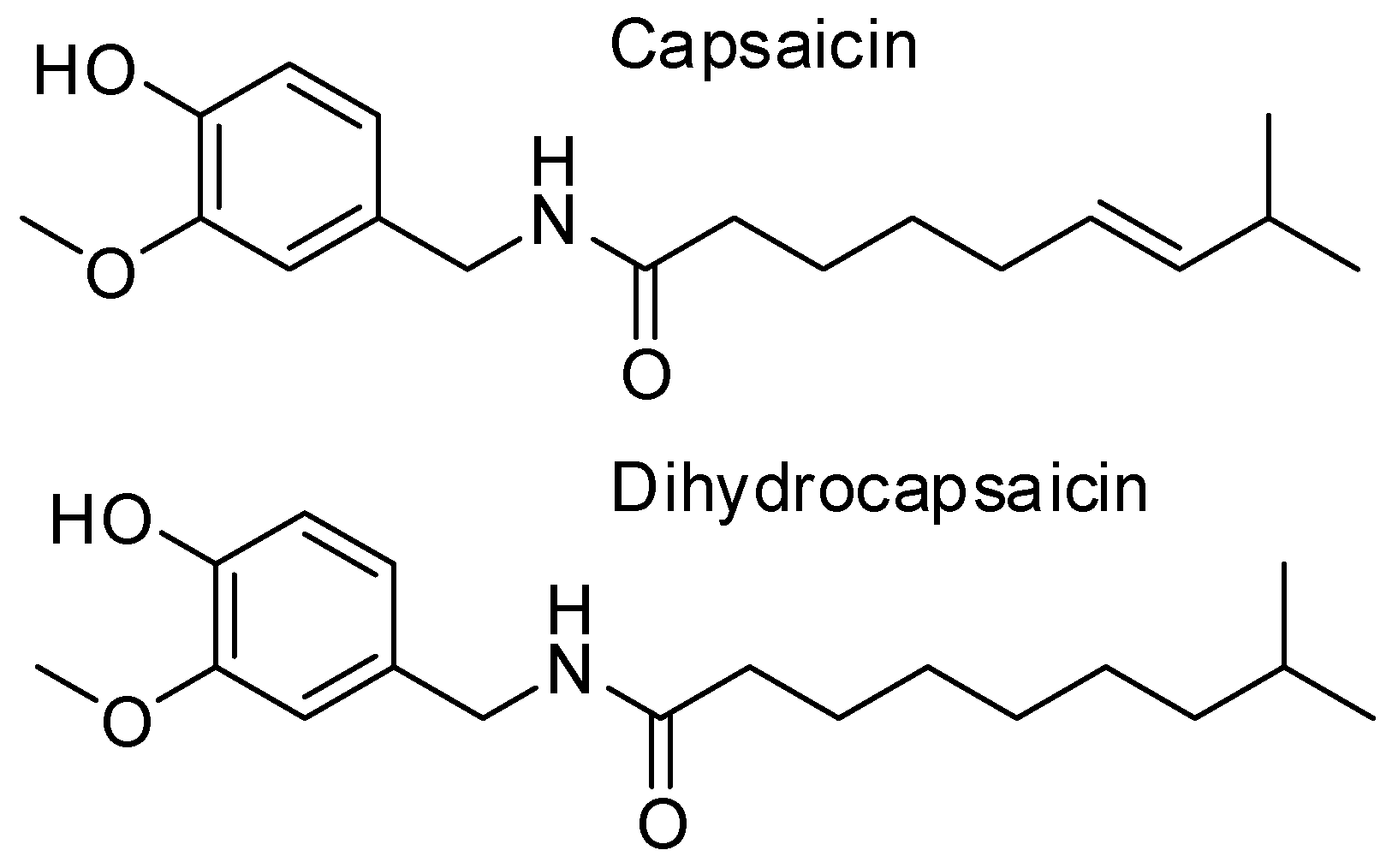
Capsaicin, a Powerful •OH-Inactivating Ligand
Abstract
1. Introduction
2. Computational Methods
2.1. Electronic Calculations
2.2. Molecular Dynamics
2.3. Reduction Reactions
