COVID-19 Vaccine Hesitancy among Patients with Inflammatory Bowel Disease Receiving Biologic Therapies in Kuwait: A Cross-Sectional Study
Abstract
:1. Introduction
2. Material and Methods
Study Design and Recruitment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cucinotta, D.; Vanelli, M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Cihan, P. Forecasting fully vaccinated people against COVID-19 and examining future vaccination rate for herd immunity in the US, Asia, Europe, Africa, South America, and the World. Appl. Soft Comput. 2021, 111, 107708. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Noviello, D.; Conforti, F.S.; Aloi, M.; Armuzzi, A.; Bossa, F.; Ficari, F.; Leone, S.; Manguso, F.; Mocci, G.; et al. COVID-19 Vaccination Willingness and Hesitancy in Patients With Inflammatory Bowel Diseases: Analysis of Determinants in a National Survey of the Italian IBD Patients’ Association. Inflamm. Bowel Dis. 2021, 1–5. Available online: https://academic.oup.com/ibdjournal/advance-article/doi/10.1093/ibd/izab172/6321213 (accessed on 30 November 2021).
- Alrashed, F.; Battat, R.; Abdullah, I.; Charabaty, A.; Shehab, M. Impact of medical therapies for inflammatory bowel disease on the severity of COVID-19: A systematic review and meta-analysis. BMJ Open Gastroenterol. 2021, 8, e000774. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-Y.; Dixon, R.; Pazos, V.M.; Gnjatic, S.; Colombel, J.-F.; Cadwell, K.; Gold, S.; Helmus, D.; Neil, J.A.; Sota, S.; et al. Serologic Response to Messenger RNA Coronavirus Disease 2019 Vaccines in Inflammatory Bowel Disease Patients Receiving Biologic Therapies. Gastroenterology 2021, 161, 715–718.e4. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. Available online: https://gut.bmj.com/content/70/10/1884 (accessed on 30 November 2021). [CrossRef] [PubMed]
- COVID-19 and IBD Reporting Database|SECURE-IBD Database. Available online: https://covidibd.org/ (accessed on 30 November 2021).
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology 2020, 159, 481–491.e3. [Google Scholar] [CrossRef]
- Rubin, D.T.; Abreu, M.T.; Rai, V.; Siegel, C.A.; Ahuja, V.; Allez, M.; Ananthakrishnan, A.N.; Bernstein, C.N.; Braun, J.G.; Chowers, Y.; et al. Management of Patients with Crohn’s Disease and Ulcerative Colitis During the Coronavirus Disease-2019 Pandemic: Results of an International Meeting. Gastroenterology 2020, 159, 6–13.e6. [Google Scholar] [CrossRef]
- Covidvax.live-Kuwait. 2021. Available online: https://covidvax.live/location/kwt (accessed on 30 November 2021).
- Gallè, F.; Sabella, E.A.; Roma, P.; De Giglio, O.; Caggiano, G.; Tafuri, S.; Da Molin, G.; Ferracuti, S.; Montagna, M.T.; Liguori, G.; et al. Knowledge and Acceptance of COVID-19 Vaccination among Undergraduate Students from Central and Southern Italy. Vaccines 2021, 9, 638. [Google Scholar] [CrossRef]
- Comirnaty and Pfizer-BioNTech COVID-19 Vaccine|FDA. 2021. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine#additional (accessed on 30 November 2021).
- ICD-10 Version: 2019. 2021. Available online: https://icd.who.int/browse10/2019/en#/K52 (accessed on 30 November 2021).
- Vieira Rezende, R.P.; Braz, A.S.; Guimarães, M.F.B.; Ribeiro, S.L.E.; Abreu Vieira, R.M.R.; Bica, B.E.; Cruz, V.A.; Machadoh, K.L.L.L.; Carvalho, J.S.; Monticielo, O.A.; et al. Characteristics associated with COVID-19 vaccine hesitancy: A nationwide survey of 1000 patients with immune-mediated inflammatory diseases. Vaccine 2021, 39, 6454–6459. [Google Scholar] [CrossRef]
- COVID-19: Science in 5: Episode #1—Herd Immunity. 2021. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-resources/science-in-5/episode-1 (accessed on 30 November 2021).
