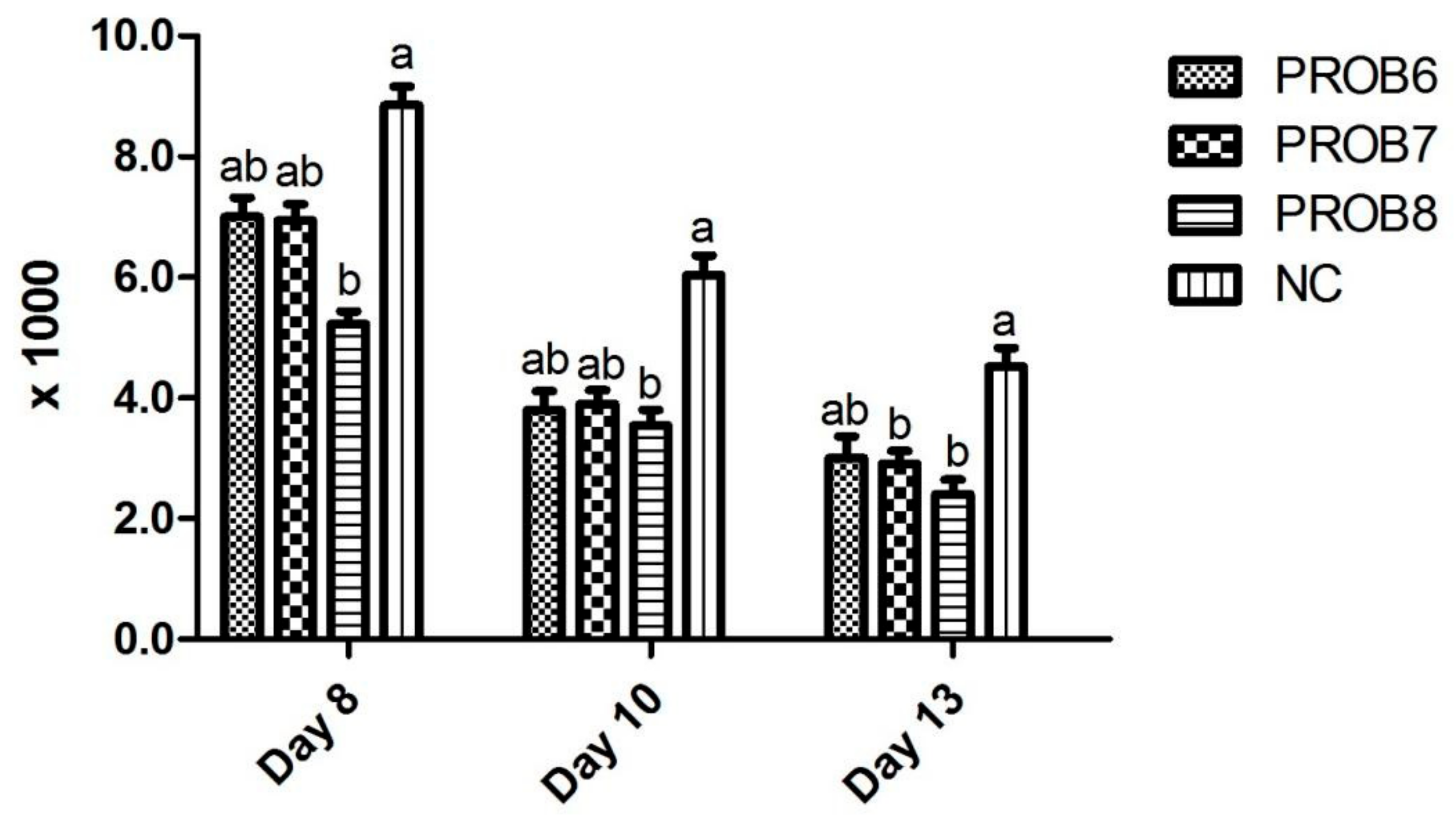
3.1. Fecal Score and OPG Assessment
3.2. Feed Conversion Ratio
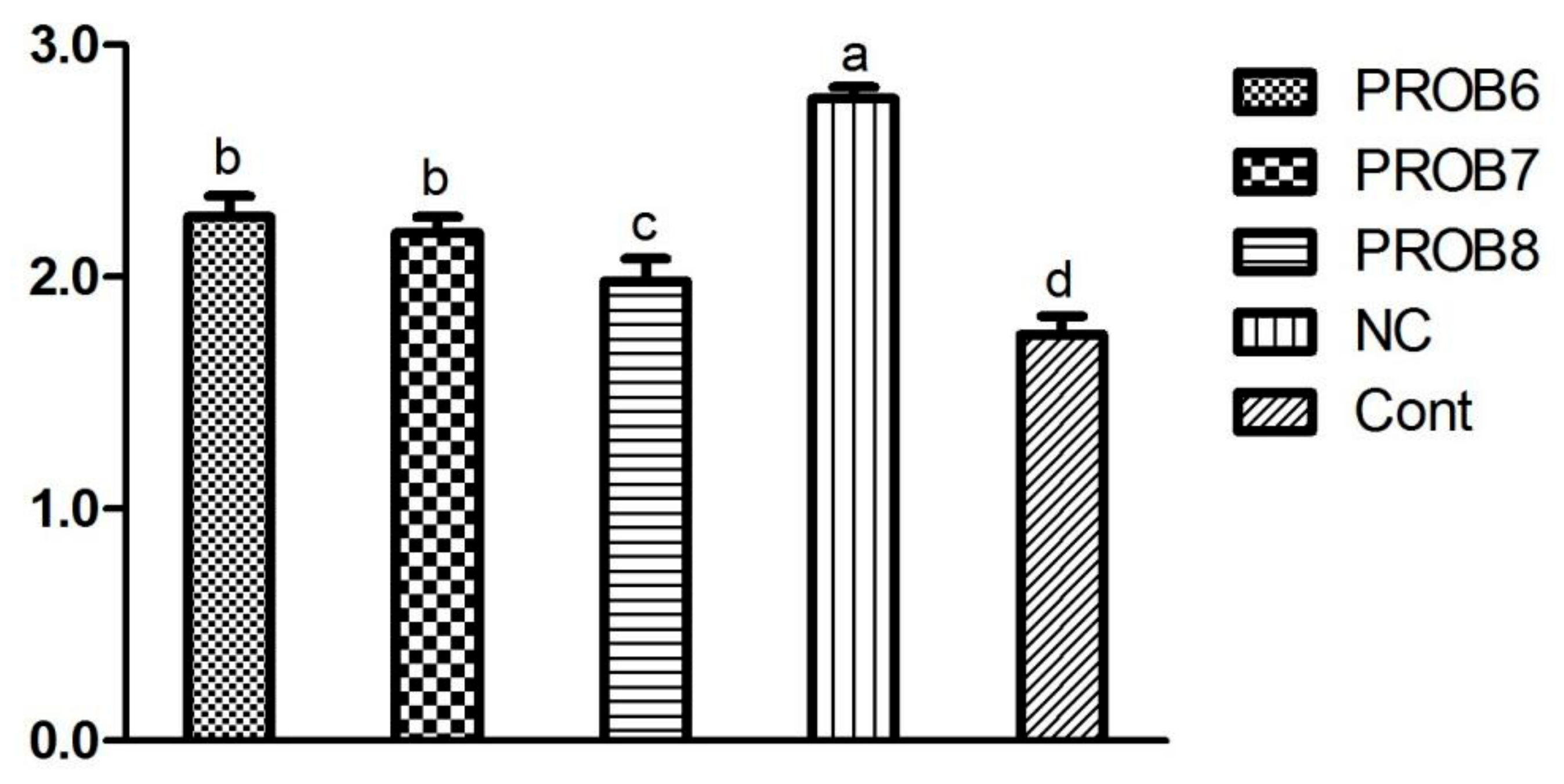
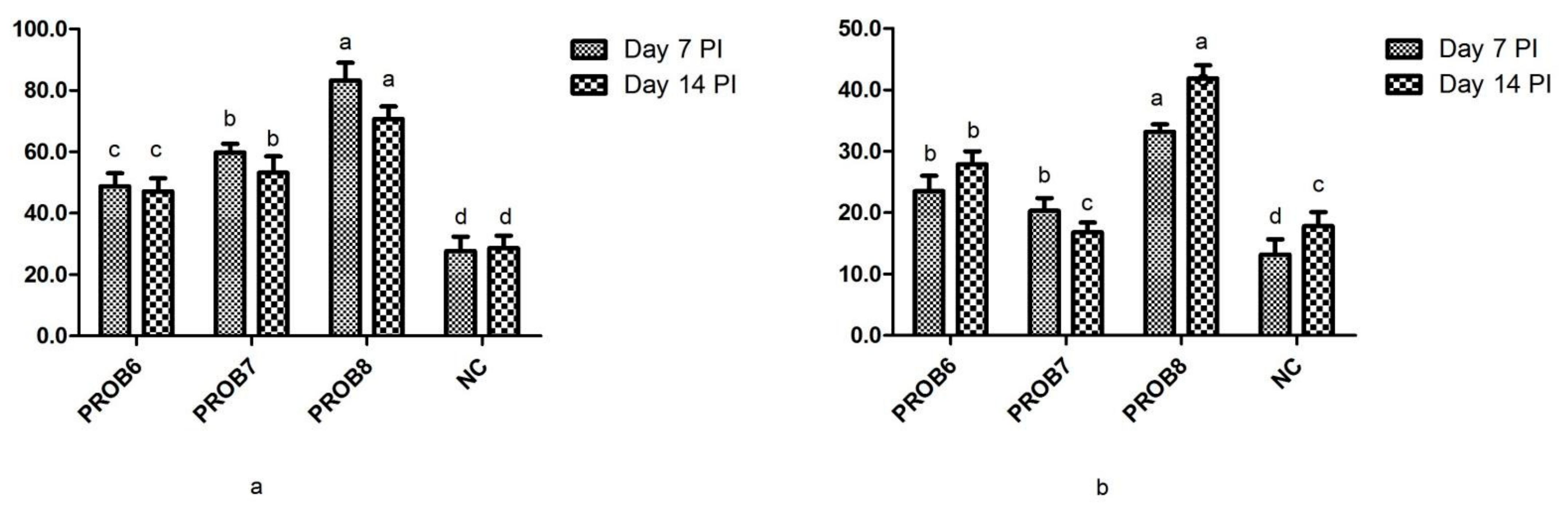
3.3. Cell-Mediated Immune Response
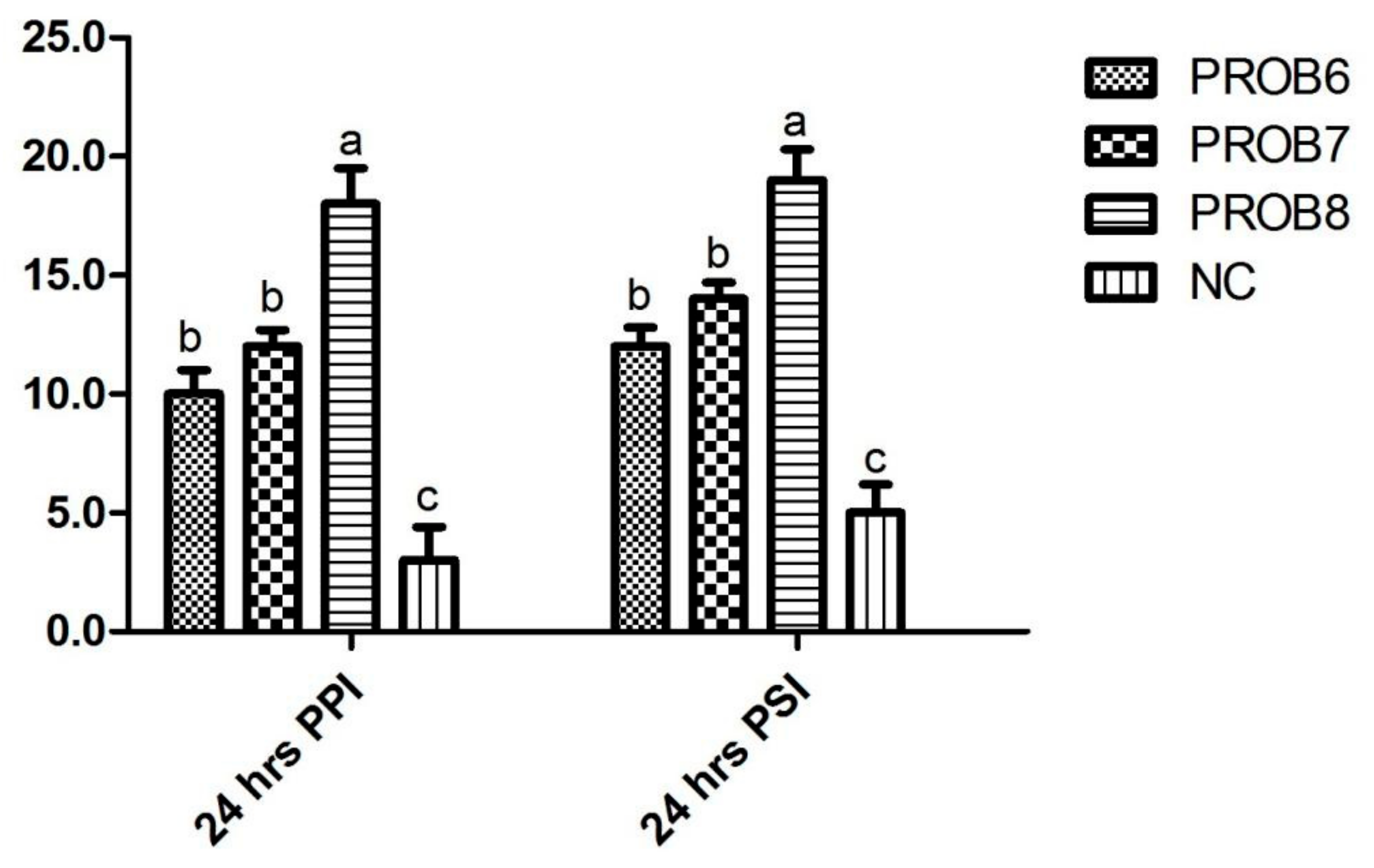
3.4. Humoral Immunity
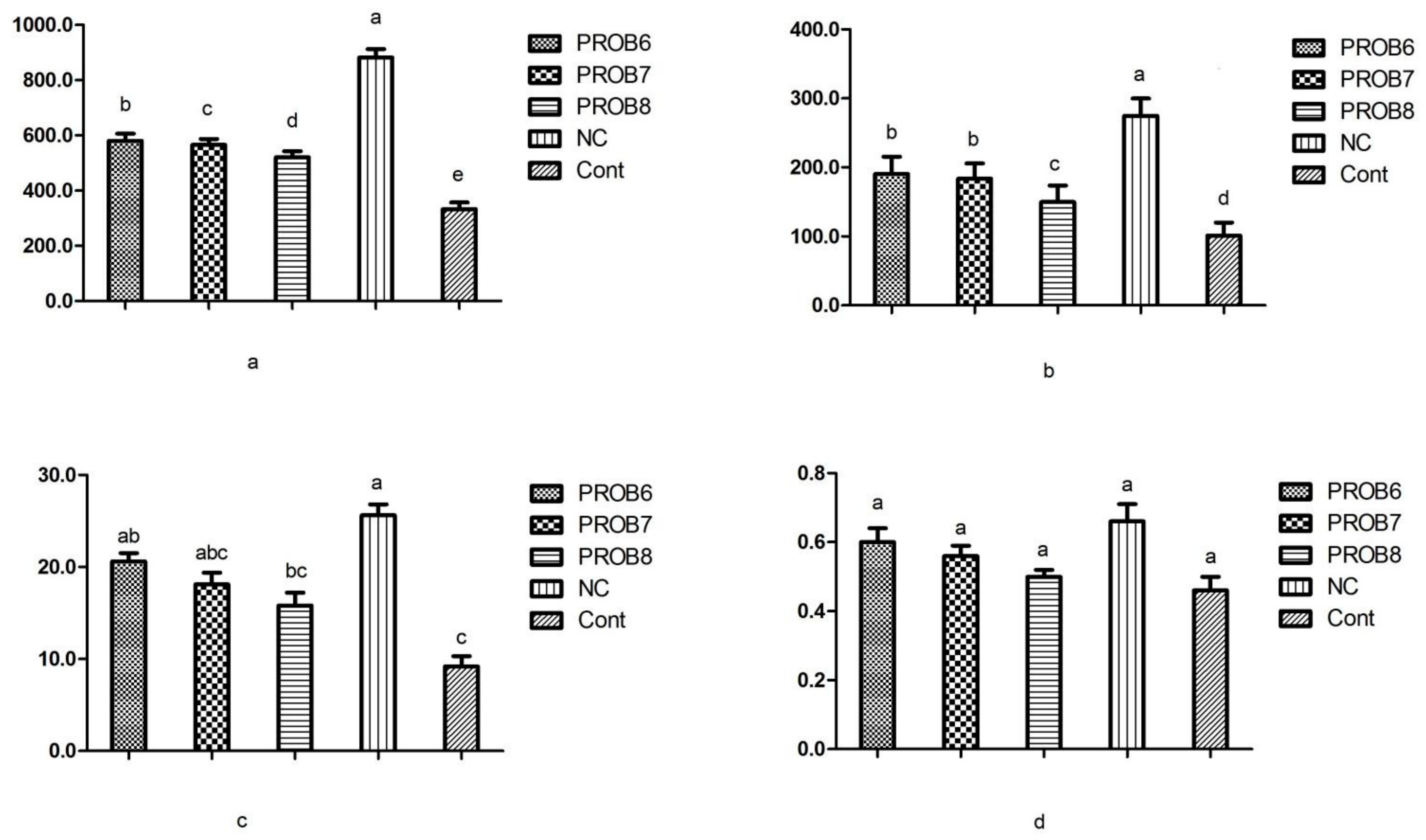
3.5. Serum Enzyme Levels for Toxicity
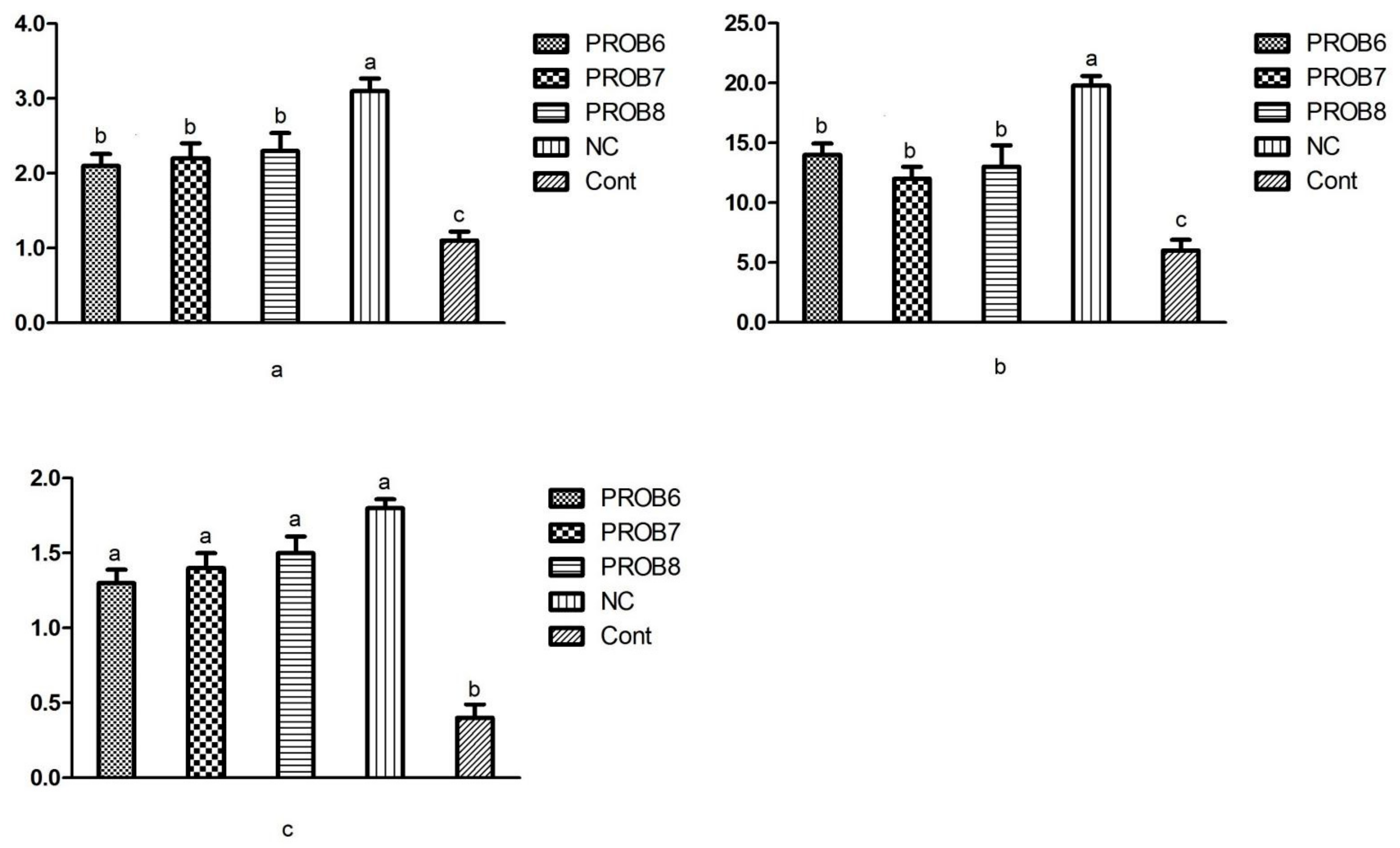
3.6. mRNA Gene Expression
3.6.1. Peptide Transporter 1 (PepT 1) and Antioxidant Enzymes mRNA Expression Levels
3.6.2. Gene Expression Levels of Tight Junction Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macpherson, A.J.; Harris, N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004, 4, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Mohsin, M.; Abbas, R.Z.; Li, L.; Chen, H.; Huang, C.; Li, Y.; Goraya, M.U.; Huang, Z.; Yin, G. Evaluation of immunogenicity and protective efficacy of Eimeria maxima immune mapped protein 1 with EDA adjuvant in chicken. Pak. Vet. J. 2020, 40, 209–213. [Google Scholar] [CrossRef]