3. Results and Discussion
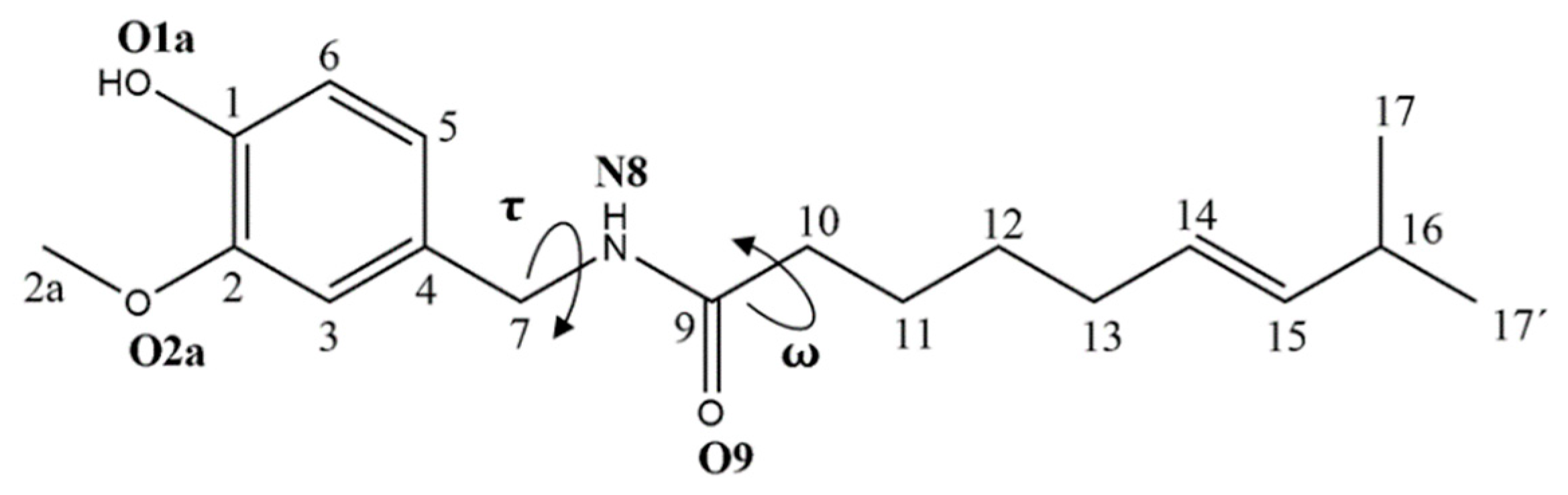
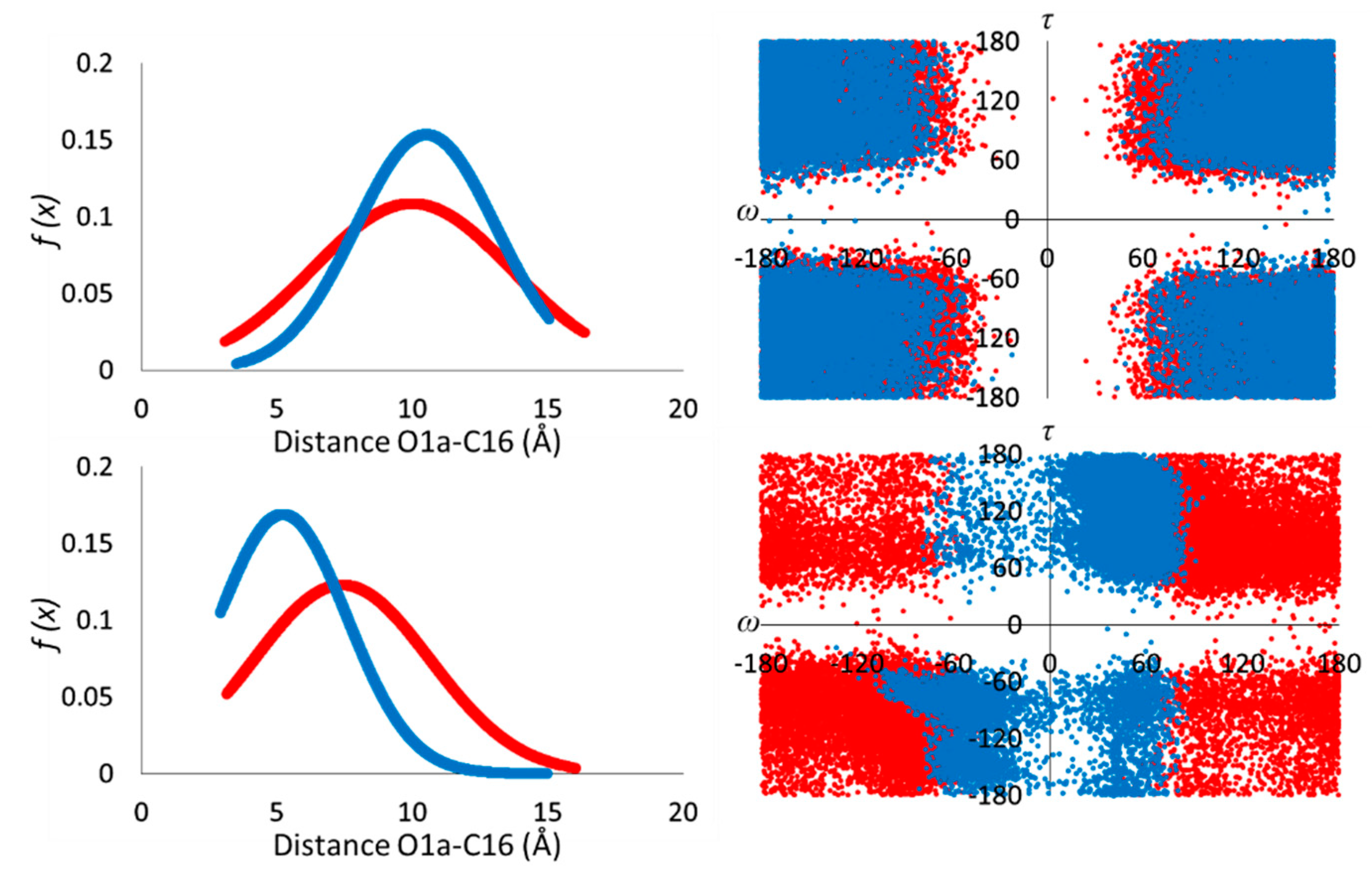
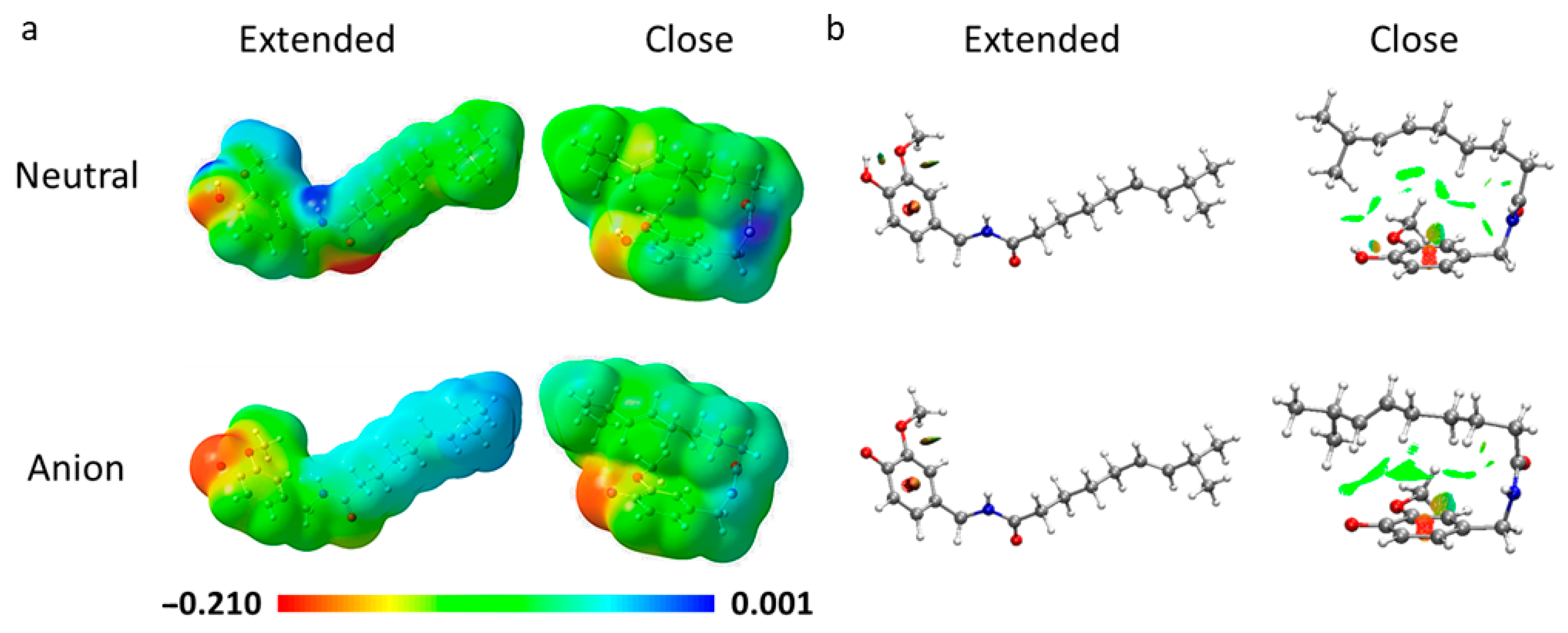
3.1. Conformational Analysis
3.2. Cu(II) Chelating Ability of CAP
3.3. OIL-1 (Inhibiting the Reduction in Metal Ions)
3.4. OIL-2 (Scavenging •OH Yielded through Fenton-Like Reactions)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: www.fao.org. (accessed on 3 July 2019).
