3.1. Morphological and Pigments Analyses
The sampled TF tomatoes showed a shape similar to SM fruits, but were smaller in length and circumference (about two-fold lower) and in weight (about five folds lower), thus supporting their nature of “Mini-San Marzano tomato” (
Table 3). Despite a smaller size, peel amount in pink and red TF fruits was two- and ten-fold higher than those of SM at the same ripening stages, respectively (
Table S1). Furthermore, at least a doubled peel amount was found in the red TF tomatoes compared to the pink ones, whereas an opposite trend was observed in SM fruits (
Table S1).
Ripeness also increased the pulp amount in both varieties. The TF pulp content per gram of fruit was at least four-fold higher with respect to SM tomatoes (
Table S1). In spite of a lower pulp amount, both pink and red SM (SM
P, SM
R) tomatoes contained high amounts of juice (pH 4.0–4.2). Conversely, the juice content was at least two to six-fold lower in pink and red TF (TF
P, TF
R) tomatoes (
Table S1). Altogether, these features support the claimed crunchy and solid consistency of TF tomatoes, likely ascribable to a high peel and pulp content, despite a significant low amount of juice.
A significant difference in the seed number and weight per gram of fruit was observed, which was about four-fold higher in TF compared to SM tomatoes at both ripening stages (
Table S1). Conversely, the size and weight of each seed were similar in both varieties, with a slight increase in the SM
R fruits (
Table S1). It is widely accepted that seed size and number are strictly linked to the plant reproductive potential. Small seeds were reported to possess lower reproductive capacity, due to a lower endosperm amount, which limits seedling survivorship and competitive ability [
42]. On the other hand, a high seed number improves the competitive ability of the plant, due to the increased probability of seedling survivorship. In this context, TF tomatoes seem to possess a higher reproductive competition related to SM, along with similar plant reproductive capacity and survival.
The color of SM tomatoes varied from pale green in the pink fruits to light red in the red ones (
Table 3 and
Figure S1). The analysis of pigments i.e., chlorophyll a, chlorophyll b, and total carotenoids, carried out on the TF and SM organic extracts from peel and pulp justified these features. The dark-green colour of TF
P tomatoes can be ascribed to the higher content in chlorophylls a and b in peel (+70% and +52% higher amount, respectively) (
p < 0.01) and in pulp (+10% and +82% higher amount, respectively) (
p < 0.01) with respect to TF
R fruits (
Table 4). On the contrary, carotenoids content (bright red color) was found about five-fold higher in TF
R peel and pulp than in pink ones (
p < 0.001) (
Figure S1).
The difference in chlorophyll a content between pink and red fruits was particularly marked in SM cultivar, where chlorophyll a amount was 90% higher in peel and pulp of SM
P compared to SM
R fruits (
p < 0.01). SM
P tomatoes displayed both chlorophyll a and b content to be double in peel with respect to pulp, while in SM
R fruits, this difference was not evident (
Table 4). The opposite trend was observed with regard to carotenoids content. SM
R peel showed a carotenoid amount 28 times higher with respect to SM
P peel (
p < 0.001), while in SM
R pulp, a 40 times higher level of total carotenoids with respect to SM
P pulp (
p < 0.001) was found.
TF fruits were characterized by higher levels of chlorophylls compared to SM ones at both ripening stages (
p < 0.01). In particular, chlorophyll a and b content was found to be 60% and 80% higher in TF
P peel and pulp fruits than in SM
P peel and pulp, respectively (
p < 0.01). A similar trend was observed in terms of total carotenoids content, which was 68% higher in TF
P fruits with respect to SM
P (
p < 0.01). These findings agree with the different shade of green color observed in the TF
P (dark green) compared to SM
P fruits (pale green) (
Figure S1). In addition, red fruits of both cultivars were characterized by different pigments proportion. In TF
R peel, chlorophyll levels were 90% higher compared to SM
R peel (+90%,
p < 0.001), the latter showed 40% higher content of carotenoids than TF
R peel (
p < 0.001;
Table 4).
Carotenoids are mainly responsible for the red color of tomatoes and are involved in the fruit protection against excessive sun irradiation and harmful UV rays. Total carotenoids amount in fruits depends on the ripening stage and other factors, such as cultivar, climate, sun exposure, agronomic practices, irrigation. The ratio of chlorophyll a and b to total carotenoids (a + b/total carotenoids) can be considered as an indicator of tomato ripening stage. During chromoplast development in fruit maturation, the ratio a + b/total carotenoids tends to decrease continuously, thus reaching a value below 1.0 [
43]. Our findings confirmed the differences in ripening stages selected for the study, revealing a ratio value of 3.16 in TF
P tomatoes and 0.18 in TF
R ones. In SM fruits, this trend was even wider, in fact, pink fruits showed a ratio value of 3.9, whereas in red fruits, this ratio was 0.02 (far below of 1).
