Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Methods
2.2. Synthesis
2.3. Pharmacokinetics Study
2.4. Animal Model of Global Cerebral Ischemia, Experimental Design, and Treatment
2.5. Evaluation of Neurological Deficits
2.6. Behavioral Tests
2.7. Brain Sections
2.8. Neuronal Death Evaluation
2.9. TUNEL Assay
2.10. Animal Model of Focal Cerebral Ischemia
2.11. Motor-Deficit Evaluation, Grip Strength Test
2.12. Infarct Volume Evaluation
2.13. Statistical Analysis
3. Results
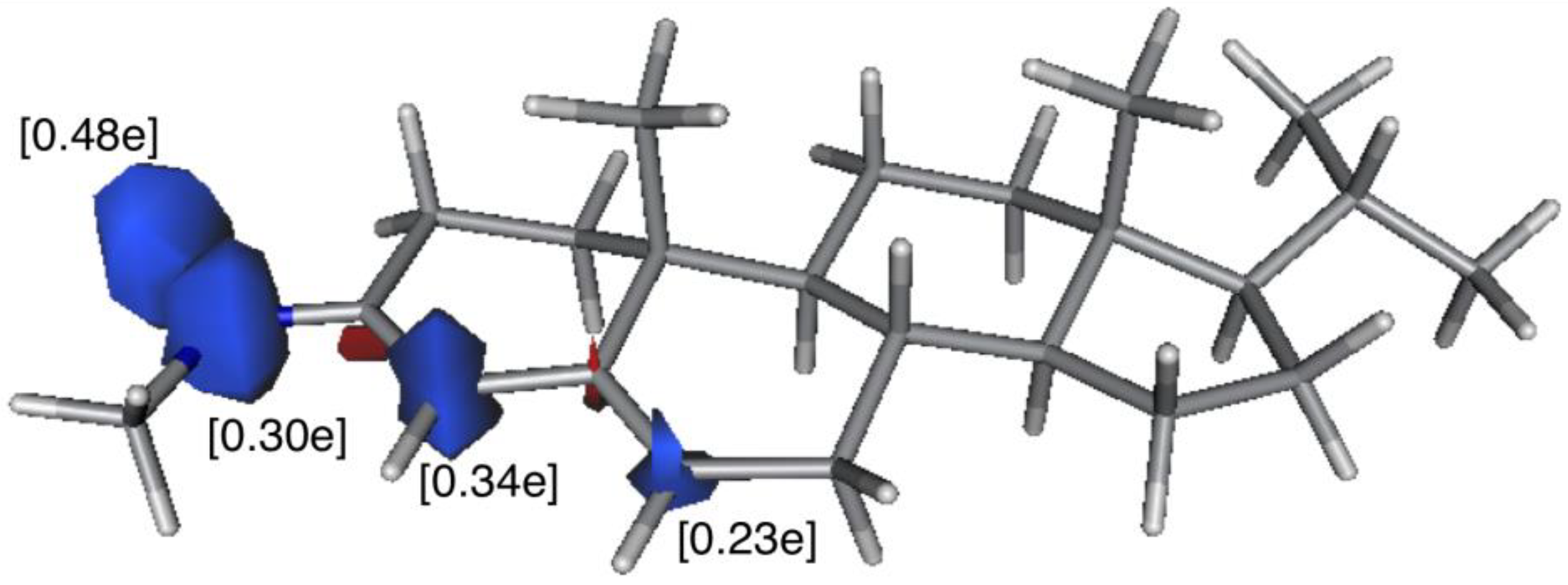
3.1. In Silico DFT-Study
3.2. Dose-Response Study
3.2.1. Analysis of Neuronal Death
3.2.2. Analysis of Neuronal Apoptosis
3.2.3. Dose-Response Curve
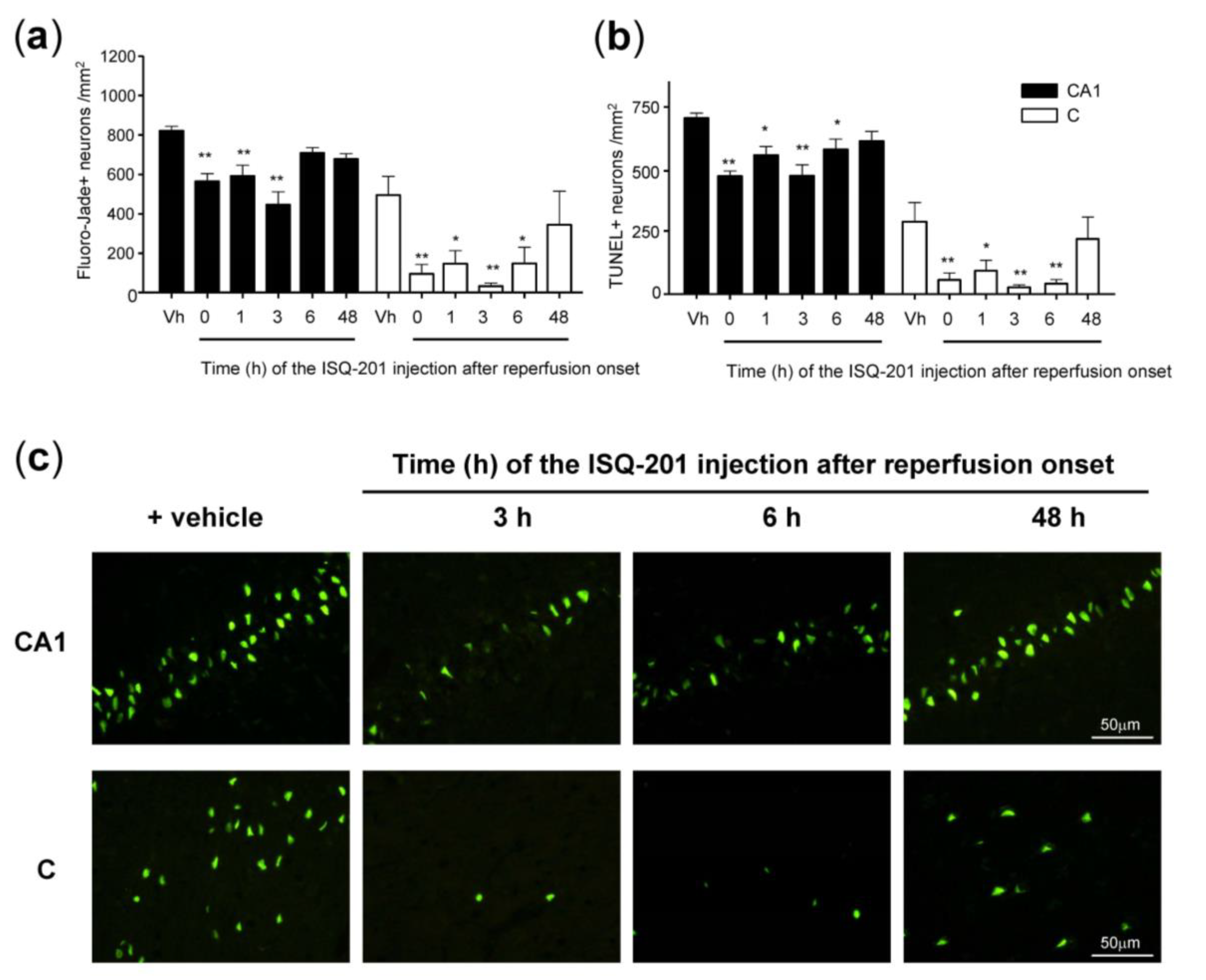
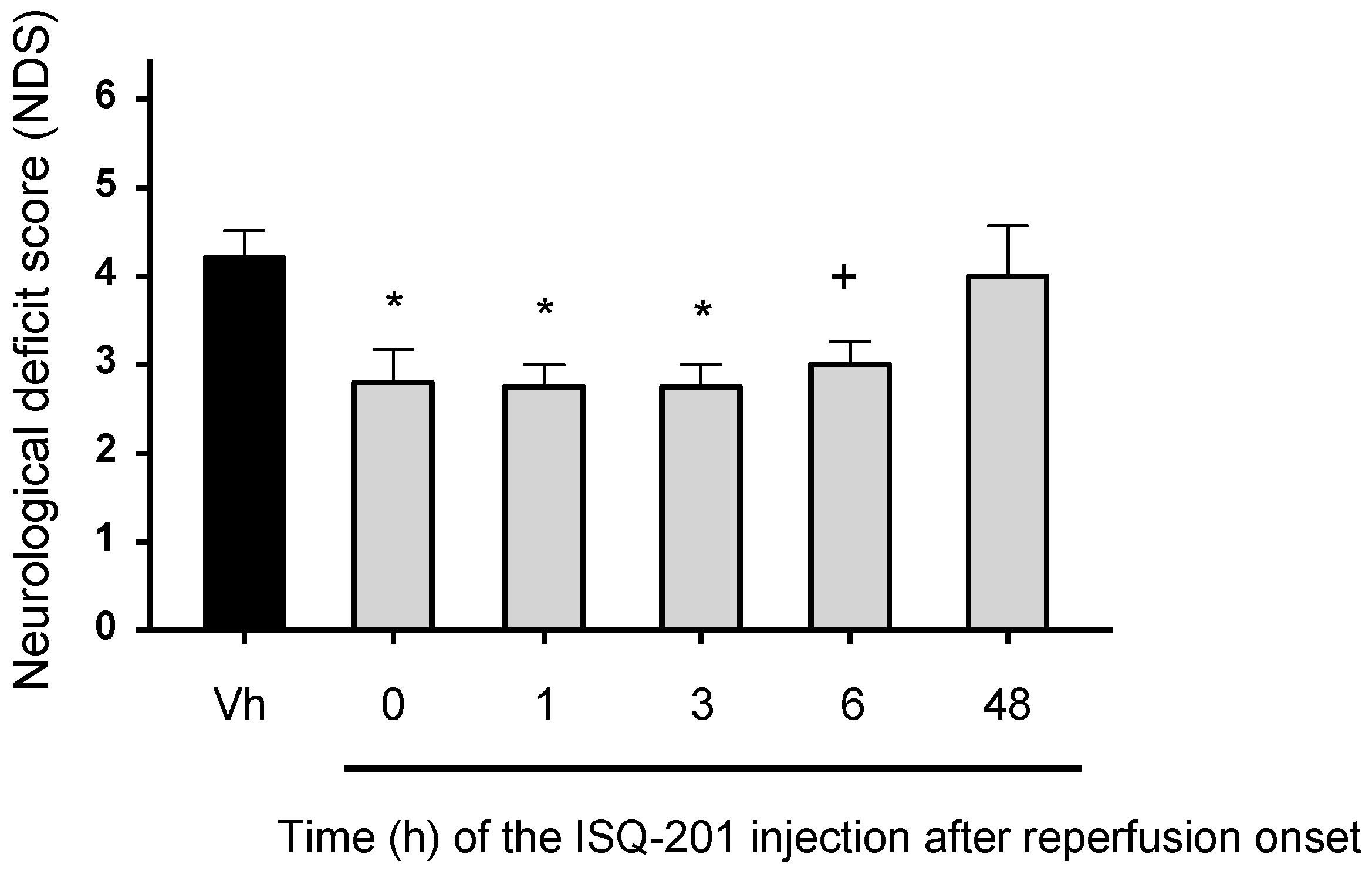
3.3. Therapeutic Window
3.3.1. Analysis of Neuronal Death
3.3.2. Analysis of Neuronal Apoptosis
3.3.3. Functional Test
3.4. Long-Term Efficacy Assessment
3.4.1. Analysis of Neuronal Viability
3.4.2. Behavioral Tests
Exploration Activity and Spatial Recognition Test
Spatial Memory Test
3.5. Pharmacokinetic Study
3.6. ISQ-201 Treatment in Transient Focal Cerebral Ischemia
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2014 Summary Tables: Deaths by Cause, Age and Sex, 2000–2016; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Brouns, R.; De Deyn, P.P. The Complexity of Neurobiological Processes in Acute Ischemic Stroke. Clin. Neurol. Neurosurg. 2009, 111, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Bach, A. Targeting Oxidative Stress in Stroke. In Neuroprotective Therapy for Stroke and Ischemic Disease; Lapchak, P.A., Zhang, H.J., Eds.; Springer: Cham, Switzerland, 2017; pp. 203–250. [Google Scholar] [CrossRef]
