Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of the Grape Extracts
2.3. Preparation of Kefir
2.4. Electrospray Ionization (ESI) Mass Spectrometry Analysis
2.5. NMR Analysis
2.6. Quantification of Total Phenolic Equivalent (Tpe) by Folin–Ciocalteu Procedure
2.7. Determination of Total Antioxidant Capacity (Tac)
2.8. Determination of Scavenging Activity on DPPH Radicals
2.9. Determination of Scavenging Effect on the ABTS Radical Cation
2.10. Cell Culture
2.11. Measurement of Trans-Epithelial Electric Resistance
2.12. Paracellular Permeability Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Grape Pomace
3.2. Evaluation of Antioxidant Activity of Extracts
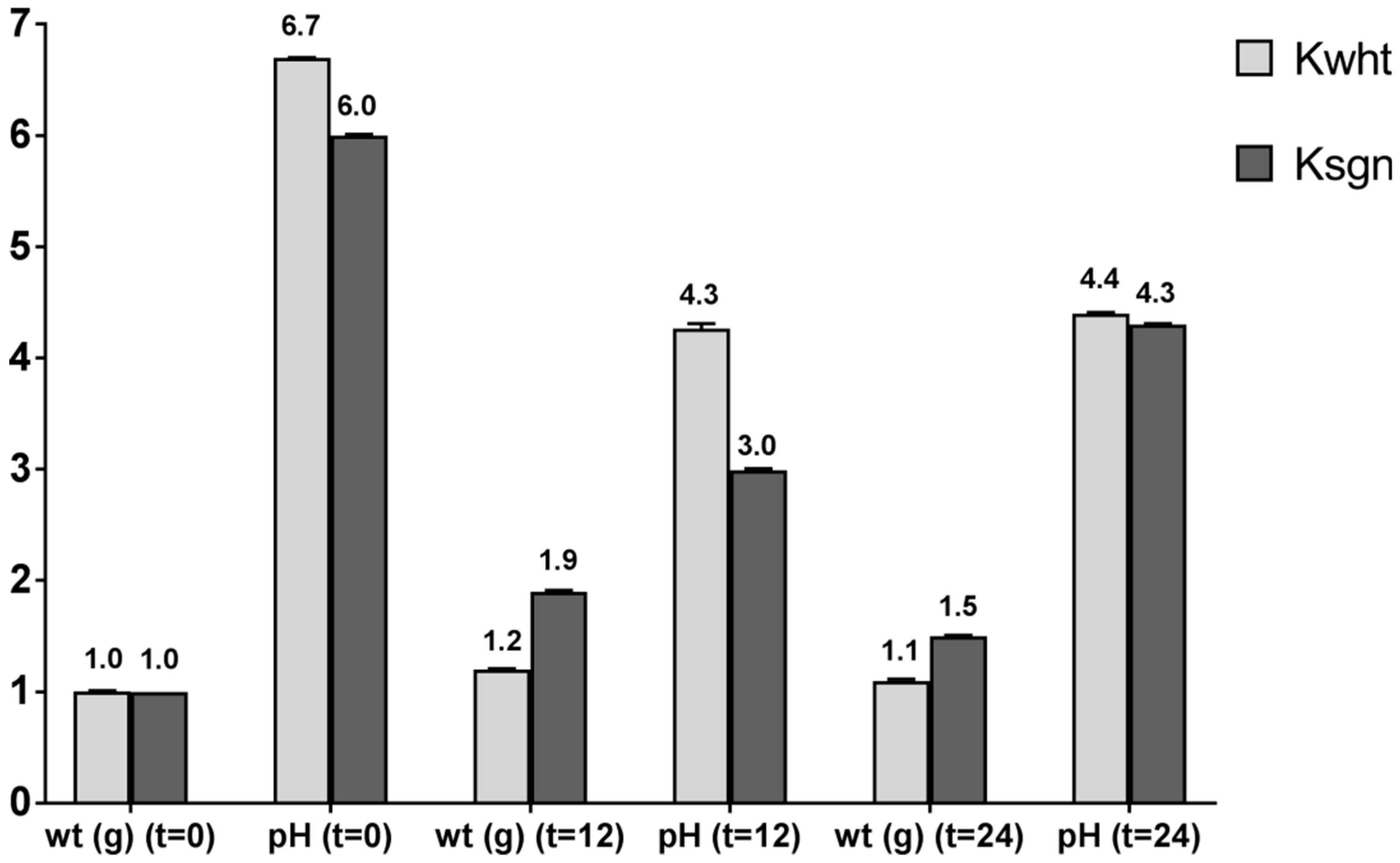
3.3. Kefir Preparation and NMR Analysis
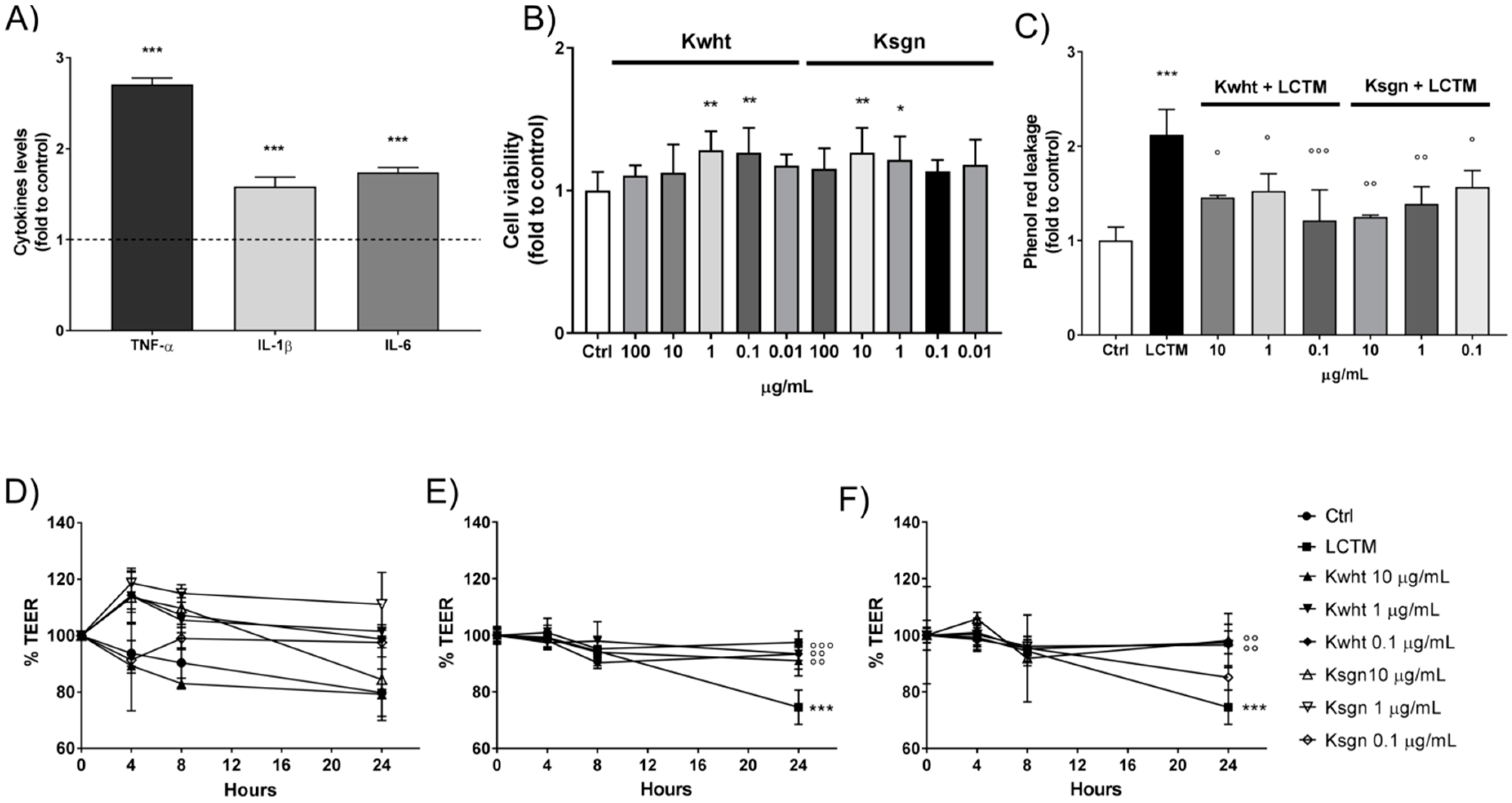
3.4. Antioxidant Activity of Kefir and Fortified Kefir
3.5. Kwht and Ksgn Protect Intestinal Barrier from Inflammatory-Impaired Permeability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvo Romero, E.; Alonso Cotoner, C.; Pardo Camacho, C.; Casado Bedmar, M.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Frattaruolo, L.; Carullo, G.; Armentano, B.; Badolato, M.; Loizzo, M.R.; Aiello, F.; Cappello, A.R. An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/anti-inflammatory agents. Nat. Prod. Res. 2019, 33, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: Identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Carullo, G.; Biagi, M.; Rago, V.; Aiello, F. Evaluation of the in vitro wound-healing activity of Calabrian honeys. Antioxidants 2019, 8, 36. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P.; et al. Polyphenols and intestinal permeability: Rationale and future perspectives. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Thumann, T.A.; Pfersch-Wenzig, E.M.; Moissl-Eichinger, C.; Bauer, R. The role of gut microbiota for the activity of medicinal plants traditionally used in the European Union for the gastrointestinal disorders. J. Ethnopharmacol. 2019, 245, 112153. [Google Scholar] [CrossRef]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Peter Pauls, K.; Deng, Z.; Tsao, R. Antioxidant and anti-inflammatory polyphenols and peptides of common bean (Phaseolus vulga L.) milk and yogurt in Caco-2 and HT-29 cell models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A.; Recio, M.C. Effect of a milk-based fruit beverage enriched with plant sterols and/or galactooligosaccharides in a murine chronic colitis model. Foods 