- Iqbal, Q.; Amjad, M.; Asi, M.R.; Ariño, A. Characterization of Capsaicinoids and Antioxidants in Hot Peppers as Influenced by Hybrid and Harvesting Stage. Plant Foods Hum. Nutr. 2013, 68, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Antonious, G.F.; Kochhar, T.S. Mobility of heavy metals from soil into hot pepper fruits: A field study. Bull. Environ. Contam. Toxicol. 2009, 82, 59–63. [Google Scholar] [CrossRef]
- Inoue, K.; Kaneko, M.; Hino, T.; Oka, H. Simple and Novel Screening Assay of Natural Antioxidants for Cu(II) Ion/Adrenaline-Mediated Oxidation of N-Terminal Amyloid β by Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 9413–9417. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Kunde, D.A.; Ball, M.J.; Geraghty, D.P. Effects of Capsaicin, Dihydrocapsaicin, and Curcumin on Copper-Induced Oxidation of Human Serum Lipids. J. Agric. Food Chem. 2006, 54, 6436–6439. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef]
- Alam, M.A.; Syazwanie, N.F.; Mahmod, N.H.; Badaluddin, N.A.; Mustafa, K.; Alias, N.; Prodhan, M.A. Evaluation of antioxidant compounds, antioxidant activities and capsaicinoid compounds of Chili (Capsicum sp.) germplasms available in Malaysia. J. Appl. Res. Med. Aromat. Plants 2018, 9, 46–54. [Google Scholar] [CrossRef]
- Almaghrabi, S.Y.; Geraghty, D.P.; Ahuja, K.D.K.; Adams, M.J. Vanilloid-like agents inhibit aggregation of human platelets. Thromb. Res. 2014, 134, 412–417. [Google Scholar] [CrossRef]
- Careaga, M.O.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J. Food Microbiol. 2003, 83, 331–335. [Google Scholar] [CrossRef]
- Prasch, S.; Duran, A.G.; Chinchilla, N.; Molinillo, J.M.G.; Macías, F.A.; Bucar, F. Resistance modulatory and efflux-inhibitory activities of capsaicinoids and capsinoids. Bioorg. Chem. 2019, 82, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Da Motta, O.V.; Machado, O.L.T.; Rodrigues, R.; Carvalho, A.O.; Teixeira-Ferreira, A.; Gomes, V.M. Thionin-like peptides from Capsicum annuum fruits with high activity against human pathogenic bacteria and yeasts. Biopolymers 2014, 102, 30–39. [Google Scholar] [CrossRef]
