Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
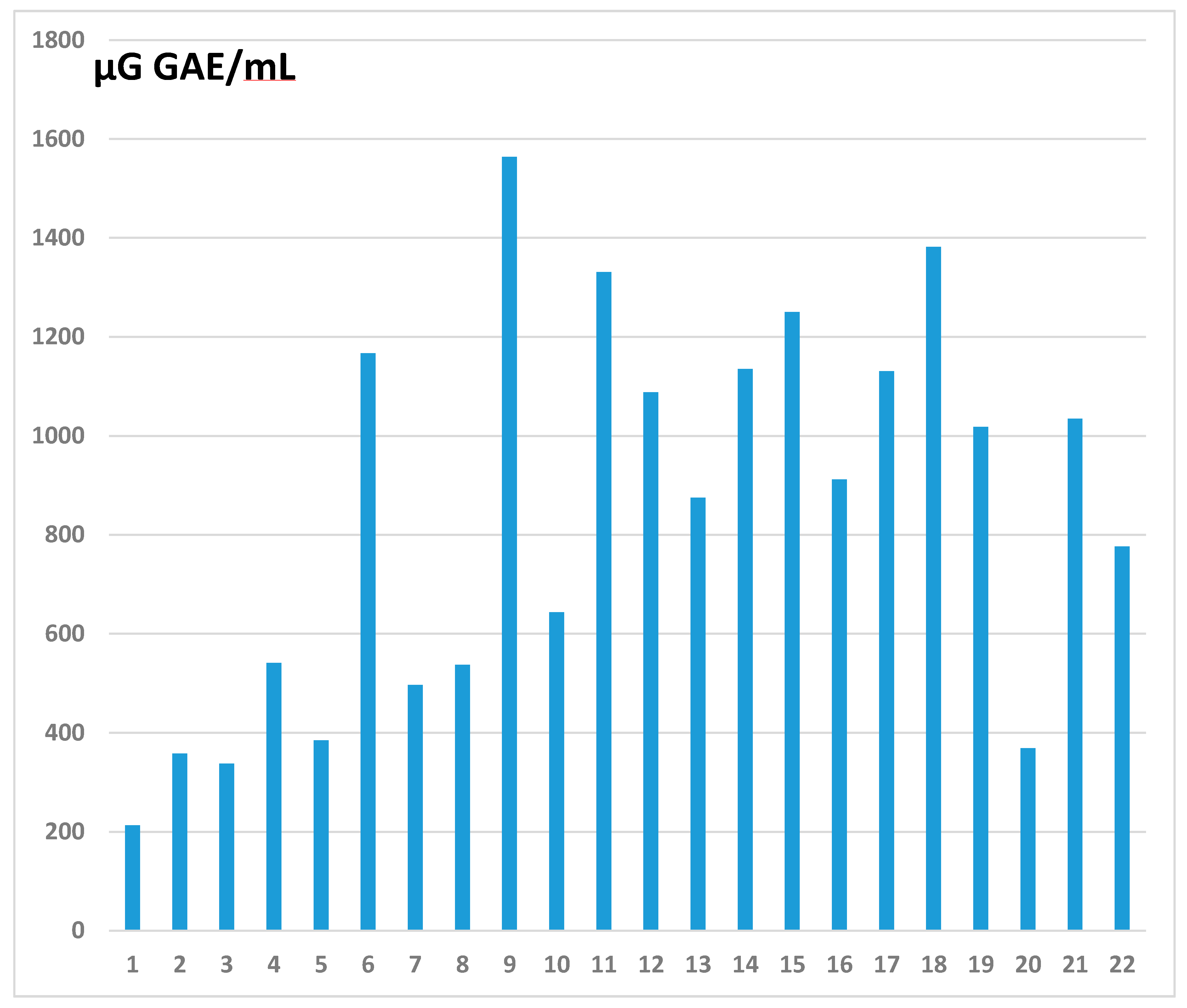
2.2. Determination of Total Phenolic Content
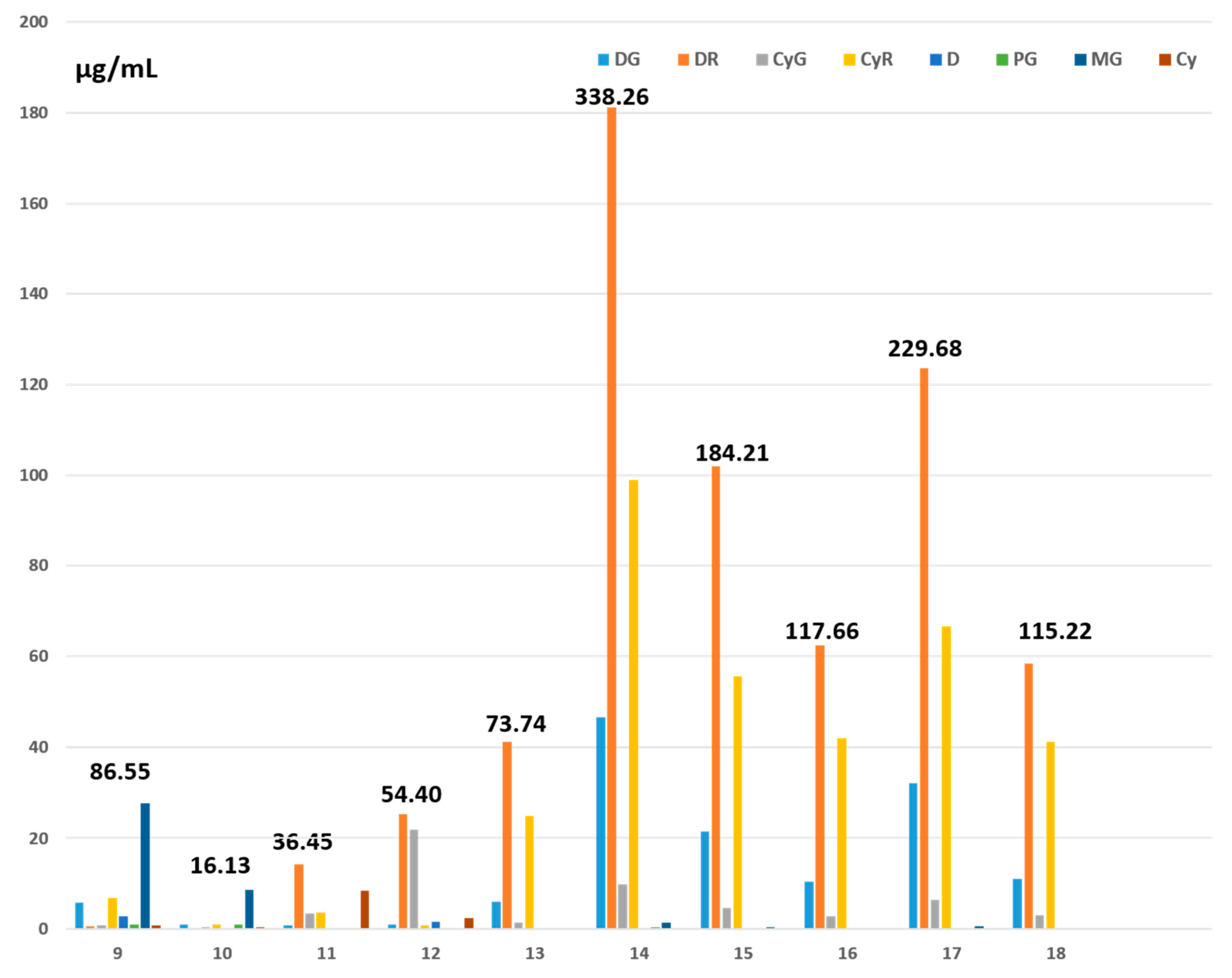
2.3. Specific Flavonoid Contents
2.4. Vascular Reactivity
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and Cardiovascular Health; MDPI: Basel, Switzerland, 2018; Volume 19, ISBN 3493227574. [Google Scholar]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: A review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen aledery sudy. Lancet 1993, 34, 1007–1011. [Google Scholar] [CrossRef]
- Mendonça, R.D.; Carvalho, N.C.; Martin-Moreno, J.M.; Pimenta, A.M.; Lopes, A.C.S.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Total polyphenol intake, polyphenol subtypes and incidence of cardiovascular disease: The SUN cohort study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 69–78. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019. [Google Scholar] [CrossRef]
- Schini-Kerth, V.B.; Étienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular protection by natural product-derived polyphenols: In vitro and in vivo evidence. Planta Med. 2011, 77, 1161–1167. [Google Scholar] [CrossRef]
- Yamagata, K. Polyphenols regulate endothelial functions and reduce the risk of cardiovascular disease. Curr. Pharm. Des. 2019. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharm. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial cells: From dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Mihalache Arion, C.; Tabart, J.; Kevers, C.; Niculaua, M.; Filimon, R.; Beceanu, D.; Dommes, J. Antioxidant potential of different plum cultivars during storage. Food Chem. 2014, 146, 485–491. [Google Scholar] [CrossRef]
- Tabart, J.; Auger, C.; Kevers, C.; Dommes, J.; Pollet, B.; Defraigne, J.O.; Schini-Kerth, V.B.; Pincemail, J. The potency of commercial blackcurrant juices to induce relaxation in porcine coronary artery rings is not correlated to their antioxidant capacity but to their anthocyanin content. Nutrition 2018, 51–52, 53–59. [Google Scholar] [CrossRef]
