Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach
Abstract
1. Introduction
| Extraction Method | TPC mg GAE/g | FRAP TEAC (µmol TE/g) | ABTS TEAC (µmol TE/g) | DPPH TEAC (µmol TE/g) | References |
|---|---|---|---|---|---|
| 2 g SCG in 100 mL pure water at 60 °C for 30 min | 6.33–19.62 * | - | - | [6] | |
| 2 g SCG in 100 mL ethanol at 60 °C for 30 min | 11.83–28.26 * | - | - | [6] | |
| Ethanol extraction 40 mL solvent/g SCG, 70% EtOH, 50 °C, 2 h | 17.09 | - | - | - | [9] |
| Subcritical water extraction 179 °C, 36 min 14.1 g SCG/L | 88.34 | - | 886.50 § | 382.8 § | [14] |
| Subcritical water extraction (different temperatures, times, and ratio solid to liquid) | 21–56 * | - | 70–320 §* | 50–220 §* | [14] |
| Autohydrolysis 15 mL water/g SCG, 200 °C, 50 min | 40.36 | - | 125.69 | 112.47 | [15] |
| Boiling water 10 g SCG/L, 10 min | 5.66 ± 0.07 | - | - | - | [19] |
| Ultrasound-assisted solid-liquid extraction 1g in 100 mL ethanol T = 30–50 °C Time= 5–45 min | 33–36 * | - | - | - | [10] |
| Ultrasound Methanol/water 0.49-1.50 w/w Time = 9–112 min Time = 0.60–7.40 min | 19–25 * | 134–174 * | 81–146 * | [11] | |
| Soxhlet 2 g in 250 mL of hexane, 5 h | 273.34 ± 34.17 | - | - | 148.40 ± 30.43 | [13] |
| Supercritical fluid extraction Pressure = 100–300 T = 40–60 °C | 17–28 * | - | 38–54 * | - | [12] |
| Soxhlet 5 g in 150 mL hexane, 6 h | 65–151 * | - | 98–381 * | - | [12] |
| Ultrasounds 7 g in 210 mL Room temperature 6 h (dichloromethane, ethanol, or ethylacecate) | 61–133.4 * | - | 128–161 * | - | [12] |
2. Materials and Methods
2.1. Materials and Standards
2.2. Sample Extraction
2.3. HPLC Analysis
2.4. Method Validation
2.5. Optimization of the Extraction Conditions
2.6. Total Phenol Content Determination
2.7. Evaluation of Antioxidant Capacity
