The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Collection and Extraction
2.2. Measurement of the Total Phenolic and Flavonoid Content
2.3. Anti-Oxidant Activity
2.3.1. DPPH• Radical Scavenging Activity
2.3.2. Ferric Reducing Anti-Oxidant Power (FRAP) Assay
2.4. Anti-Cancer Activity
2.4.1. Cell Culture
2.4.2. Cell Titer-Glo Viability Assay
2.5. Phytochemical Profiling
2.6. Data Analysis
3. Results
3.1. Anti-Oxidant Analyses
3.2. Anti-Cancer Activity
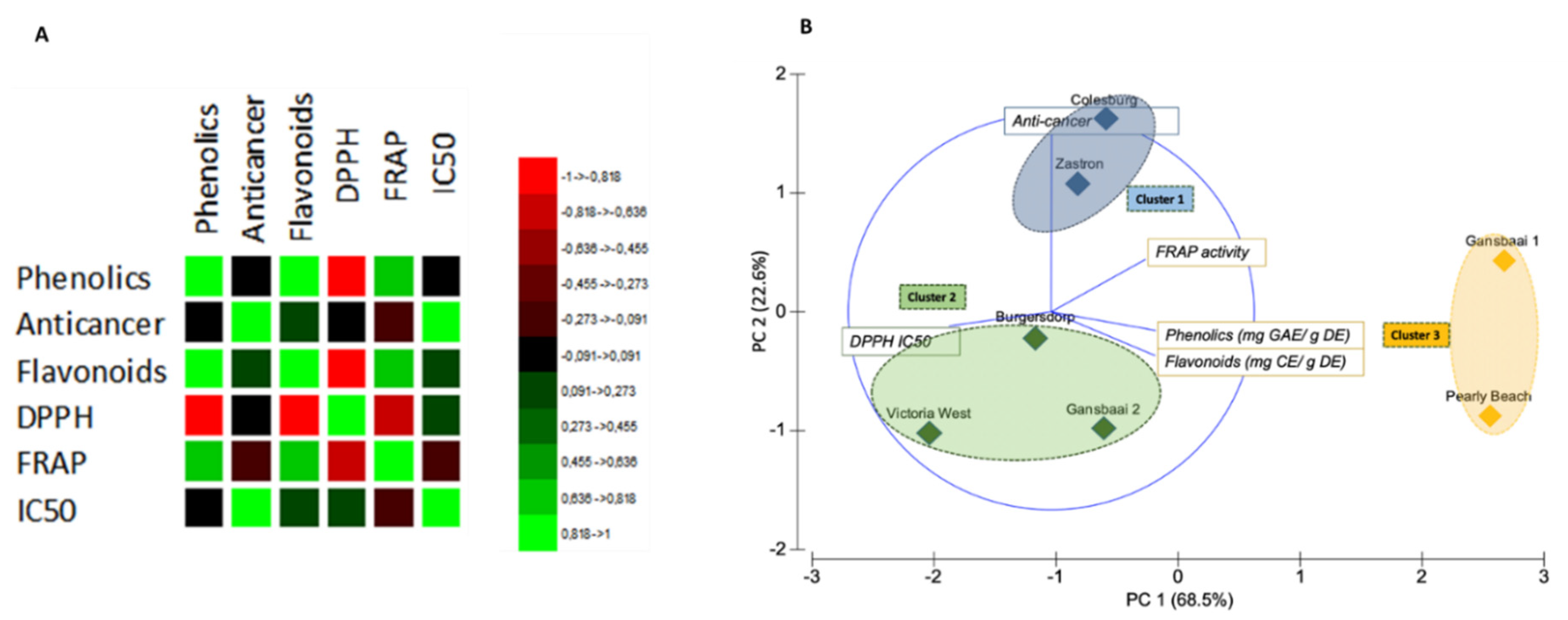
3.3. Correlation Matrix and Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Wyk, B.-E.; Albrecht, C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J. Ethnopharmacol. 2008, 119, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Umesh, C.V.; Jamsheer, A.M.; Alex, P.M. The role of flavonoids in drug discovery—Review on potential applications. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 70–77. [Google Scholar]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A review of probable mechnisms of action and potential applications. Am. J. Clin. Nutrit. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Melzig, M.F.; Fuchs, H.; Weng, A. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Botanics 2011, 1, 19–29. [Google Scholar]
- Albrecht, C.F.; Stander, M.A.; Grobbelaar, M.C.; Colling, J.; Kossmann, J.; Hills, P.N.; Makunga, N.P. LC–MS-based metabolomics assists with quality assessment and traceability of wild and cultivated plants of Sutherlandia frutescens (Fabaceae). S. Afr. J. Bot. 2012, 82, 33–45. [Google Scholar] [CrossRef]