- Iwamura, M.; Inamoto, N. Novel formation of nitroxide radicals by radical addition to nitrones. Bull. Chem. Soc. Jpn. 1967, 40, 703. [Google Scholar] [CrossRef]
- Floyd, R.A.; Kopke, R.D.; Choi, C.H.; Foster, S.B.; Doblas, S.; Towner, R.A. Nitrones as therapeutics. Free Radic. Biol. Med. 2008, 45, 1361–1374. [Google Scholar] [CrossRef]
- Novelli, G.P.; Angiolini, P.; Tani, R.; Consales, G.; Bordi, L. Phenyl-t-butyl-nitrone is active against traumatic shock in rats. Free Radic. Res. Commun. 1986, 1, 321–327. [Google Scholar] [CrossRef]
- Green, A.R.; Ashwood, T.; Odergren, T.; Jackson, D.M. Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharmacol. Ther. 2003, 100, 195–214. [Google Scholar] [CrossRef]
- Kuroda, S.; Tsuchidate, R.; Smith, M.-L.; Maples, K.R.; Siesjo, B.K. Neuroprotective Effects of a Novel Nitrone, NXY-059, After Transient Focal Cerebral Ischemia in the Rat. J. Cereb. Blood Flow Metab. 1999, 19, 778–787. [Google Scholar] [CrossRef]
- Edenius, C.; Strid, S.; Borgå, O.; Breitholtz-Emanuelsson, A.; Vallén, K.L.; Fransson, B. Pharmacokinetics of NXY-059, a nitrone- based free radical trapping agent, in healthy young and elderly subjects. J. Stroke Cerebrovasc. Dis. 2002, 11, 34–43. [Google Scholar] [CrossRef]
- Lees, K.R.; Sharma, A.K.; Barer, D.; Ford, G.A.; Kostulas, V.; Cheng, Y.F.; Odergren, T. Tolerability and pharmacokinetics of the nitrone NXY-059 in patients with acute stroke. Stroke 2001, 32, 675–680. [Google Scholar] [CrossRef][Green Version]
