Quantitative Spatiotemporal Mapping of Lipid and Protein Oxidation in Mayonnaise
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oil Samples
2.3. Preparation of Emulsions
2.4. Accelerated Oxidation of Samples
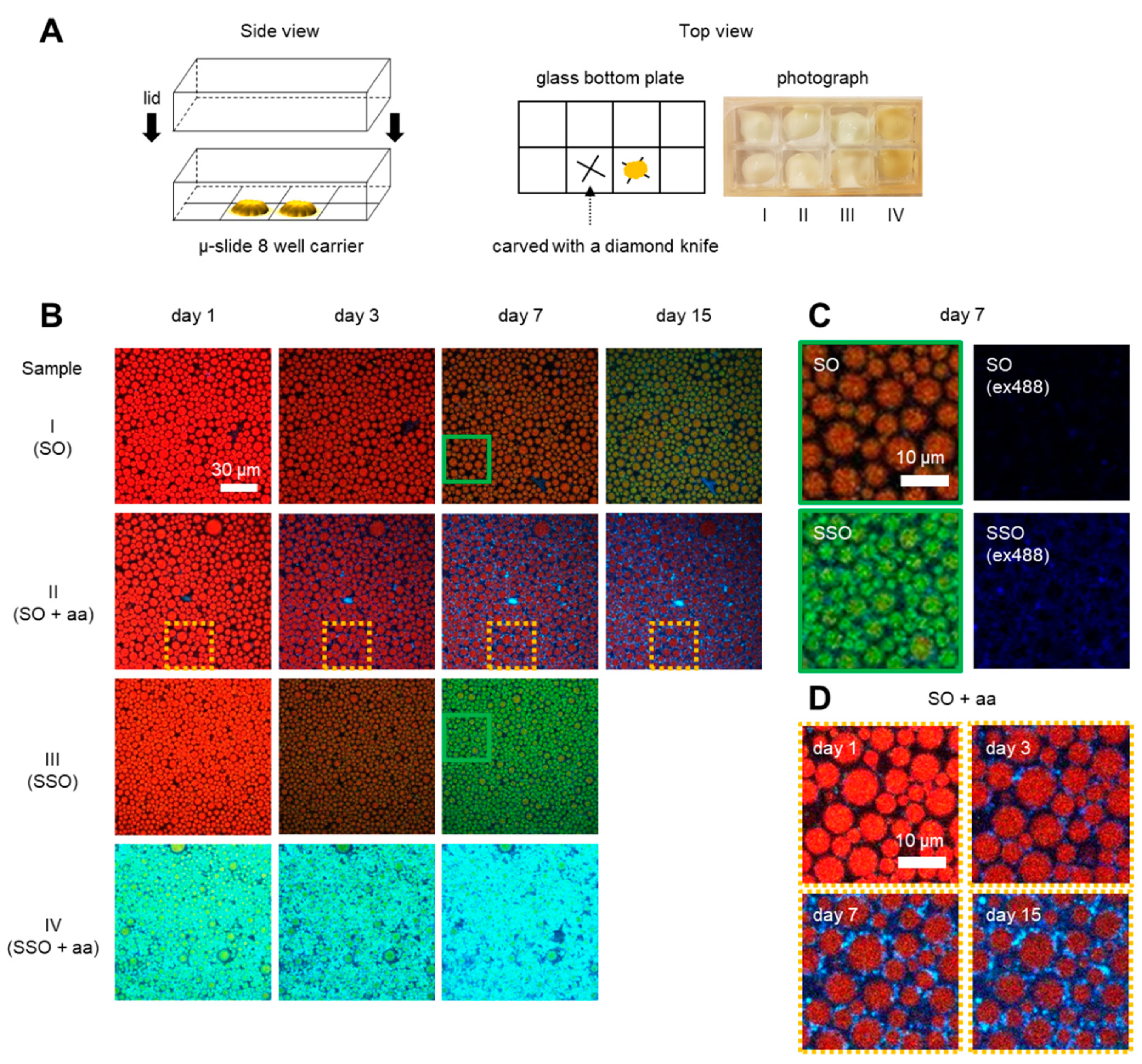
2.5. Preparation of Sample Carrier
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Spectrophotometry
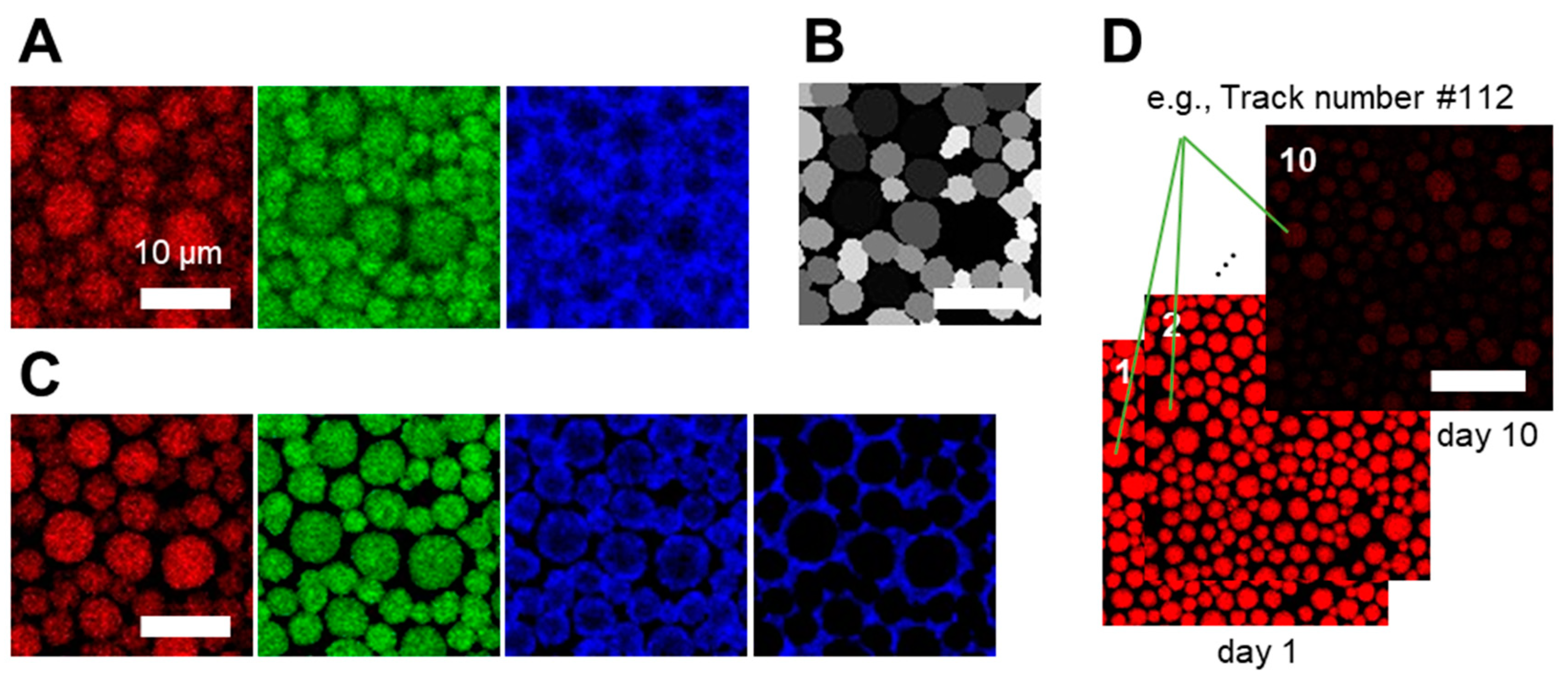
2.8. Segmentation and Tracking
2.9. Gompertz Curve Fitting
2.10. Statistical Analysis
3. Results
3.1. Confocal Microscopy Allows Spatiotemporal Mapping of Lipid and Protein Oxidation
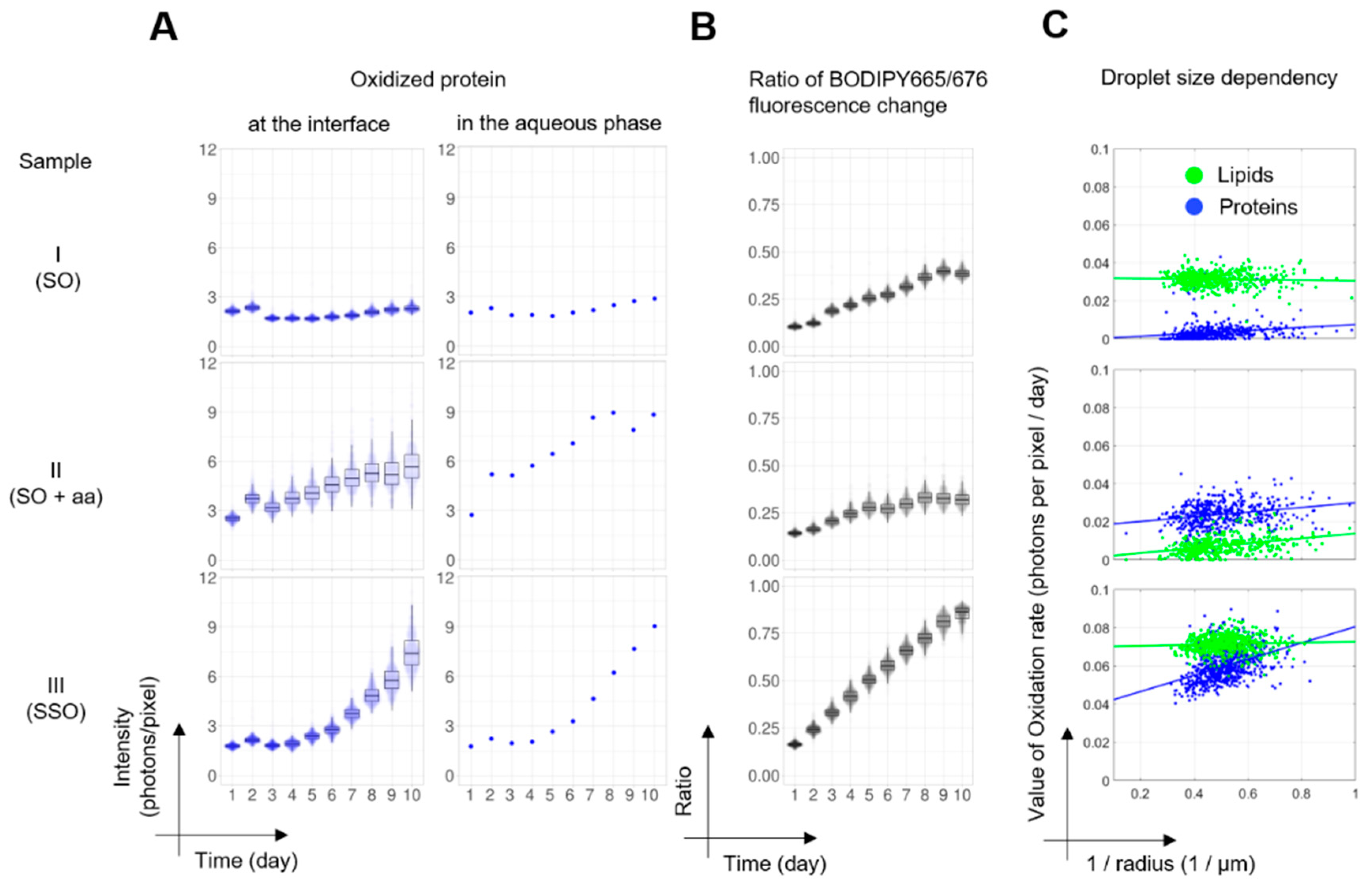
3.2. Quantitative Spatiotemporal Analysis of Lipid and Protein Oxidation Maps
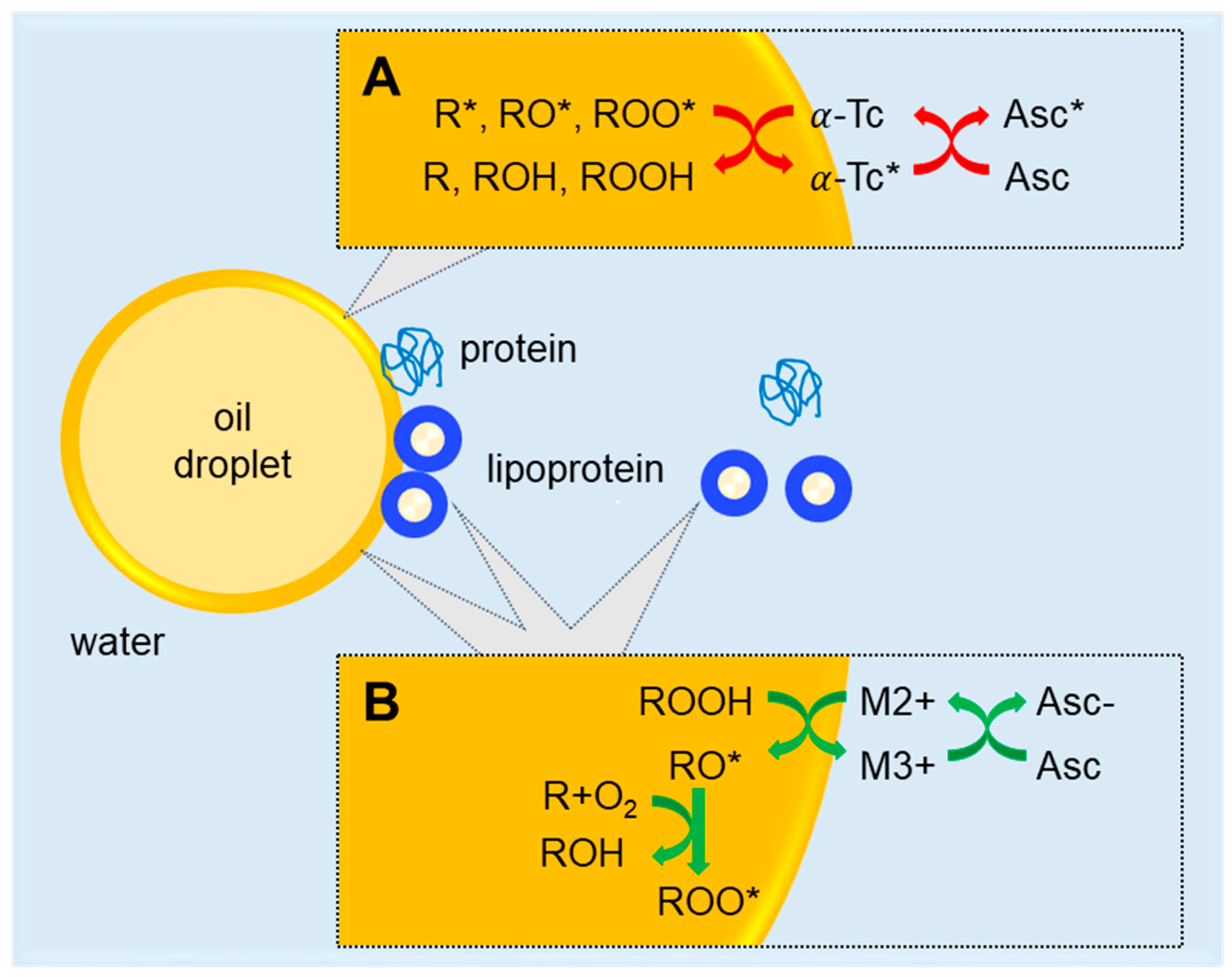
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Access to Raw Data
References
- Ganesan, B.; Brothersen, C.; McMahon, D.J. Fortification of Foods with Omega-3 Polyunsaturated Fatty Acids. Crit. Rev. Food Sci. Nutr. 2014, 54, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer: Lipid oxidation: An interface outlook…. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Berton-Carabin, C.; Schroën, K. Towards new food emulsions: Designing the interface and beyond. Curr. Opin. Food Sci. 2019, 27, 74–81. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.M.; Calviello, G. Dietary n-3 Polyunsaturated Fatty Acids and the Paradox of Their Health Benefits and Potential Harmful Effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E. Interfacial Antioxidants: A Review of Natural and Synthetic Emulsifiers