2.8. Determination of Metals and Minerals by Inductively Coupled Plasma Mass Spectrometry (ICP–MS)
2.9. Determination of Cytotoxicity and Antioxidant Activity in Cells
2.9.1. Cell Cultures
2.9.2. Cytotoxicity
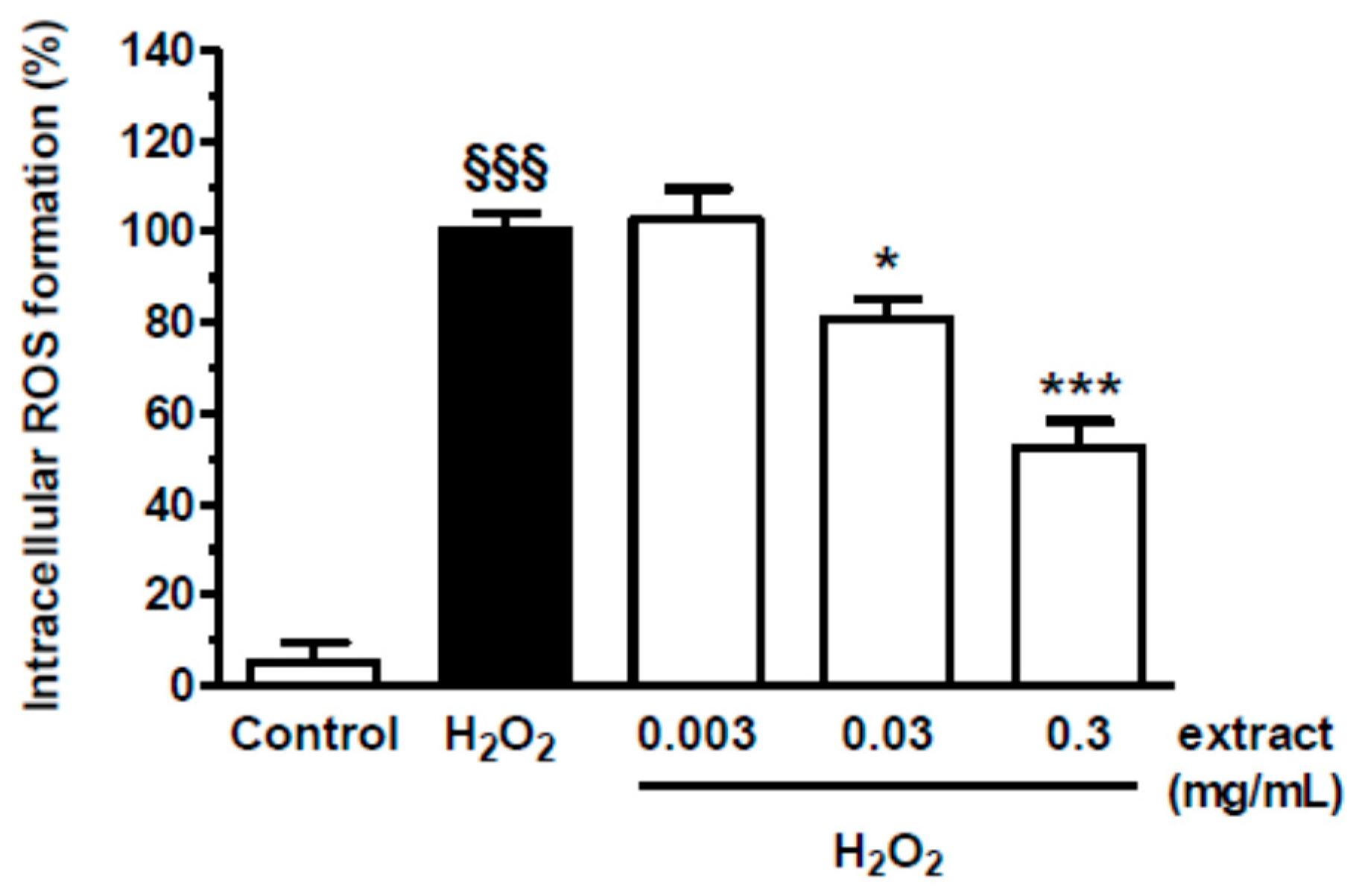
2.9.3. Intracellular Reactive Oxygen Species (ROS) Formation
2.10. Statistical Analysis
3. Results
3.1. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach
3.2. Evaluation of Total Phenol Content and Antioxidant Capacity of Spent Coffee Extracts
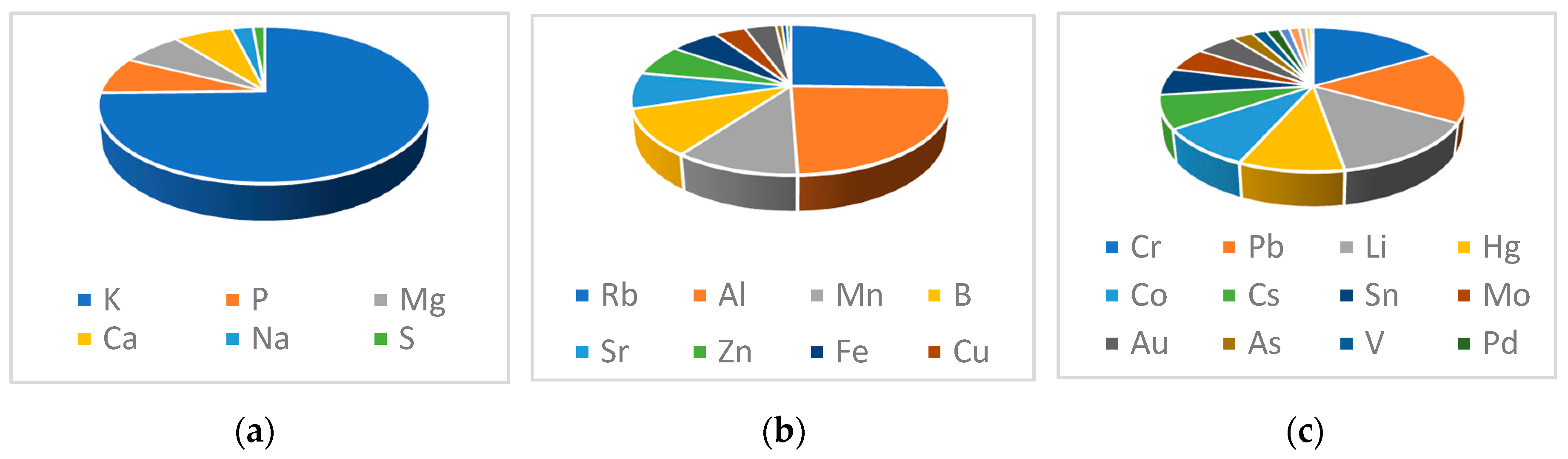
3.3. Determination of Metals and Minerals by Inductively Coupled Plasma Mass Spectrometry (ICP–MS)
3.4. In Vitro Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-GarcíaFrancisco, J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Campos, D.; Gómez-García, R.; Vilas-Boas, A.; Madureira, A.; Pintado, M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Internation Coffee Organization. Available online: http://www.ico.org/ (accessed on 26 March 2020).
- Jeszka-Skowron, M.; Stanisz, E.; De Peña, M.P. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT—Food Sci. Technol. 2016, 73, 243–250. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Taguchi, H.; Sakaguchi, M.; Shimabayashi, Y. Trigonelline Content in Coffee Beans and the Thermal Conversion of Trigonelline into Nicotinic Acid during the Roasting of Coffee Beans. Agric. Biol. Chem. 1985, 49, 3467–3471. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Naif Abdullah, A.-D.; Karuppiah, P.; Prakash, M.J. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Fiore, A.G. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: a study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2016, 243, 835–847. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira Sandra, R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Spent Coffee Grounds as a Renewable Source of Bioactive Compounds. J. Biobased Mater. Bioenergy 2013, 7, 1–9. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crop Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Optimization of autohydrolysis conditions to extract antioxidant phenolic compounds from spent coffee grounds. J. Food Eng. 2017, 199, 1–8. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants – a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Biondo, E.; Kolchinski, E.M.; da Silva Luís, F.S.; Corrêa Ana, P.F.; Bach, E.; Brandelli, A. Total Polyphenols, Antioxidant, Antimicrobial and Allelopathic Activities of Spend Coffee Ground Aqueous Extract. Waste Biomass Valorization 2017, 8, 439–442. [Google Scholar] [CrossRef]
- Box George, E.; Hunter William, G.; Hunter, J. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Zuorro, A. Optimization of polyphenol recovery from espresso coffee residues using factorial design and response surface methodology. Sep. Purif. Technol. 2015, 152, 64–69. [Google Scholar] [CrossRef]
- Ranic, M.; Nikolic, M.; Pavlovic, M.; Buntic, A.; Siler-Marinkovic, S.; Dimitrijevic-Brankovic, S. Optimization of microware-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J. Clean. Prod. 2014, 69–79. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: the influence of espresso machines and coffee cultivars. Int. J. Food Sci. Nutr. 2014, 65, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; Di Martino, P. Quantification of phenolic compounds in different types of crafts beers, worts, starting and spent ingredients by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Mathieu, D.; Phan, T.-L. Pharmaceutical Experimental Design; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F.; et al. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef]