- Lees, K.R.; Barer, D.; Ford, G.A.; Hacke, W.; Kostulas, V.; Sharma, A.K.; Odergren, T. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke 2003, 34, 482–487. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lees, K.R.; Davalos, A.; Davis, S.M.; Diener, H.-C.; Grotta, J.; Lyden, P.; Shuaib, A.; Ashwood, T.; Hårdemark, H.-G.; Wasiewski, W.; et al. Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial. Stroke 2006, 37, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chen, S.; Ganesh, A.; Hill, M.D. Long-term neurological, vascular, and mortality outcomes after stroke. Int. J. Stroke 2018, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Verheyden, G.; Brinkmann, N.; Dejaeger, E.; De Weerdt, W.; Feys, H.; Gantenbein, A.R.; Jenni, W.; Laenen, A.; Lincoln, N.; et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: Follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke 2015, 46, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Chioua, M.; Martínez-Alonso, E.; Soriano, E.; Montaner, J.; Masjuán, J.; Hadjipavlou-Litina, D.J.; Marco-Contelles, J.; Alcázar, A. CholesteroNitrones for Stroke. J. Med. Chem. 2015, 58, 6704–6709. [Google Scholar] [CrossRef] [PubMed]
- Gaussian Programs; Version 09; Computational Chemistry Software; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H.J. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- McIver, J.W.; Komornicki, A.K. Structure of transition states in organic reactions. General theory and an application to the cyclobutene-butadiene isomerization using a semiempirical molecular orbital method. J. Am. Chem. Soc. 1972, 94, 2625–2633. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Pascual-Ahuir, J.L.; Silla, E.; Tuñón, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J. Comp. Chem. 1994, 15, 1127–1138. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Martínez-Alonso, E.; Cid, C.; de Leciñana, M.A.; Alcázar, A. The translational repressor eIF4E-binding protein 2 (4E-BP2) correlates with selective delayed neuronal death after ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 1173–1181. [Google Scholar] [CrossRef]
- García-Bonilla, L.; Cid, C.; Alcázar, A.; Burda, J.; Ayuso, I.; Salinas, M. Regulation proteins of eukaryotic initiation factor 2-alpha subunit (eIF2α) phosphatase, under ischemic reperfusion and tolerance. J. Neurochem. 2007, 103, 1368–1380. [Google Scholar] [CrossRef]
- Basavarajappa, B.S.; Subanna, S. CB1 Receptor-Mediated Signaling Underlies the Hippocampal Synaptic, Learning and Memory Deficits Following Treatment with JWH-081, a New Component of Spice/K2 Preparations. Hippocampus 2014, 24, 178–188. [Google Scholar] [CrossRef]
- Morancho, A.; García-Bonilla, L.; Barceloó, V.; Giralt, D.; Campos-Martorell, M.; García, S.; Montaner, J.; Rosell, A. A new method for focal transient cerebral ischaemia by distal compression of the middle cerebral artery. Neuropathol. Appl. Neurobiol. 2012, 38, 617–627. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5- triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef]