3.2. Metabolite Profiling
FT-ICR MS and NMR untargeted analyses were carried out for a thorough metabolite profile characterization of TF and SM in relation to their pink and red states. The high mass accuracy typically achieved with FT-MS implies that elemental formulas can be determined, pertaining to a large number of metabolites, based on their accurate mass, whereas the NMR capacity of structural determination allows the unambiguous compound identification and quantification.
The ESI FT-ICR MS analysis of both Bligh–Dyer hydroalcoholic and organic fractions of TF
P, TF
R, SM
P, and SM
R fruits has allowed to detect both polar and non-polar metabolites. Each sample was analyzed in both positive and negative ionization mode (
Figures S2–S5), detecting up to 1138 molecular formulas; however, a larger number of compounds were detected in positive ionization mode (
Table 5). Overall, the TF cultivar was characterized by a smaller number of compounds with respect to SM and the ripeness process promotes a general increase in the number of putatively identified metabolites (
Table 5). An overview of all the recorded plausible compounds is available in
Tables S2 and S3.
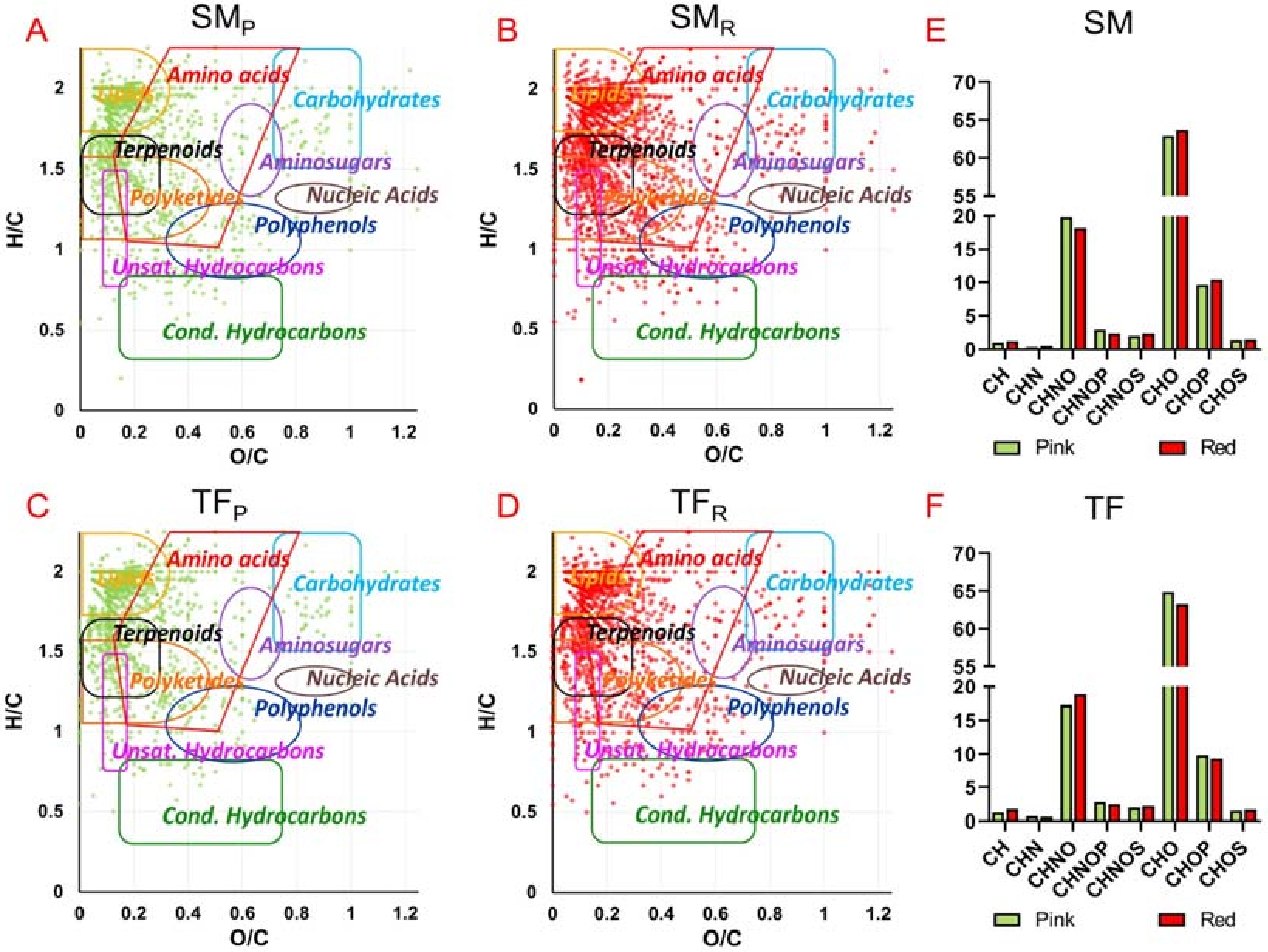
Specific data analysis allows to organize the vast amount and complexity of detected formulas to uncover interesting information. Among the detected molecular formulas, the relative frequency distribution was investigated (
Figure 1E,F) showing that all tomato extracts contain a majority of CHO species followed by CHON, CHOP and, in smaller amount, CHNOP and CHNOS. In particular, CHO components correspond mainly to polyphenols (more hits in SM
R extracts), steroids (more hits in TF), and fatty acids (more entries in TF), followed by di- and tri-glycerides (more entries in SM
P), terpenoids, organic acids, and arachidonic derivatives (
Supplementary Figure S6A). When considering CHON components, they can be ascribed mainly to amino fatty acids, amino-sugars, amines (more hits in red extracts), N-acylamines (more hits in TF
P), followed by amino acids (more entries in pink extracts), solanidines, nucleosides (more hits in SM
P), and vitamins (more hits in TF
R) as shown in
Figure S6B.