- Wang, W.; Wu, Q.; Yang, J.; Dong, K.; Chen, X.; Bai, X.; Chen, X.; Chen, Z.; Viboud, C.; Ajelli, M.; et al. Global, regional, and national estimates of target population sizes for COVID-19 vaccination: Descriptive study. BMJ 2020, 371, m4704. [Google Scholar] [CrossRef]
- Alaskar, D.; AlAmeel, T.; Al Besher, M.; Al Sulais, E. Attitude of patients with IBD toward COVID-19 vaccine in Saudi Arabia. United Eur. Gastroenterol. J. 2021, 9, 530. [Google Scholar] [CrossRef]
- Bertakis, K.D.; Azari, R.; Helms, L.J.; Callahan, E.J.; Robbins, J.A. Gender differences in the utilization of health care services. J. Fam. Pract. 2000, 49, 147. [Google Scholar]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Women’s Health 2021, 2, 761511. [Google Scholar] [CrossRef]
- Priori, R.; Pellegrino, G.; Colafrancesco, S.; Alessandri, C.; Ceccarelli, F.; Di Franco, M.; Riccieri, V.; Scrivo, R.; Scavalli, A.S.; Spinelli, F.R.; et al. Pos1219 SARS-COV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: A message for rheumatologists. Ann. Rheum. Dis. 2021, 80, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Selim, R.; Wellens, J.; Marlow, L.; Satsangi, J.J. SARS-CoV-2 vaccination uptake by patients with inflammatory bowel disease on biological therapy. Lancet Gastroenterol. Hepatol. 2021. Available online: http://www.thelancet.com/article/S2468125321003472/fulltext (accessed on 30 November 2021).
- Walldorf, J.; von Arnim, U.; Schmelz, R.; Riesner-Wehner, A.; Michl, P.; Grunert, P.C.; Stallmach, A.; Teich, N.; Reuken, P.A. SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease—Fear and Desire. Inflamm. Bowel Dis. 2021, 27, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines, Pregnancy and Breastfeeding. 2021. Available online: https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/ (accessed on 30 November 2021).
- COVID-19 Vaccination Considerations for Obstetric–Gynecologic Care|ACOG. 2021. Available online: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care (accessed on 30 November 2021).