- Censi, R.; Vargas Peregrina, D.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.G.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Maleci Bini, L.; Giuliani, C.; et al. Phytochemical Analysis, Biological Activity, and Secretory Structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from Central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [CrossRef]
- Benzie Iris, F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Papa, F.; Vittori, S.; Maggi, F.; et al. Chemopreventive and Antioxidant Activity of the Chamazulene-Rich Essential Oil Obtained from Artemisia arborescens L. Growing on the Isle of La Maddalena, Sardinia, Italy. Chem. Biodiver. 2013, 10, 1464–1474. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Hrelia, S.; Angeloni, C.; Cantelli-Forti, G.; Hrelia, P. Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. J. Neurochem. 2009, 111, 1161–1171. [Google Scholar] [CrossRef]
- Tarozzi, A.; Marchesi, A.; Hrelia, S.; Angeloni, C.; Andrisano, V.; Fiori, J.; Cantelli-Forti, G.; Hrelia, P. Protective effects of cyanidin-3-O-beta-glucopyranoside against UVA-induced oxidative stress in human keratinocytes. Photochem. Photobiol. 2005, 81, 623–629. [Google Scholar] [CrossRef]
- Pohl, P.; Stelmach, E.; Welna, M.; Szymczycha-Madeja, A. Determination of the Elemental Composition of Coffee Using Instrumental Methods. Food Anal. Methods 2013, 6, 598–613. [Google Scholar] [CrossRef]
- Hombunaka, P.; Rowell, D.L. Potassium leaching potential and fertilizer recommendations for smallholder coffee gardens of Papua New Guinea. Commun. Soil Sci. Plant Anal. 2002, 33, 1767–1778. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crop Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso Coffee Residues: A Valuable Source of Unextracted Compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef]

