Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sample and Reagents
2.2. Extraction Procedure
2.3. Total Phenolic (TFC) and Flavonoid Content (TPC)
2.4. Antioxidant Activity
2.4.1. 1,1-Diphenyl-2-Picrylhydrazyl Radical (DPPH●) Scavenging Activity
2.4.2. 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS+●) Radical Scavenging Activity
2.4.3. Determination of Inhibition of β-Carotene/Linoleic Acid Co-Oxidation
2.5. Enzyme Inhibitory Activity
Anti-Collagenase (MMP-1) and Anti-Elastase Activities
2.6. Qualitative and Quantitative Chromatographic Analysis
2.6.1. Analysis with Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) High Performance Liquid Chromatography (HPLC) Systems
2.6.2. Analysis with High Performance Liquid Chromatography (HPLC) Systems
2.7. Liposomal Formulation Studies
2.7.1. Preparation of Nanoliposomes
2.7.2. Determination of Nanoliposomes Characteristics
2.7.3. In vitro Release Study
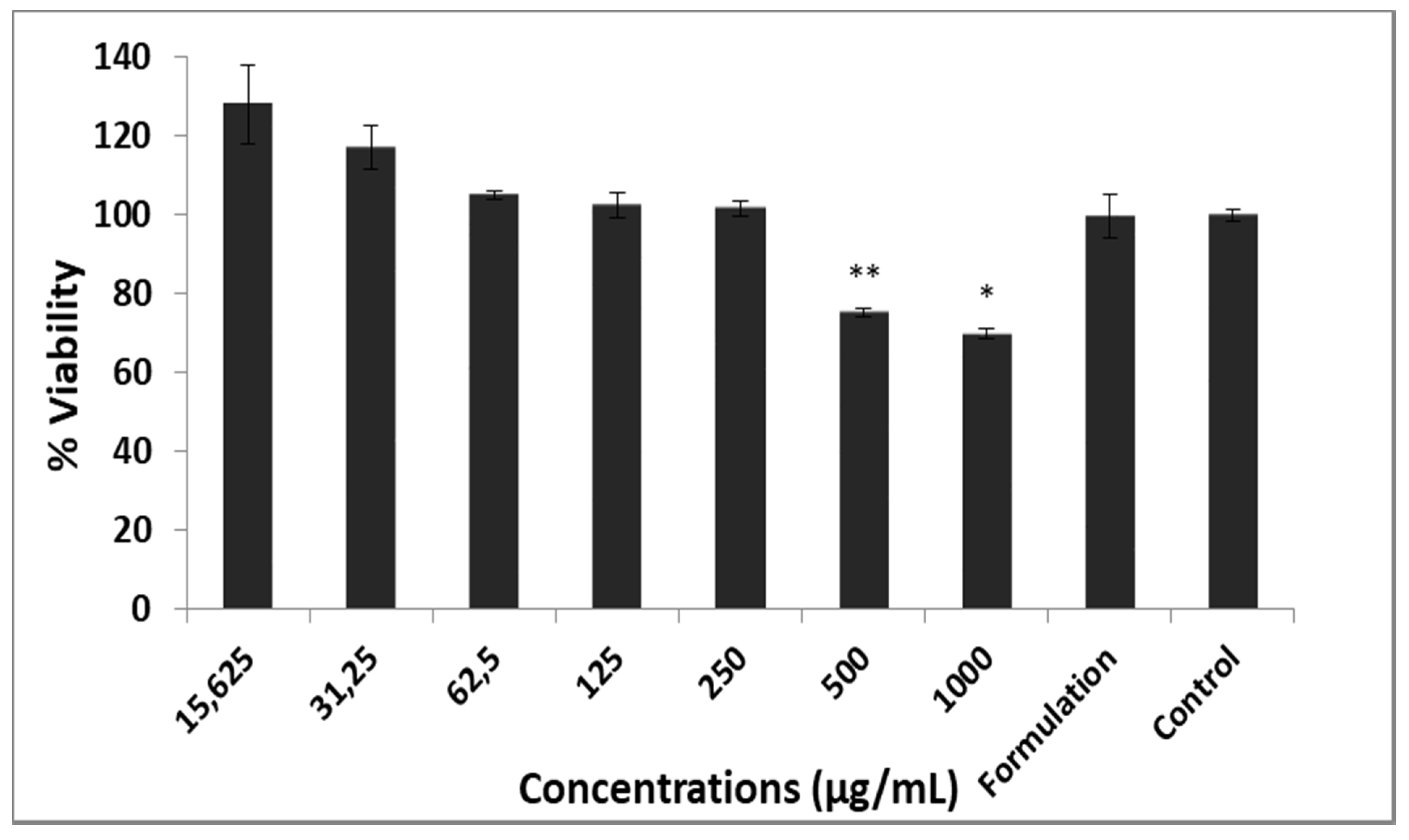
2.8. Toxicity Assessment on L929 Cell Line
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramasubramania Raja, R. Medicinally potential plants of Labiatae (Lamiaceae) Family: An owerview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar]
- Dweck, A.C. Introduction the folklore and cosmetic use of various Salvia species. In Sage, the Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic: Amsterdam, The Netherlands, 2000; pp. 1–25. [Google Scholar]
- Demirci, B.; Başer, K.H.C.; Tumen, G. Composition of the essential oil of Salvia aramiensis Rech. Fil. growing in Turkey. Flavor Frag. J. 2002, 17, 23–25. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Goto, K.; Ikeshire, Y. Traditional medicine in Turkey IV, folk medicine in Mediterranean Subdivision. J. Ethnopharmacol. 1993, 39, 31–38. [Google Scholar] [CrossRef]
- Yeşilada, E.; Honda, G.; Sezik, E.; Tabata, M.; Fujita, T.; Tanaka, T.; Takeda, Y.; Takaishi, H. Traditional medicine in Turkey V. Folk medicine in the inner Taurus Mountain. J. Ethnopharmacol. 1995, 46, 133–152. [Google Scholar] [CrossRef]
- Aşkun, T.; Başer, K.H.C.; Tümen, G.; Kürkçüoğlu, M. Characterization of essential oils of some Salvia species and their antimycobacterial activities. Turk J. Biol. 2010, 34, 89–95. [Google Scholar]
- Esquivel, B.; Sanchez, A.A.; Aranda, E. Natural Products of Agricultural Interest from Mexican Labiatae. In Phytochemicals and Phytopharmaceuticals; Shahidi, F., Ho, C.H., Eds.; AOCS Press: Champaign, IL, USA, 2009; pp. 371–385. [Google Scholar]