Van Krevelen diagrams were used to classify the detected molecular formulas in different classes of natural compounds such as lipids, terpenoids, carbohydrates, amino acids, aminosugars, nucleic acids, polyphenols, polyketides, unsaturated hydrocarbons and condensed hydrocarbons (
Figure 1A–D). TF and SM tomato extracts showed marked similarities, covering several classes of metabolite families. A relatively higher compound density is present in the area of lipids, terpenoids, and polyketides, followed by components in the areas of amino acids, unsaturated hydrocarbons, polyphenols and (relatively less) in the regions of carbohydrates, aminosugars, nucleic acids, and condensed hydrocarbons (
Figure 1A–D).
Moreover, Venn diagrams (
Figure S7) pointed out possible similarities and differences in the metabolic profile of the sampled TF and SM fruits. The combined pattern of hydroalcoholic and organic fractions of pink and red extracts of the two cultivars showed that overall only 19% of the molecular formulas were found to be common, thus suggesting a noticeable extent of chemical diversity, whereas more than 40% of molecular formulas were shared between pink and red samples of each variety.
This wide metabolomics survey supported the untargeted and targeted analyses driving the identification of selected classes of metabolites. In particular, 1D NMR spectra assignment of the TF and SM hydroalcoholic extracts solubilized in D
2O phosphate buffer and organic extracts solubilized in CDCl
3/CD
3OH (
Table 2) were obtained by means of literature data [
28,
44,
45,
46]. Furthermore, targeted analytical approaches provided the identification and quantification of total polyphenols, tannins, and flavonoids content and BAs.
Results will be presented and discussed according to compound classes.
3.2.1. Amino Acids and Derivatives
NMR spectra of both red and pink TF and SM hydroalcoholic extracts showed signals of sixteen amino acids, namely leucine, valine, isoleucine, threonine, alanine, GABA, glutamic acid, glutamine, aspartic acid, asparagine, lysine, arginine, tyrosine, phenylalanine, tryptophan, and histidine, as also confirmed by ESI FT-ICR MS. All of them were quantifiable, except arginine. In addition, ESI FT-ICR MS revealed the presence of proline, serine, the non-essential amino acid citrulline, and other amino-acids-related metabolites, like hydroxyproline and phosphoserine. Some peptides were also found. In particular, glutathione was detected in all hydroalcoholic extracts, S-nitrosoglutathione in red fruits, glutathione disulfide in pink fruits.
Alanyl-alanine (in hydroalcoholic SMP), glycyl-proline (in organic SMR), glycyl-leucine (in organic TFR), glutamyl-valine and glutamyl-glutamine (in hydroalcoholic SMR) were also revealed.
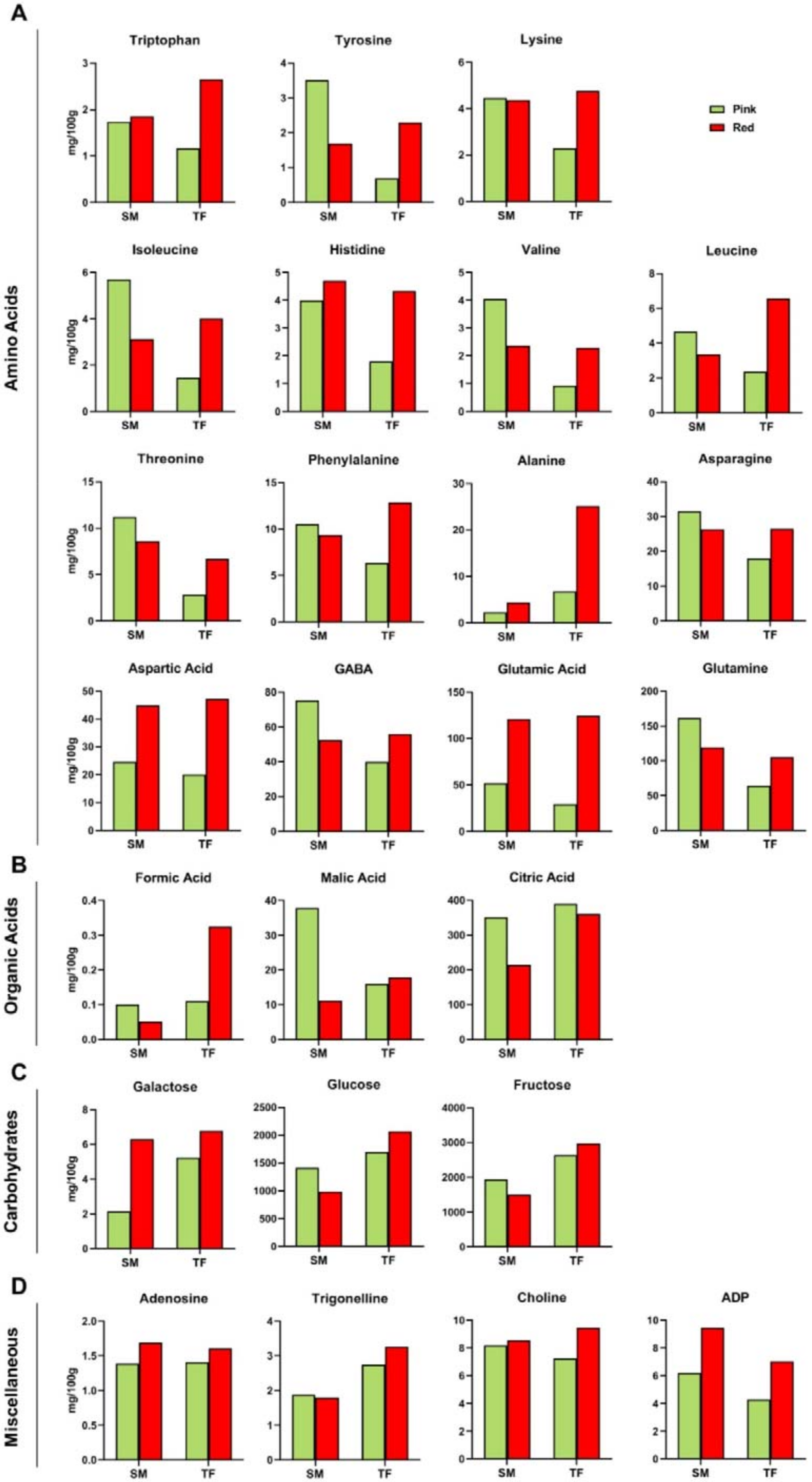
According to NMR scrutiny, TF and SM samples showed some/several similarities (
