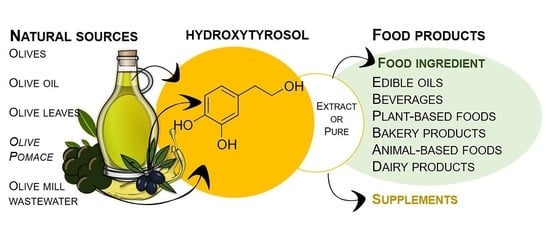
Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements
Abstract
1. Introduction
2. Antioxidant Properties of HT
3. Applicability of Hydroxytyrosol in Food Products
3.1. Consumption Recommendations
3.2. Application of HT in the Functional Food Market
Supplements and Patented HT-Rich Extracts
3.3. HT as a Food Ingredient
3.3.1. Plant-Based Products
Edible Oils
Beverages
Vegetable-Based Products
Bakery Products
3.3.2. Animal-Based Food Products
Meat-Based Products
Fishery-Based Products
Dairy Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Nova Mentis GRAS Notice (GRN) No. 876. Office of Food Additive Safety. 2019. Available online: https://www.fda.gov/media/134474/download (accessed on 17 July 2020).
- Europeo COMMISSION IMPLEMENTING DECISION (EU) 2017/2373 of 14 December 2017 authorising the placing on the market of hydroxytyrosol as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2017, 2017, 56–59.
- Parliament, E.; States, M. COMMISSION REGULATION (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 13, 22. [Google Scholar]
- Boskou, D. Phenolic Compounds in Olives and Olive Oil. In Olive oil:Minor Constituents and Health; Boskou, D., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 12–36. ISBN 9780429136900. [Google Scholar]
- García-García, M.I.; Hernández-García, S.; Sánchez-Ferrer, Á.; García-Carmona, F. Kinetic Study of Hydroxytyrosol Oxidation and Its Related Compounds by Red Globe Grape Polyphenol Oxidase. J. Agric. Food Chem. 2013, 61, 6050–6055. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Guyot, S.; Marnet, N.; Lopes-da-Silva, J.A.; Renard, C.M.G.C.; Coimbra, M.A. Characterisation of phenolic extracts from olive pulp and olive pomace by electrospray mass spectrometry. J. Sci. Food Agric. 2005, 85, 21–32. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef]
- Santos, M.M.; Piccirillo, C.; Castro, P.M.L.; Kalogerakis, N.; Pintado, M.E. Bioconversion of oleuropein to hydroxytyrosol by lactic acid bacteria. World J. Microbiol. Biotechnol. 2012, 28, 2435–2440. [Google Scholar] [CrossRef]
- Di Tommaso, D.; Calabrese, R.; Rotilio, D. Identification and Quantitation of Hydroxytyrosol in Italian Wines. J. High. Resolut. Chromatogr. 1998, 21, 549–553. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodríguez, G.R.; Chen, C.-Y.O.; Salazar-López, N.J.; Robles-Sánchez, M.; González-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Sarriá, B.; Madrona, A.; Espartero, J.L.; Escuderos, M.E.; Bravo, L.; Mateos, R. Digestive stability of hydroxytyrosol, hydroxytyrosyl acetate and alkyl hydroxytyrosyl ethers. Int. J. Food Sci. Nutr. 2012, 63, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E. Hydroxytyrosol Disposition in Humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pereira-Caro, G.; Saha, S.; Cert, R.; Redondo-Horcajo, M.; Bravo, L.; Kroon, P.A. Acetylation of hydroxytyrosol enhances its transport across differentiated Caco-2 cell monolayers. Food Chem. 2011, 125, 865–872. [Google Scholar] [CrossRef]
- Rubió, L.; Macià, A.; Valls, R.M.; Pedret, A.; Romero, M.-P.; Solà, R.; Motilva, M.-J. A new hydroxytyrosol metabolite identified in human plasma: Hydroxytyrosol acetate sulphate. Food Chem. 2012, 134, 1132–1136. [Google Scholar] [CrossRef]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; López-Villodres, J.A.; Reyes, J.J.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Role of the catechol group in the antioxidant and neuroprotective effects of virgin olive oil components in rat brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef]
- Raneva, V.; Shimasaki, H.; Ishida, Y.; Ueta, N.; Niki, E. Antioxidative activity of 3,4-dihydroxyphenylacetic acid and caffeic acid in rat plasma. Lipids 2001, 36, 1111–1116. [Google Scholar] [CrossRef]
- Deiana, M.; Incani, A.; Rosa, A.; Corona, G.; Atzeri, A.; Loru, D.; Paola Melis, M.; Assunta Dessì, M. Protective effect of hydroxytyrosol and its metabolite homovanillic alcohol on H2O2 induced lipid peroxidation in renal tubular epithelial cells. Food Chem. Toxicol. 2008, 46, 2984–2990. [Google Scholar] [CrossRef]