and Coemulsifiers That Can Inhibit Lipid Oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Birtić, S. Lipid oxidation in food. In Lipids and Edible Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–287. ISBN 978-0-12-817105-9. [Google Scholar]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in Predicting Antioxidant Efficacy in Oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Berton, C.; Genot, C.; Guibert, D.; Ropers, M.-H. Effect of lateral heterogeneity in mixed surfactant-stabilized interfaces on the oxidation of unsaturated lipids in oil-in-water emulsions. J. Colloid Interface Sci. 2012, 377, 244–250. [Google Scholar] [CrossRef]
- Laguerre, M.; Bily, A.; Roller, M.; Birtić, S. Mass Transport Phenomena in Lipid Oxidation and Antioxidation. Annu. Rev. Food Sci. Technol. 2017, 8, 391–411. [Google Scholar] [CrossRef]
- Schaich, K.M. Co-oxidations of oxidizing lipids: Reactions with proteins. In Lipid Oxidation Pathways; Kamal-Eldin, A., Min, D., Eds.; AOCS Press: Champaign, IL, USA, 2008; Volume 2, Chapter 8; pp. 183–274. [Google Scholar]
- Ghorbani Gorji, S.; Smyth, H.E.; Sharma, M.; Fitzgerald, M. Lipid oxidation in mayonnaise and the role of natural antioxidants: A review. Trends Food Sci. Technol. 2016, 56, 88–102. [Google Scholar] [CrossRef]
- Laguerre, M.; Tenon, M.; Bily, A.; Birtić, S. Toward a Spatiotemporal Model of Oxidation in Lipid Dispersions: A Hypothesis-Driven Review. Eur. J. Lipid Sci. Technol. 2020, 1900209. [Google Scholar] [CrossRef]