- Liu, R.H. Heath-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, 1–14. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Gardner, E.J.; Walker, D. Can pure fruit and vegetable juices protect against cancer and cardiovascular disease too? A review of the evidence. Int. J. Food Sci. Nutr. 2006, 57, 249–272. [Google Scholar] [CrossRef]
- Hyson, D.A. A review andc ritical analysis of the scientific literature related to 100% fruit juice and human health. Adv. Nutr. 2015, 6, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J.J.F. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct. 2010, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Gośliński, M.; Szwengiel, A. Multidimensional comparative analysis of phenolic compounds in organic juices with high antioxidant capacity. J. Sci. Food Agric. 2017, 97, 2657–2663. [Google Scholar] [CrossRef]
- Granato, D.; Karnopp, A.R.; van Ruth, S.M. Characterization and comparison of phenolic composition, antioxidant capacity and instrumental taste profile of juices from different botanical origins. J. Sci. Food Agric. 2015, 95, 1997–2006. [Google Scholar] [CrossRef]
- Wern, K.H.; Haron, H.H.; Keng, C.B. Comparison of total phenolic contents (TPC) and antioxidant activities of fresh fruit juices, commercial 100 % fruit juices and fruit drinks. Sains Malays. 2016, 45, 1319–1327. [Google Scholar]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D.; Valero, M. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wasila, H.; Liu, L.; Yuan, T.; Gao, Z.; Zhao, B.; Ahmad, I. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Pollet, B.; Arnold, C.; Marx, C.; Schini-Kerth, V.B. Great heterogeneity of commercial fruit juices to induce endothelium-dependent relaxations in isolated porcine Coronary Arteries: Role of the phenolic content and composition. J. Med. Food 2015, 18, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, A.; Hóvári, J. Antioxidant properties of commercial alcoholic and nonalcoholic beverages. Nahrung 2003, 47, 79–86. [Google Scholar] [CrossRef]
- Jakobek, L.; Seruga, M.; Medvidovic-Kosanovic, M.; Novak, I. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch. Lebensm. 2007, 103, 58–64. [Google Scholar]