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Slattery, C.; Cotter, P.D.; O’Toole, P.W. Analysis of health benefits conferred by Lactobacillus species from kefir. Nutrients 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Nijmeijer, R.M.; Sandström, P.A.; Trulsson, L.M.; Magnusson, K.; Timmerman, H.M.; van Minnen, L.P.; Rijkers, G.T.; Gooszen, H.G.; Akkermans, L.M.A.; et al. Probiotics Prevent Intestinal Barrier Dysfunction in Acute Pancreatitis in Rats via Induction of Ileal Mucosal Glutathione Biosynthesis. PLoS ONE 2009, 4, e4512. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Torres-Fuentes, C.; Heeney, D.D.; Marco, M.L. Synergy between Probiotic Lactobacillus casei and Milk to Maintain Barrier Integrity of Intestinal Epithelial Cells. J. Agric. Food Chem. 2019, 67, 1955–1962. [Google Scholar] [CrossRef]
- Gao, X.; Li, B. Chemical and microbiological characteristics of kefir grains and their fermented dairy products: A review. Cogent Food Agric. 2016, 2, 1272152. [Google Scholar] [CrossRef]
- Bourrie, B.C.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandòn, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wang, Y.; Ahmad, A.; Khan, S.T.; Nisa, M.; Ahmad, H.; Afreen, A. Kefir and health: A contemporary perspective. Crit. Rev. Food Sci. Nutr. 2013, 53, 422–434. [Google Scholar] [CrossRef]
- Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Milk fermented with Lactobacillus fermentum ameliorates indomethacin-induced intestinal inflammation: An exploratory study. Nutrients 2019, 11, 1610. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Jeong, D.; Kang, I.B.; Chon, J.W.; Kim, H.S.; Song, K.Y.; Seo, K.H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, D.H.; Jeong, D.; Seo, K.H.; Jeong, H.S.; Lee, H.G.; Kim, H. Characterization of yeasts isolated from kefir as a probiotic and its synergic interaction with the wine byproduct grape seed flour/extract. LWT Food Sci. Technol. 2018, 90, 535–539. [Google Scholar] [CrossRef]
- Carullo, G.; Durante, M.; Sciubba, F.; Restuccia, D.; Spizzirri, U.G.; Ahmed, A.; Di Cocco, M.; Saponara, S.; Aiello, F.; Fusi, F. Vasoactivity of Mantonico and Pecorello grape pomaces on rat aorta rings: An insight into nutraceutical development. J. Funct. Foods 2019, 57, 328–334. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Gambacorta, G.; La Notte, E. Phenolic content and antioxidant activity of Primitivo Wine: Comparison among winemaking technologies. J. Food Sci. 2009, 74, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Tech. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, A.; Curone, G.; Solè, M.; Capuani, G.; Sciubba, F.; Conta, G.; Miccheli, A.; Vigo, D. NMR-based metabolomics to evaluate the milk composition from Friesian and autochthonous cows of Northern Italy at different lactation times. Nat. Prod. Res. 2019, 33, 1085–1091. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Valli, E.; Farfalla, A.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Chitosan-Quercetin bioconjugate as multi-functional component of antioxidants and dual-responsive hydrogel networks. Macromol. Mater. Eng. 2019, 304, 1800728. [Google Scholar] [CrossRef]
- Governa, P.; Biagi, M. Copaifera langsdorffii Desf.: In vitro investigation on anti-Helicobacter pylori and anti-inflammatory activities of oleoresin and fruit methanolic extract. Plant Biosyst. 2019. [Google Scholar] [CrossRef]
- Cirillo, G.; Puoci, F.; Iemma, F.; Curcio, M.; Parisi, O.I.; Spizzirri, U.G.; Altimari, I.; Picci, N. Starch-quercetin conjugate by radical grafting: Synthesis and biological characterization. Pharm. Dev. Technol. 2012, 17, 466–476. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Chiocchio, I.; Poli, F.; Governa, P.; Biagi, M.; Lianza, M. Wound healing and in vitro antiradical activity of five Sedum species grown within two sites of community importance in Emilia-Romagna (Italy). Plant Biosyst. 2019, 153, 610–615. [Google Scholar] [CrossRef]
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Catanzaro, D.; Cocetta, V.; Igl, N.; Ragazzi, E.; Giron, M.C.; Cecconello, L.; Montopoli, M. Protective effects of psi taraxasterol 3-O-myristate and arnidiol 3-O-myristate isolated from Calendula officinalis on epithelial intestinal barrier. Fitoterapia 2016, 109, 230–235. [Google Scholar] [CrossRef]
- Cocetta, V.; Catanzaro, D.; Borgonetti, V.; Ragazzi, E.; Giron, M.C.; Governa, P.; Carnevali, I.; Montopoli, M.; Biagi, M. A Fixed Combination of Probiotics and Herbal Extracts Attenuates Intestinal Barrier Dysfunction from Inflammatory Stress in an In vitro Model Using Caco-2 Cells. Recent Pat. Food Nutr. Agric. 2019, 10, 62–69. [Google Scholar] [CrossRef]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef]
- Governa, P.; Marchi, M.; Cocetta, V.; De Leo, B.; Saunders, P.T.K.; Catanzaro, D.; Miraldi, E.; Montopoli, M.; Biagi, M. Effects of Boswellia serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharmaceuticals 2018, 11, 126. [Google Scholar] [CrossRef]