- Perucka, I.; Materska, M. Phenylalanine ammonia-lyase and antioxidant activities of lipophilic fraction of fresh pepper fruits Capsicum annum L. Innov. Food Sci. Emerg. Technol. 2001, 2, 189–192. [Google Scholar] [CrossRef]
- Galano, A.; Martínez, A. Capsaicin, a tasty free radical scavenger: Mechanism of action and kinetics. J. Phys. Chem. B 2012, 116, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, S.; Bouchaut, M.; Brumas, V.; Berthon, G. Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 2000, 32, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Miche, H.; Brumas, V.; Berthon, G. Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 1997, 68, 27–38. [Google Scholar] [CrossRef]
- Berthon, G. Is copper pro- or anti-inflammatory? A reconciling view and a novel approach for the use of copper in the control of inflammation. Agents Actions 1993, 39, 210–217. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-iondependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991, 273, 601–604. [Google Scholar] [CrossRef]
- Letelier, M.E.; Sanchez-Jofre, S.; Peredo-Silva, L.; Cortés-Troncoso, J.; Aracena-Parks, P. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem. Biol. Interact. 2010, 188, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Phillips, D.H. Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) Fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat. Res. 1999, 424, 23–36. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Fox, D.J. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef]
- Galano, A.; Muñoz-Rugeles, L.; Alvarez-Idaboy, J.R.; Bao, J.L.; Truhlar, D.G. Hydrogen Abstraction Reactions from Phenolic Compounds by Peroxyl Radicals: Multireference Character and Density Functional Theory Rate Constants. J. Phys. Chem. A 2016, 120, 4634–4642. [Google Scholar] [CrossRef]
- Li, R.; Peverati, R.; Isegawa, M.; Truhlar, D.G. Assessment and validation of density functional approximations for iron carbide and iron carbide cation. J. Phys. Chem. A 2013, 117, 169–173. [Google Scholar] [CrossRef]