- Gordon, M.H.; Paiva-Martins, F.; Almeida, M. Antioxidant Activity of Hydroxytyrosol Acetate Compared with That of Other Olive Oil Polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. Aronia-Enriched Lemon Juice: A New Highly Antioxidant Beverage. J. Agric. Food Chem. 2008, 56, 11327–11333. [Google Scholar] [CrossRef]
- Burke, W.J.; Li, S.W.; Williams, E.A.; Nonneman, R.; Zahm, D.S. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: Implications for Parkinson’s disease pathogenesis. Brain Res. 2003, 989, 205–213. [Google Scholar] [CrossRef]
- López de las Hazas, M.C.; Piñol, C.; Macià, A.; Romero, M.P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.J. Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Pastor, A.; Rodríguez-Morató, J.; Olesti, E.; Pujadas, M.; Pérez-Mañá, C.; Khymenets, O.; Fitó, M.; Covas, M.-I.; Solá, R.; Motilva, M.-J.; et al. Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J. Chromatogr. A 2016, 1437, 183–190. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Fonollá, J.; Lopez-Huertas, E. Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol. Res. 2010, 61, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The In Vivo Fate of Hydroxytyrosol and Tyrosol, Antioxidant Phenolic Constituents of Olive Oil, after Intravenous and Oral Dosing of Labeled Compounds to Rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Nenadis, N.; Servili, M.; García-González, D.L.; Gallina Toschi, T. Why Tyrosol Derivatives Have to Be Quantified in the Calculation of “Olive Oil Polyphenols” Content to Support the Health Claim Provisioned in the EC Reg. 432/2012. Eur. J. Lipid Sci. Technol. 2018, 120, 1800098. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Kitsati, N.; Mantzaris, M.D.; Galaris, D. Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic signaling via labile iron chelation. Redox Biol. 2016, 10, 233–242. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free Radical-Scavenging Properties of Olive Oil Polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef] [PubMed]
- O′Dowd, Y.; Driss, F.; Dang, P.M.-C.; Elbim, C.; Gougerot-Pocidalo, M.-A.; Pasquier, C.; El-Benna, J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: Scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004, 68, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R.M.M. New Insights into Controversies on the Antioxidant Potential of the Olive Oil Antioxidant Hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, B.; Yao, J.; Duan, D.; Fang, J. Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food Funct. 2015, 6, 2091–2100. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol Against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In vitro and in vivo Study. Front. Vet. Sci. 2020, 7, 136. [Google Scholar] [CrossRef]
- Goya, L.; Mateos, R.; Bravo, L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Eur. J. Nutr. 2007, 46, 70–78. [Google Scholar] [CrossRef]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Aldini, G.; Piccoli, A.; Beretta, G.; Morazzoni, P.; Riva, A.; Marinello, C.; Maffei Facino, R. Antioxidant activity of polyphenols from solid olive residues of c.v. Coratina. Fitoterapia 2006, 77, 121–128. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants 2019, 8, 188. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Giordano, E.; Dávalos, A.; Visioli, F. Chronic hydroxytyrosol feeding modulates glutathione-mediated oxido-reduction pathways in adipose tissue: A nutrigenomic study. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Liu, L.; Pan, H.; Ma, Y.; Wang, D.; Kang, K.; Wang, J.; Sun, B.; Sun, X.; Jiang, H. Protective effects of hydroxytyrosol on liver ischemia/reperfusion injury in mice. Mol. Nutr. Food Res. 2013, 57, 1218–1227. [Google Scholar] [CrossRef]