- Ulubelen, A.; Topçu, G. Flavonoids and terpenoids from Salvia verticillata and Salvia pinnata. J. Nat. Prod. 1984, 47, 1068. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topçu, G.; Bozok-Johansson, C. Norditerpenoids and diterpenoids from Salvia multicaulis with antituberculous activity. J. Nat. Prod. 1997, 60, 1275–1280. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the In Vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Radu, G.L. Assessment of acetylcholinesterase and tyrosinase inhibitory and antioxidant activity of Alchemilla vulgaris and Filipendula ulmaria extracts. J. Taiwan Inst. Chem. Eng. 2015, 52, 1–6. [Google Scholar] [CrossRef]
- Arslan, M. Cultivation potential of Salvia tomentosa and S. aramiensis under the Eastern Mediterranean conditions. Sci. Pap. Ser. A Agron. 2016, 59, 174–177. [Google Scholar]
- Baytop, T. Türkiye’de Bitkilerle Tedavi. In Geçmişte ve Bugün, 2nd ed.; Nobel Tıp Kitapevleri Ltd. Şti: İstanbul, Turkey, 1999; pp. 143–144. [Google Scholar]
- Kelen, M.; Tepe, B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bioresour. Technol. 2008, 99, 4096–4104. [Google Scholar] [CrossRef] [PubMed]
- Karaman, Ş.; Ilcim, A.; Çömlekçioğlu, N. Composition of the essential oils of Salvia aramiensis Rech. Fil. and Salvia cyanescens Boiss & Bal. Pak. J. Bot. 2007, 39, 169–172. [Google Scholar]
- Genc, Y.; Dereli, F.T.G.; Saracoglu, I.; Akkol, E.K. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharm. J. 2020, 28, 101–106. [Google Scholar]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, G.; Fouche, G.; Tselanyane, M.; Cordier, W.; Steenkamp, V. In vitro determination of the anti-aging potential of four southern African medicinal plants. BMC Complement. Altern. Med. 2013, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; Martins, J.A.; Marcos, J.C.; Cavaco-Paulo, A. Characterization of potential elastase inhibitor-peptides regulated by a molecular switch for wound dressings applications. Enzym. Microb. Technol. 2012, 50, 107–114. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Dhawan, B.N. Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytother. Res. 1999, 13, 50–54. [Google Scholar] [CrossRef]
- Aksoy, H.; Bingöl Özakpinar, Ö. Wound healing and oxidative stress. Marmara Pharm. J. 2014, 18, 153–158. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, T.H.; Kang, K.C.; Pyo, H.B.; Jeong, H.H. Microencapsulation of rosmarinic acid using polycaprolactone and various surfactants. Int. J. Cosmet. Sci. 2010, 32, 185–191. [Google Scholar] [CrossRef]