- Helen Skirrow, A.; Barnett, S.; Bell, S.; Riaposova, L.; Mounier-Jack, S.; Kampmann, B.; Holder, B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: A multi-methods study in the UK. medRxiv. 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.04.30.21256240v1 (accessed on 30 November 2021).
- International Organization for the Study of Inflammatory Bowel Disease|to Promote the Health of People with IBD Worldwide. 2021. Available online: https://ioibd.org/ (accessed on 30 November 2021).
- Botwin, G.J.; Li, D.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P.B.; Melmed, G.Y. Adverse Events After SARS-CoV-2 mRNA Vaccination Among Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2021, 116, 1746–1751. Available online: https://pubmed.ncbi.nlm.nih.gov/34047304/ (accessed on 30 November 2021). [CrossRef]
- Siegel, C.A.; Melmed, G.Y.; McGovern, D.P.B.; Rai, V.; Krammer, F.; Rubin, D.T.; Abreu, M.T.; Dubinsky, M.C. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut 2021, 70, 635. [Google Scholar] [CrossRef]
- Shehab, M.; Abu-Farha, M.; Alrashed, F.; Alfadhli, A.; Alotaibi, K.; Alsahli, A.; Thanaraj, T.A.; Channanath, A.; Ali, H.; Abubaker, J.; et al. Immunogenicity of BNT162b2 Vaccine in Patients with Inflammatory Bowel Disease on Infliximab Combination Therapy: A Multicenter Prospective Study. J. Clin. Med. 2021, 10, 5362. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alfadhli, A.; Alotaibi, K.; Alsahli, A.; Mohammad, H.; Cherian, P.; Al-Khairi, I.; Thanaraj, T.A.; Channanath, A.; et al. Serological Response to BNT162b2 and ChAdOx1 nCoV-19 Vaccines in Patients with Inflammatory Bowel Disease on Biologic Therapies. Vaccines 2021, 9, 1471. [Google Scholar] [CrossRef]
- Curigliano, G.; Eggermont, A.M. Adherence to COVID-19 vaccines in cancer patients: Promote it and make it happen! Eur. J. Cancer 2021, 153, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Falzone, L.; Torino, F.; Scandurra, G.; Russo, G.; Bordonaro, R.; Pappalardo, F.; Spandidos, D.A.; Raciti, G.; Libra, M. Immune-checkpoint inhibitors from cancer to COVID-19: A promising avenue for the treatment of patients with COVID-19. Int. J. Oncol. 2020, 58, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Trillo Aliaga, P.; Trapani, D.; Sandoval, J.L.; Crimini, E.; Antonarelli, G.; Vivanet, G.; Morganti, S.; Corti, C.; Tarantino, P.; Friedlaender, A.; et al. Safety of COVID-19 mRNA Vaccines in Patients with Cancer Enrolled in Early-Phase Clinical Trials. Cancers 2021, 13, 5829. [Google Scholar] [CrossRef]