Figure 2A): glutamine was found to be the most abundant amino acid in both cultivars at the pink stage, followed by GABA and glutamic acid, whereas, at the red stage, glutamic acid increased, becoming the most abundant amino acid. The pattern of SM developmental changes in free amino acid content was in agreement with literature data, being glutamic acid characterized by a remarkable increase in all ripe fruits [
2,
3,
4,
45,
47,
48,
49,
50,
51,
52]. Glutamic acid, aspartate, tryptophan, and alanine rose upon the fruit ripening; asparagine and phenylalanine turned out to be constant; tyrosine, isoleucine, valine, threonine, GABA, and glutamine content decreased from pink to red SM fruits. These findings reflect data reported in literature about the analyses of SM [
2,
4] and other cultivars [
3,
45,
47,
48,
49,
50,
51,
52].
Interestingly, the TF fruit ripening showed a peculiar trend in the amino acid profile, being characterized by an increase in the content of all the amino acids from the pink to red stage. In particular, except for asparagine, GABA, and glutamine, the content of the remaining twelve amino acids rose more than two-fold.
3.2.2. Organic Acids
Ascorbic, citric, chlorogenic, malic and formic acids were detected by NMR analysis. Chlorogenic acid (5-caffeoylquinic acid) was identified only in hydroalcoholic TF
P extracts. The ESI FT-ICR mass spectra in negative mode provided additional peaks corresponding to deprotonated organic acids identified in one or a few samples, like succinic and glutaric (in organic SM
R and hydroalcoholic TF
P extracts), maleic (in hydroalcoholic TF
P extracts), quinic and shikimic (in hydroalcoholic TF
R and TF
P extracts), and lactic (absent only in red hydroalcoholic samples) acids, as the most prominent signals. In addition, sugar esters of caffeic and ferulic acids, caffeoyl- and feruloyl-hexose, were revealed, with both metabolites being present in hydroalcoholic extracts. Although the present method does not allow to recognize which constitutional isomer is formed, glycosylated forms of phenolic acids have been previously identified in methanol extracts of tomato fruit by a HPLC/DAD/MS approach [
53].
Histograms reporting NMR data (
Figure 2B) showed that formic acid was always present in a minor amount, whereas citric acid represented the main organic acid, contributing to sourness [
5] and confirming literature data [
2,
3,
4,
45,
47,
48]. During the developmental process, both malic and citric acids contents stayed constant in TF fruits and decreased in SM tomatoes. Previous results concerning the organic acid trends are contradictory: Mounet et al. [
48] and Jezequel et al. [
54] described an increasing citric acid content from the pink to red stage, whereas Perez et al. [
45] found out a decrease in citric and malic acids content. These differences might be ascribed to the combination of genetic, pedoclimatic, seasonal, and agronomic factors.