- Zonyane, S.; Chen, L.; Xu, M.J.; Gong, Z.N.; Xu, S.; Makunga, N.P. Geographic-based metabolomic variation and toxicity analysis of Sutherlandia frutescens LR Br.—An emerging medicinal crop in South Africa. Ind. Crops Prod. 2019, 133, 414–427. [Google Scholar] [CrossRef]
- Vorster, C.; Stander, A.; Joubert, A. Differential signalling involved in Sutherlandia frutescens—Induced cell death in MCF-7 and MCF-12A cells. J. Ethnopharmacol. 2012, 140, 123–130. [Google Scholar] [CrossRef]
- Mqoco, T.V.; Visagie, M.H.; Albrecht, C.; Joubert, A.M. Differential cellular interaction of Sutherlandia frutescens extracts on tumorigenic and non-tumorigenic breast cells. S. Afr. J. Bot. 2014, 90, 59–67. [Google Scholar] [CrossRef][Green Version]
- Leisching, G.; Loos, B.; Nell, T.; Engelbrecht, A.-M. Sutherlandia frutescens treatment induces apoptosis and modulates the PI3-kinase pathway in colon cancer cells. S. Afr. J. Bot. 2015, 100, 20–26. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Cromarty, A.D.; Albrecht, C.; Jansen van Rensburg, C.E. The antioxidant potential of Sutherlandia frutescens. J. Ethnopharmacol. 2004, 95, 1–5. [Google Scholar] [CrossRef]
- Katerere, D.R.; Eloff, J.N. Antibacterial and anti-oxidant activity of Sutherlandia frutescens (Fabaceae), a reputed anti-HIV/AIDS phytomedicine. Phytother. Res. 2005, 19, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chuang, D.Y.; Zong, Y.; Patel, J.; Brownstein, K.; Lei, W.; Lu, C.-H.; Simonyi, A.; Gu, Z.; Cui, J.; et al. Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PLoS ONE 2014, 9, e89748. [Google Scholar] [CrossRef] [PubMed]
- Tobwala, S.; Fan, W.; Hines, C.J.; Folk, W.R.; Ercal, N. Anti-oxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Comp. Altern. Med. 2014, 14, 271. [Google Scholar]