- Rechner, A.R.; Kuhnle, G.; Hu, H.; Roedig-Penman, A.; van den Braak, M.H.; Moore, K.P.; Rice-Evans, C.A. The metabolism of dietary polyphenols and the relevance to circulating levels of conjugated metabolites. Free Radic. Res. 2002, 36, 1229–1241. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Tomás-Barberán, F.A.; García-Villalba, R. Pomegranate fruit and juice (cv. Mollar), rich in ellagitannins and anthocyanins, also provide a significant content of a wide range of proanthocyanidins. J. Agric. Food Chem. 2019, 67, 9160–9167. [Google Scholar] [CrossRef]
- Mattila, P.H.; Hellström, J.; McDougall, G.; Dobson, G.; Pihlava, J.M.; Tiirikka, T.; Stewart, D.; Karjalainen, R. Polyphenol and vitamin C contents in European commercial blackcurrant juice products. Food Chem. 2011, 127, 1216–1223. [Google Scholar] [CrossRef]
- Alamgeer, A.; Auger, C.; Chabert, P.; Lugnier, C.; Mushtaq, M.N.; Schini-Kerth, V.B. Mechanisms underlying vasorelaxation induced in the porcine coronary arteries by Thymus linearis, Benth. J. Ethnopharmacol. 2018, 225, 211–219. [Google Scholar] [CrossRef]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomized controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef]
- Mahobiya, A.; Singh, T.U.; Rungsung, S.; Kumar, T.; Chandrasekaran, G.; Parida, S.; Kumar, D. Kaempferol-induces vasorelaxation via endothelium-independent pathways in rat isolated pulmonary artery. Pharmacol. Rep. 2018, 70, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Leung, S.W.S.; Leung, G.P.H.; Man, R.Y.K. Kaempferol enhances endothelium-dependent relaxation in the porcine coronary artery through activation of large-conductance Ca2+-activated K+ channels. Br. J. Pharmacol. 2015, 172, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Aggio, A.; Grassi, D.; Onori, E.; D’Alessandro, A.; Masedu, F.; Valenti, M.; Ferri, C. Endothelium/nitric oxide mechanism mediates vasorelaxation and counteracts vasoconstriction induced by low concentration of flavanols. Eur. J. Nutr. 2013, 52, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Thilavech, T.; Abeywardena, M.Y.; Adams, M.; Dallimore, J.; Adisakwattana, S. Naturally occurring anthocyanin cyanidin-3-rutinoside possesses inherent vasorelaxant actions and prevents methylglyoxal-induced vascular dysfunction in rat aorta and mesenteric arterial bed. Biomed. Pharmacother. 