Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture and Preparation
2.3. Superoxide Anion Levels Determination Using Microchip Electrophoresis with Laser-Induced Fluorescence (ME-LIF) and MitoSOX Red Probe
2.4. HPLC Analysis of Metabolites
2.5. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
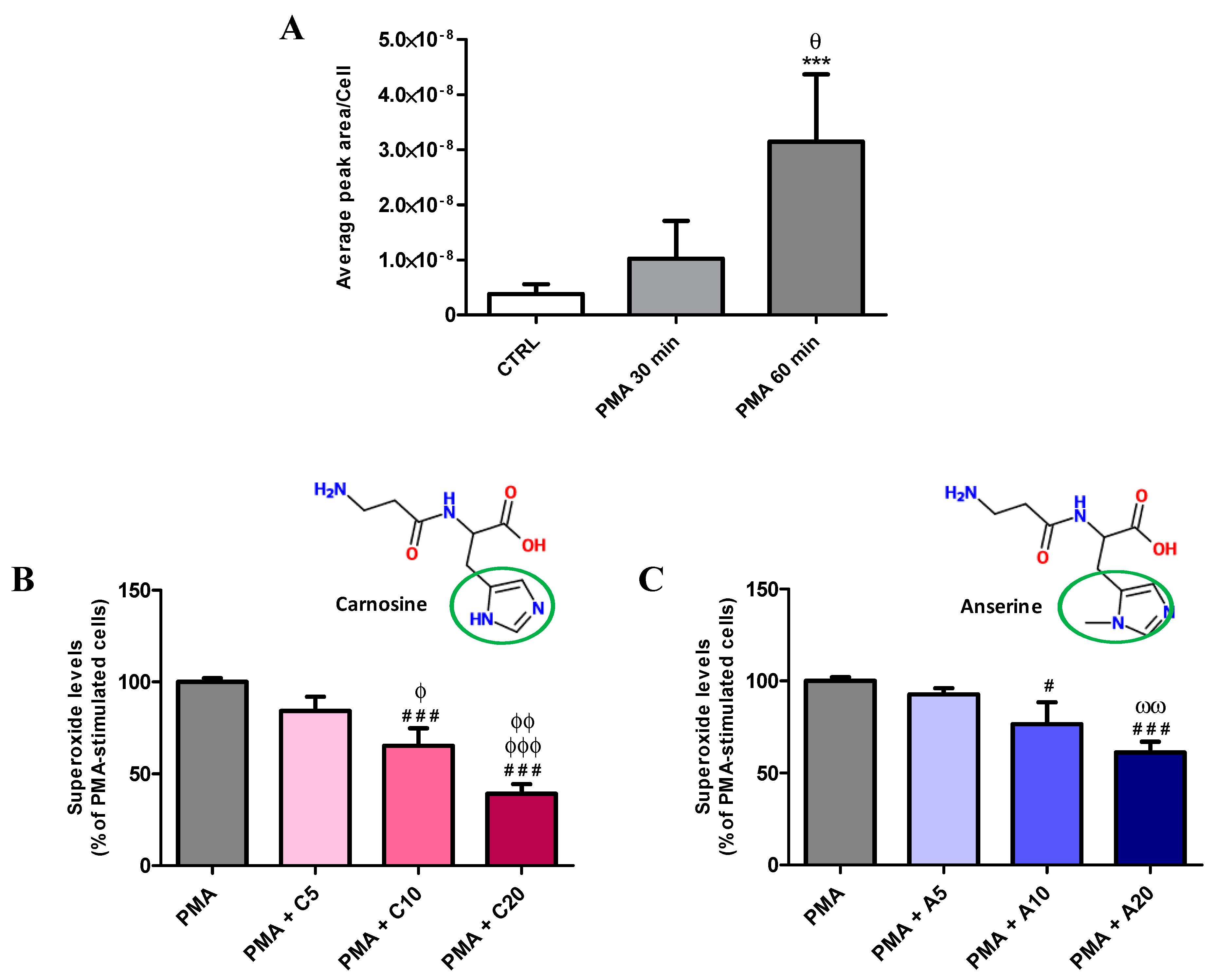
3.1. Carnosine Effects on PMA-Induced O2−• Production in Cultured Macrophages
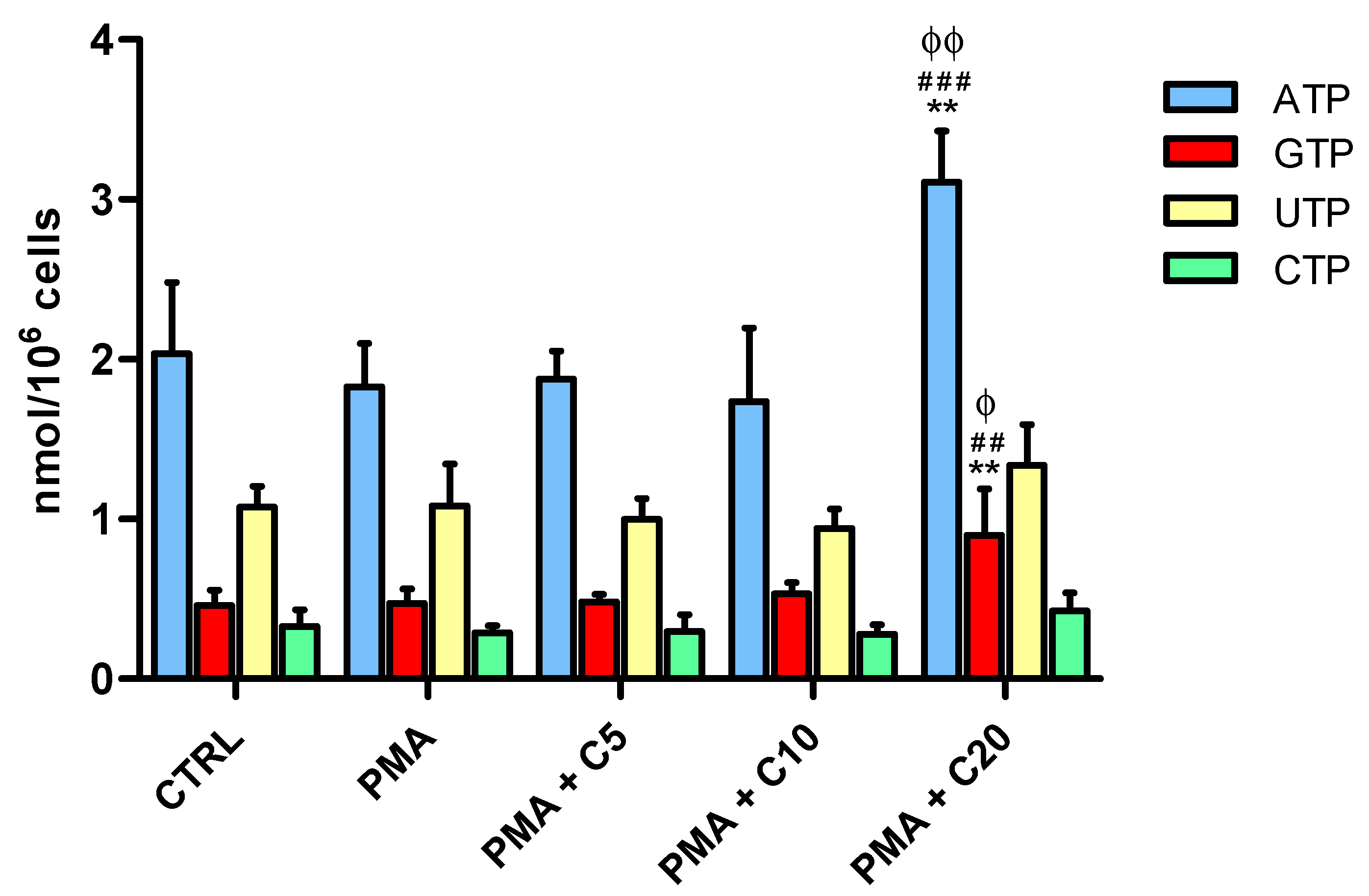