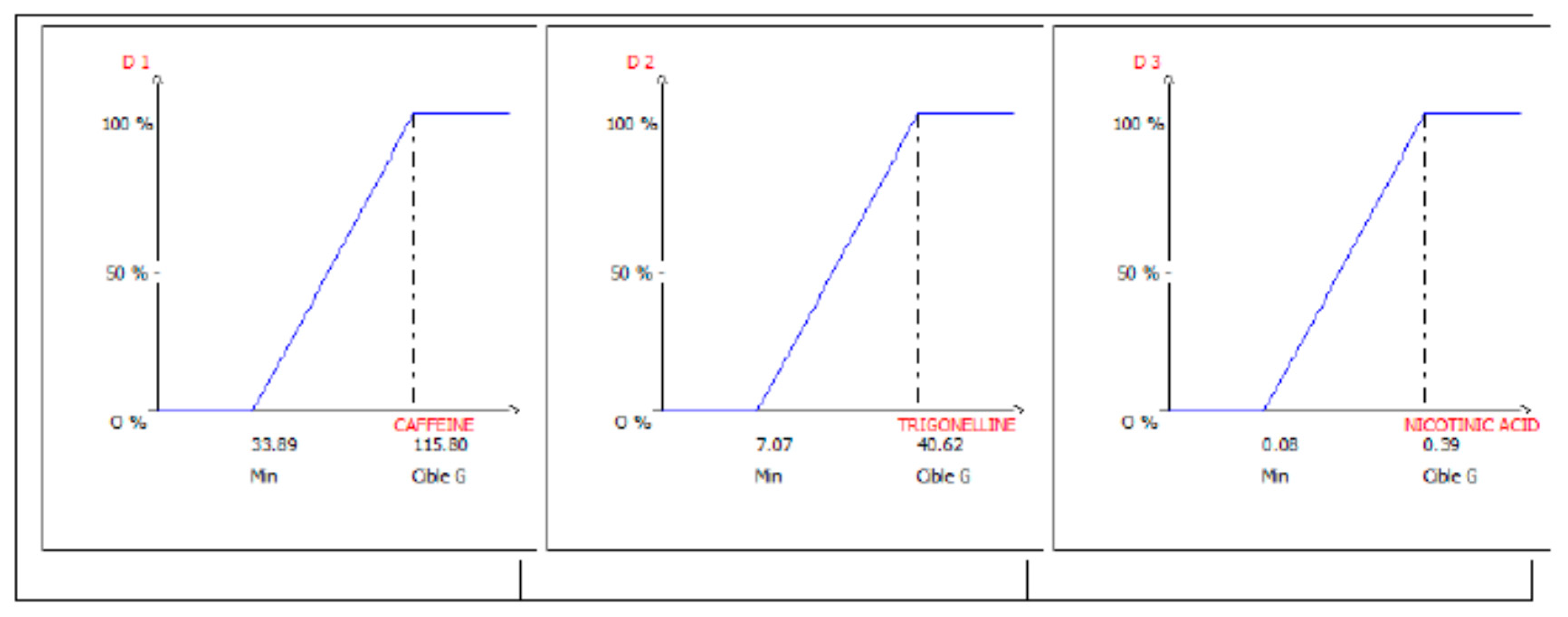
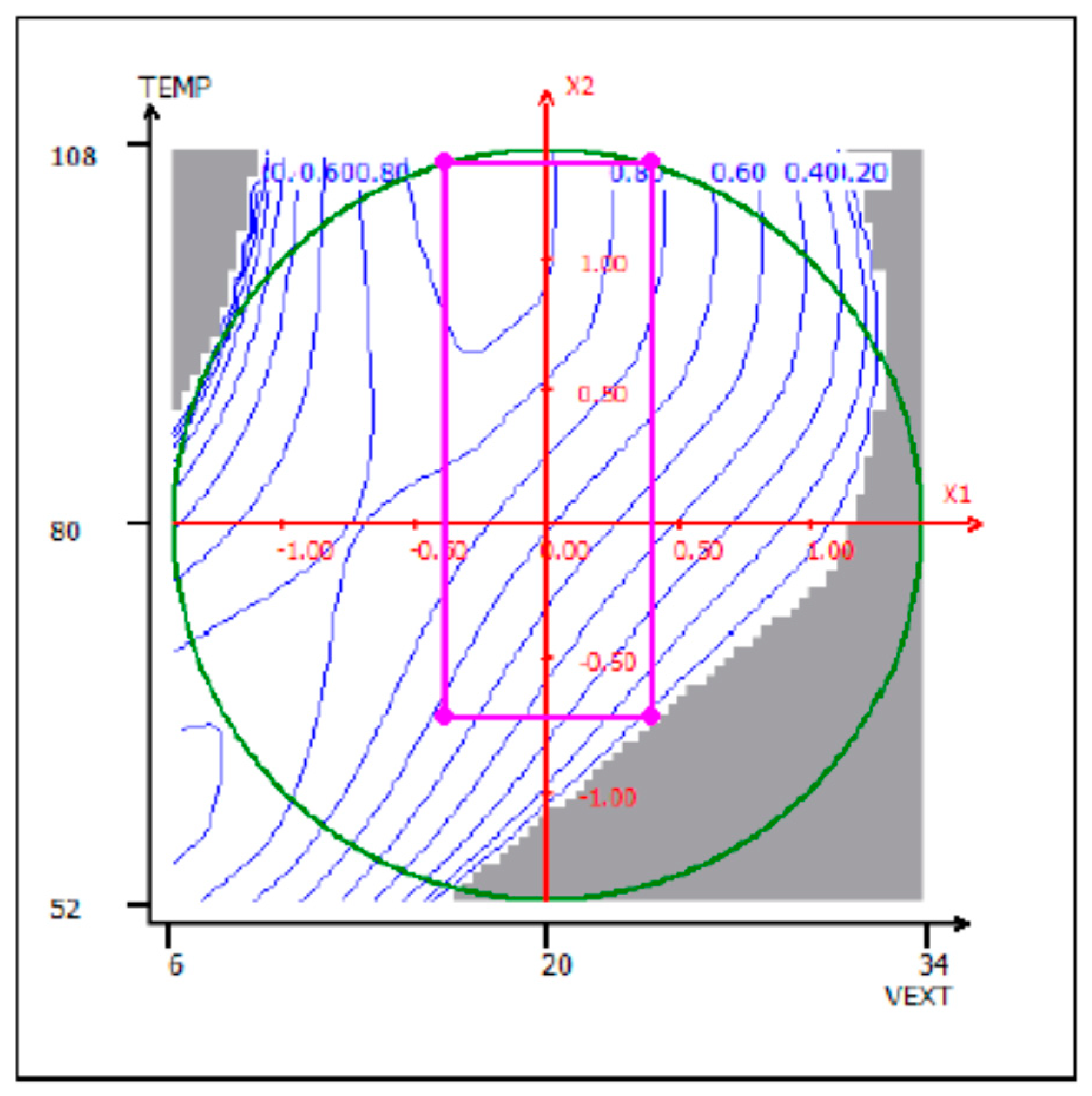

| Process Variables | Coded Variable | Original Units | Coded Units | Response Variables | ||
|---|---|---|---|---|---|---|
| U1 | Volume (mL) | X1 | 10 30 | –1 +1 | Y1 | Caffein content |
| U2 | Temperature (°C) | X2 | 60 100 | –1 +1 | ||
| Y2 Y3 | Trigonelline content Nicotinic acid content | |||||
| Experiment Number | Design (Coded Variables) | Run Order | Plan (Natural Variables) | ||
|---|---|---|---|---|---|
| Volume | Temperature | ||||
| X1 | X2 | (mL) | (°C) | ||
| 1 | −1.00 | −1.00 | 13 | 10 | 60 |
| 2 | +1.00 | −1.00 | 11 | 30 | 60 |
| 3 | −1.00 | +1.00 | 6 | 10 | 100 |
| 4 | +1.00 | +1.00 | 12 | 30 | 100 |
| 5 | −1.414 | 0.00 | 8 | 6 | 80 |
| 6 | +1.414 | 0.00 | 9 | 34 | 80 |
| 7 | 0.00 | −1.414 | 4 | 20 | 52 |
| 8 | 0.00 | +1.414 | 2 | 20 | 108 |
| 9 | 0.00 | 0.00 | 7 | 20 | 80 |
| 10 | 0.00 | 0.00 | 3 | 20 | 80 |
| 11 | −0.61 | −0.35 | 1 | 14 | 73 |
| 12 | +0.61 | −0.35 | 10 | 26 | 73 |
| 13 | 0.00 | +0.70 | 5 | 20 | 94 |
| Caffeine | Trigonelline | Nicotinic Acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | Sum of Squares | Mean Square | F-ratio | Sig. | Sum of Squares | Mean Square | F-ratio | Sig. | Sum of Squares | Mean Square | F-ratio | Sig. |
| Regression | 5 | 2.70279 × 104 | 5.40557 × 103 | 38.498 | 0.18 ** | 5.09583 × 103 | 1.01916 × 103 | 42.163 | 0.15 ** | 0.2950 | 0.0590 | 17.47 | 0.80 ** |
| Residual SSE | 4 | 5.61648 × 102 | 1.40412 × 102 | 9.66891 × 101 | 2.41723 × 101 | 0.0135 | 0.0034 | ||||||
| Lack of fit SSLOF | 3 | 4.77654 × 102 | 1.59218 × 102 | 1.896 | 48.0 | 7.75867 × 101 | 2.58622 × 101 | 1.354 | 54.7 | 0.0117 | 0.0039 | 2.169 | 45.4 |
| Error SSPE | 1 | 8.39938 × 101 | 8.39938 × 101 | 1.91024 × 101 | 1.91024 × 101 | 0.0018 | 0.0018 | ||||||