- Gohtani, S.; Sirendi, M.; Yamamoto, N.; Kajikawa, K.; Yamano, Y. Effect of droplet size on oxidation of docosahexaenoic acid in emulsion system. J. Dispers. Sci. Technol. 1999, 20, 1319–1325. [Google Scholar] [CrossRef]
- Yalçinöz, Ş.; Erçelebi, E. Effect of surfactant type and droplet size on lipid oxidation in oil-in-water nano-emulsions. QAS 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Sun, C.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Osborn, H.T.; Akoh, C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004, 84, 451–456. [Google Scholar] [CrossRef]
- Kiokias, S.; Dimakou, C.; Oreopoulou, V. Effect of heat treatment and droplet size on the oxidative stability of whey protein emulsions. Food Chem. 2007, 105, 94–100. [Google Scholar] [CrossRef]
- Roozen, J.P.; Frankel, E.N.; Kinsella, J.E. Enzymic and autoxidation of lipids in low fat foods: Model of linoleic acid in emulsified hexadecane. Food Chem. 1994, 50, 33–38. [Google Scholar] [CrossRef]
- Erdmann, M.E.; Lautenschlaeger, R.; Zeeb, B.; Gibis, M.; Weiss, J. Effect of differently sized O/W emulsions loaded with rosemary extract on lipid oxidation in cooked emulsion-type sausages rich in n-3 fatty acids. LWT Food Sci. Technol. 2017, 79, 496–502. [Google Scholar] [CrossRef]
- Jacobsen, C.; Timm, M.; Meyer, A.S. Oxidation in Fish Oil Enriched Mayonnaise: Ascorbic Acid and Low pH Increase Oxidative Deterioration. J. Agric. Food Chem. 2001, 49, 3947–3956. [Google Scholar] [CrossRef]