- Mohsin, M.; Li, L.; Huang, X.; Aleem, M.T.; Habib, Y.J.; Ismael, A. Immunogenicity and Protective Efficacy of Probiotics with EtIMP1C against Eimeria tenella Challenge. Pak. Vet. J. 2021, 41, 274–278. [Google Scholar]
- Ritzi, M.M.; Abdelrahman, W.; Mohnl, M.; Dalloul, R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014, 93, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Abbas, R.Z.; Khan, M.K.; Raza, M.A.; Mahmood, M.S.; Saleemi, M.K.; Hussain, T.; Khan, J.A.; Sindhu, Z. Anticoccidial effects of Trachyspermum ammi (Ajwain) in broiler chickens. Pak. Vet. J. 2019, 39, 301–304. [Google Scholar] [CrossRef]
- Ritzi, M.M.; Abdelrahman, W.; Van-Heerden, K.; Mohnl, M.; Barrett, N.W.; Dalloul, R.A. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 2016, 47, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Huang, C.; Chen, Y.; Mohsin, M.; Li, L.; Lin, X.; Huang, Z.; Yin, G. Efficacy of recombinant N-and C-terminal derivative of EmIMP1 against E. maxima infection in chickens. Br. Poult. Sci. 2020, 61, 518–522. [Google Scholar] [CrossRef]
- Ramadan, M.; Elmadway, R.; Lashin, A.; ELdiarby, A. Immunization of lambs against coccidiosis by using ultraviolet irradiated Eimeria oocysts for the first time. Benha. Med. J. 2018, 34, 246–258. [Google Scholar] [CrossRef]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines 2006, 5, 143–163. [Google Scholar] [CrossRef]
- Chapman, H. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef]
- Chapman, H.; Cherry, T.; Danforth, H.; Richards, G.; Shirley, M.; Williams, R. Sustainable coccidiosis control in poultry production: The role of live vaccines. Int. J. Parasitol. 2002, 32, 617–629. [Google Scholar] [CrossRef]
