The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Sample Preparation
2.2. Nutritional Value and Energy Content
2.3. Chemical Characterization
2.3.1. Organic Acids
2.3.2. Free Sugars
2.3.3. Tocopherols
2.3.4. Fatty Acids
2.4. Hydroethanolic Extracts Preparation
Extract Preparation
2.5. Phenolic Compound Determination
2.6. Bioactive Property Evaluation
2.6.1. Antioxidant Activity
2.6.2. Antimicrobial Properties
2.7. Statistical Analysis
3. Results and Discussion
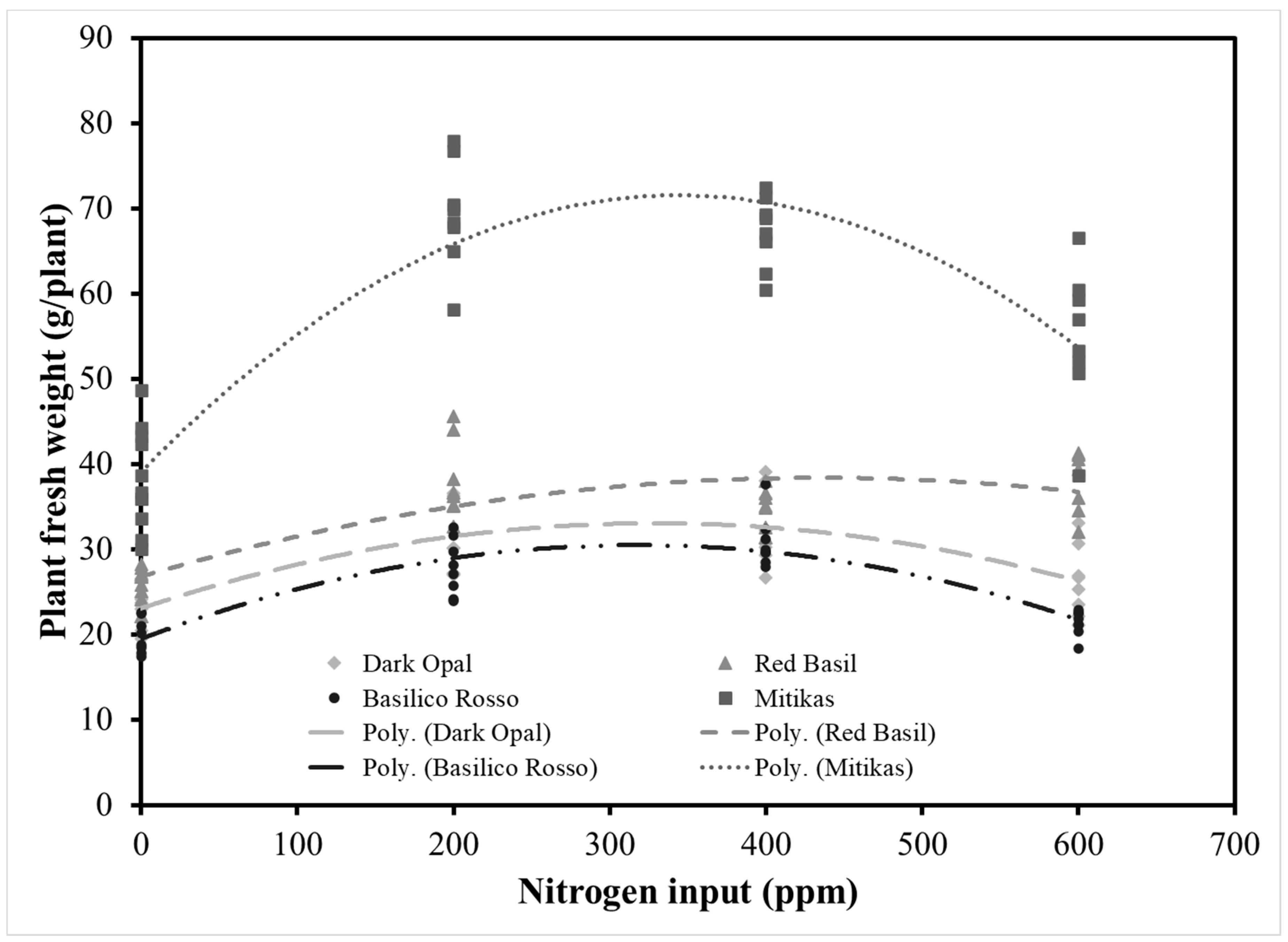
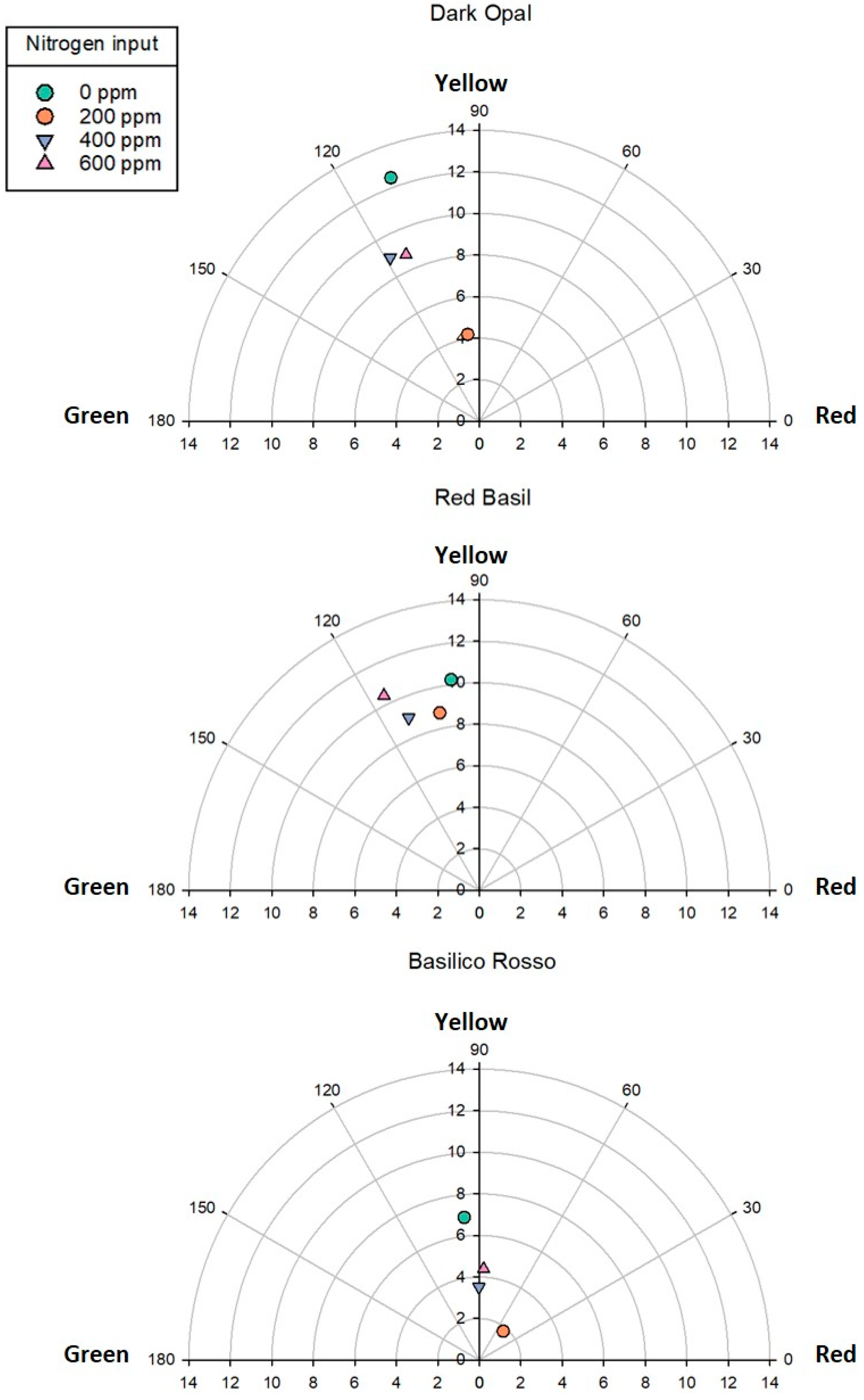
3.1. Effect of Genotype and Nitrogen Input on Basil Yield and Leaf Color
3.2. Effect of Genotype and Nitrogen Input on Basil Leaves Nutritional Value
3.3. Effect of Genotype and Nitrogen Input on Basil Leaves’ Organic Acids Content
3.4. Effect of Genotype and Nitrogen Input on Basil Leaves’ Free Sugar Content
3.5. Effect of Genotype and Nitrogen Input on Basil Leaves’ Tocopherol Content
3.6. Effect of Genotype and Nitrogen Input on Basil Leaves’ Fatty Acids Content
3.7. Effect of Genotype and Nitrogen Input on Basil Leaves’ Hydroethanolic Extract Phenolic Content
3.8. Effect of Genotype and Nitrogen Input on Basil Leaves’ Hydroethanolic Extract Bioactive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs. Spices Med. Plants 2008, 13, 37–41. [Google Scholar] [CrossRef]
- Nadeem, F.; Hanif, M.A.; Bhatti, I.A.; Jilani, M.I.; Al-Yahyai, R. Medicinal Plants of South Asia—Novel Sources for Drug Discovery; Hanif, M.A., Khan, M.M., Nawaz, H., Byrne, H.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 47–62. ISBN 9780081026595. [Google Scholar]
- Biesiada, A.; Kuś, A. The effect of nitrogen fertilization and irrigation on yielding and nutritional status of sweet basil (Ocimum basilicum L.). Acta Sci. Pol. Hortorum Cultus 2010, 9, 3–12. [Google Scholar]
- Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Lobiuc, A.; Teliban, G.C.; Stoleru, V.; Zamfirache, M.M. Fertilization modifies the essential oil and physiology of basil varieties. Ind. Crop. Prod. 2018, 121, 282–293. [Google Scholar] [CrossRef]
- Di Gioia, F.; Tzortzakis, N.; Rouphael, Y.; Kyriacou, M.C.; Sampaio, S.L.; Ferreira, I.C.F.R.; Petropoulos, S.A. Grown to be blue—Antioxidant properties and health effects of colored vegetables. Part II: Leafy, fruit, and other vegetables. Antioxidants 2020, 9, 97. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kite, G.C.; Goldstone, F.J.; Bryan, S.E.; Paton, A.; Putievsky, E. Infraspecific taxonomy and essential oil chemotypes in sweet basil, Ocimum basilicum. Phytochemistry 1996, 43, 1033–1039. [Google Scholar] [CrossRef]
- Primi, R.; Ruggeri, R.; Ronchi, B.; Bernabucci, U.; Rossini, F.; Martin-Pedrosa, M.; Danieli, P.P. Sowing date and seeding rate affect bioactive compound contents of chickpea grains. Animals 2019, 9, 571. [Google Scholar] [CrossRef]
- Beszterda, M.; Nogala-Kałucka, M. Current research developments on the processing and improvement of the nutritional quality of rapeseed (Brassica napus L.). Eur. J. Lipid Sci. Technol. 2019, 121, 1800045. [Google Scholar] [CrossRef]