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors. Ann. Intern. Med. 2006, 145, 333. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar]
- Christian, M.S.; Sharper, V.A.; Hoberman, A.M.; Seng, J.E.; Fu, L.; Covell, D.; Diener, R.M.; Bitler, C.M.; Crea, R. The Toxicity Profile of Hydrolyzed Aqueous Olive Pulp Extract. Drug Chem. Toxicol. 2004, 27, 309–330. [Google Scholar] [CrossRef]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Achmon, Y.; Fishman, A. The antioxidant hydroxytyrosol: Biotechnological production challenges and opportunities. Appl. Microbiol. Biotechnol. 2015, 99, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Novelli, E.; Esposto, S.; Taticchi, A.; Servili, M. Applications of recovered bioactive compounds in food products. In Olive Mill Waste; Elsevier: Amsterdam, The Netherlands, 2017; pp. 231–263. ISBN 9780128053140. [Google Scholar]
- Changping, L.; Zhuo, L. A Kind of Extracting Process of Hydroxytyrosol. Patent CN107382675 A, 24 November 2017. [Google Scholar]
- Más, J.A.L.; Mellado, M.P.; Ortiz, P.M.; Streitenberger, S.A. Process and Apparatus for the Production of Hydroxytyrosol Containing Extract from Olives and Solids Containing Residues of Olive Oil Extraction. Patent EP2049458 A1, 24 June 2010. [Google Scholar]
- Weiping, H. The Method that Hydroxytyrosol Is Extracted from Processing Olive Oil Waste Water. Patent CN106946662A, 14 July 2017. [Google Scholar]
- Lingxiao; Shende, J.; Xinyan, G.; Chao, X. Method for Preparing High-Purity Hydroxytyrosol. Patent CN103420804 A, 4 December 2013. [Google Scholar]
- Milczarek, R.; Larson, D.; Li, Y.O.; Sedej, I.; Wang, S. Olive. In Integrated Processing Technologies for Food and Agricultural By-Products; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128141380. [Google Scholar]
- Ulm, J.; Leuenberger, B.H. Novel Powders Based on Vegetation Water from Olive Oil Production. Patent EP2106218 A1, 7 October 2009. [Google Scholar]
- Raederstorff, D.; Richard, N.; Schwager, J.; Wertz, K. Compositions. U.S. Patent US8841264 B2, 23 September 2010. [Google Scholar]
- Martínez, N.; Herrera, M.; Frías, L.; Provencio, M.; Pérez-Carrión, R.; Díaz, V.; Morse, M.; Crespo, M.C. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clin. Transl. Oncol. 2019, 21, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ciafrè, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, N.G.; Ceccanti, M.; Boccardo, C.; et al. TNF-α and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist Super Sanità 2015, 5, 382–386. [Google Scholar] [CrossRef]
- Aponte, M.; Ungaro, F.; D’Angelo, I.; De Caro, C.; Russo, R.; Blaiotta, G.; Dal Piaz, F.; Calignano, A.; Miro, A. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int. J. Pharm. 2018, 543, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Fernández, R.; López-Plaza, B.; Bermejo, L.; Palma-Milla, S.; Gómez-Candela, C. Supplementation with Hydroxytyrosol and Punicalagin Improves Early Atherosclerosis Markers Involved in the Asymptomatic Phase of Atherosclerosis in the Adult Population: A Randomized, Placebo-Controlled, Crossover Trial. Nutrients 2019, 11, 640. [Google Scholar] [CrossRef]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef]
- Bañares, C.; Martin, D.; Reglero, G.; Torres, C.F. Protective effect of hydroxytyrosol and rosemary extract in a comparative study of the oxidative stability of Echium oil. Food Chem. 2019, 290, 316–323. [Google Scholar] [CrossRef]
- Farràs, M.; Fernández-Castillejo, S.; Rubió, L.; Arranz, S.; Catalán, Ú.; Subirana, I.; Romero, M.-P.; Castañer, O.; Pedret, A.; Blanchart, G.; et al. Phenol-enriched olive oils improve HDL antioxidant content in hypercholesterolemic subjects. A randomized, double-blind, cross-over, controlled trial. J. Nutr. Biochem. 2018, 51, 99–104. [Google Scholar] [CrossRef]