| Total SS | 9 | 2.75895 × 104 | 5.19252 × 103 | 0.3085 | |||||||||
| Model Fitting without Test Points | Model Fitting with the Complete Set of 13 Data Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caffeine | Trigonelline | Nicotinic Acid | Caffeine | Trigonelline | Nicotinic Acid | |||||||
| Coeff. | Sig. % | Coeff. | Sign. % | Coeff. | Sig. % | Coeff. | Sig. % | Coeff. | Sig. % | Coeffic. | Sig. % | |
| b0 | 118.98 | 0.0143 *** | 30.33 | 0.0950 *** | 0.39 | 0.0697 *** | 116.89 | <0.01 *** | 30.89 | <0.01 *** | 0.37 | <0.01 *** |
| b1 | −54.60 | 0.0204 *** | −19.70 | 0.0353 *** | 0.01 | 82.0 | −54.24 | <0.01 *** | −19.78 | <0.01 *** | 0.01 | 75.6 |
| b2 | 17.18 | 1.51 * | 14.70 | 0.109 ** | 0.10 | 0.783 ** | 17.78 | 0.0888 *** | 14.61 | <0.01 *** | 0.10 | 0.0338 *** |
| b11 | −2.28 | 70.5 | 6.31 | 5.4 | −0.06 | 8.1 | −1.52 | 71.1 | 6.09 | 0.614 ** | −0.05 | 2.44 * |
| b22 | −14.35 | 6.3 * | −0.22 | 93.0 | 0.04 | 25.8 | −13.16 | 1.26 * | −0.51 | 75.5 | 0.04 | 5.2 |
| b12 | 4.66 | 47.5 | −4.73 | 12.7 | 0.21 | 0.203 ** | 4.41 | 37.8 | −4.67 | 4.09 * | 0.21 | <0.01 *** |
| Caffeine | Trigonelline | Nicotinic Acid | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N° Exp | Y1,exp | Y1,cal | Difference | Y2,exp | Y2,cal | Difference | Y3,exp | Y3,cal | Difference |
| 1 | 145.860 | 144.4302 | 1.4298 | 35.692 | 36.6957 | −1.0037 | 0.5080 | 0.4616 | 0.0464 |
| 2 | 35.0080 | 25.9049 | 9.1031 | 10.7200 | 6.7551 | 3.9649 | 0.0760 | 0.0561 | 0.0199 |
| 3 | 157.822 | 169.4687 | −11.6467 | 70.9080 | 75.5516 | −4.6436 | 0.3010 | 0.2500 | 0.0510 |
| 4 | 65.6290 | 69.6024 | −3.9734 | 27.0300 | 26.7050 | 0.3250 | 0.7000 | 0.6756 | 0.0244 |
| 5 | 197.722 | 190.9433 | 6.7787 | 74.1740 | 70.2787 | 3.8953 | 0.2010 | 0.2561 | −0.0551 |
| 6 | 33.8860 | 38.0692 | −4.1832 | 11.9250 | 15.1277 | −3.2027 | 0.2530 | 0.2702 | −0.0172 |