- Raudsepp, P.; Brüggemann, D.A.; Andersen, M.L. Detection of radicals in single droplets of oil-in-water emulsions with the lipophilic fluorescent probe BODIPY665/676 and confocal laser scanning microscopy. Free Radic. Biol. Med. 2014, 70, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; McClements, D.J.; Decker, E.A. Application of flow cytometry as novel technology in studying lipid oxidation and mass transport phenomena in oil-in-water emulsions. Food Chem. 2020, 315, 126225. [Google Scholar] [CrossRef] [PubMed]
- Raudsepp, P.; Brüggemann, D.A.; Lenferink, A.; Otto, C.; Andersen, M.L. Oxidative stabilization of mixed mayonnaises made with linseed oil and saturated medium-chain triglyceride oil. Food Chem. 2014, 152, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Westberg, M.; Breitenbach, T.; Bregnhøj, M.; Ogilby, P.R. Monitoring Interfacial Lipid Oxidation in Oil-in-Water Emulsions Using Spatially Resolved Optical Techniques. Anal. Chem. 2017, 89, 6239–6247. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Kokawa, M.; Nishi, K.; Ashida, H.; Trivittayasil, V.; Sugiyama, J.; Tsuta, M. Predicting the Heating Temperature of Soymilk Products Using Fluorescence Fingerprints. Food Bioprocess Technol. 2017, 10, 462–468. [Google Scholar] [CrossRef]
- Aidyraliev, R.K.; Azizova, O.A.; Vakhrusheva, T.V.; Lopukhin, Y.M.; Mirrakhimov, M.M. Autofluorescence of low-density lipoproteins modified as a result of autooxidation. Bull. Exp. Biol. Med. 2006, 142, 433–436. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-convex Polygons. arXiv 2018, arXiv:1806.03535. [Google Scholar] [CrossRef]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practice and Techniques; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Postma, M.; Goedhart, J. PlotsOfData—A web app for visualizing data together with their summaries. PLoS Biol. 2019, 17, e3000202. [Google Scholar] [CrossRef]