In terms of cultivar, the three organic acids were comparably abundant at the pink stage, except for malic acid which was significantly higher in SM than in TF fruits. At the red stage, formic, malic and citric acids contents were found nearly doubled in TF compared to SM fruits.
3.2.3. Sugars
Fructose, glucose and galactose were the monosaccharides identified in the
1H NMR spectrum of hydroalcoholic extracts. Besides, confirming the widespread incidence of mono and disaccharides, ESI FT-ICR results reported an Amadori compound, fructosyl lysine, in all hydroalcoholic extracts, providing a sensitive marker of early modifications in food nutrients previously described also in unprocessed tomato extracts [
55].
Fructose was the most abundant sugar in both cultivars at both ripeness stages, followed by glucose; however, higher levels of both carbohydrates were found in TF compared to SM fruits (
Figure 2C). Fructose, glucose, and galactose levels increased in TF cultivar over the ripening period, whereas the opposite trend was observed in SM fruits. Loiudice et al. [
2] reported glucose and fructose contents of eleven SM tomato cultivars at three harvesting years: fructose was always found the most abundant sugar in all samples characterized by a mean content of 1.4g/100 g, whereas glucose mean content was 1.2 g/100 g. These data are consistent with the values reported in
Figure 2C: fructose was the main sugar in SM with a mean value of 1.7 g/100 g and glucose content was 1.2 g/100 g, with a total amount of 2.9 g/100 g. However, sugars content in hybrid cultivars tended to increase [
2], confirming the higher amount of both fructose and glucose in TF, which reached 5 g/100 g as mean value.
3.2.4. Other Compounds
NMR signals of choline, trigonelline, uridine, adenosine, and ADP were identified and, except for uridine, quantified (
Figure 2D). A rich variety of miscellaneous compounds were detected by ESI FT-ICR analysis, comprising small amines (serotonin), nucleosides (adenosine, methylthioadenosine, orotidine), and nucleotides (guanosine-, cytidine-, and uridine-monophosphate), sugar alcohols (sorbitol, mannitol), sugar acids (galactonic and glucuronic acids), aminosugars (glucosamine, lactosamine), terpenes (
p-cymene, caryophyllene, limonene), terpenoid (apiole, oxo-campholide), vitamins and derivatives (ascorbic acid, retinol, α-tocopherol). As expected, both cultivars contained several key secondary metabolites characteristic of tomato fruits, including alkaloids (trigonelline, narciclasine, and catharanthine, only in hydroalcoholic extracts, nicotine and sauroxine, spread in all samples), polyketides such as lycoflexine, mostly in pink fruits, and glycoalkaloids as tomatine and tomatidine, only in pink hydroalcoholic extracts [
56,
57]. Several phytohormones, recognized as key signaling molecules, were observed in most samples, like derivatives of abscisic acid, only in hydroalcoholic extracts, jasmonic acid, only in red hydroalcoholic fractions, and salicylic acid, mostly in TF
P and SM
R, and several gibberellins, mainly detected in red organic samples [
58].
The concentration of choline and adenosine were comparable in SM and TF fruits, slightly increasing at the red stage, whereas trigonelline content was found to be lower in SM with respect to TF at both developmental stages. ADP content severely increased in both cultivars at the red stage, almost doubling its level.
The presence of hydroxyl-substituted fatty acids, metabolites with strong anti-inflammatory and antioxidative effects, confirms the nutraceutical potential of tomato. Hydroxy-stearic acid was observed in all samples except for organic SM
R, hydroxy-linoleic acid, in all extract excluding hydroalcoholic SM
P, and hydroxylinolenic acid, in organic SM
R and all TF extracts [
59].