- Nsangou, M.; Dhaouadi, Z.; Jaidane, N.; Lakhdar, Z.B. DFT study of the structure of hydroxybenzoic acids and their reactions with HO• and O2•– radicals. J. Mol. Struct. THEOCHEM 2008, 850, 135–143. [Google Scholar] [CrossRef]
- Nasha, K.M.; Schieferb, I.T.; Shah, Z.A. Development of a reactive oxygen species-sensitive nitric oxide synthase inhibitor for the treatment of ischemic stroke. Free Radic. Biol. Med. 2018, 115, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, M.I.; Martínez-Alonso, E.; Regidor, I.; Alcázar, A. Stress granule induction after brain ischemia is independent of eukaryotic translation initiation factor (eIF) 2α phosphorylation and is correlated with a decrease in eIF4B and eIF4E proteins. J. Biol. Chem. 2016, 291, 27252–27264. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Hara, Y. Brain Plasticity and Rehabilitation in Stroke Patients. J. Nippon Med. Sch. 2015, 82, 4–13. [Google Scholar] [CrossRef]
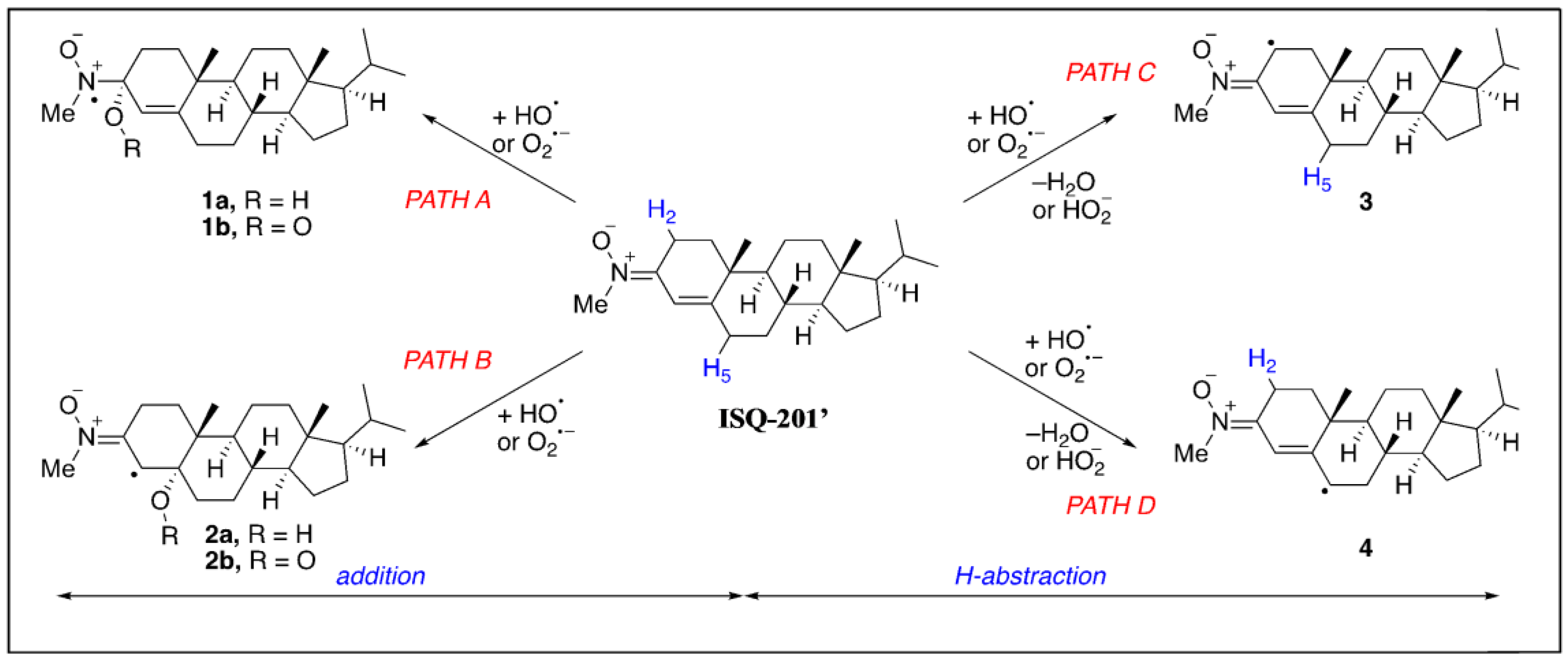






| Reaction Path | HO• | O2•− |
|---|---|---|
| PATH A | −36.5 | +14.5 |
| PATH B | −37.1 | +6.9 |
| PATH C | −41.1 | +15.6 |
| PATH D | −48.7 | +18.5 |
| Dose (mg/kg) | C0 1 (ng/mL) | Cmax (ng/mL) | AUC0–8h (ng·h/mL) | Clast (ng/mL) | Vd (mL) | kel (h−1) | Cl (mL/h) | t1/2 (h) |
|---|---|---|---|---|---|---|---|---|
| 0.07 | 74.6 ± 12.2 | 50.6 ± 7.4 | 81.1 ± 9.5 | 1.53 ± 0.09 | 364 ± 110 | 0.317 ± 0.021 | 105.0 ± 20.5 | 1.66 ± 0.10 |