- Cavanna, L.; Citterio, C.; Biasini, C.; Madaro, S.; Bacchetta, N.; Lis, A.; Cremona, G.; Muroni, M.; Bernuzzi, P.; Cascio, G.L.; et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. Eur. J. Cancer 2021, 157, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, S.; Falzone, L.; Grillo, C.M.; Scandurra, G.; Torino, F.; Libra, M. Cancer Management during COVID-19 Pandemic: Is Immune Checkpoint Inhibitors-Based Immunotherapy Harmful or Beneficial? Cancers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Biswas, M.R.; Alzubaidi, S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z.; Barattucci, M. A Scoping Review to Find Out Worldwide COVID-19 Vaccine Hesitancy and Its Underlying Determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef]
- Dubé, E.; Vivion, M.; MacDonald, N.E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: Influence, impact and implications. Expert Rev. Vaccines 2015, 14, 99–117. [Google Scholar] [CrossRef]
- Troiano, G.; Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef]
| Variable | Study Group (n = 280) |
|---|---|
| Mean age (years) | 33.2 |
| Sex n (%) | |
| Male | 157 (56.0%) |
| Female | 123 (44.0%) |
| BMI (Median) | 24.8 |
| Smoking n (%) | 58 (20.0%) |
| Comorbidities n (%) | |
| Diabetes | 19 (6.7%) |
| OSA | 5 (1.7%) |
| Hypertension | 9 (3.2%) |
| Cardiovascular Disease | 9 (3.2%) |
| Arthritis | 14 (5.0%) |
| Kidney | 9 (3.2%) |
| Asthma | 38 (13.6%) |
| Hyperlipidemia | 9 (3.2%) |
| Median infliximab therapy (months) Median vedolizumab therapy (months) | 12 11 |
| Disease extent, n (%) | |
| Ulcerative colitis (UC) | 112 (40.0%) |
| E1: ulcerative proctitis | 20 (17.8%) |
| E2: left sided colitis | 32 (28.5%) |
| E3: extensive colitis | 60 (53.6%) |
| Crohn’s disease (CD) | 168 (60.0%) |
| L1: ileal | 84 (50.0%) |
| L2: colonic | 20 (11.9%) |