- Smith, T.; Eckl, V.; Morton, C.; Gillespie, M.; Knepper, J. Herbal supplement sales in US increase 9.4% in 2018. HerbalGram 2019, 123, 62–73. [Google Scholar]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Santamaria, P. Agronomic, physiological and quality response of romaine and red oak-leaf lettuce to nitrogen input. Ital. J. Agron. 2017, 12, 47–58. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Polyzos, N.; Antoniadis, V.; Barros, L.; Ferreira, I.C.F.R. The Impact of fertilization regime on crop performance and chemical composition of potato (Solanum tuberosum L.) cultivated in Central Greece. Agronomy 2020, 10, 474. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Olympios, C.M.; Passam, H.C. The effect of nitrogen fertilization on plant growth and the nitrate content of leaves and roots of parsley in the Mediterranean region. Sci. Hortic. 2008, 118, 255–259. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Daferera, D.; Polissiou, M.G.; Passam, H.C. Effect of nitrogen-application rate on the biomass, concentration, and composition of essential oils in the leaves and roots of three types of parsley. J. Plant. Nutr. Soil Sci. 2009, 172, 210–215. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Xyrafis, E.; Polyzos, N.; Antoniadis, V.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R. The optimization of nitrogen fertilization regulates crop performance and quality of processing tomato (Solanum lycopersicum L. cv. Heinz 3402). Agronomy 2020, 10, 715. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Antoniadis, V.; Barros, L.; Ferreira, I.C.F.R.F.R. Nutrient solution composition and growing season affect yield and chemical composition of Cichorium spinosum plants. Sci. Hortic. 2018, 231, 97–107. [Google Scholar] [CrossRef]
- Di Gioia, F.; Gonnella, M.; Buono, V.; Ayala, O.; Cacchiarelli, J.; Santamaria, P. Calcium cyanamide effects on nitrogen use efficiency, yield, nitrates, and dry matter content of lettuce. Agron. J. 2017, 109, 354–362. [Google Scholar] [CrossRef]
- Ozores-hampton, M.; Di Gioia, F.; Sato, S.; Simonne, E.; Morgan, K. Effects of nitrogen rates on nitrogen, phosphorous, and potassium partitioning, accumulation, and use efficiency in seepage-irrigated fresh market tomatoes. HortScience 2015, 50, 1636–1643. [Google Scholar] [CrossRef]
- Groenveld, T.; Kohn, Y.Y.; Gross, A.; Lazarovitch, N. Optimization of nitrogen use efficiency by means of fertigation management in an integrated aquaculture-agriculture system. J. Clean. Prod. 2019, 212, 401–408. [Google Scholar] [CrossRef]
- Acharya, T.P.; Reiter, M.S.; Welbaum, G.; Arancibia, R.A. Nitrogen uptake and use efficiency in sweet basil production under low tunnels. HortScience 2020, 55, 429–435. [Google Scholar] [CrossRef]
- Rahimikhoob, H.; Sohrabi, T.; Delshad, M. Development of a critical nitrogen dilution curve for basil (Ocimum basilicum L.) under greenhouse conditions. J. Soil Sci. Plant. Nutr. 2020, 20, 881–891. [Google Scholar] [CrossRef]
- Mclellan, M.R.; Lind, L.R.; Kime, R.W. Hue angle determinations and statistical. J. Food Qual. 1994, 18, 235–240. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Soković, M.; Rosa, E.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327, 127043. [Google Scholar] [CrossRef]
- Prinsi, B.; Negrini, N.; Morgutti, S.; Espen, L. Nitrogen starvation and nitrate or ammonium availability differently affect phenolic composition in green and purple basil. Agronomy 2020, 10, 498. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, E.; Círić, A.; Soković, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Ocimum basilicum var. purpurascens leaves (red rubin basil): A source of bioactive compounds and natural pigments for the food industry. Food Funct. 2019, 10, 3161–3171. [Google Scholar] [PubMed]