| 7 | 58.7620 | 66.8046 | −8.0426 | 7.0710 | 9.3247 | −2.2537 | 0.2840 | 0.3169 | −0.0329 |
| 8 | 125.558 | 114.9199 | 10.6381 | 53.4350 | 50.4888 | 2.9462 | 0.5630 | 0.6024 | −0.0394 |
| 9 | 125.414 | 118.9854 | 6.4286 | 33.4090 | 30.3324 | 3.0766 | 0.4200 | 0.3886 | 0.0314 |
| 10 | 112.453 | 118.9854 | −6.5324 | 27.2280 | 30.3324 | −3.1044 | 0.3600 | 0.3886 | −0.0286 |
| 11 | 134.492 | 144.1289 | −8.6369 | 40.1900 | 38.2579 | 1.9321 | 0.3400 | 0.3749 | −0.0449 |
| 12 | 73.3240 | 76.6523 | −3.3283 | 17.3240 | 38.2579 | 0.7171 | 0.2600 | 0.2937 | −0.0437 |
| 13 | 125.544 | 123.9834 | 1.5606 | 40.6470 | 40.5170 | 0.1300 | 0.4520 | 0.4777 | −0.0257 |
| Caffeine | Trigonelline | Nicotinic Acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | d.f. | Sum of Squares | Mean Square | F-ratio | Sig. | Sum of Squares | Mean Square | F-ratio | Sig. | Sum of Squares | Mean Square | F-ratio | Sig. |
| Regression | 5 | 2.92657 × 104 | 5.85315 × 103 | 65.297 | <0.01 *** | 5.46235 × 103 | 1.09247× 103 | 76.80 | <0.01 *** | 0.3126 | 0.0625 | 28.92 | 0.00 *** |
| Residual SSE | 7 | 6.27473 × 102 | 8.96391 × 101 | 9.95740 × 101 | 1.42248× 101 | 0.0151 | 0.0022 | ||||||
| Lack of fit SSLOF | 6 | 5.43480 × 102 | 9.05800 × 101 | 1.078 | 62.7 | 8.04716 × 101 | 1.34119× 101 | 0.702 | 72.2 | 0.0133 | 0.0022 | 1.235 | 59.7 |
| Error SSPE | 1 | 8.39938 × 101 | 8.39938 × 101 | 1.91024 × 101 | 1.91024× 101 | 0.0018 | 0.0018 | ||||||
| Total SS | 12 | 2.98932 × 104 | 5.56192 × 103 | 0.3277 | |||||||||
| TPC mg GAE/g | FRAP TEAC (µmol TE/g) | ABTS TEAC (µmol TE/g) | DPPH TEAC (µmol TE/g) |
|---|---|---|---|
| 61.49 ± 1.36 | 311.62 ± 22.65 | 735.47 ± 0.60 | 324.51 ± 13.58 |
| mg/g | ELEMENTS | µg/g | ELEMENTS | ng/g | |
|---|---|---|---|---|---|
| K | 6.03 | Rb | 7.98 | Cr | 38.86 |