- Perugini, P.; Genta, I.; Pavanetto, F.; Conti, B.; Scalia, S.; Baruffini, A. Study on glycolic acid delivery by liposomes and microspheres. Int. J. Pharm. 2000, 196, 51–61. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, M.C.; Tsai, W.J. A fibrin encapsulated liposomes-in-chitosan matrix (FLCM) for delivering water-soluble drugs influences of the surface properties of liposomes and the crosslinked fibrin network. Int. J. Pharm. 2006, 311, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Beatriz Oliveira, M.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Huang, M.H.; Huang, S.S.; Wang, B.S.; Sheu, M.J.; Hou, W.C. Antioxidant and anti-inflammatory properties of Cardiospermum halicacabum and its reference compounds ex vivo and in vivo. J. Ethnopharmacol. 2011, 133, 743–750. [Google Scholar] [CrossRef]
- Velioğlu, Y.S.; Mazza, G.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Seker Karatoprak, G.; Ilgün, S.; Koşar, M. Phenolic composition, anti-inflammatory, antioxidant, and antimicrobial activities of Alchemilla mollis (Buser) Rothm. Chem. Biodivers. 2017, 14, e1700150. [Google Scholar] [CrossRef]
- Koşar, M.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and phenolic composition of Salvia halophila Hedge from Turkey. Food Chem. 2011, 129, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Şeker Karatoprak, G.; İlgün, S.; Koşar, M. Antioxidant properties and phenolic composition of Salvia virgata Jacq. Turk. J. Pharm. Sci. 2016, 13, 201–212. [Google Scholar] [CrossRef]
- Akkol, E.K.; Göger, F.; Koşar, M.; Başer, K.H.C. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008, 108, 942–949. [Google Scholar] [CrossRef]
- Süntar, İ.; Küpeli Akkol, E.; Şenol, F.S.; Keleş, H.; Erdoğan Orhan, İ. Investigating wound healing, tyrosinase inhibitory and antioxidant activities of the ethanol extracts of Salvia cryptantha and Salvia cyanescens using in vivo and in vitro experimental models. J. Ethnopharmacol. 2011, 135, 71–77. [Google Scholar] [CrossRef]
- Hatamia, T.; Emamic, S.A.; Miraghaeea, S.S.; Mojarrab, M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iran. J. Pharm. Res. 2014, 13, 551–558. [Google Scholar]
- Hnarayan, S.; Sasmal, D.; Mazumder, P.M. Evaluation of the wound healing effect of herbal ointment formulated with Salvia splendens (Scarlet sage). Int. J. Pharm. Pharm. Sci. 2011, 3, 195–199. [Google Scholar]