- Murillo-Amador, B.; Nieto-Garibay, A.; Troyo-Diéguez, E.; Flores-Hernández, A.; Cordoba-Matson, M.V.; Villegas-Espinoza, A. Proximate analysis among 24 Ocimum cultivars under two cultivation environments: A comparative study. J. Food Agric. Environ. 2013, 11, 2842–2848. [Google Scholar]
- Pereira, C.; Barros, L.; Ferreira, I.C.F.R. A Comparison of the nutritional contribution of thirty-nine aromatic plants used as condiments and/or herbal infusions. Plant. Foods Hum. Nutr. 2015, 70, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Siti Mahirah, Y.; Rabeta, M.S.; Antora, R.A. Effects of different drying methods on the proximate composition and antioxidant activities of Ocimum basilicum leaves. Food Res. 2018, 2, 421–428. [Google Scholar]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Di Gioia, F.; Kolovou, P.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of Cichorium spinosum L. in relation to nitrate/ammonium nitrogen ratio. J. Sci. Food Agric. 2019, 99, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Ivanov, M.; Sokovic, M.D.; Ferreira, I.C.F.R.; Barros, L. The effect of nitrogen fertigation and harvesting time on plant growth and chemical composition of Centaurea raphanina subsp. mixta (DC.) runemark. Molecules 2020, 25, 3175. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C.F.R. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Di Gioia, F.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and cultivated Centaurea raphanina subsp. mixta: A valuable source of bioactive compounds. Antioxidants 2020, 9, 314. [Google Scholar]
- Sgherri, C.; Cecconami, S.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Levels of antioxidants and nutraceuticals in basil grown in hydroponics and soil. Food Chem. 2010, 123, 416–422. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef] [PubMed]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical characterization and bioactive properties of cinnamon basil (Ocimum basilicum cv. ‘Cinnamon’) and lemon basil (Ocimum × citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Złotek, U.; Kordowska-Wiater, M.; Świeca, M. Effect of basil leaves and wheat bran water extracts on antioxidant capacity, sensory properties and microbiological quality of shredded iceberg lettuce during storage. Antioxidants 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed]

| Cultivar | ppm | Nutritional Value | ||||
|---|---|---|---|---|---|---|
| Fat | Proteins | Ash | Carbohydrates | Energy | ||
| Dark Opal | 0 | 1.9 ± 0.2 A,* | 47 ± 14 A | 12.9 ± 0.2 B | 38 ± 14 B | 358 ± 1 A |
| 2.17 ± 0.01 a,b | 26 ± 2 h | 12.8 ± 0.1 h | 59 ± 1 b | 359.8 ± 0.2 b | ||
| 200 | 1.69 ± 0.01 g | 46.8 ± 0.2 g | 12.8 ± 0.3 h | 38.71 ± 0.08 c | 357 ± 1 e,f,g | |
| 400 | 1.79 ± 0.02 e | 51.1 ± 0.6 d,e | 13.1 ± 0.1 e,f | 34.0 ± 0.5 g | 356.5 ± 0.3 g,h | |
| 600 | 1.97 ± 0.03 d | 63.6 ± 0.6 a | 13.0 ± 0.3 f,g | 21.4 ± 0.7 j | 357.7 ± 0.8 e,f | |
| Red Basil | 0 | 1.9 ± 0.3 A | 45 ± 11 B | 13.0 ± 0.6 A | 40 ± 11 A | 357 ± 2 A |
| 2.19 ± 0.01 a,b | 25.4 ± 0.8 h,i | 12.9 ± 0.1 f,g,h | 59.5 ± 0.6 a,b | 359.3 ± 0.3 b,c | ||
| 200 | 1.40 ± 0.02 i | 51.5 ± 0.5 d | 12.1 ± 0.2 i | 35.0 ± 0.2 d,e,f | 358.7 ± 0.7 c,d | |
| 400 | 1.78 ± 0.01 e,f | 50.4 ± 0.8 e | 13.6 ± 0.5 c | 34.3 ± 0.2 f,g | 355 ± 1 i | |
| 600 | 2.06 ± 0.07 c | 52.3 ± 0.1 c | 13.3 ± 0.1 d,e | 32.3 ± 0.1 h | 356.9 ± 0.4 f,g | |
| Basilico Rosso | 0 | 1.9 ± 0.2 A | 45 ± 11 B | 12.7 ± 0.6 A,B | 40 ± 12 A | 359 ± 3 A |
| 2.17 ± 0.02 b | 25.5 ± 0.9 h,i | 12.2 ± 0.4 i | 60.1 ± 0.9 a | 362 ± 1 a | ||