- Mohsin, M.; Li, Y.; Zhang, X.; Wang, Y.; Huang, Z.; Yin, G.; Zhang, Z. Development of CRISPR-CAS9 based RNA drugs against Eimeria tenella infection. Genomics 2021, 113, 4126–4135. [Google Scholar] [CrossRef]
- Abbas, A.; Iqbal, Z.; Abbas, R.Z.; Khan, M.K.; Khan, J.A. Immunomodulatory activity of Pinus radiata extract against coccidiosis in broiler chicken. Pak. Vet. J. 2017, 37, 145–149. [Google Scholar]
- Mohsin, M.; Abbas, R.Z.; Yin, G.; Sindhu, Z.-U.-D.; Abbas, A.; Huang, Z.; Aleem, M.T.; Saeed, Z.; Afzal, M.Z.; Ejaz, A. Probiotics as therapeutic, antioxidant and immunomodulatory agents against poultry coccidiosis. World’s Poult. Sci. J. 2021, 77, 1–15. [Google Scholar] [CrossRef]
- Awais, M.M.; Jamal, M.A.; Akhtar, M.; Hameed, M.R.; Anwar, M.I.; Ullah, M.I. Immunomodulatory and ameliorative effects of Lactobacillus and Saccharomyces based probiotics on pathological effects of eimeriasis in broilers. Microb. Pathog. 