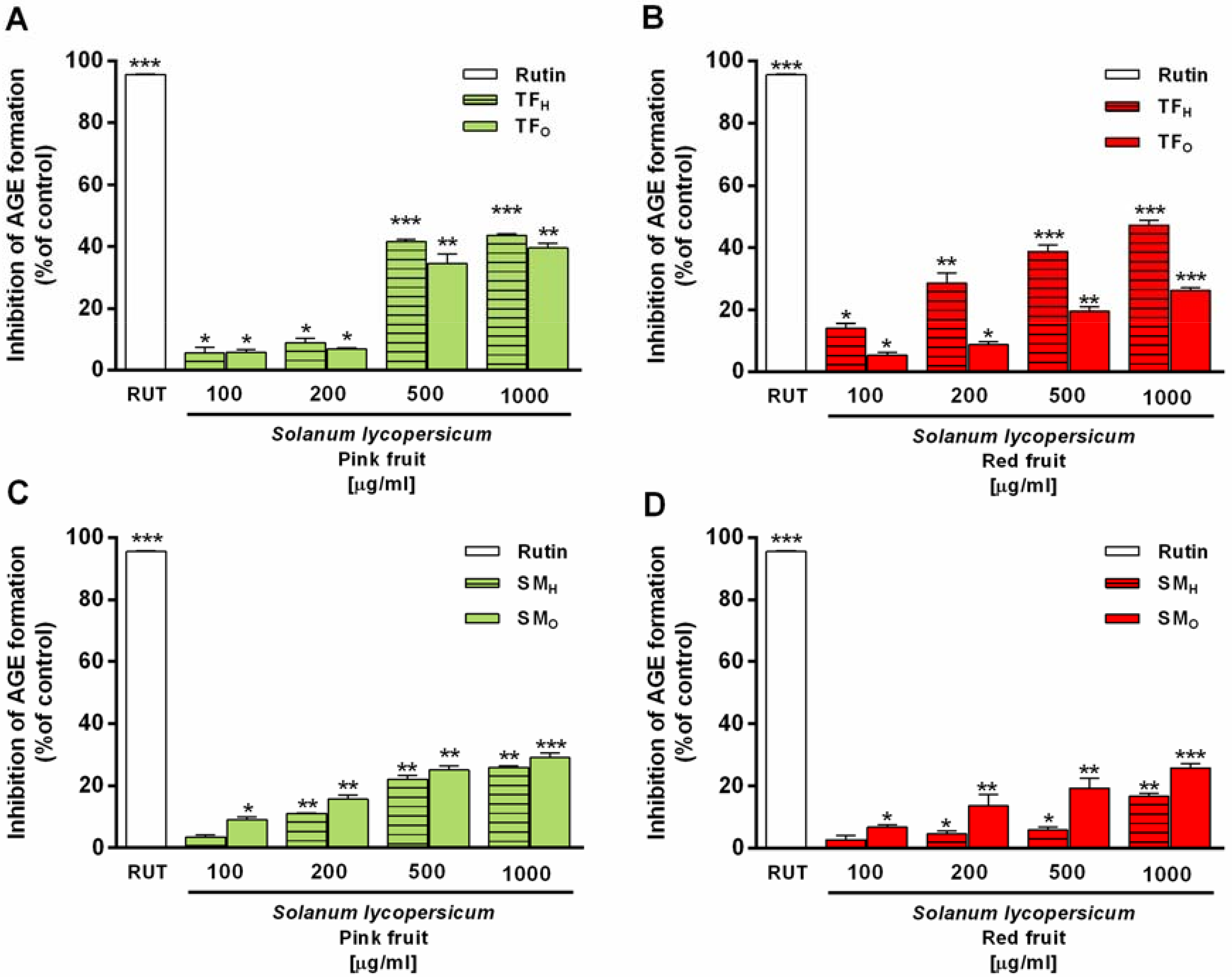
3.2.5. Phenolic Compounds (Polyphenols, Tannins, and Flavonoids)
Spectrophotometric targeted analyses provided the total content of phenolic compounds expressed as polyphenols, tannins, and flavonoids. Highest levels of total polyphenols were found in hydroalcoholic extracts from pink fruits of both cultivars, being TF
P the most enriched sample (triple content compared SM
P); conversely, their levels were reduced of about 14- and 6-fold with ripeness (
Table 6). Similarly, a 1.6-fold reduction in total polyphenols was found in TF
R organic extracts with respect to those from TF
P, whereas an opposite trend occurred in the SM variety (
Table 6).
TF
P and SM
P hydroalcoholic extracts contained similar levels of tannins, which slightly increased with ripening (
Table 6). Comparing the ripening stages, both SM
P and SM
R organic extracts contained an analogue content of tannins, whereas a marked 8-fold reduction in their levels occurred in TF
R organic extracts compared to TF
P (
Table 6).
Flavonoids were mainly concentrated in the hydroalcoholic extracts of both TF and SM tomatoes at both the ripening stages, although high levels were also found in the organic extracts of TF
P and SM
R fruits (
Table 6). TF
P hydroalcoholic extract resulted in the most enriched sample in flavonoids, being three times more concentrated than SM
P; similarly, the same trend was observed in pink organic fractions, being TF the cultivar characterized by an almost 30-fold higher flavonoid content compared to SM (
Table 6). At the red stage, TF fruits showed a flavonoid reduction of about 1.5- and 4-fold in organic and hydroalcoholic extracts respectively, whereas an opposite trend was registered for SM tomatoes. Indeed, a 2- and 66-fold flavonoid increase was found in the hydroalcoholic and organic SM
R with respect to SM
P (
Table 6). This evidence revealed that the highest levels of total polyphenols, tannins, and flavonoids were concentrated in the TF
P tomatoes, although a high flavonoid content was retained in TF
R and SM
R fruits. Our data agree with previous evidence that highlighted a total flavonoid content of 200 μg/g (calculated as quercetin equivalents) in SM
R tomatoes [
60]. Conversely, to the best of our knowledge, no comparison data are available in the literature regarding TF landrace.