2017, 95, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, M.; Burns, J.; Crozier, A.; Kelly, I.E.; Lean, M.E.J. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur. J. Clin. Nutr. 2000, 54, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Coletta, W.; Rapisarda, P.; Donati, M.B.; Rotilio, D. Development and validation of an LC-MS/MS analysis for simultaneous determination of delphinidin-3-glucoside, cyanidin-3-glucoside and cyanidin-3-(6-malonylglucoside) in human plasma and urine after blood orange juice administration. J. Sep. Sci. 2007, 30, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, J.; Huang, K.; Michel, D.; Fang, J. HPLC-MS/MS analysis of anthocyanins in human plasma and urine using protein precipitation and dilute-and-shoot sample preparation methods, respectively. Biomed. Chromatogr. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Kay, D.J.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef]
- Birringer, M. Hormetics: Dietary triggers of an adpatative stress response. Pharm. Res. 2011, 28, 2680–2694. [Google Scholar] [CrossRef]
- Pincemail, J.; Defraigne, J.O.; Courtois, A.; Albert, A.; Cheramy-Bien, J.P.; Sakalihasan, N. Abdominal Aortic Aneurysm (AAA): Is There a Role for the Prevention and Therapy Using Antioxidants? Curr. Drug Targets 2018, 19, 1256–1264. [Google Scholar] [CrossRef]
- Ray, S.; Miglio, T.B.; Del Rio, D. Assessment of vascular and endothelial dysfunction in nutritional studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bao, Y.; Wang, X.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. The effect of anthocyanins on blood pressure. A PRISMA-compliant meta-analysis of randomized clinical trials. Medicine 2016, 95, 1–7. [Google Scholar]
| number | List of Juices | Myricetin | Quercetin | Kaempferol | Total Flavonols | EGC | EGCG | ECG | GC | C | EC | Total Flavanols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tomato (Carrefour) | 0.3 | 2.7 | 0.1 | 3.1 | 2.74 | 8.86 | 1.64 | 53.55 | 0.05 | 4.34 | 71.19 |
| 2 | Tomato (Biotta) | 0.1 | 1.6 | 0.2 | 1.9 | 14.40 | 8.34 | 3.89 | 26.70 | 0.06 | 2.67 | 56.06 |