- Chen, L.; Xu, M.; Gong, Z.; Zonyane, S.; Xu, S.; Makunga, N.P. Comparative cardio and developmental toxicity induced by the popular medicinal extract of Sutherlandia frutescens (L.) R.Br. detected using a zebrafish Tuebingen embryo model. BMC Comp. Altern. Med. 2018, 18, 273. [Google Scholar] [CrossRef]
- Camille, N.; Dealtry, G.B. Regulation of M1/M2 macrophage polarization by Sutherlandia frutescens via NFKB and MAPK signaling pathways. S. Afr. J. Bot. 2018, 116, 42–51. [Google Scholar] [CrossRef]
- Ntuli, S.S.B.N.; Gelderblom, W.C.A.; Katerere, D.R. The mutagenic and antimutagenic activity of Sutherlandia frutescens extracts and marker compounds. BMC Comp. Altern. Med. 2018, 18, 93. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Jackson, G.A.; Lu, Y.; Drenkhahn, S.K.; Brownstein, K.J.; Starkey, N.J.; Lamberson, W.R.; Fritsche, K.L.; Mossine, V.V.; Besch-Williford, C.L.; et al. Inhibition of Gli/hedgehog signaling in prostate cancer cells by “cancer bush” Sutherlandia frutescens extract. Cell Biol. Internat. 2016, 40, 131–142. [Google Scholar] [CrossRef]
- Van der Walt, N.B.; Zakeri, Z.; Cronjé, M.J. The induction of apoptosis in A375 malignant melanoma cells by Sutherlandia frutescens. Evid. Comp. Altern. Med. 2016, 2016, 4921067. [Google Scholar]
- Makkar, H.P.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 74–75. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating anti-oxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Fawole, O.A.; Umezuruike, L.O.; Theron, K.I. Chemical and phytochemical properties and anti-oxidant activities of three pomegranate cultivars grown in South Africa. Food Bioprocess Technol. 2011, 5, 2934–2940. [Google Scholar] [CrossRef]