2019, 126, 101–108. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Ofongo, R.T. The effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: A review. Int. J. Anim. Vet. Adv. 2012, 4, 135–143. [Google Scholar]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef] [Green Version]
- Simmering, R.; Blaut, M. Pro-and prebiotics—The tasty guardian angels? Appl. Microbiol. Biotechnol. 2001, 55, 19–28. [Google Scholar] [CrossRef]
- Pourakbari, M.; Seidavi, A.; Asadpour, L.; Martínez, A. Probiotic level effects on growth performance, carcass traits, blood parameters, cecal microbiota, and immune response of broilers. An. Da Acad. Bras. Ciências 2016, 88, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Maldonado Galdeano, M.C.; Lemme Dumit, J.M.; Thieblemont, N.; Carmuega, E.; Weill, R.; Perdigon, G.d.V. Stimulation of Innate Immune Cells Induced by Probiotics: Participation of Toll-Like Receptors. 2015. Available online: https://www.longdom.org/open-access/stimulation-of-innate-immune-cells-induced-by-probiotics-participation-of-toll-like-receptors-2155-9899-1000283.pdf (accessed on 4 January 2022).
- Azad, M.; Kalam, A.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [Green Version]
- West, C.E.; Gothefors, L.; Granström, M.; Käyhty, H.; Hammarström, M.L.K.; Hernell, O. Effects of feeding probiotics during weaning on infections and antibody responses to diphtheria, tetanus and Hib vaccines. Pediatr. Allergy Immunol. 2008, 19, 53–60. [Google Scholar] [CrossRef]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Tewari, A.; Maharana, B. Control of poultry coccidiosis: Changing trends. J. Parasit. Dis. 2011, 35, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Praharaj, I.; John, S.M.; Bandyopadhyay, R.; Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140144. [Google Scholar] [CrossRef] [Green Version]
- Wakelin, D.; Rose, M.E. Immunity to coccidiosis. In Coccidiosis of Man and Domestic Animals; CRC Press: Boca Raton, FL, USA, 2019; pp. 281–306. [Google Scholar]
- Yadav, S.; Teng, P.-Y.; Dos Santos, T.S.; Gould, R.L.; Craig, S.W.; Fuller, A.L.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Lillehoj, E.P. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. 2000, 44, 408–425. [Google Scholar] [CrossRef]
- Yin, G.; Qin, M.; Liu, X.; Suo, J.; Tang, X.; Tao, G.; Han, Q.; Suo, X.; Wu, W. An Eimeria vaccine candidate based on Eimeria tenella immune mapped protein 1 and the TLR-5 agonist Salmonella typhimurium FliC flagellin. Biochem. Biophys. Res. Commun. 2013, 440, 437–442. [Google Scholar] [CrossRef]
- Ministry of Agriculture Großbritannien. Manual of Veterinary Parasitological Laboratory Techniques: 160 S.: Ill; HM Stationery Office: London, UK, 1986; 129p.