| 3 | Carrot (Biotta) | 0.1 | 0.5 | 0.2 | 0.8 | 0.71 | 29.64 | 1.58 | 0.00 | 0.32 | 0.85 | 33.09 |
| 4 | Orange d’Espagne (Carrefour) | 0.5 | 0.9 | 0.1 | 1.5 | 20.47 | 7.91 | 1.57 | 0.60 | 0.21 | 0.22 | 30.97 |
| 5 | Pure Orange (Vitamont) | 1.3 | 0.8 | 0.7 | 2.8 | 7.69 | 2.67 | 2.19 | 1.53 | 0.09 | 1.09 | 15.25 |
| 6 | Lemon (Bonneterre) | 0.7 | 1.3 | 1.9 | 4.0 | 0.52 | 0.86 | 0.42 | 0.11 | 0.03 | 0.68 | 2.62 |
| 7 | Grapefruit | 1.8 | 2.0 | 1.1 | 4.9 | 4.18 | 1.48 | 0.85 | 0.24 | 0.14 | 3.15 | 10.03 |
| 8 | Pure Grapefruit (Vitamont) | 0.6 | 0.9 | 0.5 | 2.0 | 9.67 | 1.99 | 1.38 | 0.03 | 0.04 | 4.66 | 17.76 |
| 9 | Grape Materne (Materne) | 13.0 | 2.7 | 0.4 | 16.0 | 86.68 | 41.37 | 14.43 | 3.89 | 1.11 | 7.51 | 154.99 |
| 10 | Pure Grape (Vitamont) | 7.6 | 3.3 | 0.9 | 11.9 | 5.63 | 11.79 | 8.85 | 0.27 | 0.36 | 1.81 | 28.71 |
| 11 | Pomegranate (Biotta) | 8.5 | 4.0 | 0.2 | 12.7 | 849.59 | 45.95 | 3.00 | 294.60 | 0.09 | 0.77 | 1194 |
| 12 | Blackcurrant (Biotta) | 14.4 | 2.5 | 0.7 | 17.7 | 13.85 | 37.80 | 7.11 | 2.00 | 0.02 | 0.63 | 61.41 |
| 13 | Blackcurrant (Natreen) | 4.8 | 3.5 | 0.0 | 8.3 | 23.00 | 18.42 | 2.10 | 1.20 | 0.10 | 1.39 | 46.22 |
| 14 | Blackcurrant (Jacoby Bio) | 1.6 | 2.2 | 0.3 | 4.1 | 21.81 | 33.17 | 1.73 | 4.29 | 0.26 | 2.17 | 63.43 |
| 15 | Blackcurrant (Van Nahmen) | 1.8 | 2.7 | 0.5 | 4.9 | 22.60 | 66.02 | 2.78 | 10.76 | 0.33 | 1.71 | 104.20 |
| 16 | Blackcurrant (Schlör nectar) | 2.0 | 1.9 | 0.3 | 4.2 | 22.68 | 41.27 | 1.96 | 6.41 | 0.22 | 0.84 | 73.39 |
| 17 | Blackcurrant (Gut and Günstig) | 1.9 | 2.7 | 0.6 | 5.1 | 21.90 | 95.06 | 4.46 | 7.77 | 0.35 | 2.31 | 131.85 |
| 18 | Blackcurrant (Jacoby) | 3.2 | 3.3 | 0.7 | 7.2 | 44.05 | 181.42 | 3.25 | 4.65 | 0.60 | 2.07 | 236.02 |
| 19 | Pineapple juice (Carrefour) | 7.7 | 1.6 | 1.2 | 10.5 | 1.74 | 4.66 | 1.12 | 0.32 | 0.12 | 3.59 | 11.55 |
| 20 | Pineapple juice (De Drie Wilgen) | 1.6 | 0.9 | 0.4 | 2.9 | 8.32 | 14.06 | 4.94 | 18.94 | 0.02 | 2.40 | 48.67 |
| 21 | Apple (Carrefour) | 1.3 | 2.3 | 1.0 | 4.5 | 1.26 | 3.17 | 1.37 | 0.03 | 0.11 | 0.45 | 6.39 |
| 22 | Pure Apple (Vitamont) | 3.5 | 4.0 | 0.3 | 7.9 | 1.32 | 2.87 | 2.80 | 0.04 | 0.14 | 0.74 | 7.91 |