- Stankovic, N.; Mihajilov-Krstev, T.; Zlatkovic, B.; Stankov-Jovanovic, V.; Mitic, V.; Jovic, J.; Comic, L.; Kocic, B.; Bernstein, N. Antibacterial and anti-oxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS Wagening. J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and non-enzymatic anti-oxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Skerman, N.B.; Joubert, A.M.; Cronjé, M.J. The apoptosis inducing effects of Sutherlandia spp. extracts on an oesophageal cancer cell line. J. Ethnopharmacol. 2011, 137, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Tai, J.; Cheung, S.; Chan, E.; Hasman, D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J. Ethnopharmacol. 2004, 93, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Stander, A.; Marais, S.; Stivaktas, V.; Vorster, C.; Albrecht, C.; Lottering, M.-L.; Joubert, A.M. In vitro effects of Sutherlandia frutescens water extracts on cell numbers, morphology, cell cycle progression and cell death in a tumorigenic and a non-tumorigenic epithelial breast cell line. J. Ethnopharmacol. 2009, 124, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Chinkwo, K.A. Sutherlandia frutescens extracts can induce apoptosis in cultured carcinoma cells. J. Ethnopharmacol. 2005, 98, 163–170. [Google Scholar] [CrossRef]
- Mncwangi, N.P.; Viljoen, A.M. Quantitative variation of amino acids in Sutherlandia frutescens (Cancer bush)—Towards setting parameters for quality control. S. Afr. J. Bot. 2012, 82, 46–52. [Google Scholar] [CrossRef]