- Tongtong, L.; Chang, L.; Uss, A.; Chu, I.; Morrison, R.A.; Wang, L.; Prelusky, D.; Cheng, K.; Li, C. The impact of protein on Caco-2 permeability of low mass balance compounds for absorption projection and efflux substrate identification. J. Pharm. Biomed. Anal. 2010, 51, 1069–1077. [Google Scholar]
- Vidau, C.; Brunet, J.; Badiou, A.; Belzunces, L.P. Phenylpyrazole insecticides induce cytotoxicity by altering mechanisms involved in cellular energy supply in the human epithelial cell model Caco-2. Toxicol. In Vitro 2009, 23, 589–597. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111. [Google Scholar] [CrossRef]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 cell culture practices. Toxicol. Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Reddy Kolli, A.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Smetanová, L.; Štětinová, V.; Svoboda, Z.; Květina, J. Caco-2 cells, biopharmaceutics classification system (BCS) and biowaiver. Acta Med. 2011, 54, 3–8. [Google Scholar]
- Gujral, N.; Won Suh, J.; Sunwoo, H.H. Effect of anti-gliadin IgY antibody on epithelial intestinal integrity and inflammatory response induced by gliadin. BMC Immunol. 2015, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Wood, R.J.; James, C.; Wood, R.J. Specific 1,25(OH)2D3-mediated regulation oftranscellular calcium transport in Caco-2 cells. Am. J. Physiol. 1999, 276, G958–G964. [Google Scholar] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Perez-Magarino, S.; Ortega-Heras, M.; Cano-Mozo, E.; Gonzalez-San Jose, M.L. The influence of oak wood chips, micro-oxygenation treatment, and grape variety on colour, and anthocyanin and phenolic composition of red wines. J. Food Compos. Anal. 2009, 22, 204–211. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Potential of grape wastes as a natural source of bioactive compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Novak, I.; Janeiro, P.; Seruga, M.; Oliveira-Brett, A.M. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Anal. Chim. Acta 2008, 630, 107–115. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Jeon, Y.J. Squalene isolated from marine macroalgae Caulerpa racemosa and its potent antioxidant and anti-inflammatory activities. Food Biochem. 2018, 42, e12628. [Google Scholar] [CrossRef]