- Shil, S.; Bhattacharya, D.; Sarkar, S.; Misra, A. Performance of the widely used Minnesota density functionals for the prediction of heat of formations, ionization potentials of some benchmarked first row transition metal complexes. J. Phys. Chem. A 2013, 117, 4945–4955. [Google Scholar] [CrossRef]
- Yu, H.; Truhlar, D.G. Components of the Bond Energy in Polar Diatomic Molecules, Radicals, and Ions Formed by Group-1 and Group-2 Metal Atoms. J. Chem. Theory Comput. 2015, 11, 2968–2983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density functional theory for reaction energies: Test of meta and hybrid meta functionals, range-separated functionals, and other high-performance functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Prejanò, M.; Marino, T.; Russo, N. On the inhibition mechanism of glutathione transferase P1 by piperlongumine. Insight from theory. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Prejanò, M.; Romeo, I.; Sgrizzi, L.; Russo, N.; Marino, T. Why hydroxy-proline improves the catalytic power of the peptidoglycan: N-deacetylase enzyme: Insight from theory. Phys. Chem. Chem. Phys. 2019, 21, 23338–23345. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Kollman, P.A. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current status of transition-state theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry: Theory and experiment (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Pure Appl. Chem. 1997, 69, 13–29. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry Theory and experiment. J. Electroanal. Chem. 1997, 438, 251–259. [Google Scholar] [CrossRef]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid. Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Smoluchowski, M. Mathematical theory of the kinetics of the coagulation of colloidal solutions. Z Phys. Chem. 1917, 92, 1839950. [Google Scholar]
- Stokes, G.G. Mathematical and Physical Papers; Cambridge University Press: Cambridge, UK, 1903; Volume III, pp. 1–55. [Google Scholar]