| Number | Juices Origin | With Endothelium | Without Endothelium | ||||
|---|---|---|---|---|---|---|---|
| E+ (1% v/v) | E+ (5 % v/v) | E+ (10 % v/v) | E− (1% v/v) | E− (5% v/v) | E− (10% v/v) | ||
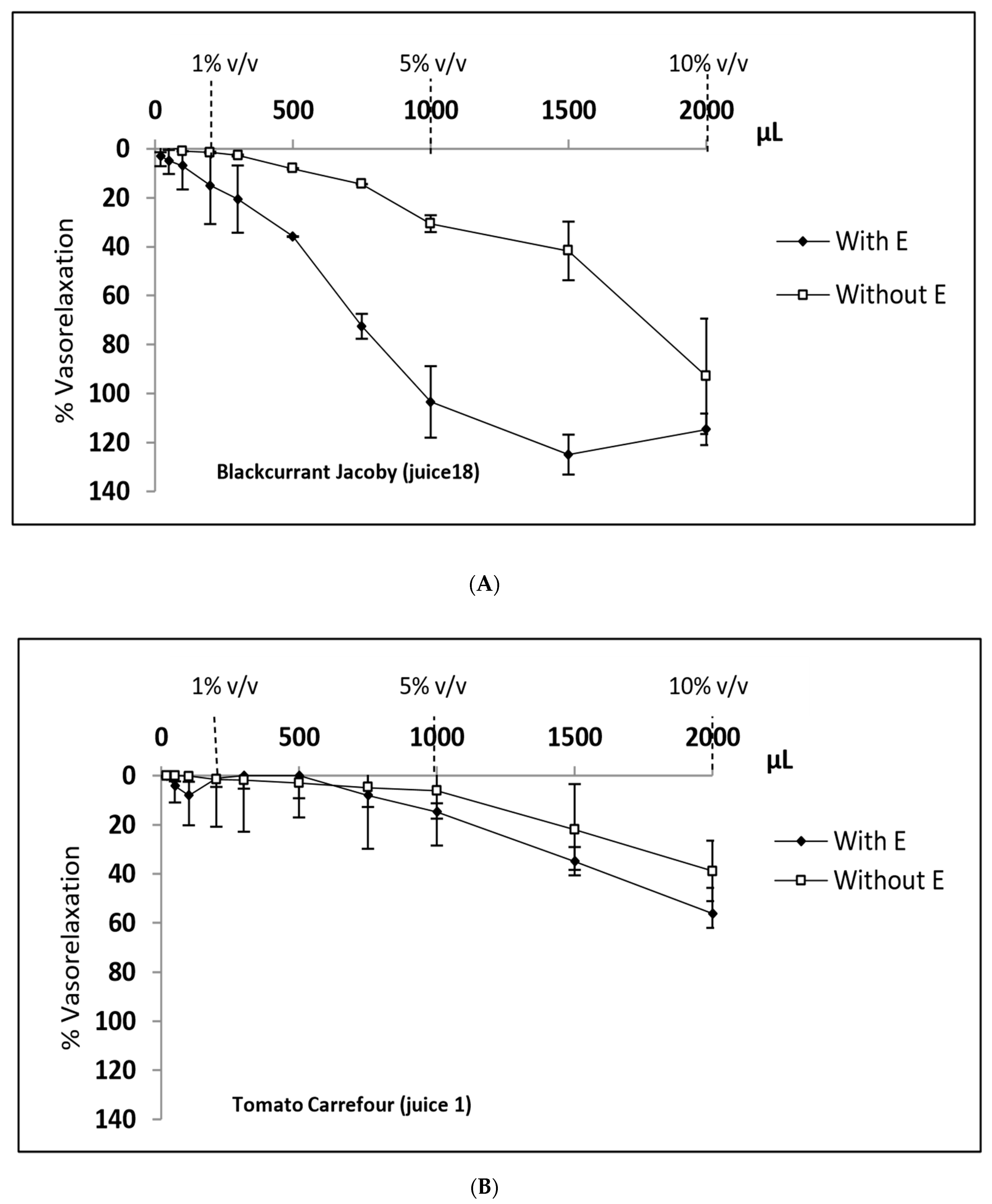
| 1 | Tomato (Carrefour) | 1.19 ± 22.87 | 14.73 ± 3.44 | 56.19 ± 10.44 | 1.51 ± 3.1 | 6.07 ± 11.49 | 38.83 ± 12.3 |
| 2 | Tomato (Biotta) | 6.9 ± 7.73 | 28.82 ± 9.63 | 30.58 ± 5.85 | 79.82 ± 7.50 | ||
| 3 | Carrot (Biotta) | 4.28 ± 6.7 | 7.72 ± 6.39 | 44.94 ± 18.26 | 8.52 ± 1.84 | 35.24 ± 9.45 | 63.41 ± 22.19 |
| 4 | Orange d’Espagne (Carrefour) | 27.86 ± 27.04 | 61.70 ± 30.32 | 6.1 ± 14.35 | 25.79 ± 16.05 | 49.68 ± 24.24 | |
| 5 | Pure Orange (Vitamont) | 40.63 ± 30.89 | 83.27 ± 21.00 | 4.11 ± 4.75 | 122.9 ± 66.61 | 173.14 ± 89.22 | |
| 6 | Lemon (Bonneterre) | 81.07 ± 9.9 | 95.06 ± 13.4 | 87.32 ± 10.97 | 79.35 ± 1.49 | 102.04 ± 1.25 | 98.48 ± 1.11 |
| 7 | Grapefruit | 16.64 ± 17.04 | 74.39 ± 1.34 | 36.17 ± 10.46 | 89.68 ± 2.70 | ||
| 8 | Pure Grapefruit (Vitamont) | 15.01 ± 0.00 | 106.56 ± 6.86 | 120.43 ± 7.10 | 102.49 ± 6.86 | 105.41 ± 7.10 | |
| 9 | Grape Materne (Materne) | 8.81 ± 0.80 | 22.98 ± 5.50 | 45.11 ± 9.58 | 16.77 ± 4.51 | 40.49 ± 4.12 | |
| 10 | Pure Grape (Vitamont) | 15.01 ± 0.23 | 20.06 ± 12.54 | 56.19 ±11.05 | 0.18 ± 17.56 | 25.76 ± 22.59 | 38.83 ± 1.92 |
| 11 | Pomegranate (Biotta) | 93.13 ± 8.42 | 106.78 ± 5.67 | 2.09 ± 1.91 | 59.35 ± 17.94 | 92.34 ± 19.73 | |
| 12 | Blackcurrant (Biotta) | 44.75 ± 22.76 | 97.29 ± 26.8 | 110.15 ± 15.87 | 4.56 ± 7.66 | 40.56 ± 22.31 | 94.35 ± 27.06 |
| 13 | Blackcurrant (Natreen) | 9.47 ± 16.59 | 106.55 ± 4.19 | 114.65 ± 13.14 | 42.59 ± 31.06 | 66.56 ± 30.63 | |
| 14 | Blackcurrant (Jacoby Bio) | 5.12± 0.92 | 96.59 ± 12.4 | 191.68 ± 3.81 | 44.21 ± 12.37 | 91.57 ± 18.88 | |
| 15 | Blackcurrant (Van Nahmen) | 19.9 ± 0.97 | 100.53 ± 5.08 | 109.73 ± 7.52 | 2.76 ± 42.66 | 64.48 ± 25.82 | 127.59 ± 18.8 |
| 16 | Blackcurrant (Schörl nectar) | 23.75 ± 3.59 | 85.57 ± 20.41 | 117.41 ± 8.91 | 1.64 ± 0.14 | 42.07 ± 20.44 | 87.59 ± 26.57 |
| 17 | Blackcurrant (Gut & Günstig) | 19.99 ± 14.51 | 85.88 ± 8.10 | 107.18 ± 2.61 | 52.29 ± 9.00 | 110.93 ± 7.56 | |