| 200 | 1.58 ± 0.03 h | 51.08 ± 0.06 d,e | 12.2 ± 0.3 i | 35.1 ± 0.3 d,e | 359.0 ± 0.8 b,c,d | |
| 400 | 1.79 ± 0.04 e,f | 50.6 ± 0.7 e | 13.4 ± 0.2 c,d | 34.1 ± 0.6 g | 355.2 ± 0.7 i | |
| 600 | 2.06 ± 0.01 c | 52.9 ± 0.9 c | 13.0 ± 0.1 f,g | 32.0 ± 0.6 h | 358.2 ± 0.1 d,e | |
| Mitikas | 0 | 1.9 ± 0.2 A | 46 ± 14 A | 13.6 ± 0.5 A | 38 ± 13 B | 355 ± 3 B |
| 2.21 ± 0.05 a | 24.8 ± 0.4 i | 13.9 ± 0.1 b | 59.1 ± 0.4 b | 355.5 ± 0.1 h,i | ||
| 200 | 1.60 ± 0.07 h | 48.9 ± 0.6 f | 14.2 ± 0.2 a | 35.3 ± 0.5 d | 351.0 ± 0.8 j | |
| 400 | 1.75 ± 0.03 f | 50.4 ± 0.8 e | 13.4 ± 0.3 c,d | 34.4 ± 0.8 e,f,g | 355.0 ± 0.8 i | |
| 600 | 2.08 ± 0.01 c | 61.5 ± 0.8 b | 12.86 ± 0.04 g,h | 23.5 ± 0.5 i | 358.9 ± 0.1 b,c,d | |
| Cultivar | ppm | Organic acids | ||||
| Oxalic acid | Quinic acid | Shikimic acid | Ascorbic acid | Total organic acids | ||
| Dark Opal | 0 | 5.7 ± 0.8 A,* | 9 ± 3 B | 0.11 ± 0.01 A | 15 ± 4 A,B | |
| 4.67 ± 0.01 i | 4.45 ± 0.01 n | 0.120 ± 0.001 b | tr | 9.24 ± 0.01 m | ||
| 200 | 5.27 ± 0.05 h | 9.80 ± 0.08 h | 0.100 ± 0.003 e,f | tr | 15.18 ± 0.02 i | |
| 400 | 6.01 ± 0.09 c,d | 10.57 ± 0.06 f | 0.100 ± 0.001 e | tr | 16.68 ± 0.03 g | |
| 600 | 6.78 ± 0.07 a | 12.41 ± 0.02 b | 0.100 ± 0.001 e,f | tr | 19.29 ± 0.05 b | |
| Red Basil | 0 | 5 ± 1 B | 10 ± 3 A,B | 0.09 ± 0.04 B | 15 ± 4 A,B | |
| 3.17 ± 0.01 j | 4.50 ± 0.03 m | 0.020 ± 0.001 j | tr | 7.69 ± 0.03 n | ||
| 200 | 5.33 ± 0.02 g | 10.37 ± 0.02 g | 0.100 ± 0.007 f | tr | 15.80 ± 0.05 h | |
| 400 | 5.76 ± 0.06 f | 11.07 ± 0.02 e | 0.110 ± 0.001 d | tr | 16.94 ± 0.04 f | |
| 600 | 6.04 ± 0.01 c | 12.41 ± 0.04 b | 0.130 ± 0.001 a | tr | 18.59 ± 0.05 c | |
| Basilico Rosso | 0 | 5 ± 2 B | 11 ± 4 A | 0.09 ± 0.04 B | 16 ± 5 A | |
| 2.41 ± 0.01 l | 4.95 ± 0.06 l | 0.030 ± 0.001 i | tr | 7.39 ± 0.06 o | ||
| 200 | 5.97 ± 0.01 d,e | 11.40 ± 0.02 d | 0.110 ± 0.002 c | tr | 17.48 ± 0.02 e | |
| 400 | 6.31 ± 0.09 b | 11.50 ± 0.05 c | 0.110 ± 0.001 c,d | tr | 17.92 ± 0.04 d | |
| 600 | 6.78 ± 0.01 a | 14.90 ± 0.06 a | 0.120 ± 0.001 b | tr | 21.80 ± 0.07 a | |
| Mitikas | 0 | 5 ± 1 B | 6 ± 1 C | 0.04 ± 0.02 C | 12 ± 3 C | |
| 2.90 ± 0.02 k | 4.14 ± 0.04 o | 0.020 ± 0.001 j | tr | 7.06 ± 0.01 p | ||
| 200 | 5.25 ± 0.01 h | 6.81 ± 0.02 k | 0.030 ± 0.001 i | tr | 12.09 ± 0.02 l | |
| 400 | 5.77 ± 0.03 f | 7.14 ± 0.02 j | 0.060 ± 0.003 h | tr | 12.97 ± 0.02 k | |
| 600 | 5.95 ± 0.07 e | 7.89 ± 0.01 i | 0.070 ± 0.003 g | tr | 13.92 ± 0.09 j | |
| Cultivar | ppm | Free Sugars | |||
|---|---|---|---|---|---|
| Fructose | Glucose | Sucrose | Total Free Sugars | ||
| Dark Opal | 0 | 1.7 ± 0.5 A,* | 3 ± 1 C | 0.9 ± 0.2 B | 5 ± 2 C |
| 1.65 ± 0.04 f | 0.87 ± 0.01 l | 0.62 ± 0.01 i | 3.14 ± 0.06 j | ||
| 200 | 0.92 ± 0.01 j | 2.51 ± 0.01 j | 0.95 ± 0.01 g | 4.38 ± 0.01 i | |
| 400 | 2.05 ± 0.09 b | 3.25 ± 0.06 h | 1.04 ± 0.05 f | 6.34 ± 0.07 f | |
| 600 | 2.32 ± 0.03 a | 3.45 ± 0.02 g | 1.07 ± 0.01 e | 6.84 ± 0.06 c | |
| Red Basil | 0 | 1.7 ± 0.4 A | 3 ± 1 C | 0.6 ± 0.2 C | 5 ± 1 B,C |
| 1.40 ± 0.02 h | 0.64 ± 0.02 m | 0.53 ± 0.05 j | 2.57 ± 0.09 k | ||
| 200 | 1.55 ± 0.01 g | 2.69 ± 0.04 i | 0.878 ± 0.004 h | 5.11 ± 0.04 h | |
| 400 | 1.40 ± 0.05 h | 3.73 ± 0.01 f | 0.442 ± 0.006 k | 5.57 ± 0.05 g | |
| 600 | 2.29 ± 0.02 a | 3.76 ± 0.02 e | 0.51 ± 0.03 j | 6.57 ± 0.07 e | |
| Basilico Rosso | 0 | 0.9 ± 0.4 B | 3.5 ± 0.6 A,B | 2.2 ± 0.1 A | 7 ± 1 A |
| 0.55 ± 0.04 k | 2.48 ± 0.04 j | 2.03 ± 0.04 d | 5.06 ± 0.05 h | ||