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chem. 2005, 93, 197–204. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Prior, Á.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Synergistic effect of 3,4-dihydroxyphenylglycol with hydroxytyrosol and α-tocopherol on the Rancimat oxidative stability of vegetable oils. Innov. Food Sci. Emerg. Technol. 2019, 51, 100–106. [Google Scholar] [CrossRef]
- Sordini, B.; Veneziani, G.; Servili, M.; Esposto, S.; Selvaggini, R.; Lorefice, A.; Taticchi, A. A quanti-qualitative study of a phenolic extract as a natural antioxidant in the frying processes. Food Chem. 2019, 279, 426–434. [Google Scholar] [CrossRef]
- Valls, R.-M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; López-Miranda, J.; Giralt, M.; Covas, M.-I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Macciola, V. Heat-oxidation stability of palm oil blended with extra virgin olive oil. Food Chem. 2012, 135, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Más, J.A.L.; Streitenberger, S.A.; Mellado, M.P.; Ortiz, P.M. Fortification of Nutritional Products with Olive Extracts Containing Hydroxytyrosol and Hydroxytyrosol Fortified Nutritional Products. Patent WO2009013596 A2, 29 January 2009. [Google Scholar]
- Bulbarello, A.; Leuthardt, B. Fortification of Edible Oils with Hyrdoxytyrosol. Patent WO2016087428 A1, 9 June 2016. [Google Scholar]
- De Hierro, A.G.-G.L.; Sánchez, A.P. Olive Oil Composition and Use Thereof as Functional Food. Patent WO2008102047 A1, 28 August 2008. [Google Scholar]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Zafrilla, P.; Gonzalo-Diago, A.; Guerrero, R.F.; Cantos-Villar, E. Replacement of sulfur dioxide by hydroxytyrosol in white wine: Influence on both quality parameters and sensory. LWT Food Sci. Technol. 2016, 65, 214–221. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Raposo, R.; Moreno-Rojas, J.M.; Zafrilla, P.; Cayuela, J.M.; Mulero, J.; Puertas, B.; Guerrero, R.F.; Piñeiro, Z.; Giron, F.; et al. Efficacy of olive oil mill extract in replacing sulfur dioxide in wine model. LWT Food Sci. Technol. 2015, 61, 117–123. [Google Scholar] [CrossRef]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Gonzalo-Diago, A.; Guerrero, R.F.; Ortiz, V.; Cantos-Villar, E. Effect of hydroxytyrosol on quality of sulfur dioxide-free red wine. Food Chem. 2016, 192, 25–33. [Google Scholar] [CrossRef]
- Kranz, P.; Braun, N.; Schulze, N.; Kunz, B. Sensory quality of functional beverages: Bitterness perception and bitter masking of olive leaf extract fortified fruit smoothies. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef]
- Larrosa, M.; Espín, J.C.; Tomás-Barberán, F.A. Antioxidant capacity of tomato juice functionalised with enzymatically synthesised hydroxytyrosol. J. Sci. Food Agric. 2003, 83, 658–666. [Google Scholar] [CrossRef]
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285. [Google Scholar] [CrossRef] [PubMed]
- Takashi, F.; Yuki, N.; Hideki, M. Beverage Containing Hydroxytyrosol. Patent WO2017094654 A1, 8 June 2017. [Google Scholar]
- Lalas, S.; Athanasiadis, V.; Gortzi, O.; Bounitsi, M.; Giovanoudis, I.; Tsaknis, J.; Bogiatzis, F. Enrichment of table olives with polyphenols extracted from olive leaves. Food Chem. 2011, 127, 1521–1525. [Google Scholar] [CrossRef]
- Caponio, F.; Difonzo, G.; Calasso, M.; Cosmai, L.; De Angelis, M. Effects of olive leaf extract addition on fermentative and oxidative processes of table olives and their nutritional properties. Food Res. Int. 2019, 116, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Schaide, T.; Cabrera-Bañegil, M.; Pérez-Nevado, F.; Esperilla, A.; Martín-Vertedor, D. Effect of olive leaf extract combined with Saccharomyces cerevisiae in the fermentation process of table olives. J. Food Sci. Technol. 2019, 56, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- De Toffoli, A.; Monteleone, E.; Bucalossi, G.; Veneziani, G.; Fia, G.; Servili, M.; Zanoni, B.; Pagliarini, E.; Gallina Toschi, T.; Dinnella, C. Sensory and chemical profile of a phenolic extract from olive mill waste waters in plant-base food with varied macro-composition. Food Res. Int. 2019, 119, 236–243. [Google Scholar] [CrossRef]