- Karimzadeh, S.; Farahpour, M.R. Topical application of Salvia officinalis hydroethanolic leaf extract improves wound healing process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar]
- Guaouguaou, F.; Taghzouti, K.; Oukabli, M.; Es-Safi, N.E. The effect of Salvia verbenaca extracts for healing of second-degree burn wounds in rats current bioactive compounds. Curr. Bioact. Compd. 2018, 14, 419–427. [Google Scholar] [CrossRef]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Vuorela, H.; Raal, A. Commercial peppermint (Mentha × piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. 2018, 8, 116–122. [Google Scholar]
- Kahil, W.K.D.; Salvia, K. Effects of Salvia officinalis extract and its nano-encapsulated form on methylmercury induced neurotoxic-stress in male rats. World Appl. Sci. J. 2013, 24, 826–837. [Google Scholar]
- Wanfeng, S.; Rongling, S.; Weiling, J. Compound Cordyceps sinensis liposome oral liquid in treatment of patients with Chronic Hepatitis B. China Pharm. 2003, 6, 438–439. [Google Scholar]
- Aisha, A.F.; Majid, A.M.; Ismail, Z. Preparation and characterization of nano liposomes of Orthosiphon stamineus ethanolic extract in soybean phospholipids. BMC Biotechnol. 2014, 14, 23–33. [Google Scholar] [CrossRef]
- Theerakittayakorn, K.; Bunprasert, T. Differentiation capacity of mouse L929 fibroblastic cell line compare with human dermal fibroblast. World Acad. Sci. Eng. Technol. 2011, 50, 373–376. [Google Scholar]
- Karamustafa, F.; Çelebi, N.; Değim, Z.; Yılmaz, Ş. Evaluation of the viability of L-929 cells in the presence of alendronate and absorption enhancers. FABAD J. Pharm. Sci. 2006, 31, 1–5. [Google Scholar]


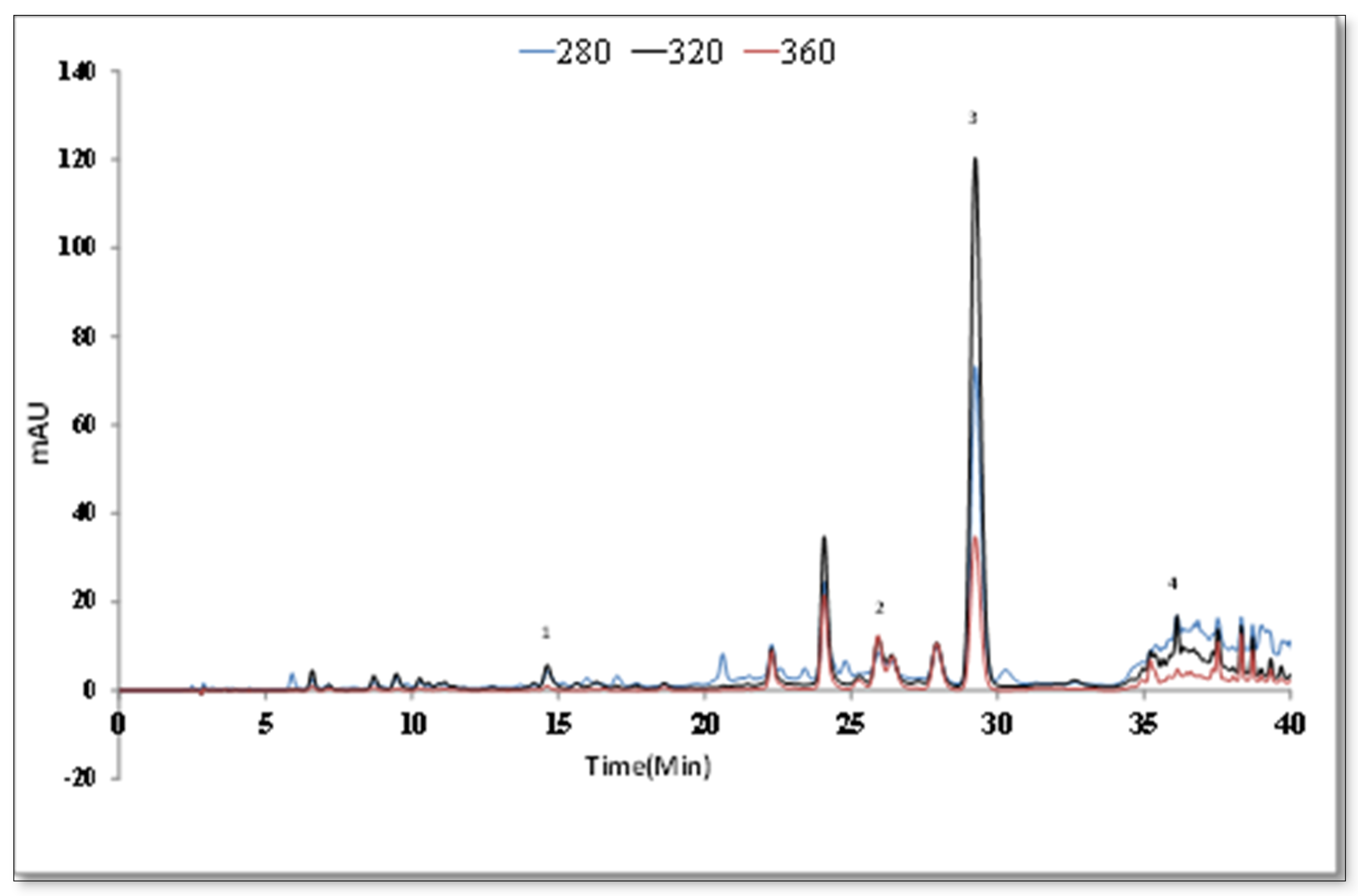


| Extracts | Total Phenol (mgGAE/gextract) a | Total Flavonoids (mgCA/gextract) b |
|---|---|---|
| S.a 70% MeOH | 198.07 ± 2.77 | 141.76 ± 1.64 |
| S.a 70% EtOH | 153.24 ± 0.94 | 93.28 ± 9.53 |
| S.a inf | 124.75 ± 1.58 | 118.85 ± 4.47 |
| Extracts/Standard | DPPH● EC50 (µg/mL) | ABTS●+ (mmol/ L /Trolox) | |
|---|---|---|---|
| 50 µg/mL | 100 µg/mL | ||
| S.a 70% MeOH | 28.4 ± 0.002 b | 1.15 ± 0.101 a | 1.77 ± 0.09 b |
| S.a 70% EtOH | 36.6 ± 0.004 c | 1.59 ± 0.091 c | 1.65 ± 0.05 b,c |
| S.a inf | 32.6 ± 0.001 b | 1.24 ± 0.113 a | 1.37 ± 0.071 a,d |
| Rosmarinic acid | 4.4 ± 0.00 a | 2.16 ± 0.044 b,e | 2.56 ± 0.001 e |
| S.a 70% MeOH/Standard | Collagenase Enzyme Inhibition (%) | Elastase Enzyme Inhibition (%) |