In addition, ESI FT-ICR MS experiments have revealed flavanols (dihydroxy-methoxy-isoflavanol), flavan-3-ols (epigallocatechin sulfate), flavonoids (apigeniflavan, tetrahydroxyflavanone glucoside), and polyphenol derivatives like catechin-O-glucoside, catechin-O-rutinoside, dihydrokaempferol, trihydroxy-prenyldihydrochalcone glucosyl-coumarate, quercetin glucoside-glucuronide [
61].
3.2.6. Sterols
β-Sitosterol and stigmasterol were detected and quantified by NMR analysis in the organic extracts of both cultivars at pink and red developmental stages.
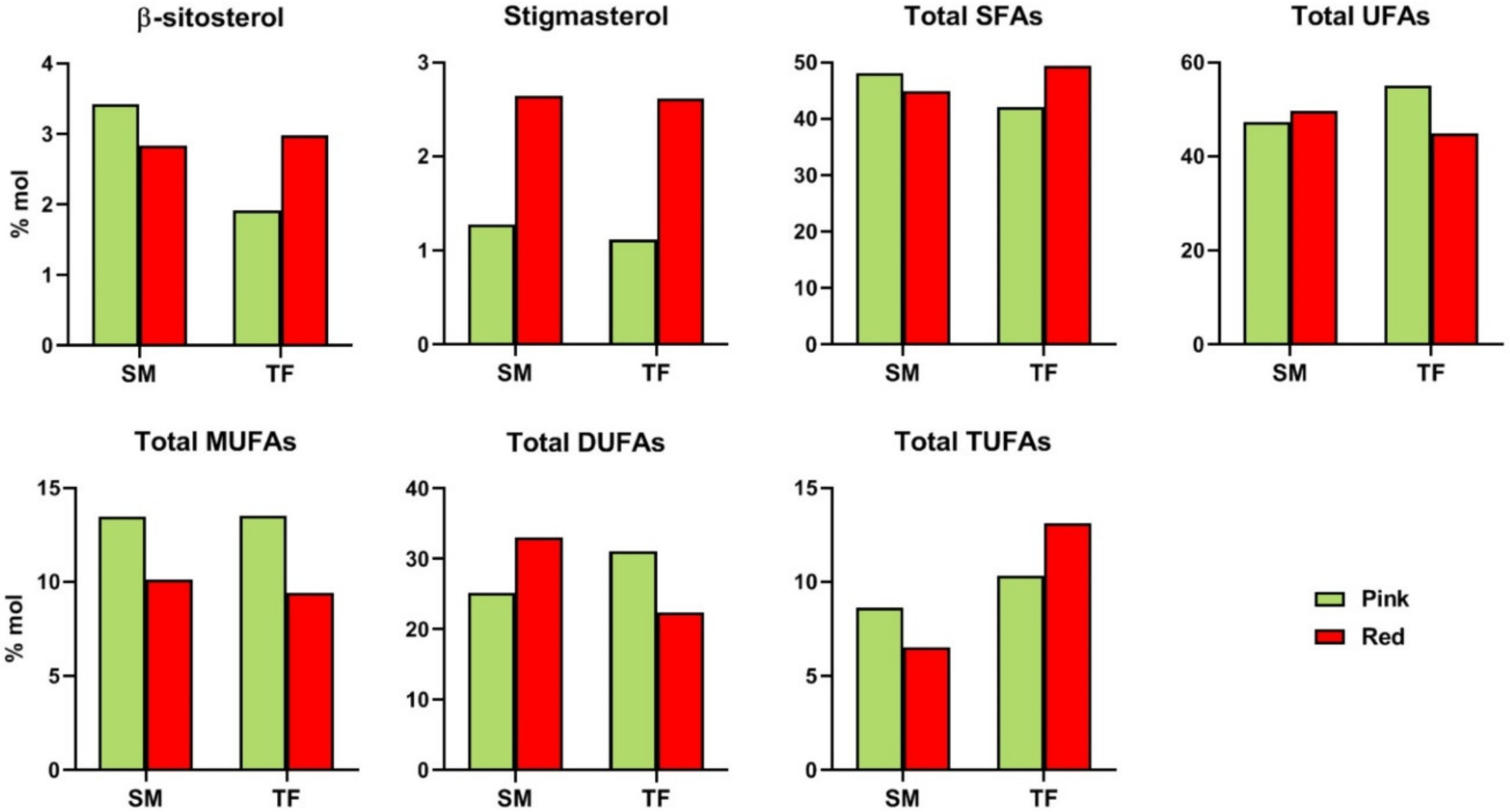
β-Sitosterol content showed a decreasing trend in SM
R fruits, opposite to TF fruits. Conversely, stigmasterol content significantly increased (3-fold higher) over the ripening stage in both cultivars (
Figure 3). According to ESI FT-ICR results, cholesterol- and hydroxycholesterol-sulfate were found in all samples, whereas methylstigmasterol was detected in the hydroalcoholic TF
R sample.