- Merkx, D.W.H.; Delić, F.; Wierenga, P.A.; Hennebelle, M.; van Duynhoven, J.P.M. 31P NMR assessment of the phosvitin-iron complex in mayonnaise. Magn. Reson. Chem. 2019, 57, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Anton, M. Egg yolk: Structures, functionalities and processes. J. Sci. Food Agric. 2013, 93, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Sirvente, H.; Beaumal, V.; Gaillard, C.; Bialek, L.; Hamm, D.; Anton, M. Structuring and Functionalization of Dispersions Containing Egg Yolk, Plasma and Granules Induced by Mechanical Treatments. J. Agric. Food Chem. 2007, 55, 9537–9544. [Google Scholar] [CrossRef]
- Pinchuk, I.; Lichtenberg, D. The effect of compartmentalization on the kinetics of transition metal ions-induced lipoprotein peroxidation. Chem. Phys. Lipids 2016, 195, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasit, W.; McClements, D.J.; Decker, E.A. The Relationship between the Physicochemical Properties of Antioxidants and Their Ability to Inhibit Lipid Oxidation in Bulk Oil and Oil-in-Water Emulsions. J. Agric. Food Chem. 2005, 53, 4982–4988. [Google Scholar] [CrossRef] [PubMed]
- Samdani, G.K.; McClements, D.J.; Decker, E.A. Impact of Phospholipids and Tocopherols on the Oxidative Stability of Soybean Oil-in-Water Emulsions. J. Agric. Food Chem. 2018, 66, 3939–3948. [Google Scholar] [CrossRef]