| 18 | Blackcurrant (Jacoby) | 56.76 ± 24.49 | 103.82 ± 5.07 | 106.1 ± 7.97 | 0.87 ± 1.23 | 67.53 ± 15.57 | 103.43 ± 4.16 |
| 19 | Pineapple juice (Carrefour) | 5.66 ± 14.96 | 13.04 ± 6.9 | 35.46 ± 2.12 | 9.1 ± 7.54 | 38.19 ± 20.4 | |
| 20 | Pineapple juice (De Drie Wilgen) | 12.95 ± 2.19 | 92.05 ± 0.72 | 5.88 ± 1.58 | 34.15 ± 5.00 | ||
| 21 | Apple (Carrefour) | 29.23 ± 6.97 | 0.98 ± 3.23 | 17.83 ± 1.34 | |||
| 22 | Pure Apple (Viamont | 15.28 ± 4.75 | 64.01 ± 4.29 | 86.64 ± 11.96 | 32.38 ± 19.4 | 68.68 ± 27.3 | |
| Compounds | E+ (1% v/v) | E+ (5% v/v) | E+ (10% v/v) | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| TPC-J | 0.32 | 0.14 | 0.58 | 0.04 | 0.55 | 0.007 |
| TPC-OB | 0.32 | 0.14 | 0.58 | 0.04 | 0.55 | 0.007 |
| Flavonols-OB | 0.19 | 0.39 | 0.17 | 0.44 | 0.02 | 0.99 |
| Flavanols-OB | 0.08 | 0.69 | 0.27 | 0.2 | 0.17 | 0.44 |
| Anthocyanins-OB | 0.54 | 0.2 | 0.28 | 0.5 | 0.75 | 0.05 |
| Myricetin | 0.1 | 0.6 | 0.06 | 0.7 | 0.07 | 0.7 |
| Quercetin | 0.11 | 0.61 | 0.35 | 0.1 | 0.19 | 0.38 |
| Kaempferol | 0.56 | 0.009 | 0.11 | 0.96 | 0.19 | 0.4 |
| EGC | 0.14 | 0.51 | 0.22 | 0.31 | 0.13 | 0.54 |
| EGCG | 0.41 | 0.05 | 0.45 | 0.03 | 0.31 | 0.15 |
| ECG | 0.004 | 0.98 | 0.13 | 0.5 | 0.17 | 0.44 |
| GC | 0.19 | 0.3 | 0.14 | 0.5 | 0.09 | 0.68 |
| C | 0.07 | 0.75 | 0.03 | 0.87 | 0.09 | 0.67 |
| EC | 0.19 | 0.37 | 0.2 | 0.35 | 0.16 | 0.45 |
| DG | 0.17 | 0.6 | 0.3 | 0.39 | 0.70 | 0.02 |
| DR | 0.06 | 0.85 | 0.49 | 0.14 | 0.81 | 0.004 |
| CyG | 0.38 | 0.26 | 0.37 | 0.28 | 0.39 | 0.25 |
| CyR | 0.06 | 0.86 | 0.44 | 0.2 | 0.76 | 0.01 |
| PG | 0.49 | 0.28 | 0.96 | 0.00001 | 0.61 | 0.1 |
| MG | 0.36 | 0.2 | 0.8 | 0.05 | 0.61 | 0.11 |
| Compounds | Title | ||
|---|---|---|---|
| Vjuice/VOBS 1% | Vjuice/VOBS 5% | Vjuice/VOBS 10% | |
| flavonols (µM) | |||
| myricetin | 0.12 ± 0.13 (0.005–0.11) | 0.56 ± 0.62 (0.022–2.60) | 1.06 ± 1.18 (0.042–4.12) |
| quercetin | 0.07 ± 0.03 (0.17–0.62) | 0.34 ± 0.16 (0.080–0.623) | 0.66 ± 0.31 (0.15–1.2) |
| kaempferol | 0.02 ± 0.02 (0.006–0.67) | 0.09 ± 0.07 (0.024–0.32) | 0.18 ± 0.14(0.046–0.615) |
| flavanols (µM) | |||
| EGC | 0.51 ± 0.60 (0.02–2.8)* | 2.5 ± 3.0 (0.08–6.8)* | 4.8 ± 5.9 (0.21–6.8)* |