- Jaiswal, S.G.; Patel, M.; Saxena, D.K.; Naik, S. Comparison of Measurements of Antioxidant Activity in the Selected Leafy Vegetables Depending on Extraction Solvent. J. Hortic. Res. 2017, 25, 75–80. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Tabasco, R.; Sánchez-Patán, F.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T. Effect of grape polyphenols on lactic acid bacteria and bifidobacteria growth: Resistance and metabolism. Food Microbiol. 2011, 28, 1345–1352. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef]
- Güzel-Seydim, Z.B.; Seydim, A.C.; Greene, A.K.; Bodine, A.B. Determination of organic acids and volatile flavor substances in kefir during fermentation. J. Food Compos. Anal. 2013, 13, 35–43. [Google Scholar] [CrossRef]
- Delgado-Fernandez, P.; Corzo, N.; Lizasoain, S.; Olano, A.; Moreno, F.J. Fermentative properties of starter culture during manufacture of kefir with new prebiotics derived from lactulose. Int. Dairy J. 2019, 93, 22–29. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Leite, D.C.A.; Del Aguila, E.M.; Alvares, T.S.; Peixoto, R.S.; Miguel, M.A.L.; Silva, J.T.; Paschoalin, V.M.F. Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes. J. Dairy Sci. 2013, 96, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Yusupova, A.I.; Dedyukhina, E.G.; Chistyakova, T.I.; Kozyreva, T.M.; Morgunov, I.G. Succinic acid synthesis by ethanol-grown yeasts. Food Technol. Biotechnol. 2009, 47, 144–152. [Google Scholar]
- Yuksekdag, Z.N.; Beyath, Y.; Aslim, B. Metabolic activities of Lactobacillus spp. strains isolated from kefir. Nahrung. 2004, 48, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Cagindi, O. Kefir: A probiotic dairy composition, nutritional and therapeutic aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Guzel-Seydim, Z.B.; Seydim, A.C.; Greene, A.K. Comparison of amino acid profiles of milk, yoghurt and Turkish Kefir. Milchwiss 2003, 58, 158–160. [Google Scholar]
- Liut Kevicius, A.; Sarkinas, A. Studies on the growth conditions and composition of kefir grains as a food and forage biomass. Dairy Sci. Abstr. 2003, 66, 903. [Google Scholar]
- Reguant, C.; Bordons, A.; Arola, L.; Rozès, N. Influence of phenolic compounds on the physiology of Oenococcus oeni from wine. J. Appl. Microbiol. 2000, 88, 1065–1071. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Coda, R.; Larena, A.; Trani, A.; Gobbetti, M.; Cagno, R.D. Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Gambacorta, E. Phenolic content and antioxidant activity of donkey milk kefir fortified with sulla honey and rosemary essential oil during refrigerated storage. Int. J. Dairy Technol. 2012, 72, 74–81. [Google Scholar] [CrossRef]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelpathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Hong, Z.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Wang, F.; Schwarz, B.T.; Vallen Graham, W.; Wang, Y.; Su, L.; Clayburgh, D.R.; Abraham, C.; Turner, J.R. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology 2006, 131, 1153–1163. [Google Scholar] [CrossRef]