| 200 | 0.52 ± 0.02 k | 3.71 ± 0.03 f | 2.09 ± 0.03 c | 6.31 ± 0.02 f | |
| 400 | 1.08 ± 0.01 i | 3.96 ± 0.02 d | 2.17 ± 0.02 b | 7.21 ± 0.05 b | |
| 600 | 1.40 ± 0.05 h | 3.98 ± 0.01 d | 2.41 ± 0.01 a | 7.79 ± 0.04 a | |
| Mitikas | 0 | 1.8 ± 0.2 A | 4 ± 2 A | 0.11 ± 0.03 D | 6 ± 2 A,B |
| 1.52 ± 0.04 g | 0.95 ± 0.06 k | 0.096 ± 0.004 m | 2.57 ± 0.09 k | ||
| 200 | 1.74 ± 0.05 e | 4.48 ± 0.05 c | 0.092 ± 0.003 m | 6.32 ± 0.01 f | |
| 400 | 1.85 ± 0.06 d | 4.82 ± 0.03 b | 0.109 ± 0.001 m | 6.78 ± 0.09 d | |
| 600 | 2.01 ± 0.06 c | 4.99 ± 0.01 a | 0.160 ± 0.004 l | 7.16 ± 0.05 b | |
| Cultivar | ppm | Tocopherols | |||
| α-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | ||
| Dark Opal | 0 | 4 ± 1 A,* | 0.8 ± 0.3 A | 0.8 ± 0.3 A | 5 ± 2 A |
| 3.60 ± 0.03 d | 0.44 ± 0.03 f | 0.325 ± 0.008 h | 4.37 ± 0.05 d | ||
| 200 | 6.07 ± 0.03 b | 1.32 ± 0.01 a | 1.18 ± 0.03 a | 8.58 ± 0.01 b | |
| 400 | 2.71 ± 0.01 g | 0.94 ± 0.02 c | 0.97 ± 0.02 c | 4.63 ± 0.01 c | |
| 600 | 2.41 ± 0.02 h | 0.60 ± 0.03 e | 0.76 ± 0.03 e | 3.77 ± 0.03 f | |
| Red Basil | 0 | 4 ± 2 A,B | 0.6 ± 0.3 B | 0.5 ± 0.4 B | 5 ± 3 A |
| 3.14 ± 0.02 e | 0.447 ± 0.001 f | 0.31 ± 0.02 h | 3.90 ± 0.01 e | ||
| 200 | 7.09 ± 0.02 a | 1.13 ± 0.01 b | 1.12 ± 0.01 b | 9.34 ± 0.02 a | |
| 400 | 3.80 ± 0.03 c | 0.41 ± 0.01 g | 0.40 ± 0.02 f | 4.61 ± 0.01 c | |
| 600 | 1.71 ± 0.01 j | 0.340 ± 0.002 h | 0.259 ± 0.003 i | 2.31 ± 0.01 j | |
| Basilico Rosso | 0 | 0.8 ± 0.3 D | 0.5 ± 0.1 B,C | 0.5 ± 0.3 B | 1.8 ± 0.6 C |
| 0.55 ± 0.02 o | 0.44 ± 0.01 f | 0.313 ± 0.004 h | 1.30 ± 0.01 n | ||
| 200 | 1.257 ± 0.001 l | 0.49 ± 0.02 e | 0.91 ± 0.04 d | 2.66 ± 0.01 i | |
| 400 | 0.87 ± 0.02 m | 0.67 ± 0.03 d | 0.370 ± 0.002 g | 1.92 ± 0.02 l | |
| 600 | 0.66 ± 0.02 n | 0.35 ± 0.04 h | 0.32 ± 0.02 h | 1.33 ± 0.08 n | |
| Mitikas | 0 | 1.9 ± 0.7 C | 0.36 ± 0.07 D | 0.27 ± 0.06 C | 2.6 ± 0.8 B |
| 2.36 ± 0.04 i | 0.445 ± 0.003 f | 0.315 ± 0.002 h | 3.13 ± 0.05 h | ||
| 200 | 2.76 ± 0.05 f | 0.396 ± 0.004 g | 0.325 ± 0.001 h | 3.48 ± 0.04 g | |
| 400 | 1.55 ± 0.01 k | 0.321 ± 0.005 i | 0.255 ± 0.004 i | 2.12 ± 0.01 k | |
| 600 | 1.13 ± 0.01 m | 0.278 ± 0.004 j | 0.171 ± 0.002 j | 1.58 ± 0.01 m | |
| Cultivar | ppm | Fatty Acids | Fatty Acid Groups | |||||
|---|---|---|---|---|---|---|---|---|
| C16:0 | C18:2n6c | C18:3n3 | SFA | MUFA | PUFA | n6/n3 | ||
| Dark Opal | 0 | 20.8 ± 0.8 A,B,* | 14.4 ± 0.7 A | 46 ± 3 B | 31 ± 2 A | 7.8 ± 0.4 B,C | 61 ± 2 B | 0.34 ± 0.01 A |
| 20.9 ± 0.2 d | 14.6 ± 0.4 c | 44.6 ± 0.3 i | 32.1 ± 0.1 c | 8.2 ± 0.1 g | 59.7 ± 0.2 h | 0.45 ± 0.01 a | ||
| 200 | 21.8 ± 0.2 b | 15.2 ± 0.2 b | 41.8 ± 0.1 j | 34.1 ± 0.2 b | 8.2 ± 0.2 g | 57.8 ± 0.4 i | 0.36 ± 0.01 b | |
| 400 | 20.5 ± 0.7 e | 13.4 ± 0.4 i | 48.9 ± 0.2 d,e | 29.7 ± 0.6 d | 7.5 ± 0.1 h,i | 62.8 ± 0.6 e | 0.274 ± 0.006 j | |
| 600 | 19.87 ± 0.07 f | 14.4 ± 0.4 c,d,e | 49.08 ± 0.04 c,d | 28.8 ± 0.4 e,f | 7.3 ± 0.1 j | 64.0 ± 0.5 c | 0.292 ± 0.008 g,h | |
| Red Basil | 0 | 18.5 ± 0.6 C | 14.4 ± 0.4 A | 49.5 ± 0.6 A | 27.9 ± 0.8 C | 7.7 ± 0.5 B,C | 64.4 ± 0.7 A | 0.29 ± 0.01 B |
| 18.89 ± 0.05 h | 14.17 ± 0.07 e,f,g | 48.6 ± 0.4 e | 28.5 ± 0.2 f | 8.12 ± 0.1 g | 63.3 ± 0.4 d | 0.29 ± 0.01 h | ||
| 200 | 19.4 ± 0.1 g | 14.06 ± 0.07 f,g | 50.03 ± 0.06 b | 28.5 ± 0.1 f | 7.0 ± 0.1 l | 64.5 ± 0.1 b | 0.281 ± 0.002 h,i | |
| 400 | 18.06 ± 0.01 i | 14.29 ± 0.06 d,e,f | 50.08 ± 0.07 b | 27.69 ± 0.02 g | 7.43 ± 0.04 i | 64.9 ± 0.1 a,b | 0.285 ± 0.002 h | |
| 600 | 17.78 ± 0.08 j | 15.13 ± 0.01 b | 49.3 ± 0.1 c | 26.7 ± 0.1 j | 8.31 ± 0.02 f | 65.0 ± 0.1 a | 0.307 ± 0.001 e | |