| EGCG | 0.6 ± 0.9 (0.02–3.9) | 3.10 ± 4.4 (0.09–19) | 6.0 ± 8.3 (0.17–36) |
| ECG | 0.1 ± 0.1 (0.01–0.32) | 0.4 ± 0.4 (0.05–1.60) | 0.7 ± 0.7 (0.09–3) |
| GC | 0.23 ± 0.4 (0.01–1.7)* | 1.1 ± (0.01–8.3)* | 2.03 ± 3.8 (0.01–16)* |
| C | 0.01 ± 0.01 (0.002–0.38) | 0.04 ± 0.04 (0.004–0.18) | 0.1 ± 0.1 (0.007–0.35) |
| EC | 0.1 ± 0.1 (0.02–0.26) | 0.3 ± 0.3 (0.04–1.20) | 0.7 ± 0.6 (0.07–2.40) |
| anthocyanins (µM) | |||
| DG | 0.29 ± 0.3 (0.02-0.99) | 1.39 ± 1.6 (0.08-4.76) | 2.66 ± 3.0 (0.14-9.10) |
| DR | 1.97 ± 1.9 (0.01–5.90) | 9.48 ± 9.1 (0.03–28) | 18.22 ± 17.6 (0.06–54) |
| CyG | 0.12 ± 0.10 (0.01–0.48) | 0.57 ± 0.7 (0.05–2.30) | 1.10 ± 1.3 (0.09–4.42) |
| CyR | 0.45 ± 0.40 (0.01–1.30) | 2.15 ± 2.10 (0.05–6.20) | 4.11 ± 4.0 (0.10–12) |
| PG | 0.01 ± 0.01 (0.01–0.02) | 0.04 ± 0.04 (0.02–0.11) | 0.08 ± 0.07 (0.04–0.21) |
| MG | >10 | >20 | >20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matute, A.; Tabart, J.; Cheramy-Bien, J.-P.; Pirotte, B.; Kevers, C.; Auger, C.; Schini-Kerth, V.; Dommes, J.; Defraigne, J.-O.; Pincemail, J. Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection. Antioxidants 2020, 9, 92. https://doi.org/10.3390/antiox9020092
Matute A, Tabart J, Cheramy-Bien J-P, Pirotte B, Kevers C, Auger C, Schini-Kerth V, Dommes J, Defraigne J-O, Pincemail J. Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection. Antioxidants. 2020; 9(2):92. https://doi.org/10.3390/antiox9020092
Chicago/Turabian StyleMatute, Alexis, Jessica Tabart, Jean-Paul Cheramy-Bien, Bernard Pirotte, Claire Kevers, Cyril Auger, Valérie Schini-Kerth, Jacques Dommes, Jean-Olivier Defraigne, and Joël Pincemail. 2020. "Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection" Antioxidants 9, no. 2: 92. https://doi.org/10.3390/antiox9020092
APA StyleMatute, A., Tabart, J., Cheramy-Bien, J.-P., Pirotte, B., Kevers, C., Auger, C., Schini-Kerth, V., Dommes, J., Defraigne, J.-O., & Pincemail, J. (2020). Compared Phenolic Compound Contents of 22 Commercial Fruit and Vegetable Juices: Relationship to Ex-Vivo Vascular Reactivity and Potential In Vivo Projection. Antioxidants, 9(2), 92. https://doi.org/10.3390/antiox9020092