- Youn, H.-J.; Kang, Y.-B.; Jang, D.-H. Effects of γ-irradiation from cobalt-60 on pathogenicity of Eimeria tenella. Korean J. Vet. Res. 1993, 33, 649–655. [Google Scholar]
- Bleumink, E.; Nater, J.P.; Koops, H.S.; The, T. A standard method for DNCB sensitization testing in patients with neoplasms. Cancer 1974, 33, 911–915. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lillehoj, H.S.; Park, D.W.; Hong, Y.H.; Lin, J. Effects of Pediococcus-and Saccharomyces-based probiotic (MitoMax®) on coccidiosis in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Iqbal, Z.; Mansoor, M.; Sindhu, Z.; Zia, M.; Khan, J. Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J. 2013, 33, 401–407. [Google Scholar]
- Idris, M.; Abbas, R.; Masood, S.; Rehman, T.; Farooq, U.; Babar, W.; Hussain, R.; Raza, A.; Riaz, U. The potential of antioxidant rich essential oils against avian coccidiosis. World’s Poult. Sci. J. 2017, 73, 89–104. [Google Scholar] [CrossRef]
- Shen, X.; Yi, D.; Ni, X.; Zeng, D.; Jing, B.; Lei, M.; Bian, Z.; Zeng, Y.; Li, T.; Xin, J. Effects of Lactobacillus plantarum on production performance, immune characteristics, antioxidant status, and intestinal microflora of bursin-immunized broilers. Can. J. Microbiol. 2014, 60, 193–202. [Google Scholar] [CrossRef]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zuo, W.; Li, F. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning. J. Anim. Sci. 2019, 97, 1693–1700. [Google Scholar] [CrossRef]
- Memon, F.; Yang, Y.; Lv, F.; Soliman, A.; Chen, Y.; Sun, J.; Wang, Y.; Zhang, G.; Li, Z.; Xu, B. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J. Appl. Microbiol. 2021, 131, 425–434. [Google Scholar] [CrossRef]
- Mountzouris, K.; Tsirtsikos, P.; Kalamara, E.; Nitsch, S.; Schatzmayr, G.; Fegeros, K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007, 86, 309–317. [Google Scholar] [CrossRef]
- Crittenden, R.; Bird, A.R.; Gopal, P.; Henriksson, A.; Lee, Y.; Playne, M. Probiotic research in Australia, New Zealand and the Asia-Pacific region. Curr. Pharm. Des. 2005, 11, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Dalloul, R.A.; Lillehoj, H.S. Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis. 2005, 49, 1–8. [Google Scholar] [CrossRef]
- Rahman, M.; Mustari, A.; Salauddin, M.; Rahman, M. Effects of probiotics and enzymes on growth performance and haematobiochemical parameters in broilers. J. Bangladesh Agril. Univ. 2013, 11, 111–118. [Google Scholar] [CrossRef] [Green Version]

| Genes | Primer Sequence (5′–3′) | Gene Bank | |
|---|---|---|---|
| CAT | F | GAGGAACCCTCAGACTCATTTG | NM_001031215.2 |
| R | CCATCAGGAATACCACGATCAC | ||
| SOD 1 | F | AGATGGCAGTGGGAAATGAG | NM_205064.1 |
| R | ACTCAAGACAGCAGAGTAGTAATG | ||
| PepT 1 | F | CCCCTGAGGAGGATCACTGTT | KF366603.1 |
| R | CAAAAGAGCAGCAGCAACGA | ||
| CLDN 1 | F | ACTCCTGGGTCTGGTTGGT | AY750897.1 |
| R | CAGGTCAAACAGAGGTACAGG | ||
| ZO 1 | F | CTTCAGGTGTTTCTCTTCCTCCTC | XM_413773 |
| R | CTGTGGTTTCATGGCTGGATC | ||
| β-actin | F | GAGAAATTGTGCGTGACATCA | L08165 |
| R | CCTGAACCTCTCATTGCCA | ||
| Groups | Day 5th | Day 6th | Day 8th |
|---|---|---|---|
| PROB6 | 2.50 ± 0.33 b | 2.83 ± 0.27 b | 2.33 ± 0.54 b |
| PROB7 | 2.33 ± 0.54 b | 2.66 ± 0.14 b | 2.00 ± 0.51 b |
| PROB8 | 1.67 ± 0.14 c | 1.67 ± 0.15 c | 1.33 ± 0.21 c |
| NC | 3.33 ± 0.15 a | 3.50 ± 0.16 a | 3.33 ± 0.15 a |
| Cont | _ | _ | _ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsin, M.; Zhang, Z.; Yin, G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines 2022, 10, 97. https://doi.org/10.3390/vaccines10010097
Mohsin M, Zhang Z, Yin G. Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines. 2022; 10(1):97. https://doi.org/10.3390/vaccines10010097
Chicago/Turabian StyleMohsin, Muhammad, Ziping Zhang, and Guangwen Yin. 2022. "Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella" Vaccines 10, no. 1: 97. https://doi.org/10.3390/vaccines10010097
APA StyleMohsin, M., Zhang, Z., & Yin, G. (2022). Effect of Probiotics on the Performance and Intestinal Health of Broiler Chickens Infected with Eimeria tenella. Vaccines, 10(1), 97. https://doi.org/10.3390/vaccines10010097