| L3: ileocolonic | 59 (35.2%) |
| L4: upper gastrointestinal | 5 (2.8%) |
| B1: inflammatory | 75 (44.6%) |
| B2: stricturing | 44 (26.2%) |
| B3: penetrating | 49 (29.2%) |
| Demographics/Vaccination Status | Total Number of Patients (280) | Vaccinated Patients (117) | Unvaccinated Patients (163) |
|---|---|---|---|
| Ulcerative Colitis | 112 | 49 (43.8%) | 63 (56.2%) |
| Crohn’s Disease | 168 | 68 (40.5%) | 100 (59.5%) |
| One dose | 25 | 25 | N/A |
| Two doses | 92 | 92 | N/A |
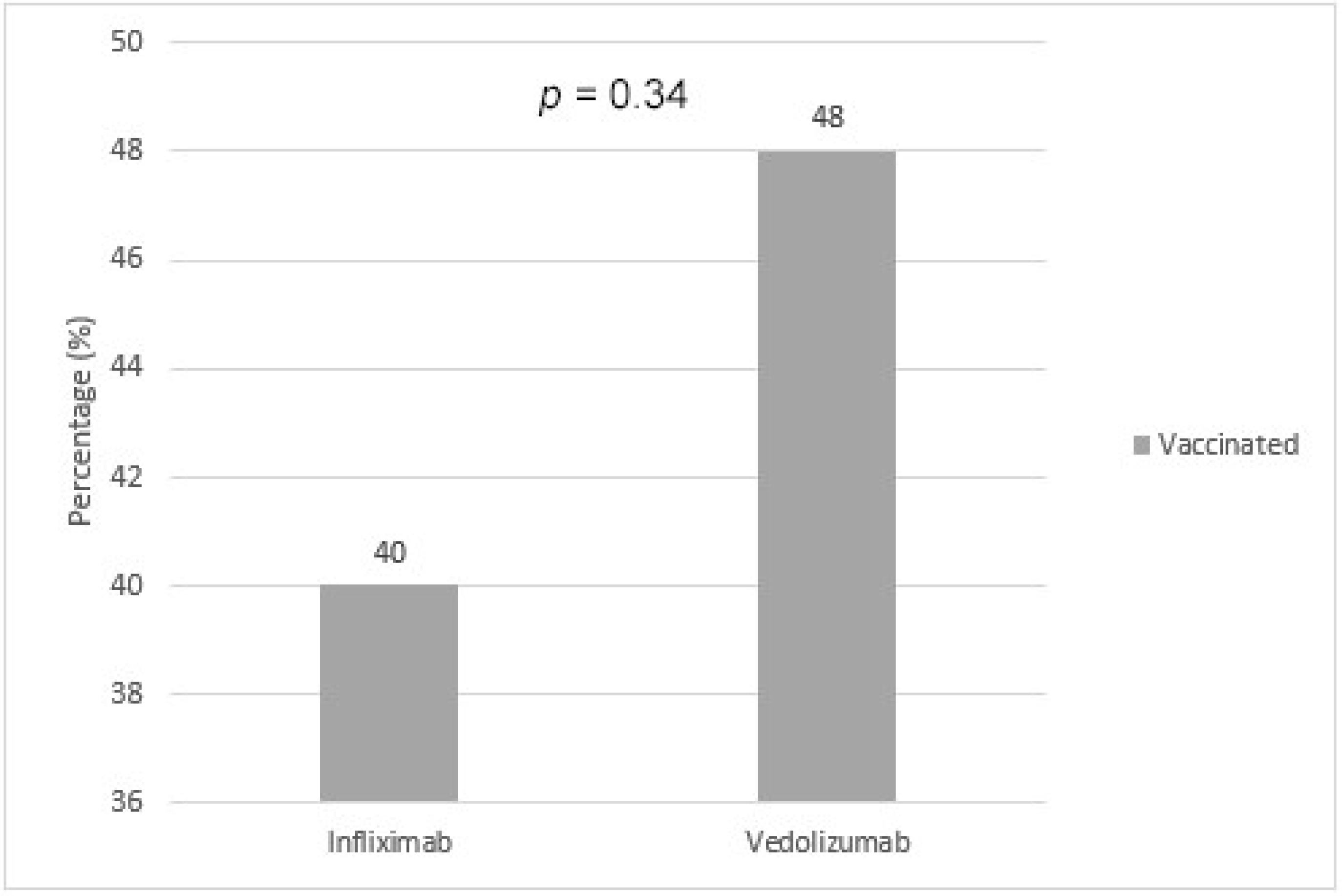
| Infliximab | 232 | 94 (40.0%) | 138 (60.0%) |
| Vedolizumab | 48 | 23 (48.0%) | 25 (52.0%) |
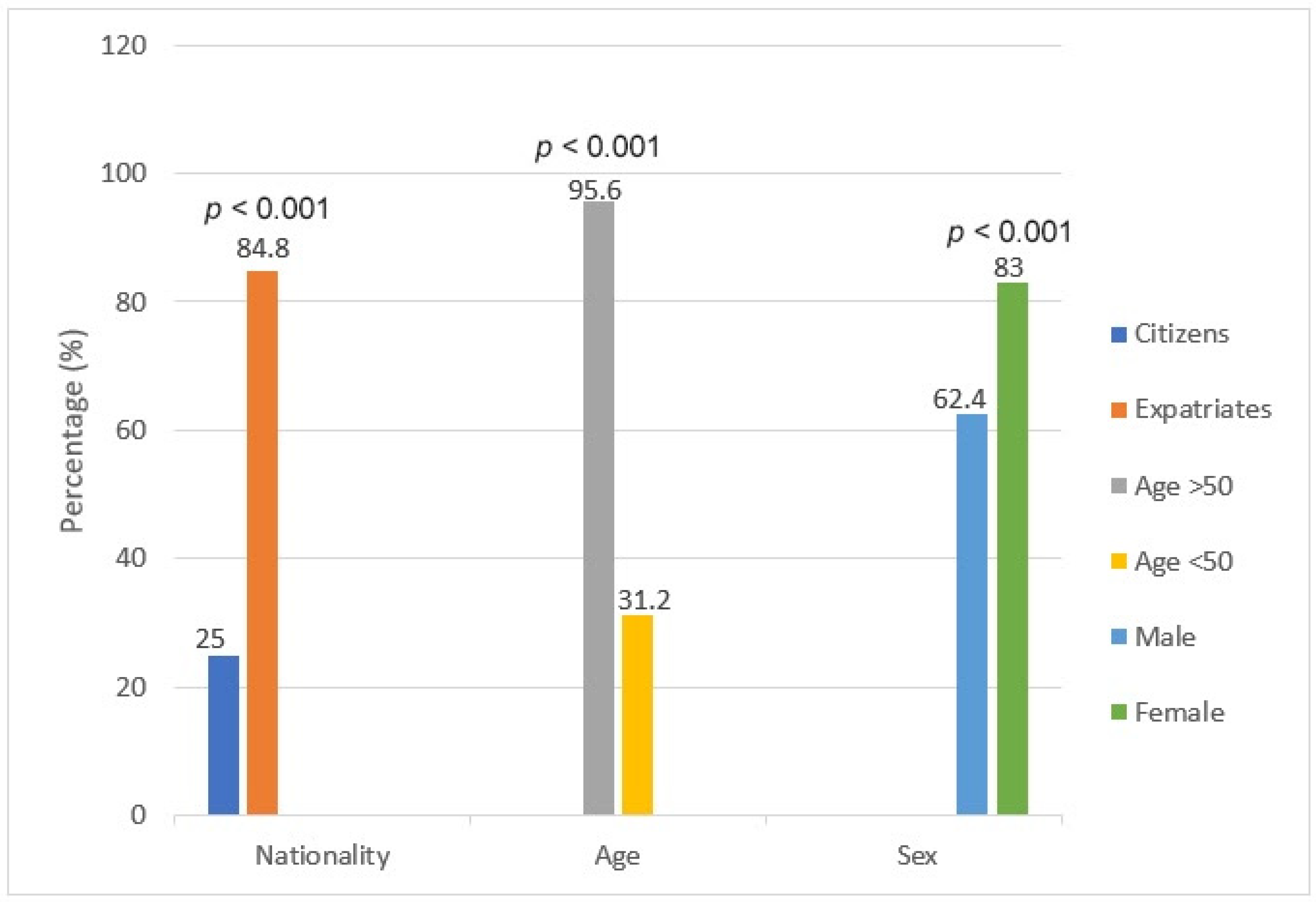
| Age > 50 | 46 | 44 (95.6%) | 2 (4.4%) |
| Age < 50 | 234 | 73 (31.2%) | 161 (68.8%) |
| Citizens | 201 | 50 (25.0%) | 151 (75.0%) |
| Expatriates | 79 | 67 (84.8%) | 12 (15.2%) |
| Male | 157 | 98 (62.4%) | 59 (37.6%) |
| Female | 123 | 102 (83.0%) | 21 (17.1%) |
| Pregnant | 5 | 0 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehab, M.; Zurba, Y.; Al Abdulsalam, A.; Alfadhli, A.; Elouali, S. COVID-19 Vaccine Hesitancy among Patients with Inflammatory Bowel Disease Receiving Biologic Therapies in Kuwait: A Cross-Sectional Study. Vaccines 2022, 10, 55. https://doi.org/10.3390/vaccines10010055
Shehab M, Zurba Y, Al Abdulsalam A, Alfadhli A, Elouali S. COVID-19 Vaccine Hesitancy among Patients with Inflammatory Bowel Disease Receiving Biologic Therapies in Kuwait: A Cross-Sectional Study. Vaccines. 2022; 10(1):55. https://doi.org/10.3390/vaccines10010055
Chicago/Turabian StyleShehab, Mohammad, Yasmin Zurba, Ali Al Abdulsalam, Ahmad Alfadhli, and Sara Elouali. 2022. "COVID-19 Vaccine Hesitancy among Patients with Inflammatory Bowel Disease Receiving Biologic Therapies in Kuwait: A Cross-Sectional Study" Vaccines 10, no. 1: 55. https://doi.org/10.3390/vaccines10010055
APA StyleShehab, M., Zurba, Y., Al Abdulsalam, A., Alfadhli, A., & Elouali, S. (2022). COVID-19 Vaccine Hesitancy among Patients with Inflammatory Bowel Disease Receiving Biologic Therapies in Kuwait: A Cross-Sectional Study. Vaccines, 10(1), 55. https://doi.org/10.3390/vaccines10010055