| Basilico Rosso | 0 | 20 ± 1 B | 14 ± 1 A | 47 ± 2 B | 29 ± 1 A,B | 8.3 ± 0.5 B | 62 ± 2 B | 0.30 ± 0.01 B |
| 21.5 ± 0.5 b,c | 15.5 ± 0.2 a | 45.4 ± 0.4 h | 30.0 ± 0.5 d | 8.8 ± 0.1 d | 61.2 ± 0.6 g | 0.341 ± 0.002 c | ||
| 200 | 18.43 ± 0.01 i,j | 13.3 ± 0.1 i,n | 51.0 ± 0.2 a | 27.2 ± 0.1 h | 7.6 ± 0.1 h | 65.2 ± 0.1 a | 0.261 ± 0.002 k | |
| 400 | 20.5 ± 0.8 e | 15.2 ± 0.1 b | 45.5 ± 0.7 h | 29.8 ± 0.8 d | 8.5 ± 0.1 e | 61.7 ± 0.8 f | 0.33 ± 0.01 d | |
| 600 | 20.3 ± 0.6 e | 13.17 ± 0.05 i | 47.3 ± 0.5 f | 29.9 ± 0.6 d | 8.4 ± 0.1 f | 61.8 ± 0.6 f | 0.278 ± 0.002 j | |
| Mitikas | 0 | 22 ± 3 A | 13.9 ± 0.4 A,B | 43 ± 7 C | 30 ± 5 A | 12 ± 2 A | 57 ± 7 C | 0.33 ± 0.01 A |
| 27.36 ± 0.01 a | 13.4 ± 0.3 i | 31.3 ± 0.1 k | 38.5 ± 0.1 a | 16.2 ± 0.3 a | 45.3 ± 0.3 j | 0.43 ± 0.01 a | ||
| 200 | 19.12 ± 0.06 g,h | 14.04 ± 0.57 g | 48.5 ± 0.5 e | 26.2 ± 0.1 i | 10.8 ± 0.1 c | 63.0 ± 0.1 d,e | 0.29 ± 0.01 h | |
| 400 | 19.8 ± 0.2 f | 14.45 ± 0.04 c,d | 46.5 ± 0.1 g | 27.3 ± 0.2 g,h | 11.3 ± 0.1 b | 61.4 ± 0.1 f,g | 0.311 ± 0.002 e | |
| 600 | 21.2 ± 0.6 c,d | 13.7 ± 0.2 h | 45.7 ± 0.6 h | 29.1 ± 0.6 e | 10.8 ± 0.2 c | 60.1 ± 0.8 h | 0.300 ± 0.001 f | |
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 8.91 | 323 | 179 | 135(100) | Caffeic acid |
| 2 | 14.96 | 323 | 473 | 313(61), 293(100) | Chicoric acid |
| 3 | 16.8 | 334 | 609 | 301(100) | Quercetin-O-deoxyhexoside-hexoside |
| 4 | 19.5 | 290/325 | 535 | 491(100), 287(34) | Eriodictyol-O-malonylhexoside |
| 5 | 20.76 | 282/327 | 719 | 359(100), 197(31), 179(42), 161(50), 135(5) | Sagerinic acid |
| 6 | 35.36 | 287/333 | 313 | 269(51), 203(12), 179(5), 161(100), 135(5) | Salvianolic acid F |
| Cultivar | ppm | Peak | TPA | TF | TPC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| Dark Opal | 0 | 1.6 ± 0.6 A,* | 2 ± 1 A | 1 ± 2 A,B | 6 ± 2 A | 11 ± 8 A | 2.9 ± 0.5 A | 18 ± 10 A | 7 ± 4 A | 25 ± 13 A |
| 2.55 ± 0.07 b | 2.84 ± 0.01 c | 3.58 ± 0.05 a | 8.0 ± 0.4 c | 24.6 ± 0.4 a | 3.49 ± 0.06 c | 33.5 ± 0.2 b | 11.6 ± 0.5 b | 45.0 ± 0.3 b | ||
| 200 | 1.51 ± 0.06 e | 3.98 ± 0.06 a | 0.275 ± 0.006 i | 8.1 ± 0.1 c | 10.56 ± 0.03 d | 3.17 ± 0.02 d | 19.2 ± 0.1 d | 8.4 ± 0.1 c | 27.6 ± 0.2 d | |
| 400 | 1.59 ± 0.01 d | 0.761 ± 0.003 h | tr | 3.86 ± 0.06 f | 5.08 ± 0.09 f | 2.33 ± 0.01 g | 9.76 ± 0.08 f | 3.86 ± 0.06 g | 13.62 ± 0.02 g | |
| 600 | 0.901 ± 0.007 j | 1.73 ± 0.04 e | tr | 3.1 ± 0.2 g | 4.61 ± 0.03 h,i | 2.43 ± 0.03 f | 9.67 ± 0.05 f | 3.1 ± 0.2 h | 12.8 ± 0.1 h | |
| Red Basil | 0 | 1.7 ± 0.8 A | 2 ± 1 A | 1 ± 1 A | 7 ± 4 A | 11 ± 8 | 3 ± 2 A | 18 ± 12 A | 8 ± 5 A | 25 ± 17 A |
| 2.68 ± 0.04 a | 3.06 ± 0.02 b | 3.55 ± 0.01 b | 11.5 ± 0.4 a | 23.2 ± 0.4 b | 4.93 ± 0.04 b | 33.9 ± 0.4 a | 15.1 ± 0.4 a | 48.9 ± 0.8 a | ||
| 200 | 2.23 ± 0.02 c | 2.12 ± 0.02 d | 0.226 ± 0.001 j | 8.4 ± 0.3 b | 14.9 ± 0.4 c | 4.93 ± 0.03 b | 24.2 ± 0.4 c | 8.6 ± 0.3 c | 32.8 ± 0.7 c | |
| 400 | 1.15 ± 0.01 h | 1.00 ± 0.01 g | 0.34 ± 0.01 g | 4.43 ± 0.02 e | 4.43 ± 0.02 g | 1.227 ± 0.005 l | 7.807 ± 0.004 h | 4.77 ± 0.03 f | 12.58 ± 0.03 h | |
| 600 | 0.61 ± 0.02 n | 0.51 ± 0.01 j | 0.074 ± 0.004 k | 1.74 ± 0.09 i | 2.782 ± 0.03 l | 1.12 ± 0.03 m | 5.0 ± 0.1 j | 1.82 ± 0.09 j | 6.8 ± 0.1 l | |
| Basilico Rosso | 0 | 0.9 ± 0.2 B | 0.9 ± 0.2 B | 1 ± 1 A | 4 ± 1 B | 3.8 ± 0.7 B | 1.6 ± 0.4 B | 7 ± 1 B | 5 ± 2 B | 12 ± 3 B |
| 1.20 ± 0.02 g | 1.00 ± 0.01 g | 2.89 ± 0.01 c | 4.42 ± 0.07 e | 4.42 ± 0072 h | 1.46 ± 0.04 k | 8.4 ± 0.2 g | 7.3 ± 0.1 d | 15.7 ± 0.2 f | ||