- Mateos, R.; Martínez-López, S.; Baeza Arévalo, G.; Amigo-Benavent, M.; Sarriá, B.; Bravo-Clemente, L. Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem. 2016, 205, 248–256. [Google Scholar] [CrossRef]
- Conterno, L.; Martinelli, F.; Tamburini, M.; Fava, F.; Mancini, A.; Sordo, M.; Pindo, M.; Martens, S.; Masuero, D.; Vrhovsek, U.; et al. Measuring the impact of olive pomace enriched biscuits on the gut microbiota and its metabolic activity in mildly hypercholesterolaemic subjects. Eur. J. Nutr. 2019, 58, 63–81. [Google Scholar] [CrossRef]
- Navarro, M.; Morales, F.J. Effect of hydroxytyrosol and olive leaf extract on 1,2-dicarbonyl compounds, hydroxymethylfurfural and advanced glycation endproducts in a biscuit model. Food Chem. 2017, 217, 602–609. [Google Scholar] [CrossRef]
- Cedola, A.; Palermo, C.; Centonze, D.; Del Nobile, M.A.; Conte, A. Characterization and Bio-Accessibility Evaluation of Olive Leaf Extract-Enriched “Taralli”. Foods 2020, 9, 1268. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Hayata, M.; özuğur, G. Phytochemical fortification of flour and bread. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 293–298. ISBN 978-0-12-380886-8. [Google Scholar]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile N -nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Nakao, T.; Hiraga, K. Species and Strain Differences in the Butylated Hydroxytoluene (BHT)-Producing Induction of Hepatic Drug Oxidation Enzymes. Jpn. J. Pharmacol. 1980, 30, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.-Y.; Lim, W.; You, S.; Song, G. Butylated Hydroxyanisole Exerts Neurotoxic Effects by Promoting Cytosolic Calcium Accumulation and Endoplasmic Reticulum Stress in Astrocytes. J. Agric. Food Chem. 2019, 67, 9618–9629. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Cofrades, S.; Salcedo Sandoval, L.; Delgado-Pando, G.; López-López, I.; Ruiz-Capillas, C.; Jiménez-Colmenero, F. Antioxidant activity of hydroxytyrosol in frankfurters enriched with n-3 polyunsaturated fatty acids. Food Chem. 2011, 129, 429–436. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez, L.; Castillo, J.; Ros, G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017, 97, 3761–3771. [Google Scholar] [CrossRef]
- De Leonardis, A.; Macciola, V.; Lembo, G.; Aretini, A.; Nag, A. Studies on oxidative stabilisation of lard by natural antioxidants recovered from olive-oil mill wastewater. Food Chem. 2007, 100, 998–1004. [Google Scholar] [CrossRef]
- Balzan, S.; Taticchi, A.; Cardazzo, B.; Urbani, S.; Servili, M.; Di Lecce, G.; Zabalza, I.B.; Rodriguez-Estrada, M.T.; Novelli, E.; Fasolato, L. Effect of phenols extracted from a by-product of the oil mill on the shelf-life of raw and cooked fresh pork sausages in the absence of chemical additives. LWT Food Sci. Technol. 2017, 85, 89–95. [Google Scholar] [CrossRef]
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; Fuente, J. de la Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processes. J. Clean. Prod. 2017, 140, 924–932. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Serio, A.; Mazzarrino, G.; Martuscelli, M.; Scarpone, E.; Paparella, A. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters. Int. J. Food Microbiol. 2015, 207, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Santos-López, J.A.; Garcimartín, A.; Bastida, S.; Bautista-Ávila, M.; González-Muñoz, M.J.; Benedí, J.; Sánchez-Muniz, F.J. Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets. Nutrients 2018, 10, 1830. [Google Scholar] [CrossRef]
- Rounds, L.; Havens, C.M.; Feinstein, Y.; Friedman, M.; Ravishankar, S. Concentration-dependent inhibition of Escherichia coli O157:H7 and heterocyclic amines in heated ground beef patties by apple and olive extracts, onion powder and clove bud oil. Meat Sci. 2013, 94, 461–467. [Google Scholar] [CrossRef]