| 0.25 | 490 ± 80.2 | 329.8 ± 51.2 | 477 ± 42 | 7.49 ± 0.75 | 166 ± 27 | 0.343 ± 0.025 | 55.4 ± 6.3 | 1.51 ± 0.12 |
| 1.00 | 1686 ± 424 | 1206.2 ± 299.5 | 2030 ± 429 | 42.03 ± 4.42 | 300 ± 103 | 0.286 ± 0.064 | 53.7 ± 6.1 | 2.20 ± 0.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Alonso, E.; Escobar-Peso, A.; Ayuso, M.I.; Gonzalo-Gobernado, R.; Chioua, M.; Montoya, J.J.; Montaner, J.; Fernández, I.; Marco-Contelles, J.; Alcázar, A. Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke. Antioxidants 2020, 9, 291. https://doi.org/10.3390/antiox9040291
Martínez-Alonso E, Escobar-Peso A, Ayuso MI, Gonzalo-Gobernado R, Chioua M, Montoya JJ, Montaner J, Fernández I, Marco-Contelles J, Alcázar A. Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke. Antioxidants. 2020; 9(4):291. https://doi.org/10.3390/antiox9040291
Chicago/Turabian StyleMartínez-Alonso, Emma, Alejandro Escobar-Peso, Maria I. Ayuso, Rafael Gonzalo-Gobernado, Mourad Chioua, Juan J. Montoya, Joan Montaner, Israel Fernández, José Marco-Contelles, and Alberto Alcázar. 2020. "Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke" Antioxidants 9, no. 4: 291. https://doi.org/10.3390/antiox9040291
APA StyleMartínez-Alonso, E., Escobar-Peso, A., Ayuso, M. I., Gonzalo-Gobernado, R., Chioua, M., Montoya, J. J., Montaner, J., Fernández, I., Marco-Contelles, J., & Alcázar, A. (2020). Characterization of a CholesteroNitrone (ISQ-201), a Novel Drug Candidate for the Treatment of Ischemic Stroke. Antioxidants, 9(4), 291. https://doi.org/10.3390/antiox9040291