3.2.7. Fatty Acid Chains
NMR analysis allowed the identification and quantification of the total saturated (SFA) and unsaturated (UFA) fatty acid chains, the latter ones including mono- (MUFA), di- (DUFA) and tri-unsaturated (TUFA) fatty acid chains. The amount of the selected metabolites was comparable in SM and TF cultivars at both ripening stages, except for the case of TUFA, more abundant in TF fruits (
Figure 3). Regarding the ripening stages, total UFA content was found to slightly increase in SM fruits and decrease in TF cultivar, conversely total SFA showed the opposite trend.
A drop from pink to red fruits was noticed in MUFA (both SM and TF), DUFA (TF), and TUFA (SM) content, whereas an opposite trend/increase with the ripening stage emerged DUFA (SM) and TUFA (TF) amount.
Despite untargeted investigation, direct infusion ESI FT-ICR MS analysis delivers consistent solution composition and maximum metabolome coverage, ion-suppression effects and differences of signal response are a concern in view of an accurate quantitation. However, careful tuning of experimental conditions has recently allowed a successful quantification of numerous isomeric groups of intact wax esters, where relative ionization efficiency was found to be influenced only by lipid class and saturation degree, while independent on carbon chain length [
62]. On this basis, the abundances of molecular formulas classified as free fatty acids (FA) and presenting the expected (CH
2)
2 increments were obtained from the lists of organic extracts in the negative ionization mode, relatively richer in lipids (
Table S4). Then, these values were summed up within each specific class, namely saturated SFA, containing the series 12:0-20:0, MUFA, with the series 14:1–20:1, DUFA and TUFA, including compounds 18:2, 20:2, 18:3, 20:3, 18:4, 20:4, respectively, to evaluate their relative abundance. Notably, four FAs at
m/z 227, 255, 277, and 279 have been assigned to myristic (14:0), palmitic (16:0), linolenic (18:3), and linoleic (18:2) acids, respectively, on the basis of their characteristic fragmentation in CID experiments. As shown in
Figure 4, an overall similar composition is highlighted in both organic SM
R and TF
R extracts, with the highest percentages found for: (i) 16:0 (ca. 60%) and 18:0 (ca. 28%) among SFAs; (ii) 16:1 (ca. 58%) and 18:1 (ca. 39%) among MUFAs; (iii) 18:2 (ca. 77%) and 20:2 (ca. 17%) among DUFAs; (iv) 18:3 (ca. 37%) and 20:3 (ca. 64%), among TUFAs.
The presence and chemical diversity of long chain fatty acids pointed out in both cultivars is intriguing, since these compounds are known to have a wide range of biological properties, including the promotion of type 2 immune responses [
63].
Noteworthy, SMR organic fraction contains both the lowest percentage of relatively shorter chain di- and tri-unsaturated FA, including 18:2 (ca. 62% vs. the above reported value of 77%) and 18:3 (ca. 21% vs. 37% above reported), and the highest amount of longer chain FA, including 20:2 (ca. 33% vs. 17%) and 20:3 (ca. 78% vs. 64%).
The availability of appropriate unsaturated fatty acids is reported as a significant factor responsible for specific fruit flavor and aroma development, due to the action of lipase enzymes that may release a rich milieu of metabolites from acyl lipids during the ripeness processes.
3.2.8. Biogenic Amines
High performance liquid chromatography allowed to detect seven BAs (
Table 7), namely putrescine (PUT), cadaverine (CAD), histidine (HIS), serotonin (SER), spermidine (SPD), and spermine (SPM). All BAs investigated are found in tomato samples, except for tyramine (TYM), an amine with negative health effect, which was not detected in any samples. By contrast, HIS, another BA with negative health effect, was detected in all samples. Histamine presence is regulated in some food, but none in tomatoes. The tolerance presence of histamine in wine and fish is up to 100 μg/g [
64]. The maximum HIS level was found in TFR at 1.463 ± 0.015 μg/g, far less than the regulation limits (Commission Regulation (EU) No 1019/2013 of 23 October 2013 amending Annex I to Regulation (EC) No 2073/2005 as regards to histamine in fishery products.) For these reasons, these tomato cultivars appear to be safe for human health. The rest of BAs are usually related to cultivar and storage condition of samples. Indeed, a great variability of BAs concentration was found between SM and TF. In all the tomatoes, SER, an important neurotransmitter, was also detected in high concentration. This BA has positive effects on human health; SER plays an important role in regulating mood, sleep, body temperature, sexuality, and appetite. SER is involved in many neuropsychiatric disorders such as migraine, bipolar disorder; serotonin deficiency causes obsessive-compulsive disorder, repetition, and mania. So, its assumption by diet is highly recommended [
65]. About the different level of the BAs in pink with respect to red tomatoes, is possible to highlight that in almost all samples, the highest concentration was found in red fruits (
Table 7). This is in accordance with previously studies which demonstrated the accumulation of BAs during ripening of meat [
66] or dairy products [
67]. Finally, based on the obtained data, BAs could be also used as ripening markers of tomatoes.