- Einstein, A. On the movement of small particles suspended in a stationary liquid demanded by the molecular-kinetic theory of heat. Ann. Phys. 1905, 17, 559–560. [Google Scholar]
- Galano, A. Free Radicals Induced Oxidative Stress at a Molecular Level: The Current Status, Challenges and Perspectives of Computational Chemistry Based Protocols. J. Mex. Chem. Soc. 2015, 59, 231–262. [Google Scholar] [CrossRef]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum. Chem. 2019, 119. [Google Scholar] [CrossRef]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and noradrenaline: Protectors against oxidative stress or molecular targets? J. Phys. Chem. B 2015, 119, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal. Res. 2015, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- McLatchie, L.M.; Bevan, S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br. J. Pharmacol. 2001, 132, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
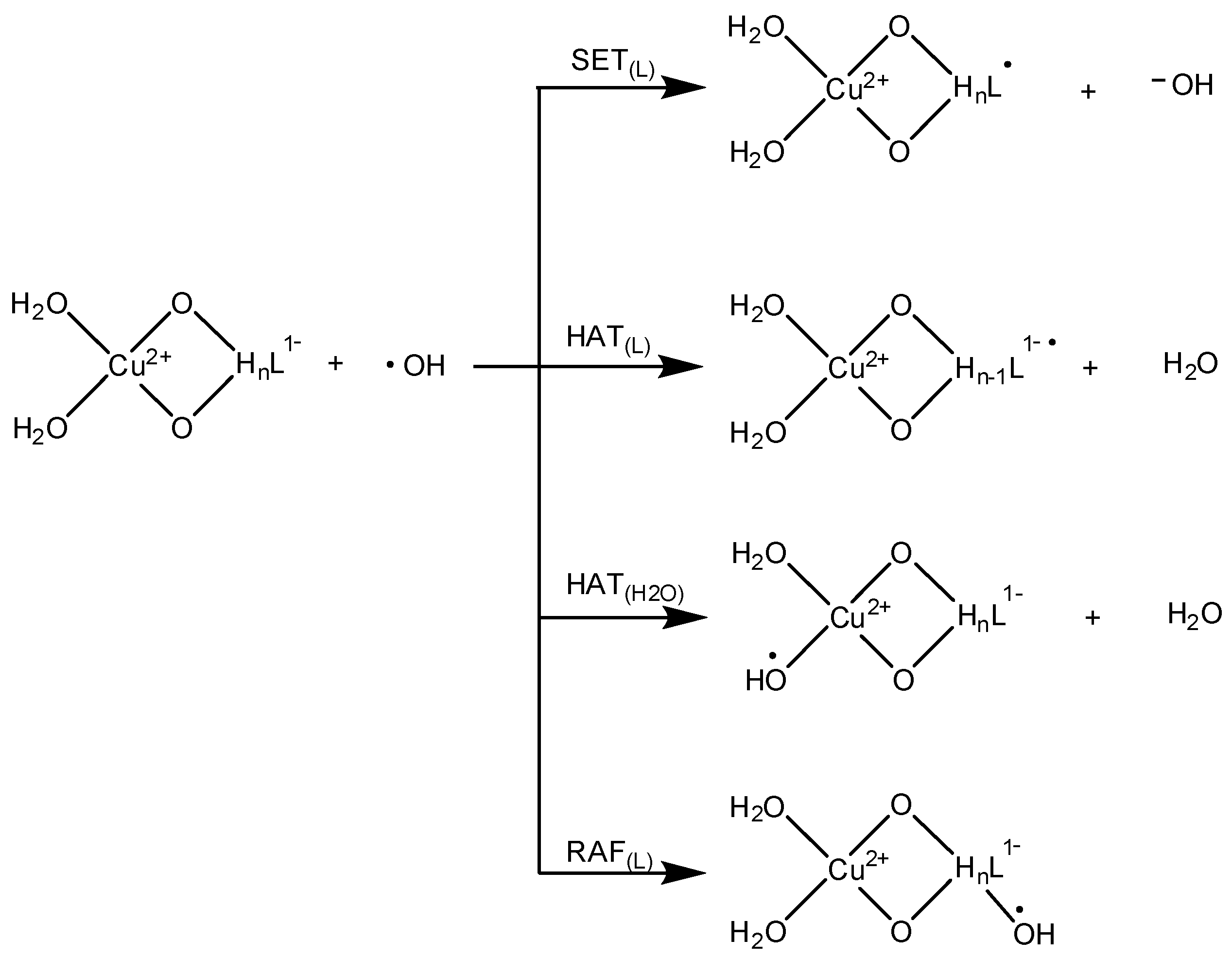
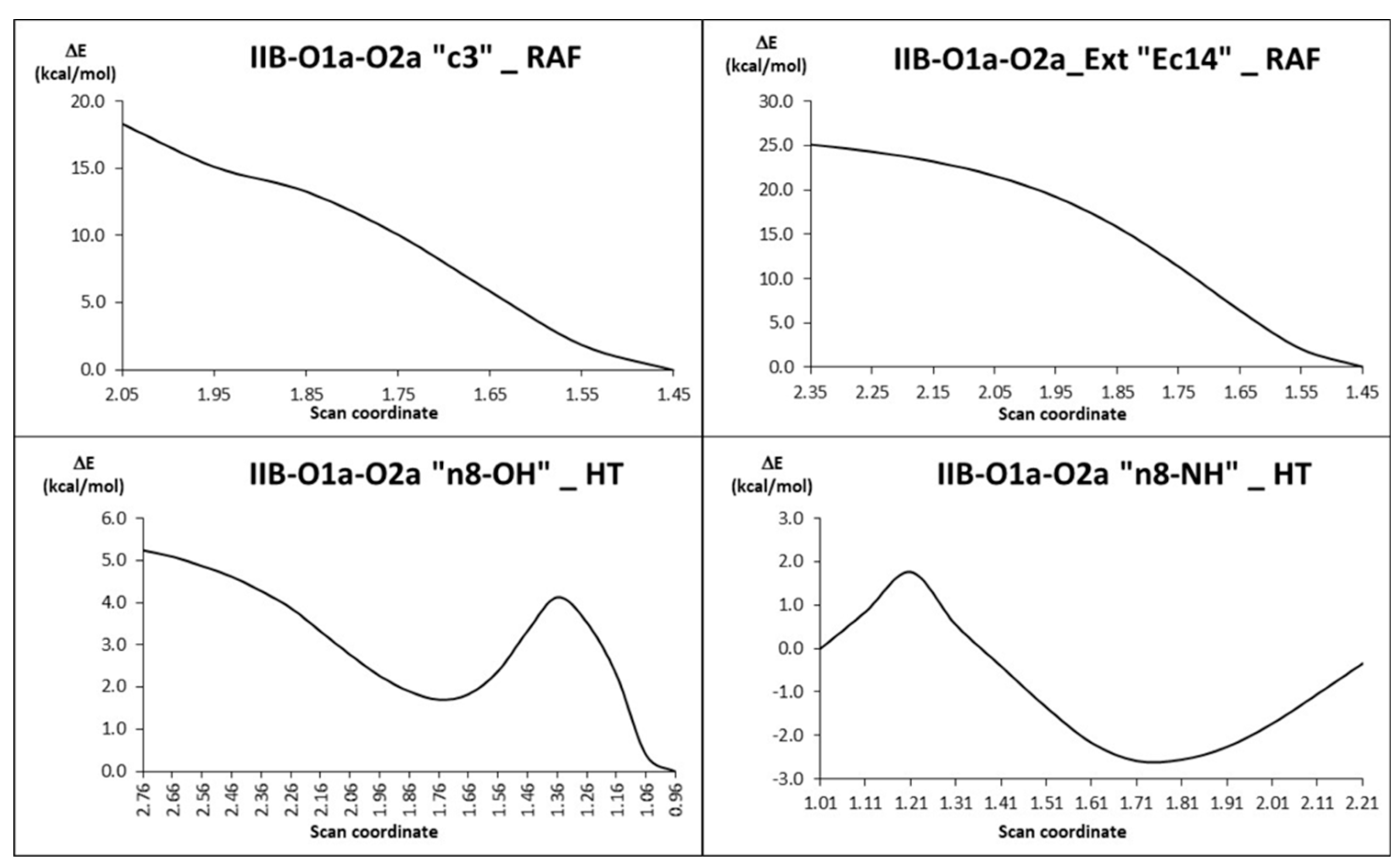
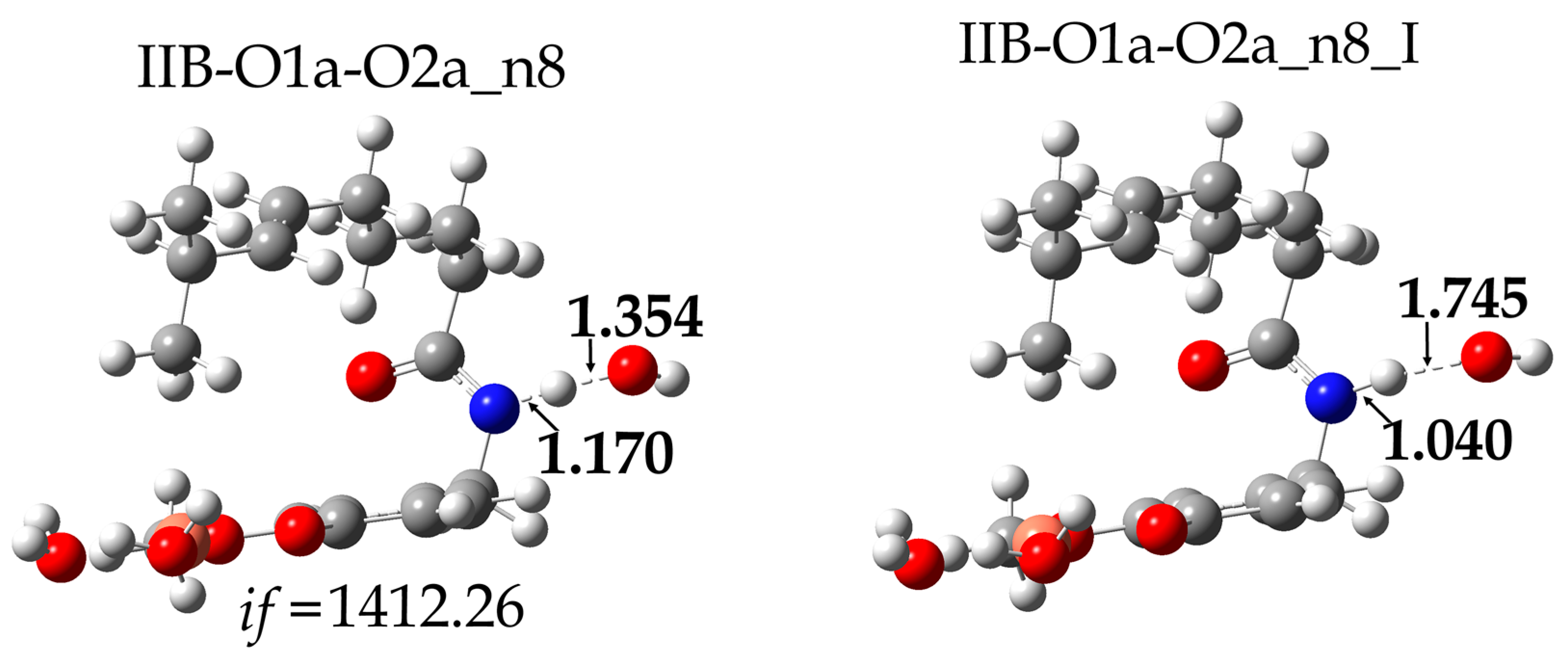
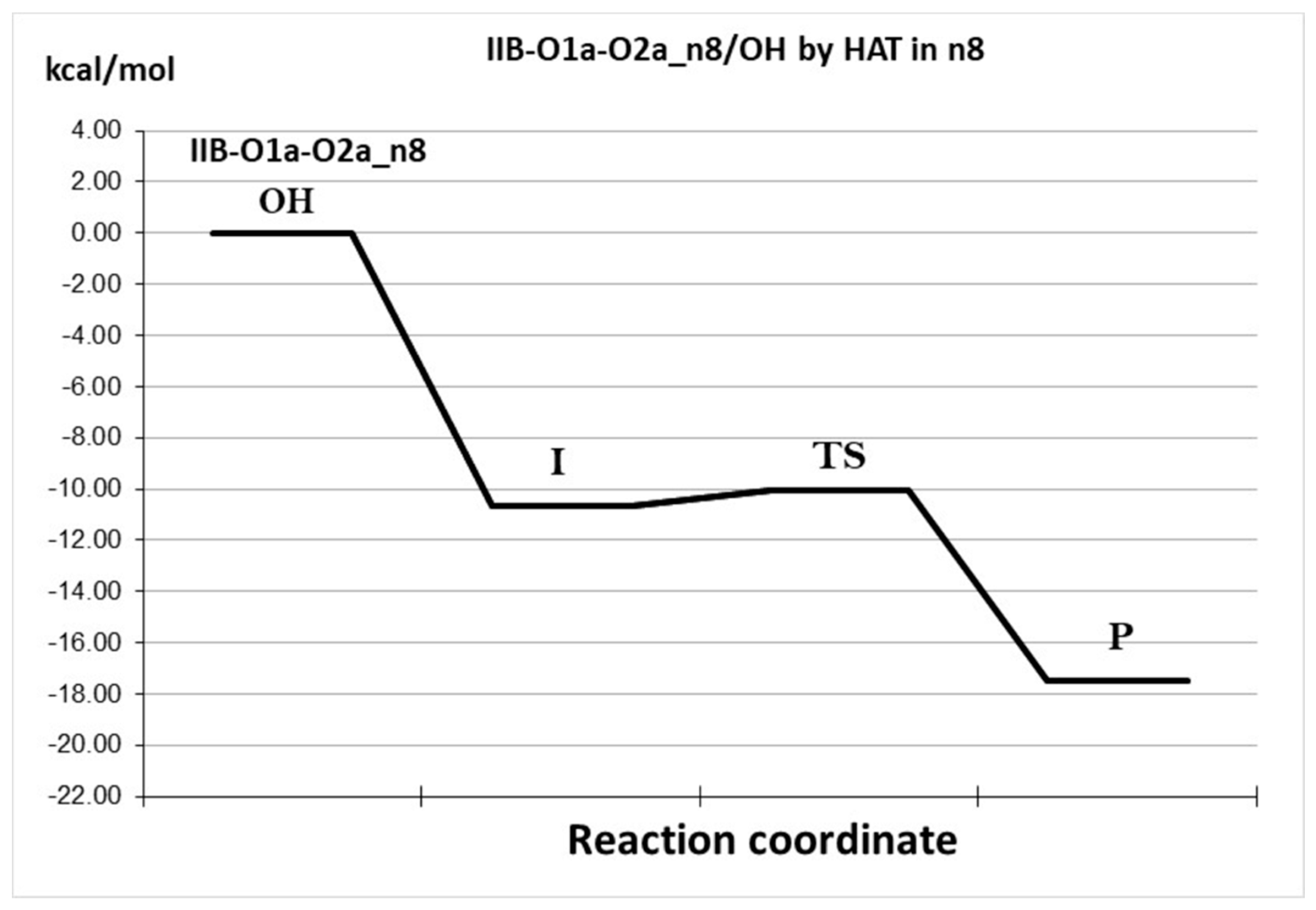
| Species * | ΔE (kcal/mol) | MB% |
|---|---|---|
| capN | 0.00 | 89.01 |
| capN_ext | 1.24 | 10.99 |
| capA | 0.00 | 67.66 |
| capA_ext | 0.44 | 32.34 |
| Complex | ∆G° | MB% |
|---|---|---|
| IA-O1a | 3.29 | - |
| IA-O1a_ext | 5.92 | - |
| IA-O2a | 5.91 | - |
| IA-O2a_ext | 7.84 | - |
| IA-O9 | 0.61 | - |
| IA-O9_ext | 2.74 | - |
| IB-O1a-O2a | 1.33 | - |
| IB-O1a-O2a_ext | 3.97 | - |
| IIA-O1a | −17.10 | 0.03 |
| IIA-O1a_ext | −17.07 | 0.03 |
| IIA-O2a_ext | −5.12 | - |
| IIA-O9_ext | 2.26 | - |
| IIB-O1a-O2a | −21.88 | 91.96 |
| IIB-O1a-O2a_ext | −20.43 | 7.98 |
| IIIA-O1a | −8.90 | - |
| IIIA-O1a_ext | −8.35 | - |
| IIIA-N8 | 3.25 | - |
| IIIA-N8_ext | 5.64 | - |
| IIIB-O1a-O2a | −13.68 | - |
| IIIB-O1a-O2a_ext | −11.71 | - |
| IIIB-N8-O9 | −3.00 | - |
| IVA-N8 | 2.79 | - |
| IVA-N8_ext | 5.24 | - |
| IVB-N8-O9 | −3.00 | - |
| Complex | ΔG° | Ki | |
|---|---|---|---|
| IIB-O1a-O2a | −21.88 | 1.09 × 1016 | 2.16 × 1013 |