- Acharya, D.; Enslin, G.; Weiyang, C.; Sandasi, M.; Mavimbela, T.; Viljoen, A. A chemometric approach to the quality control of Sutherlandia (cancer bush). Biochem. Syst. Ecol. 2014, 56, 221–230. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Venkata, N.; Kalyan, C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.; Becker, K. The biological action of saponins in animal systems: A review. Braz. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef] [PubMed]
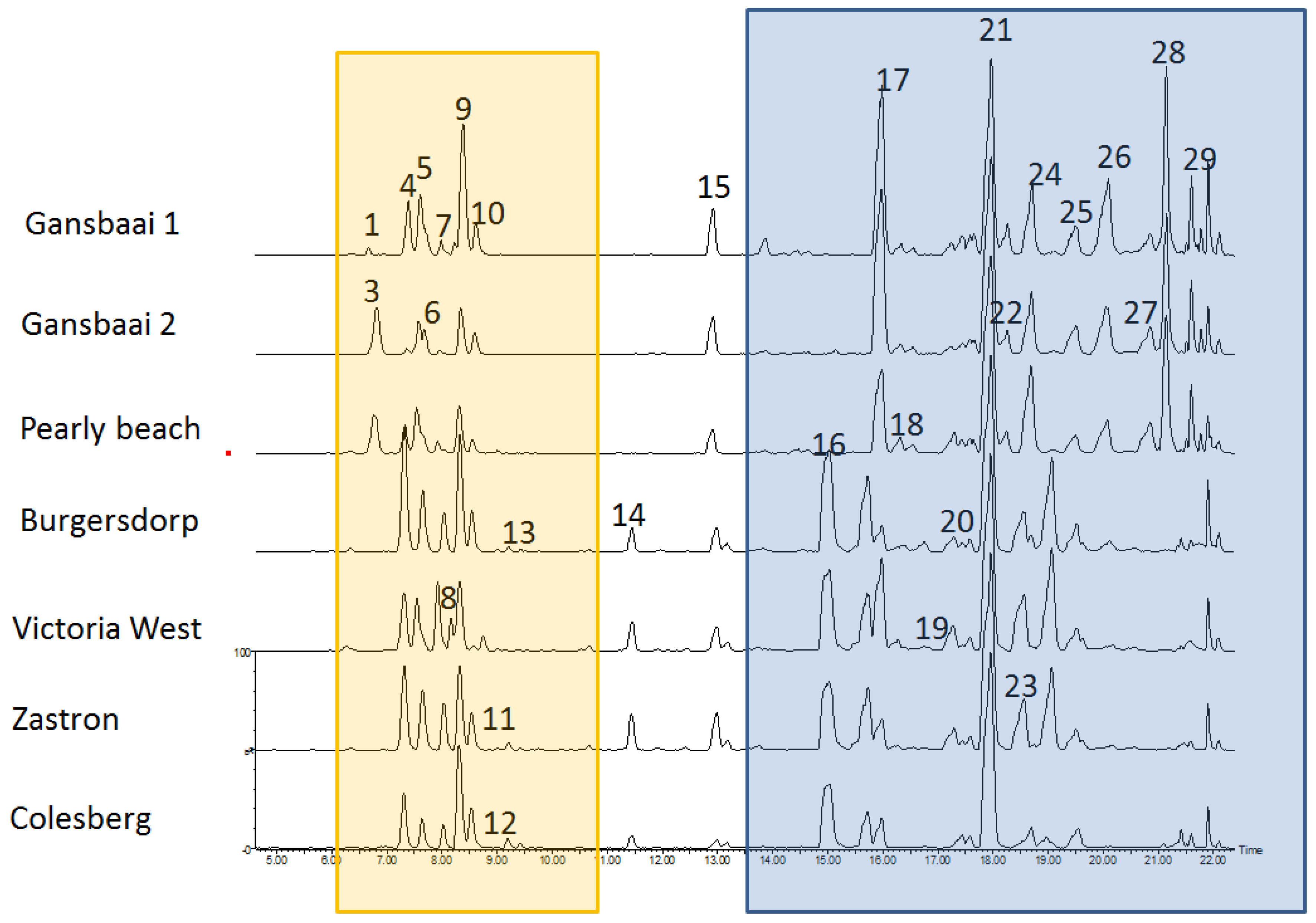
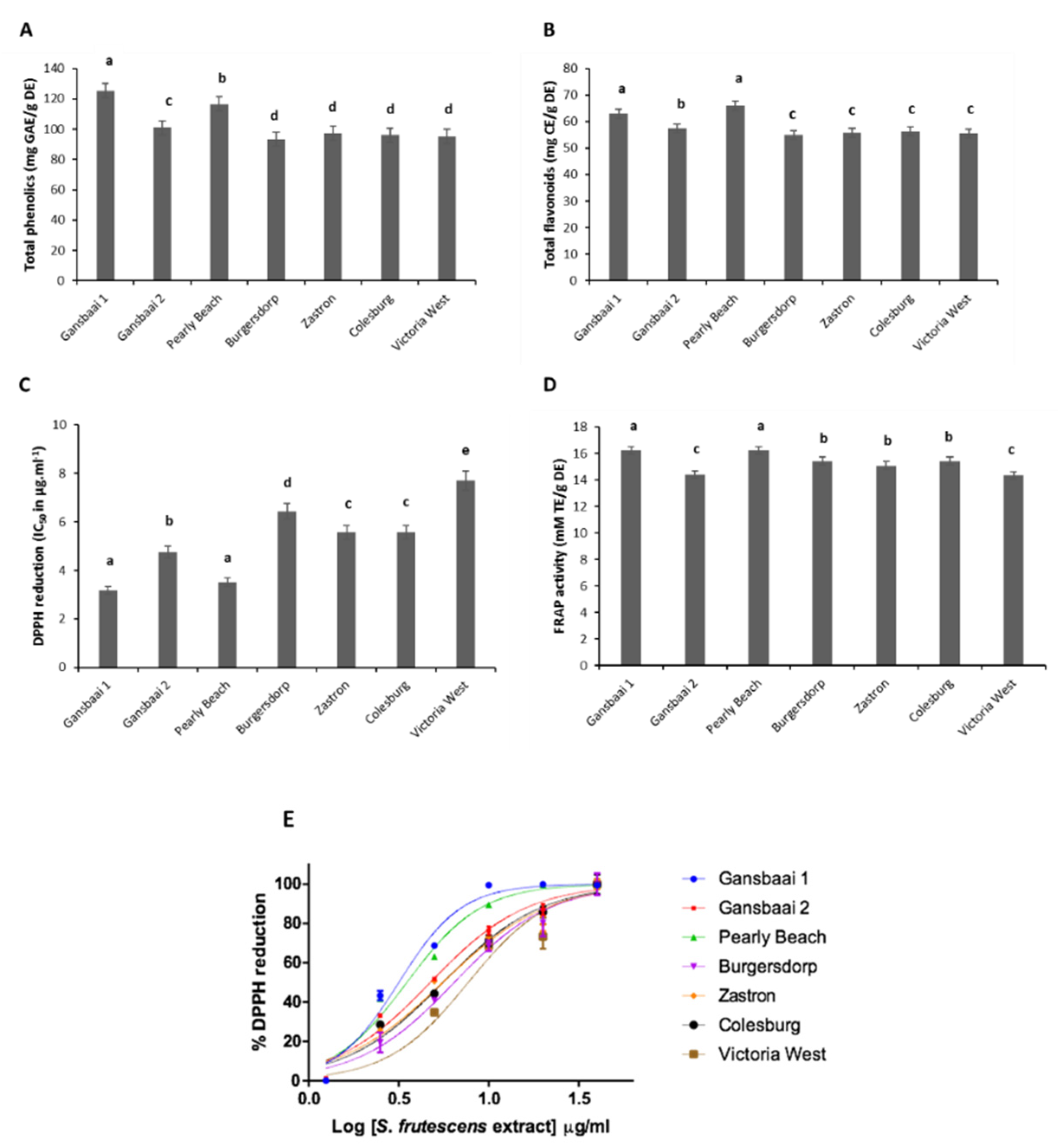
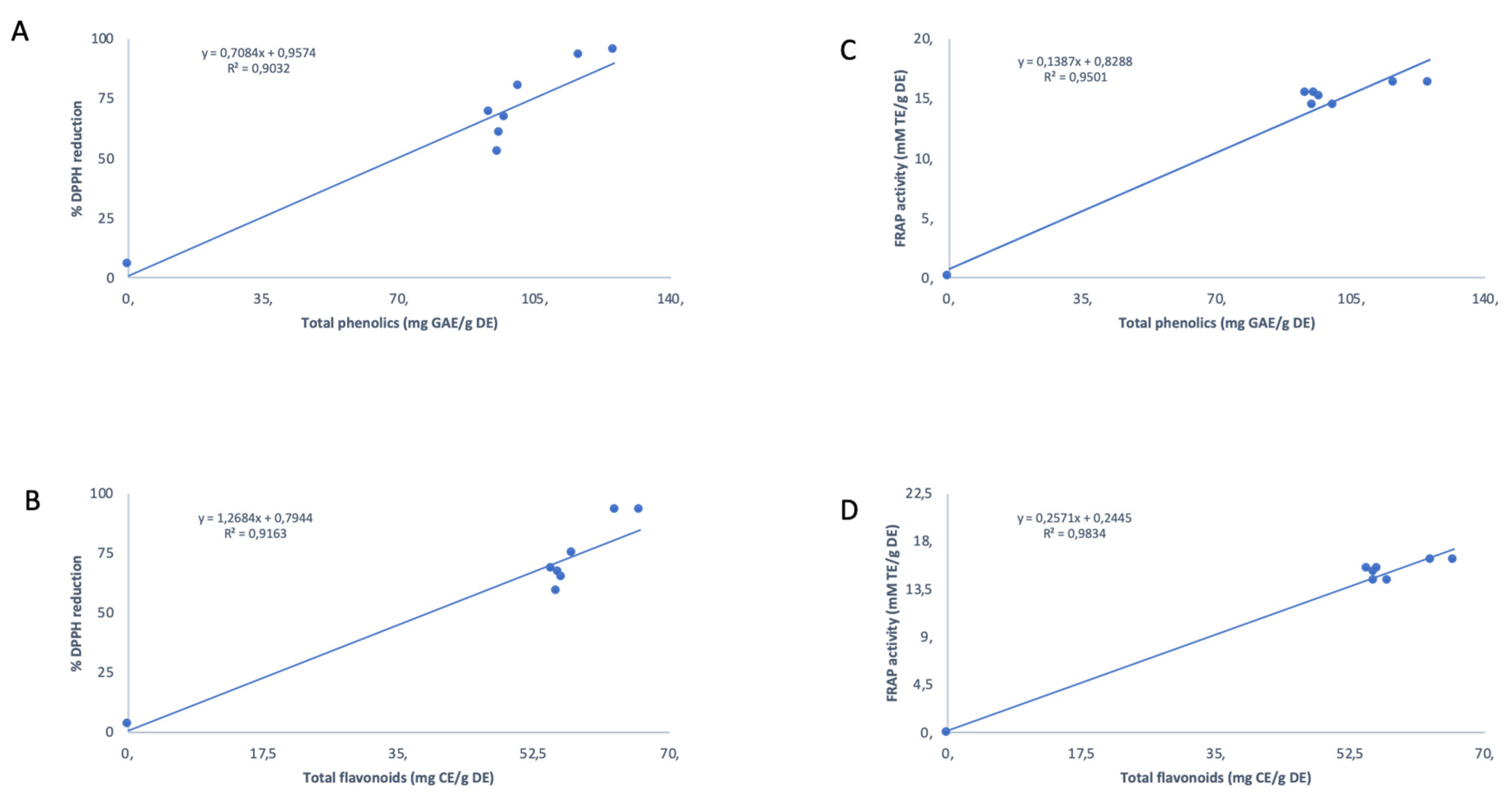
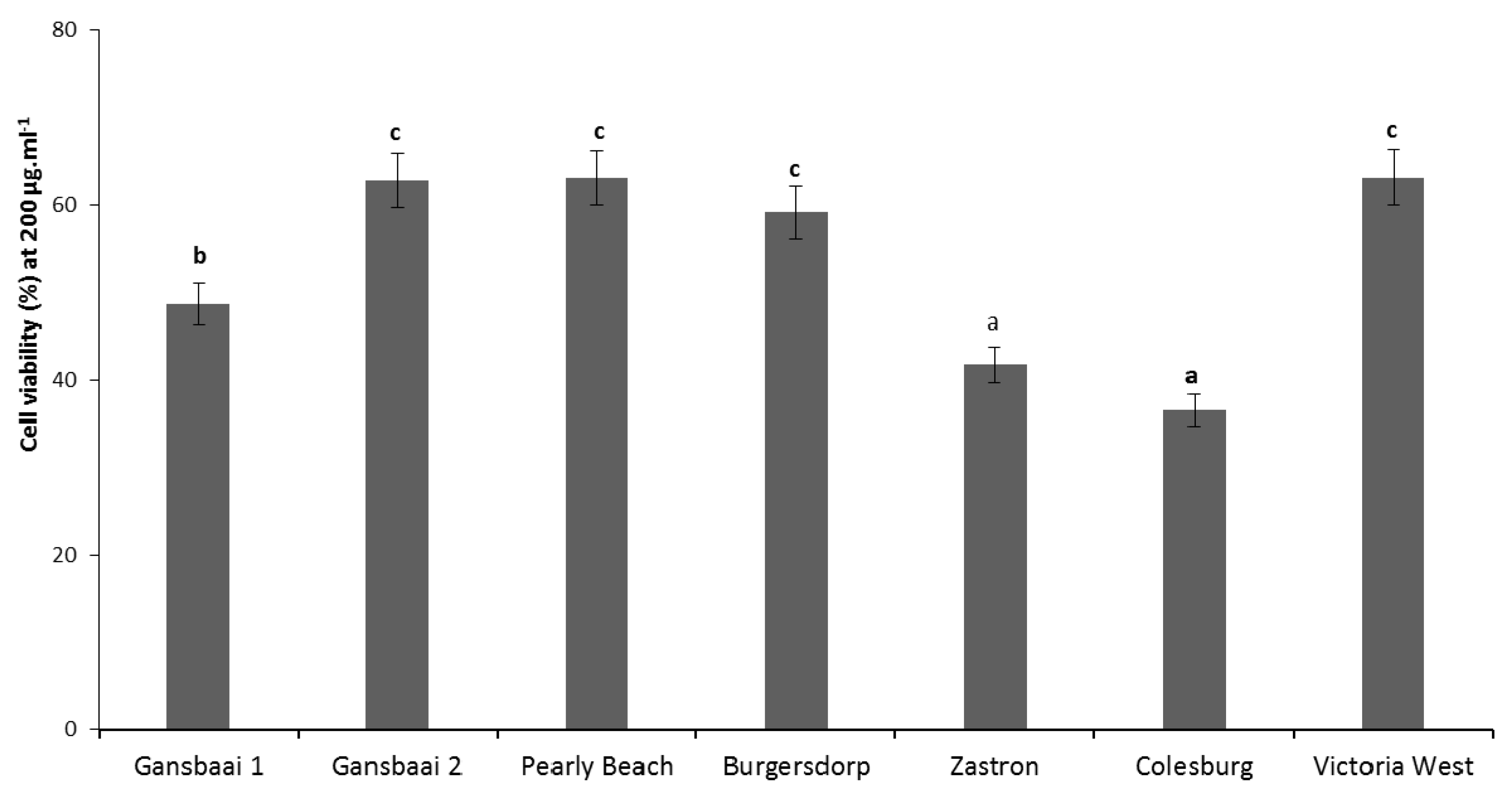
| Province | Geographic Location | Quercetin and Kaempferol Derived Flavonoids | Terpenoid Saponins (Cycloartanol Glycosides) |
|---|---|---|---|
| Western Cape | Gansbaai 1 34°32′09.4″S 19°24′26.0″E | Sutherlandin B 7.66_755.2021 * | 18.69_737.4104 * |
| Gansbaai 2 34°32′34.8″S 19°24′38.6″E | Sutherlandin B 7.66_755.2021 * | 18.69_737.4104 * | |
| Pearly Beach 34°40′47.3″S 19°33′20.0″E | Sutherlandin B 7.66_755.2021 * | 18.69_737.4104 * | |
| Eastern Cape | Burgersdorp 31°02′26.0″S 25°44′08.0″E | Sutherlandin A Sutherlandin B | Sutherlandioside A Sutherlandioside B |
| Free State | Zastron 30°29′49″S 27°09′71″E | 8.73_593.1501 * 7.99_609.1448 * | Sutherlandioside A Sutherlandioside B |
| Northern Cape | Colesberg 30°48′17.3″S 24°58′52.4″E | Sutherlandin A Sutherlandin C Sutherlandin D | Sutherlandioside A Sutherlandioside B |
| Victoria West 31°32′48.2″S 23°35′00.4″E | Sutherlandin A Sutherlandin C Sutherlandin D | Sutherlandioside B Sutherlandioside C |
| Plant Type | Provincial Location | IC50 (µg·mL−1) |
|---|---|---|
| Gansbaai 1 | Western Cape | 176.7 |
| Gansbaai 2 | Western Cape | >200 |
| Pearly Beach | Western Cape | >200 |
| Burgersdorp | Eastern Cape | >200 |
| Zastron | Free State | 172.7 |
| Colesburg | Northern Cape | 158.7 |
| Victoria West | Northern Cape | >200 |