| Sample | TPE (meq GA/g Extract) | TAC (meq CT/g Extract) | IC50 (mg ml−1) | |
|---|---|---|---|---|
| DPPH Radical | ABTS Radical | |||
| sgnbs | 0.549 ± 0.019 | 0.859 ± 0.018 | 0.160 ± 0.005 | 0.085 ± 0.001 |
| sgnss | 1.620 ± 0.054 | 0.520 ± 0.014 | 0.012 ± 0.001 | 0.004 ± 0.001 |
| m/z | Formula [M + H]+ | RDB | Error (ppm) | MS2 | Tentative Identification |
|---|---|---|---|---|---|
| 130.0863 | C6H12NO2 | 1.5 | 0.1 | Pipecolic acid | |
| 130.0498 | C5H8N | 2.5 | −0.5 | Oxoproline | |
| 189.0758 | C8H13O5 | 2.5 | 0.1 | ||
| 280.2636 | C18H34NO | 2.5 | 0.4 | Too many possible compounds. Octadecadienamide or | |
| 284.2952 | C18H38NO | 0.5 | 1.5 | Stearamide | |
| 302.3054 | C18H40NO2 | −0.5 | 0.1 | MS2: 284.2948, 266.2819, 254.2834, 240.2679, 109.1007 C8H13+ 97.0993 C7H13+ 95.0841 C7H11+ 83.0859 C6H11+ 81.0689 C6H9+ 69.0683 C5H9+ 67.0538 C5H7+ 60.0435 C2H6ON+ | Dihydrosphingosine or isomer |
| 318.3006 | C18H40NO3 | −0.5 | 1.0 | Phytosphingosine | |
| 331.2340 | C15H31N4O4 | 2.5 | 0.1 | Too many possible compounds | |
| 439.1387 | C24H23O8 | 13.5 | −0.1 | MS2: 277.0891 (−162) | Flavonoid glycoside |
| 463.1240 | C22H23O11 | 11.5 | 1.1 | Peonidin glucoside | |
| 493.1335 | [C23H25O12]+ | 11.5 | −1–1 | MS2: 331, 315, 287,137 | Malvidin-3-O-glucoside |
| 639.1714 | C32H31O14 | 17.5 | 0.9 | Malvidin cumaroyl glucoside |
| m/z | Formula [M − H]− | RDB | Error (ppm) | MS2 | Tentative Identification |
|---|---|---|---|---|---|
| 133.0145 | C4H5O5 | 2.5 | 1.9 | Malic acid | |
| 149.0094 | C4H5O6 | 2.5 | 1.6 | Tartaric acid | |
| 163.0249 | C5H7O6 | 2.5 | 0.5 | 2-Dehydro-D-xylonate | |
| 191.0198 | C6H7O7 | 3.5 | 0.3 | Citric acid | |
| 191.0561 | C7H11O6 | 2.5 | −0.2 | MS2 191: 173 (−18); 147 (−44 CO2); 136 (−58); 111 (−80); 85 (−106) MS3 191->173: 155 (−18), 129 (−44), 111 (−62) | Quinic acid |
| 193.0352 | C6H9O7 | 2.5 | −0.9 | Glucuronic acid | |
| 193.0564 | C10H9O4 | 6.5 | 0.1 | Ferulic acid | |
| 205.0352 | C7H9O7 | 3.5 | −0.8 | Acid methyl citric/Acid homocitric | |
| 207.0509 | C7H11O7 | 2.5 | −0.4 | Methyl glucuronic acid | |
| 221.0666 | C8H13O7 | 2.5 | −0.3 | Ethyl glucuronic acid | |