| IIB-O1a-O2a_ext | −20.43 | 9.43 × 1014 | 1.88 × 1012 |
| Kapp | 2.35 × 1013 |
| O2•− | ΔG° | λ | ΔG≠ | kiapp | kCu(II)app/kiapp |
|---|---|---|---|---|---|
| O2•− | |||||
| Cu(II) | −24.01 | 51.87 | 3.74 | 4.46 × 109 | |
| IIB-O1a-O2a | −13.21 | 50.11 | 6.79 | 5.92 × 107 | 75.39 |
| IIB-O1a-O2a_ext | −13.96 | 50.09 | 6.52 | 8.14 × 106 | 548.32 |
| koverall | 6.73 × 107 | 66.28 | |||
| Asc− | |||||
| Cu(II) | −4.67 | 34.14 | 6.36 | 1.33 × 108 | |
| IIB-O1a-O2a | 6.12 | 32.38 | 11.45 | 2.31 × 104 | 5744.24 |
| IIB-O1a-O2a_ext | 5.38 | 32.37 | 11.00 | 4.25 × 103 | 31,316.16 |
| koverall | 2.74 × 104 | 4853.91 |
| IIB-O1a-O2a | IIB-O1a-O2a_ext | |
|---|---|---|
| SET | ||
| O1a-O2a_ct | −23.85 | −24.63 |
| HAT | ||
| c2a | −22.35 | −22.84 |
| c7 | −41.97 | −41.46 |
| n8 | −17.48 | −17.99 |
| c10 | −29.71 | −31.44 |
| c11 | −27.34 | −27.07 |
| c12 | −27.07 | −27.36 |
| c13 | −39.82 | −42.34 |
| c17 | −22.14 | −22.18 |
| c17p | −20.05 | −22.77 |
| o1a | −36.19 | −33.99 |
| o2a | −40.28 | −38.86 |
| RAF | ||
| c1 | −16.30 | −16.51 |
| c2 | −19.19 | −21.73 |
| c3 | −13.16 | −13.72 |
| c4 | −17.26 | −18.51 |
| c5 | −14.89 | −15.32 |
| c6 | −16.40 | −16.21 |
| c14 | −26.15 | −26.44 |
| c15 | −26.23 | −24.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-González, A.; Prejanò, M.; Russo, N.; Marino, T.; Galano, A. Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants 2020, 9, 1247. https://doi.org/10.3390/antiox9121247
Pérez-González A, Prejanò M, Russo N, Marino T, Galano A. Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants. 2020; 9(12):1247. https://doi.org/10.3390/antiox9121247
Chicago/Turabian StylePérez-González, Adriana, Mario Prejanò, Nino Russo, Tiziana Marino, and Annia Galano. 2020. "Capsaicin, a Powerful •OH-Inactivating Ligand" Antioxidants 9, no. 12: 1247. https://doi.org/10.3390/antiox9121247
APA StylePérez-González, A., Prejanò, M., Russo, N., Marino, T., & Galano, A. (2020). Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants, 9(12), 1247. https://doi.org/10.3390/antiox9121247