| RT (min) | [M + H]+ Found | MSE Fragment Ions | Elemental Formula | UV Max | Tentative Identity | |
|---|---|---|---|---|---|---|
| 1 | 6.31 | 903.2404 | 303.051, 465.100, 597.142, 771.187 | C38H46O25 | 256; 347 | Quercetin glycoside (Sutherlandin A/B derivative) |
| 2 | 6.66 | 427.1911 | 149.069, 287.059, 177.07, 120.091, 225.163, 207.126, 163.056, 303.059, 387.197, 427.194 | C28H26O4 | 245; 323 | Unknown flavonoid |
| 3 | 6.80 | 771.1998 | 303.051, 609.143, 771.198 | C33H38O21 | 256; 347 | Quercetin-glycoside (Sutherlandin A/B derivative) |
| 4 | 7.32 | 741.1874 | 303.05, 609.144, 741.186, 287.057, 127.041 | C32H36O20 | 256; 352 | Sutherlandin A |
| 5 | 7.54 | 741.1855 | 303.05, 609.148, 741.19, 187.06, 127.039 | C32H36O20 | 255; 354 | Sutherlandin B |
| 6 | 7.66 | 755.2021 | 287.055, 755.200, 777.1755 | C33H38O20 | 265; 351 | Kaempferol glycoside (Sutherlandin C/D derivative) |
| 7 | 7.99 | 609.1469 | 303.051, 609.107, 287.059, 187.065 | C27H28O16 | 256; 347 | Quercetin glycoside (Sutherlandin A/B derivative) |
| 8 | 8.16 | 725.1927 | 287.055, 725.211, 593.146, 303.035, 187.061, 593.148 | C32H36O19 | 264; 347 | Sutherlandin C isomer (small peak) |
| 9 | 8.32 | 725.1920 | 287.055, 593.149, 725.192, 127.039, 187.06 | C32H36O19 | 265; 347 | Sutherlandin C |
| 10 | 8.53 | 725.1899 | 287.055, 593.15, 725.191, 127.042, 187.049 | C32H36O19 | 266; 348 | Sutherlandin D |
| 11 | 8.73 | 593.1501 | 287.055, 593.1500, 615.130 | C27H28O15 | 265,349 | Kaempferol glycoside (Sutherlandin C/D derivative) |
| 12 | 9.01 | 1079.2913 | 177.056, 303.048, 641.152, 1079.287, 947.248, 145.035 | C66H45O15 | 255; 347 | Quercetin glycoside (Sutherlandin A/B derivative) |