|---|---|---|
| 50 µg/mL | 66.64 ± 1.53 b | 76.68 ± 1.21 a |
| 100 µg/mL | 69.54 ± 1.49 a,b | 83.70 ± 1.72 b |
| 200 µg/mL | 72.66 ± 0.70 a | 86.91 ± 0.81 c |
| RA 25 µg/mL | 72.89 ± 0.99 a | 80.58 ± 0.89 a,b |
| Rt | [M − H]− | Fragments | Identified Compounds | Ref |
|---|---|---|---|---|
| 7.2 | 387 | 207, 163 | Icariside b5/medioresinol | [42] |
| 8.7 | 179 | 135 | Caffeic acid | [43] |
| 8.9 | 463 | 300, 271 | Quercetin glucoside/hydroxyluteolin glucoside | [43] |
| 10.7 | 447 | 285 | Luteolin glucoside | [43] |
| 11.2 | 477 | 315, 300, 285 | Isorhamnetin hexoside | [44] |
| 12.3 | 717 | 537, 519, 493, 321 | Salvianolic acid E | [45] |
| 13.3 | 359 | 197, 179, 161, | Rosmarinic acid | [43] |
| 14.9 | 563 | 387, 207 | Icariside b5/Medioresinol O-glucoronide | [46] |
| 17.3 | 285 | 151, 133 | Luteolin | [43] |
| Compounds | Extract * | Standards | ||
|---|---|---|---|---|
| S.a 70% MeOH | Equation and r2 | LOD | LOQ | |
| Caffeic acid | 1.84 ± 0.09 | y = 44.283x + 40.553 r2 = 0.998 | 0.0067 | 0.0204 |
| Luteolin-7-o-glucoside | 3.70 ± 0.22 | y = 39.422x + 78.434 r2 = 0.997 | 0.0085 | 0.0257 |
| Rosmarinic acid | 122.32 ± 0.21 | y = 23.777x − 11.797 r2 = 0.997 | 0.2595 | 0.7865 |
| Luteolin | 4.06 ± 0.03 | y = 41.981x − 70.752 r2 = 0.997 | 0.1407 | 0.4263 |
| Formulation | PS ± SD (µm) | ZP ± SD (mV) | PDI ± SD | EE ± SD (%) | RA ± SD (%) |
|---|---|---|---|---|---|
| Liposome | 0.395 ± 0.004 | −35.2 ± 2.1 | 0.107 ± 0.017 | 13.83 ± 1.25 | 60 ± 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatoprak, G.Ş.; Yücel, Ç.; Göger, F.; Sobarzo-Sánchez, E.; Küpeli Akkol, E. Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract. Antioxidants 2020, 9, 293. https://doi.org/10.3390/antiox9040293
Karatoprak GŞ, Yücel Ç, Göger F, Sobarzo-Sánchez E, Küpeli Akkol E. Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract. Antioxidants. 2020; 9(4):293. https://doi.org/10.3390/antiox9040293
Chicago/Turabian StyleKaratoprak, Gökçe Şeker, Çiğdem Yücel, Fatih Göger, Eduardo Sobarzo-Sánchez, and Esra Küpeli Akkol. 2020. "Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract" Antioxidants 9, no. 4: 293. https://doi.org/10.3390/antiox9040293
APA StyleKaratoprak, G. Ş., Yücel, Ç., Göger, F., Sobarzo-Sánchez, E., & Küpeli Akkol, E. (2020). Potential Antioxidant and Enzyme Inhibitory Effects of Nanoliposomal Formulation Prepared from Salvia aramiensis Rech. f. Extract. Antioxidants, 9(4), 293. https://doi.org/10.3390/antiox9040293