- Djenane, D.; Gómez, D.; Yangüela, J.; Roncalés, P.; Ariño, A. Olive Leaves Extract from Algerian Oleaster (Olea europaea var. sylvestris) on Microbiological Safety and Shelf-life Stability of Raw Halal Minced Beef during Display. Foods 2018, 8, 10. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants 2020, 9, 851. [Google Scholar] [CrossRef]
- Pazos, M.; Alonso, A.; Sánchez, I.; Medina, I. Hydroxytyrosol Prevents Oxidative Deterioration in Foodstuffs Rich in Fish Lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef]
- Testa, B.; Lombardi, S.J.; Macciola, E.; Succi, M.; Tremonte, P.; Iorizzo, M. Efficacy of olive leaf extract (Olea europaea L. cv Gentile di Larino) in marinated anchovies (Engraulis encrasicolus, L.) process. Heliyon 2019, 5, e01727. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef]
- Servili, M.; Rizzello, C.G.; Taticchi, A.; Esposto, S.; Urbani, S.; Mazzacane, F.; Di Maio, I.; Selvaggini, R.; Gobbetti, M.; Di Cagno, R. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. Int. J. Food Microbiol. 2011, 147, 45–52. [Google Scholar] [CrossRef]
- Georgakouli, K.; Mpesios, A.; Kouretas, D.; Petrotos, K.; Mitsagga, C.; Giavasis, I.; Jamurtas, A. The Effects of an Olive Fruit Polyphenol-Enriched Yogurt on Body Composition, Blood Redox Status, Physiological and Metabolic Parameters and Yogurt Microflora. Nutrients 2016, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Fei, P.; Xu, Y.; Zhao, S.; Gong, S.; Guo, L. Olive oil polyphenol extract inhibits vegetative cells of Bacillus cereus isolated from raw milk. J. Dairy Sci. 2019, 102, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; Brenes, M.; de Castro, A. Antimicrobial Activity of Olive Oil, Vinegar, and Various Beverages against Foodborne Pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Villanova, A.; Villanova, L.; Merendino, A.; Fasiello, G. Yoghurt Containing Hydroxytyrosol and Other Biophenols with a Preventive Nutritional Activity Beneficial to Human Beings. Patent EP2170089 A2, 7 April 2010. [Google Scholar]
| Formulation | Dosage Applied to Food Products | Main Findings | Ref |
|---|---|---|---|
| Edible oils | |||
| HT Rosemary EXT | 200 mg/kg HT in Echium oil | ↑ oil antioxidant status (1.4-fold) HT > rosemary EXT | [69] |
| Olive cake/dried Thymus zygis EXT | VOO supplementation Three oils doses tested (25 mL): 0.01 a, 0.12 b and 0.21 c mg of HT | Thirty-three hypercholesterolemic individuals ingested VOO a, olive EXT supplemented VOO b and olive/thyme EXT supplemented VOO c (25 mL of olive oil/day, 3 weeks): ↑ HDL antioxidant compounds; ↑ blood plasma antioxidant activity; No consequences on levels of fat intake | [70] |
| OMWW EXTHT | 200 mg/kg of HT and EXT in husk and olive oils (EXT with 1225.6 mg HT/L) | ↓ oxidation rate; ↓ peroxide values HT antioxidant effect > BHT | [71] |
| Olivefen® DHPG, HT, and α-tocopherol mixes | 8, 20, 40 and 80 mg Olivefen® to 50 g vegetable oil; Olivefen (37.75% HT) Mixes (0.125–3 mmol/kg vegetable oil) | ↑ oxidative stability of oils (DHPG/HT combination > Olivefen®); Antioxidant synergic effect in HT/DHPG mixtures | [72] |
| OMWW EXT | 350 to 531 mg HT/kg refined olive oil for French fries | ↓ α-tocopherol oxidation; ↓ formation acrolein and hexanal Preservation of French fries sensory and nutritional values; ↑ color, texture score and taste | [73] |
| Olive cake EXT | Olive oil supplementation Olive cake EXT = 6.64 mg HT/kg olive oil cake | Single-dose of 30 mL enriched olive oil administered to thirteen pre- and stage-1 hypertensive patients: ↓ oxidation of LDL; more benefits on endothelial function than a standard VOO - ↑ ischemic reactive hyperemia | [74] |
| PO + gallic acid, caffeic acid and HT | PO/EVO: 20:80, 40:60 and 80:20 (100 mg each phenol /kg PO) | No PO oxidation stability by HT (gallic acid > caffeic acid > HT); PO stabilize mixtures < 20% EVO | [75] |
| HT | Patented application of HT to supplements and edibles oils 30–300 mg HT/kg edible oils 300–30,000 mg HT/kg dietary supplements | Prevention/treatment of cardiovascular diseases, plaque build-up, arterial hypertension, metabolic syndrome | [76] |