| 200 | 1.08 ± 0.01 i | 1.03 ± 0.02 f | 0.41 ± 0.01 f | 5.1 ± 0.2 d | 3.98 ± 0.05 j | 2.12 ± 0.02 h | 8.20 ± 0.01 g | 5.5 ± 0.2 e | 13.7 ± 0.2 g | |
| 400 | 0.726 ± 0.003 m | 1.01 ± 0.01 f,g | 0.624 ± 0.005 d | 3.2 ± 0.2 g | 3.7 ± 0.1 k | 1.88 ± 0.04 i | 7.3 ± 0.1 h | 3.8 ± 0.2 g | 11.1 ± 0.3 i | |
| 600 | 0.80 ± 0.03 l | 0.59 ± 0.02 i | 0.298 ± 0.004 h | 2.32 ± 0.01 h | 2.7 ± 0.1 l | 1.05 ± 0.01 n | 5.17 ± 0.03 j | 2.61 ± 0.01 i | 7.8 ± 0.1 k | |
| Mitikas | 0 | 1.0 ± 0.4 B | 0.8 ± 0.6 B | - | 1 ± 1 C | 3 ± 3 B | 3 ± 2 A | 8 ± 5 B | 2 ± 1 C | 9 ± 6 C |
| 1.34 ± 0.03 f | 1.72 ± 0.02 e | 0.52 ± 0.01 e | 3.22 ± 0.03 g | 7.6 ± 0.5 e | 4.97 ± 0.02 a | 15.6 ± 0.5 e | 3.74 ± 0.04 g | 19.4 ± 0.5 e | ||
| 200 | 1.31 ± 0.01 f | 0.45 ± 0.01 k | tr | 1.49 ± 0.01 j | 2.21 ± 0.03 m | 3.02 ± 0.04 e | 6.99 ± 0.01 i | 1.49 ± 0.01 k | 8.48 ± 0.01 j | |
| 400 | 0.84 ± 0.02 k | 0.51 ± 0.01 j | tr | 1.08 ± 0.01 k | 1.77 ± 0.06 n | 1.50 ± 0.02 j | 4.6 ± 0.1 k | 1.08 ± 0.01 l | 5.7 ± 0.1 m | |
| 600 | 0.33 ± 0.02 o | 0.41 ± 0.01 l | tr | 0.065 ± 0.006 l | 0.98 ± 0.01 o | 1.25 ± 0.02 l | 3.0 ± 0.1 l | 0.065 ± 0.006 m | 3.0 ± 0.1 n | |
| Cultivar | ppm | TBARS (EC50, µg/mL) | OxHLIA (IC50 Values, µg/mL) | |
|---|---|---|---|---|
| Δt = 60 min | Δt = 120 min | |||
| Dark Opal | 0 | 32 ± 16 D,* | 106 ± 56 B | 198 ± 89 B |
| 34 ± 3 d | 79 ± 3 g | 145 ± 4 f | ||
| 200 | 13.1 ± 0.3 g | 30.8 ± 0.9 l | 82 ± 1 i | |
| 400 | 25.7 ± 0.5 f | 144 ± 2 d | 270 ± 3 d | |
| 600 | 55.4 ± 0.6 b | 171 ± 4 c | 293 ± 4 c | |
| Red Basil | 0 | 50 ± 15 B | 68 ± 26 D | 170 ± 84 C |
| 55.7 ± 0.2 b | 60 ± 2 h | 109 ± 2 h | ||
| 200 | 25.2 ± 0.2 f | 40.0 ± 0.9 j,k | 79 ± 3 i | |
| 400 | 60 ± 1 a | 64 ± 3 h | 202 ± 4 e | |
| 600 | 61 ± 2 a | 109 ± 6 f | 289 ± 6 c | |
| Basilico Rosso | 0 | 43 ± 12 C | 77 ± 44 C | 151 ± 76 D |
| 32 ± 1 d | 38 ± 1 k,l | 65 ± 2 j | ||
| 200 | 31.0 ± 0.2 e | 46 ± 3 i,j | 136 ± 3 f,g | |
| 400 | 54.9 ± 0.7 b | 147 ± 4 d | 271 ± 7 d | |
| 600 | 55.1 ± 0.8 b | 79 ± 3 g | 130 ± 5 g | |
| Mitikas | 0 | 57 ± 4 A | 183 ± 106 A | 269 ± 254 A |
| 54.3 ± 0.8 b | 51 ± 2 i | 105 ± 2 h | ||
| 200 | 52.0 ± 0.9 c | 119 ± 7 e | 314 ± 15 b | |
| 400 | 59.7 ± 0.5 a | 250 ± 11 b | na | |
| 600 | 61.2 ± 0.2 a | 313 ± 13 a | 655 ± 19 a | |
| Trolox | 5.4 ± 0.3 | 19.6 ± 0.7 | 41 ± 1 | |
| Cultivar | Nitrogen Level (ppm) | MIC/MBC | S. aureus | B. cereus | L. monocytogenes | E. coli | S. Typhimurium | E. cloacae |
|---|---|---|---|---|---|---|---|---|
| Dark Opal | 0 | MIC | 4 | 1 | 2 | 2 | 2 | 4 |
| MBC | 8 | 2 | 4 | 4 | 4 | 8 | ||
| 200 | MIC | 2 | 1 | 1 | 2 | 2 | 2 | |
| MBC | 4 | 2 | 2 | 4 | 4 | 4 | ||
| 400 | MIC | 1 | 1 | 2 | 1 | 2 | 1 | |
| MBC | 2 | 2 | 4 | 2 | 4 | 2 | ||
| 600 | MIC | 2 | 1 | 2 | 2 | 2 | 2 | |
| MBC | 4 | 2 | 4 | 4 | 4 | 4 | ||
| Red Basil | 0 | MIC | 2 | 1 | 2 | 1 | 1 | 2 |
| MBC | 4 | 2 | 4 | 2 | 2 | 4 | ||
| 200 | MIC | 1 | 1 | 1 | 1 | 2 | 1 | |
| MBC | 2 | 2 | 2 | 2 | 4 | 2 | ||
| 400 | MIC | 2 | 1 | 2 | 2 | 1 | 2 | |
| MBC | 4 | 2 | 4 | 4 | 2 | 4 | ||
| 600 | MIC | 2 | 0.5 | 2 | 1 | 1 | 2 | |
| MBC | 4 | 1 | 4 | 2 | 2 | 4 | ||
| Basilico Rosso | 0 | MIC | 1 | 1 | 1 | 2 | 2 | 1 |
| MBC | 2 | 2 | 2 | 4 | 4 | 2 | ||
| 200 | MIC | 1 | 0.5 | 1 | 2 | 1 | 1 | |
| MBC | 2 | 1 | 2 | 4 | 2 | 2 | ||
| 400 | MIC | 2 | 1 | 2 | 2 | 2 | 2 | |
| MBC | 4 | 2 | 2 | 4 | 4 | 4 | ||
| 600 | MIC | 1 | 1 | 2 | 2 | 2 | 1 | |
| MBC | 2 | 2 | 4 | 4 | 4 | 2 | ||
| Mitikas | 0 | MIC | 2 | 1 | 2 | 2 | 2 | 2 |