| 13 | 9.21 | 593.1508 | 287.055, 593.150, 615.134 | C27H28O15 | 265; 347 | Kaempferol glycoside (Sutherlandin C/D derivative) |
| 14 | 11.79 | 829.4537 | 505.348, 487.337, 177.067, 846.464, 851.438 | C49H64O11 | None | Unknown triterpenoid |
| 15 | 12.90 | 831.4672 | 505.353, 487.342, 689.387, 203.152, 471.349, 853.458, 705.363 | C49H66O11 | None | Similar to compound 505 at RT 11.71, Unknown triterpenoid |
| 16 | 15.02 | 653.4250 | 473.363, 455.353, 437.342, 491.373, 419.331, 635.416, 653.427, 675.408 | C36H60O10 | None | Sutherlandioside A |
| 17 | 15.97 | 813.4630 | 489.358, 471.347, 651.410, 813.461, 830.489, 835.447 | C42H68O15 | None | Cycloartanol glycoside |
| 18 | 16.31 | 899.4630 | 489.358, 471.347, 916.491, 719.400, 657.397, 453.336, 921.447 | C45H70O18 | None | Unknown cycloartanol glycoside |
| 19 | 17.3 | 653.4252 | 437.341, 455.352, 419.330, 489.357, 617.404, 635.415, 675.407, 653.424 | C36H60O10 | None | Sutherlandioside A isomer |
| 20 | 17.57 | 809.4454 | 489.357, 471.347, 177.06, 437.339, 827.457 | C46H65O12 | None | Cycloartanol glycoside |
| 21 | 17.97 | 651.4094 | 489.358, 471.346, 453.336, 668.437, 873.392 | C36H58O10 | None | Sutherlandioside C |
| 22 | 18.21 | 825.4257 | 437.341, 419.331, 455.353, 541.355, 789.407, 771.396, 807.414, 523.343, 842.455, 848.409 | C42H65O16 | None | Cycloartanol glycoside |
| 23 | 18.53 | 653.4268 | 437.342, 455.353, 473.363, 489.358, 419.33, 635.416, 653.426, 675.408 | C36H60O10 | None | Sutherlandioside B |
| 24 | 18.69 | 737.4104 | 489.359, 471.348, 453.338, 737.412, 719.401, 657.402, 701.391, 759.395 | C39H60O13 | None | Cycloartanol glycoside |
| 25 | 19.49 | 635.4160 | 455.353, 437.342, 419.333, 657.398, 473.364635.419 | C36H58O9 | None | Sutherlandioside D |