| Beverages | |||
| Olive by-products HT EXT | 80 mg HT or EXT/L white wine (Sauvignon blanc) | ↑ yellowness; ↑ visual appearance score (first six months of bottling); preservation of VC; ↓ Sauvignon blanc wines character (≠ aromas) | [80] |
| Olive by-products HT EXT | 50 mg HT or EXT/L red wine (Syrah) | better red wine chromatic parameters (at bottling); ↓ fruit aroma scents and oxidation odors; ↓ Syrah characteristics after six months | [82] |
| Olive leaf EXT (OLE) | 30 and 60 mg OLE/100 mL fruit smoothies “strawberry-banana” | ↑ bitterness perception; recommended < 20 mg OLE/100 g (to prevent bitterness) | [83] |
| HT | 0.02, 0.2 and 1 mg HT/mL tomato juice | 0.2 and 1 mg/mL of HT ↑ antioxidant activity (3 and 8-fold, respectively); 1 mg/mL of HT ↓ lipid peroxidation (> BHA); No changes in sensory quality and color. Favorable olive oil taste. | [84] |
| Olive leaves (dry, infusion, atomized extract) | 0.34,1.01, 1.74 and 3 g atomized/L; 0.34, 1.01 and 132 mL infusion/L, 9.9 g leaves/L dry leaves (1.27 mg HT/g), infusion (3.43 mg HT/g), and atomized extract (5.58 mg HT/g) | ↑ PC, antioxidant, HT content; Sour/astringent taste, herbal aroma; colloidal instability of beer | [85] |
| Vegetable-based products | |||
| OLE | Table olives fermentation (0.7 kg of olives and 0.7 L of brine solution with 200 mg OLE/L) | ↑ antioxidant, anti-inflammatory, and antimicrobial substances; ↓ bitter taste; No adverse effect on their sensorial qualities | [88] |
| OLE | 30 kg table olives for 30 L OLE with 8% NaCl brine solution and starter culture | ↑ PC, mostly HT (1700 mg/kg flesh olives vs. 900 mg/kg control olives) | [89] |
| HT/OLEUR EXT | Table olives submerged in EXT (200 mg HT/kg EXT) | ↑ HT content in table olive (↑ 109%); ↑ bitterness; good overall acceptability | [87] |
| OMWW EXT | 0.44, 1.00, 2.25 or 5.06 g EXT/kg vegetable meals: bean purée, potato purée, tomato juice | ↑ PC (from 3.7 to 13%); ↑ bitterness, sourness, astringency and pungency | [90] |
| Bakery products | |||
| HT | 5.25 mg HT/30 g Biscuit (20% of HT is lost during baking) | ↓ oxidized LDL levels; No changes in antioxidant activity of human serum (pharmacokinetic assay) | [91] |
| Olive pomace EXT* | Biscuit enriched in olive pomace extract (PreBiÒ®) (8.11 µg HT/g biscuit), | Human intake study (90 g biscuit/day, 8 weeks): subtle change on bacteria abundance of gut microbiota ↑ homovanillic acid and DOPAC; ↓ oxidative LDL cholesterol | [92] |
| HT/OLE | 2.55, 5.11 and 10.22 mg of HT/g dough; 0.127 and 0.537 mg of OLE/g dough OLE (0.15 mg HT/mL) | ↑ slight the darkens the biscuits; ↑ antiglycative effect | [93] |
| OLE | 17% OLE (weight/weight) OLE (24.08 mg gallic acid equivalent/g dry weight) | ↑ antioxidant activity, total flavonoids and PC ↑ dark color | [94] |
| OMWW EXT Ascorbic acid α-Tocopherol (powder and emulsion) | 50, 200, 500, 1000, 2000, 3000 mg olive PC/kg bread 100, 200, 400 mg olive PC/kg Rusk | 200 mg PC/kg was the most efficient as antimicrobial; ↑ antimicrobial emulsification effect and shelf life of both bread (from 10 to 15 days) and rusk samples (up to 12 weeks) | [95] |
| Formulation | Dosage Applied to Food Products | Main Findings | Ref |
|---|---|---|---|
| Meat products | |||
| Olive EXT | 100 mg HT/kg low-fat frankfurters sausages | ↓ lipid oxidation; HT antioxidant potential > BHA/BHT | [102] |
| OMWW EXT/OLE/Olive oil, walnut | 50 mg of HT/kg chicken sausages | ↓ lipid and protein oxidation; ↓ sausage acceptability with olive oil. ↓ stale odor of sausages with OMWW EXT and walnuts (7 and 14 days); ↑ cooking loss, change the color, texture, and taste | [103] |
| OMWW EXT | 150 mg OMWW (66.8% of HT)/kg lard | ↓ peroxidation | [104] |
| Olive vegetative water EXT | 0.075 and 0.15 g EXT/100 g pork sausage | ↓ pH, diacylglycerols, peroxide value and cholesterol oxidation; no unpleasant sensory properties | [105] |