| P | 0.62 | Al | 7.56 | Pb | 38.10 |
| Mg | 0.57 | Mn | 3.35 | Li | 32.97 |
| Ca | 0.55 | B | 3.15 | Hg | 21.76 |
| Na | 0.20 | Sr | 2.45 | Co | 20.71 |
| S | 0.11 | Zn | 2.13 | Cs | 17.39 |
| Fe | 1.85 | Sn | 14.06 | ||
| Cu | 1.20 | Mo | 12.54 | ||
| Ba | 1.19 | Au | 11.88 | ||
| Ni | 0.23 | As | 6.08 | ||
| Ga | 0.19 | V | 4.28 | ||
| Ti | 0.15 | Pd | 3.90 | ||
| Ag | 2.95 | ||||
| Cd | 2.85 | ||||
| Tl | 2.00 | ||||
| Sb | 1.43 | ||||
| U | 0.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigliobianco, M.R.; Campisi, B.; Vargas Peregrina, D.; Censi, R.; Khamitova, G.; Angeloni, S.; Caprioli, G.; Zannotti, M.; Ferraro, S.; Giovannetti, R.; et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants 2020, 9, 370. https://doi.org/10.3390/antiox9050370
Gigliobianco MR, Campisi B, Vargas Peregrina D, Censi R, Khamitova G, Angeloni S, Caprioli G, Zannotti M, Ferraro S, Giovannetti R, et al. Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants. 2020; 9(5):370. https://doi.org/10.3390/antiox9050370
Chicago/Turabian StyleGigliobianco, Maria Rosa, Barbara Campisi, Dolores Vargas Peregrina, Roberta Censi, Gulzhan Khamitova, Simone Angeloni, Giovanni Caprioli, Marco Zannotti, Stefano Ferraro, Rita Giovannetti, and et al. 2020. "Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach" Antioxidants 9, no. 5: 370. https://doi.org/10.3390/antiox9050370
APA StyleGigliobianco, M. R., Campisi, B., Vargas Peregrina, D., Censi, R., Khamitova, G., Angeloni, S., Caprioli, G., Zannotti, M., Ferraro, S., Giovannetti, R., Angeloni, C., Lupidi, G., Pruccoli, L., Tarozzi, A., Voinovich, D., & Di Martino, P. (2020). Optimization of the Extraction from Spent Coffee Grounds Using the Desirability Approach. Antioxidants, 9(5), 370. https://doi.org/10.3390/antiox9050370
