| 26 | 19.99 | 721.4149 | 437.341, 455.352, 703.404, 419.330, 685.393, 667.382, 641.407, 721.415, 743.397 | C39H60O12 | None | Cycloartanol glycoside |
| 27 | 20.84 | 635.4144 | 437.341, 455.351, 419.330, 657.396, 489.358, 473.362, 635.414 | C36H58O9 | None | Sutherlandioside D isomer |
| 28 | 21.13 | 721.4154 | 437.341, 455.352, 721.416, 419.331, 703.404, 473.362, 685.392, 738.443 | C39H60O12 | None | Cycloartanol glycoside |
| 29 | 21.61 | 703.4058 | 703.406, 437.342, 455.352, 419.332, 229.160, 685.396 | C39H58O11 | None | Cycloartanol glycoside |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonyane, S.; Fawole, O.A.; la Grange, C.; Stander, M.A.; Opara, U.L.; Makunga, N.P. The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations. Antioxidants 2020, 9, 152. https://doi.org/10.3390/antiox9020152
Zonyane S, Fawole OA, la Grange C, Stander MA, Opara UL, Makunga NP. The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations. Antioxidants. 2020; 9(2):152. https://doi.org/10.3390/antiox9020152
Chicago/Turabian StyleZonyane, Samkele, Olaniyi A. Fawole, Chris la Grange, Maria A. Stander, Umezuruike L. Opara, and Nokwanda P. Makunga. 2020. "The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations" Antioxidants 9, no. 2: 152. https://doi.org/10.3390/antiox9020152
APA StyleZonyane, S., Fawole, O. A., la Grange, C., Stander, M. A., Opara, U. L., & Makunga, N. P. (2020). The Implication of Chemotypic Variation on the Anti-Oxidant and Anti-Cancer Activities of Sutherlandia frutescens (L.) R.Br. (Fabaceae) from Different Geographic Locations. Antioxidants, 9(2), 152. https://doi.org/10.3390/antiox9020152