| MBC | 4 | 2 | 4 | 4 | 4 | 4 | ||
| 200 | MIC | 2 | 1 | 2 | 2 | 1 | 2 | |
| MBC | 4 | 2 | 4 | 4 | 2 | 4 | ||
| 400 | MIC | 2 | 1 | 2 | 2 | 1 | 2 | |
| MBC | 4 | 2 | 4 | 4 | 2 | 4 | ||
| 600 | MIC | 2 | 1 | 2 | 1 | 2 | 2 | |
| MBC | 4 | 2 | 4 | 2 | 4 | 4 | ||
| Positive controls | E211 | MIC | 4.0 | 0.5 | 1.0 | 1.0 | 1.0 | 2.0 |
| MBC | 4.0 | 0.5 | 2.0 | 2.0 | 2.0 | 4.0 | ||
| E224 | MIC | 1.0 | 2.0 | 0.5 | 0.5 | 1.0 | 0.5 | |
| MBC | 1.0 | 4.0 | 1.0 | 1.0 | 1.0 | 0.5 |
| Cultivar | Nitrogen Level (ppm) | MIC/MFC | A. fumigatus | A. niger | A. versicolor | P. funiculosum | P. v. var. cyclopium | T. viride |
|---|---|---|---|---|---|---|---|---|
| Dark Opal | 0 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 200 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 400 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 600 | MIC | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 0.5 | 1 | 1 | 1 | 1 | 0.5 | ||
| Red Basil | 0 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 200 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 400 | MIC | 0.25 | 0.5 | 0.25 | 0.5 | 1 | 0.25 | |
| MFC | 0.5 | 1 | 0.5 | 1 | 2 | 0.5 | ||
| 600 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| Basilico Rosso | 0 | MIC | 0.25 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 |
| MFC | 0.5 | 1 | 1 | 1 | 1 | 0.5 | ||
| 200 | MIC | 0.5 | 0.5 | 0.5 | 1 | 1 | 0.5 | |
| MFC | 1 | 1 | 1 | 2 | 2 | 1 | ||
| 400 | MIC | 0.25 | 0.5 | 0.25 | 0.5 | 1 | 0.25 | |
| MFC | 0.5 | 1 | 0.5 | 1 | 2 | 0.5 | ||
| 600 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| Mitikas | 0 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.125 |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.25 | ||
| 200 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 400 | MIC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | |
| MFC | 1 | 1 | 1 | 1 | 1 | 0.5 | ||
| 600 | MIC | 0.25 | 0.5 | 0.5 | 0.5 | 1 | 0.25 | |
| MFC | 0.5 | 1 | 1 | 1 | 2 | 0.5 | ||
| Positive controls | E211 | MIC | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 |
| MFC | 2.0 | 2.0 | 2.0 | 2.0 | 4.0 | 2.0 | ||
| E224 | MIC | 1.0 | 1.0 | 1.0 | 0.5 | 1.0 | 0.5 | |
| MFC | 1.0 | 1.0 | 1.0 | 0.5 | 1.0 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, L.R.O.; Fernandes, Â.; Di Gioia, F.; Petropoulos, S.A.; Polyzos, N.; Dias, M.I.; Pinela, J.; Kostić, M.; Soković, M.D.; Ferreira, I.C.F.R.; et al. The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars. Antioxidants 2020, 9, 1036. https://doi.org/10.3390/antiox9111036
Cruz LRO, Fernandes Â, Di Gioia F, Petropoulos SA, Polyzos N, Dias MI, Pinela J, Kostić M, Soković MD, Ferreira ICFR, et al. The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars. Antioxidants. 2020; 9(11):1036. https://doi.org/10.3390/antiox9111036
Chicago/Turabian StyleCruz, Luís R. O., Ângela Fernandes, Francesco Di Gioia, Spyridon A. Petropoulos, Nikolaos Polyzos, Maria Inês Dias, José Pinela, Marina Kostić, Marina D. Soković, Isabel C. F. R. Ferreira, and et al. 2020. "The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars" Antioxidants 9, no. 11: 1036. https://doi.org/10.3390/antiox9111036
APA StyleCruz, L. R. O., Fernandes, Â., Di Gioia, F., Petropoulos, S. A., Polyzos, N., Dias, M. I., Pinela, J., Kostić, M., Soković, M. D., Ferreira, I. C. F. R., & Barros, L. (2020). The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars. Antioxidants, 9(11), 1036. https://doi.org/10.3390/antiox9111036