| 223.0460 | C7H11O8 | 2.5 | 0.5 | MS2 223: 205 (−18); 191 (−32 CH3OH); 163 (−60); 131 (−92 (60 + 32)); 113 (131 − 18); 103 (131 − 28); 87 (−136: 131+44)) MS3: 223->131: 103 (−28); 87 (−44); 59(−(44 + 28)) MS3: 223->113: 85 (−28) | Hydroxymethyl monoglycosylpyranosonic acid |
| 311.0622 | C10H15O11 | 3.5 | 0.7 | Glycosyl tartrate | |
| 359.0757 | C12H20O10Cl | 2.5 | 3.1 | Anhydrodisaccharide |
| Molecule | Kwht | Ksgn |
|---|---|---|
| Amino Acids (mg L−1) | ||
| Valine | 28.12 | 28.82 |
| Isoleucine | 17.84 | 20.59 |
| Alanine | 33.50 | 4.28 |
| Tyrosine | 9.78 | 9.24 |
| Phenylalanine | 22.80 | 40.20 |
| Carbohydrates (g L−1) | ||
| Galactose | 4.22 | 10.72 |
| Glucose | 3.65 | 8.31 |
| Lactose | 0.0 | 2.26 |
| Organic Acids (mg L−1) | ||
| Lactic acid | 1480 | 2610 |
| 2-hydroxy-isobutyrric acid | 13.45 | 6.41 |
| Acetic acid | 7.21 | 10.09 |
| Pyruvic acid | 31.00 | 16.82 |
| Succinic acid | 21.72 | 2.83 |
| Citric acid | 278.96 | 1006 |
| Fumaric acid | 4.87 | 11.72 |
| Formic acid | 3.31 | 3.31 |
| Sample | TPE (meq GA/L) | TAC (meq CT/L) | IC50 (mg mL−1) |
|---|---|---|---|
| DPPH Radical | |||
| Kwht | 1.615 ± 0.005 | 0.558 ± 0.004 | 0.107 ± 0.001 |
| Ksgn | 1.975 ± 0.004 | 0.785 ± 0.003 | 0.090 ± 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, G.; Governa, P.; Spizzirri, U.G.; Biagi, M.; Sciubba, F.; Giorgi, G.; Loizzo, M.R.; Di Cocco, M.E.; Aiello, F.; Restuccia, D. Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells. Antioxidants 2020, 9, 54. https://doi.org/10.3390/antiox9010054
Carullo G, Governa P, Spizzirri UG, Biagi M, Sciubba F, Giorgi G, Loizzo MR, Di Cocco ME, Aiello F, Restuccia D. Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells. Antioxidants. 2020; 9(1):54. https://doi.org/10.3390/antiox9010054
Chicago/Turabian StyleCarullo, Gabriele, Paolo Governa, Umile Gianfranco Spizzirri, Marco Biagi, Fabio Sciubba, Gianluca Giorgi, Monica Rosa Loizzo, Maria Enrica Di Cocco, Francesca Aiello, and Donatella Restuccia. 2020. "Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells" Antioxidants 9, no. 1: 54. https://doi.org/10.3390/antiox9010054
APA StyleCarullo, G., Governa, P., Spizzirri, U. G., Biagi, M., Sciubba, F., Giorgi, G., Loizzo, M. R., Di Cocco, M. E., Aiello, F., & Restuccia, D. (2020). Sangiovese cv Pomace Seeds Extract-Fortified Kefir Exerts Anti-Inflammatory Activity in an In Vitro Model of Intestinal Epithelium Using Caco-2 Cells. Antioxidants, 9(1), 54. https://doi.org/10.3390/antiox9010054