| Sample | Lipid Soluble Antioxidant | |
|---|---|---|
| I | Soybean oil (SO) | + |
| II | Soybean oil + ascorbic acid (SO + aa) | + |
| III | Stripped soybean oil (SSO) | − |
| IV | Stripped soybean oil + ascorbic acid (SSO + aa) | − |
| Results | Number of Analyzed Droplets | Average Radius Size (µm) 3 | ||||
|---|---|---|---|---|---|---|
| Sample | I | II | III | I | II | III |
| StarDist 1 | 600 | 601 | 910 | 2.2 ± 0.6 | 2.1 ± 0.6 | 1.8 ± 0.4 |
| Re-assigned after Trackmate 2 | 484 | 445 | 641 | 2.2 ± 0.5 | 2.1 ± 0.5 | 2.0 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Verhoeff, A.A.; Merkx, D.W.H.; van Duynhoven, J.P.M.; Hohlbein, J. Quantitative Spatiotemporal Mapping of Lipid and Protein Oxidation in Mayonnaise. Antioxidants 2020, 9, 1278. https://doi.org/10.3390/antiox9121278
Yang S, Verhoeff AA, Merkx DWH, van Duynhoven JPM, Hohlbein J. Quantitative Spatiotemporal Mapping of Lipid and Protein Oxidation in Mayonnaise. Antioxidants. 2020; 9(12):1278. https://doi.org/10.3390/antiox9121278
Chicago/Turabian StyleYang, Suyeon, Aletta A. Verhoeff, Donny W. H. Merkx, John P. M. van Duynhoven, and Johannes Hohlbein. 2020. "Quantitative Spatiotemporal Mapping of Lipid and Protein Oxidation in Mayonnaise" Antioxidants 9, no. 12: 1278. https://doi.org/10.3390/antiox9121278
APA StyleYang, S., Verhoeff, A. A., Merkx, D. W. H., van Duynhoven, J. P. M., & Hohlbein, J. (2020). Quantitative Spatiotemporal Mapping of Lipid and Protein Oxidation in Mayonnaise. Antioxidants, 9(12), 1278. https://doi.org/10.3390/antiox9121278