| Olive waste EXT (Hytolive®) | Hytolive®: 10.5% HT in EXT 100, 200 or 400 mg GAE/kg lamb meat patties | ↓ lipid oxidation and protein carbonylation; ↑ loss of thiol groups during 6 days of storage; ↓ meat discoloration; change in odor and flavor; texture stable | [106] |
| Olive pomace HT with grape seed or chestnut EXT | 10 g EXT/kg sausages (EXT with11.65 g of HT/L) | ↓ lipid oxidation; ↓ microbial growth, comparable to nitrites Changes in profile, color, and texture; good acceptability | [107] |
| OMWW EXT | 100.23 ± 5.25 mg HT/g EXT Fermented sausages dipped 2.5% of OMWW | ↓ undesired fungal species growth; ↓ VC (from fatty acids oxidative degradation) | [108] |
| Olive oil EXT | Olive oil, 1 and 3% to ground beef | ↓ E. coli cell count; ↓ amine formation | [110] |
| HT | 3.6 g HT/kg fresh pork | Aged rats fed with high cholesterol/high saturated fat diets: ↓ body weight; ↓ cholesterol content in very-low-density lipoprotein (VLDL) (74% less); ↓ VLDL total mass (63% less) | [109] |
| OLE | 1, and 5% of OLE with 7.32% of HT | ↑ antioxidant activity; ↓ microbial growth no impact on overall acceptability | [111] |
| HT and OMWW extract | 200 mg/kg lamb burgers patties HT 99.2% purity; OMWW extract: 7.3% HT | Both induced ↓ lipid oxidation and ↓ the microbiological growth; good color maintenance and general acceptability; ↑ loss of thiol maintenance and general acceptability. HT more efficient | [112] |
| Fishery products | |||
| HT | 50 and 100 mg HT/kg fresh horse mackerel (Trachurus trachurus), cod (Gadus morhua) liver oil | ↓ lipid oxidation in bulk oil and minced horse mackerel during frozen storage; ↓ generation of oxidation products; preservation of PUFAs content | [113] |
| OLE | Anchovy fillets: marinade (1:1) marinade = 10 mg/mL of OLE | in vitro antimicrobial activity, inclusive psychrophilic bacteria ↓ oxidation; preservation of texture, appearance, and organoleptic characteristics | [114] |
| OLE Fruit EXT | 200 mg OLE/kg fish patties | ↑ antioxidant activity; ↓ VC (oxidation indicators); ↓ viable count patties, after 11 days (S. Aureus > E. coli and L. monocytogenes) | [115] |
| Dairy products | |||
| OMWW EXT | 100 or 200 mg PC/mL functional milk beverage (yogurt-like) | No interference with the fermentation/lactic acid bacteria; No impact on the VC | [116] |
| Medoliva© (Polyhealth S.A. Larissa, Greece) | olive PC encapsulated in maltodextrin (0.5 mg HT/g powder) Greek yogurt 2% fat | Human intake of 50 mg olive PC + 400 g yogurt/day, 2 weeks: ↓ body weight, body mass index, hip circumference and systolic blood pressure; ↓ LDL cholesterol levels In yogurt: ↑ lactic acid bacteria during fermentation, ↓ spoilage | [117] |
| Olive oil extract | 0.625 mg EXT/mL milk | ↓ vegetative cells to undetectable levels | [118] |
| VOO EXT Vinegar Wine | VOO (52.8 mg HT/kg); olive oil (14.1 mg HT/kg) VOO EXT (36.9 mg HT/kg); olive oil EXT (13.5 mg HT/kg to milk and egg mayonnaises | ↑ bactericidal effect (vinegar > aqueous VOO EXT > wines > olive oil EXT); ↓ Salmonella Enteritidis and Listeria monocytogenes (VOO mayonnaise ~ 3 log CFU/g > olive oil mayonnaise) | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants 2020, 9, 1246. https://doi.org/10.3390/antiox9121246
Silva AFR, Resende D, Monteiro M, Coimbra MA, Silva AMS, Cardoso SM. Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants. 2020; 9(12):1246. https://doi.org/10.3390/antiox9121246
Chicago/Turabian StyleSilva, Andreia F. R., Daniela Resende, Mariana Monteiro, Manuel A. Coimbra, Artur M. S. Silva, and Susana M. Cardoso. 2020. "Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements" Antioxidants 9, no. 12: 1246. https://doi.org/10.3390/antiox9121246
APA StyleSilva, A. F. R., Resende, D., Monteiro, M., Coimbra, M. A., Silva, A. M. S., & Cardoso, S. M. (2020). Application of Hydroxytyrosol in the Functional Foods Field: From Ingredient to Dietary Supplements. Antioxidants, 9(12), 1246. https://doi.org/10.3390/antiox9121246