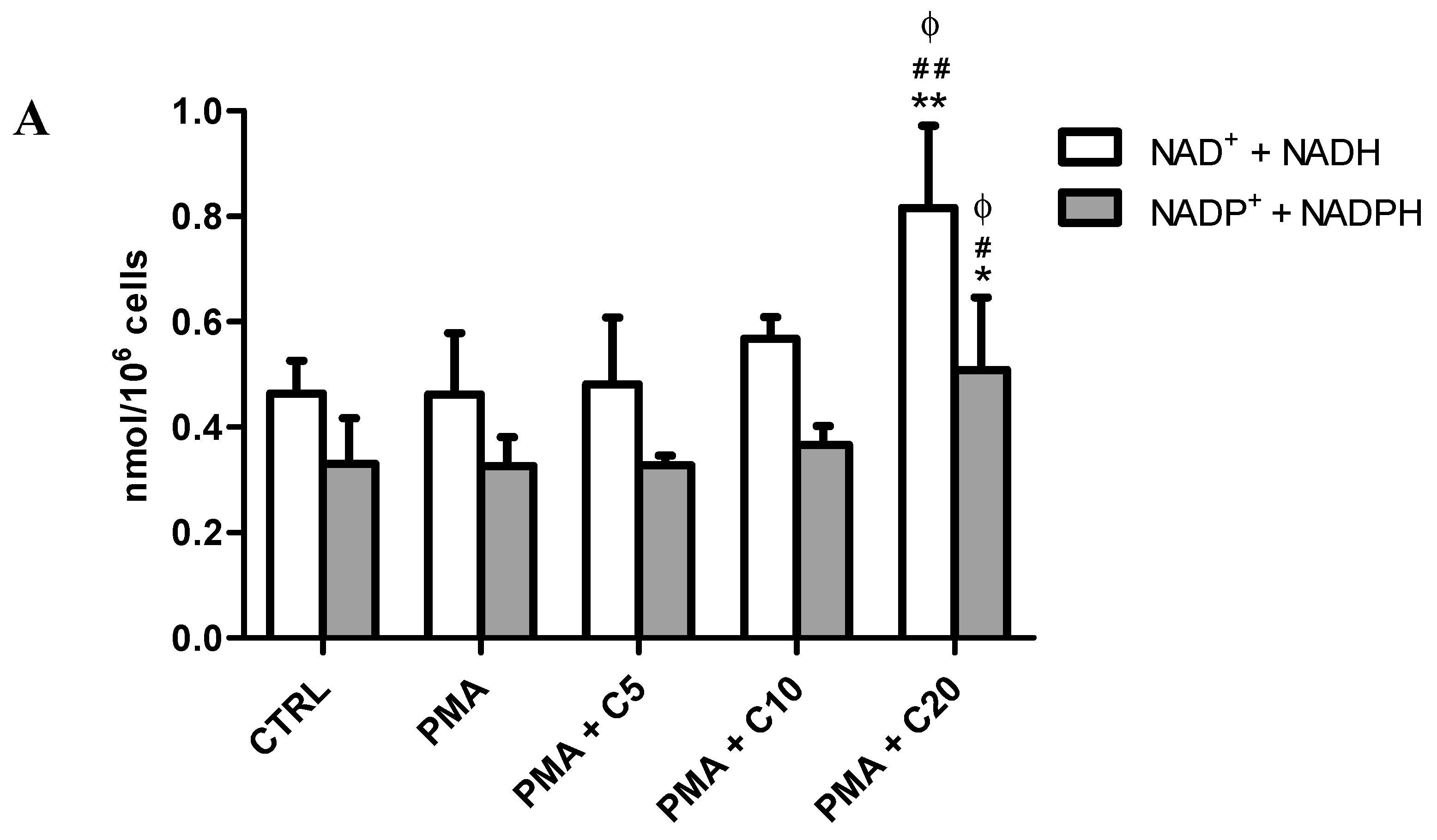
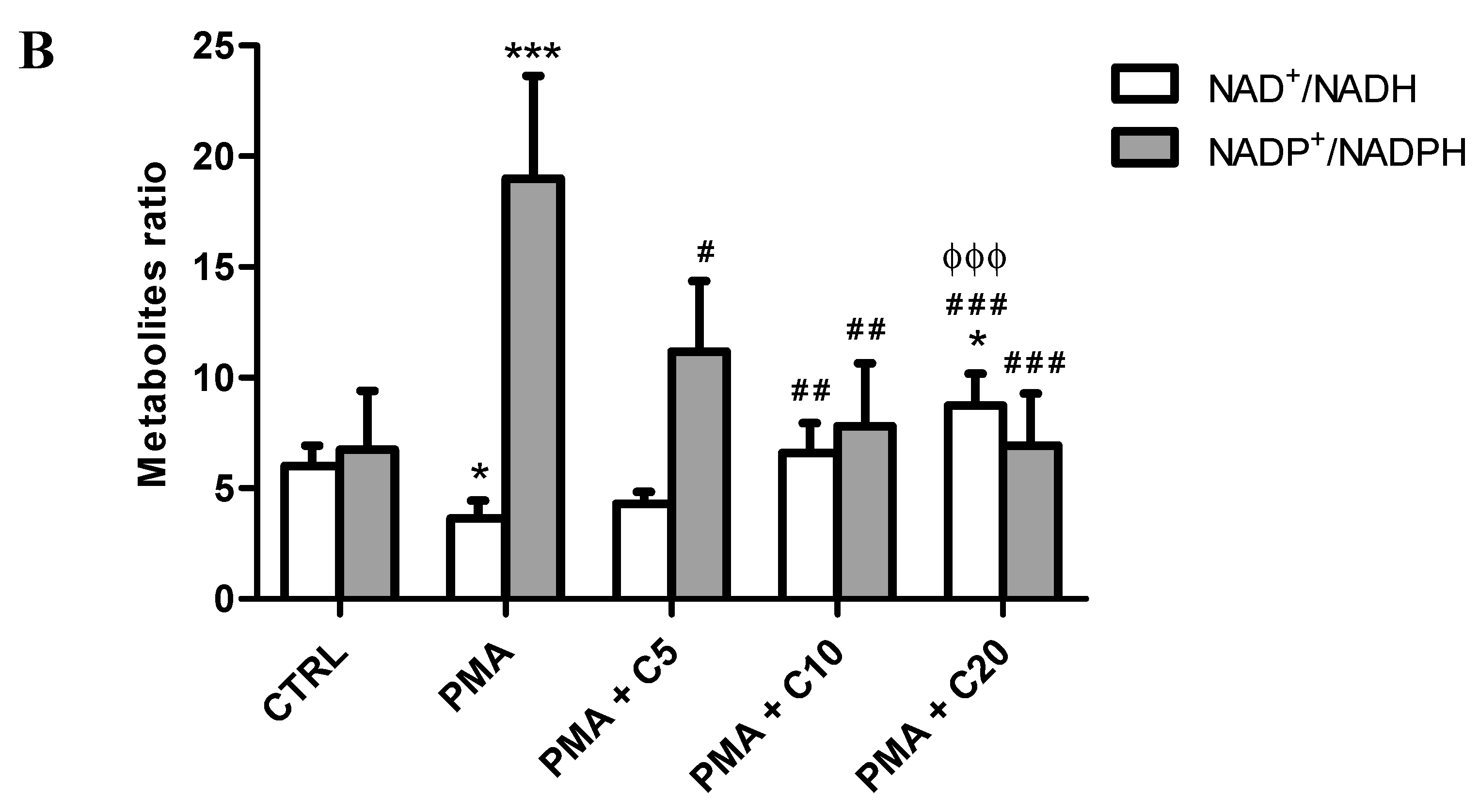
3.2. Influence of Carnosine on Energy Metabolism of Cultured Macrophages
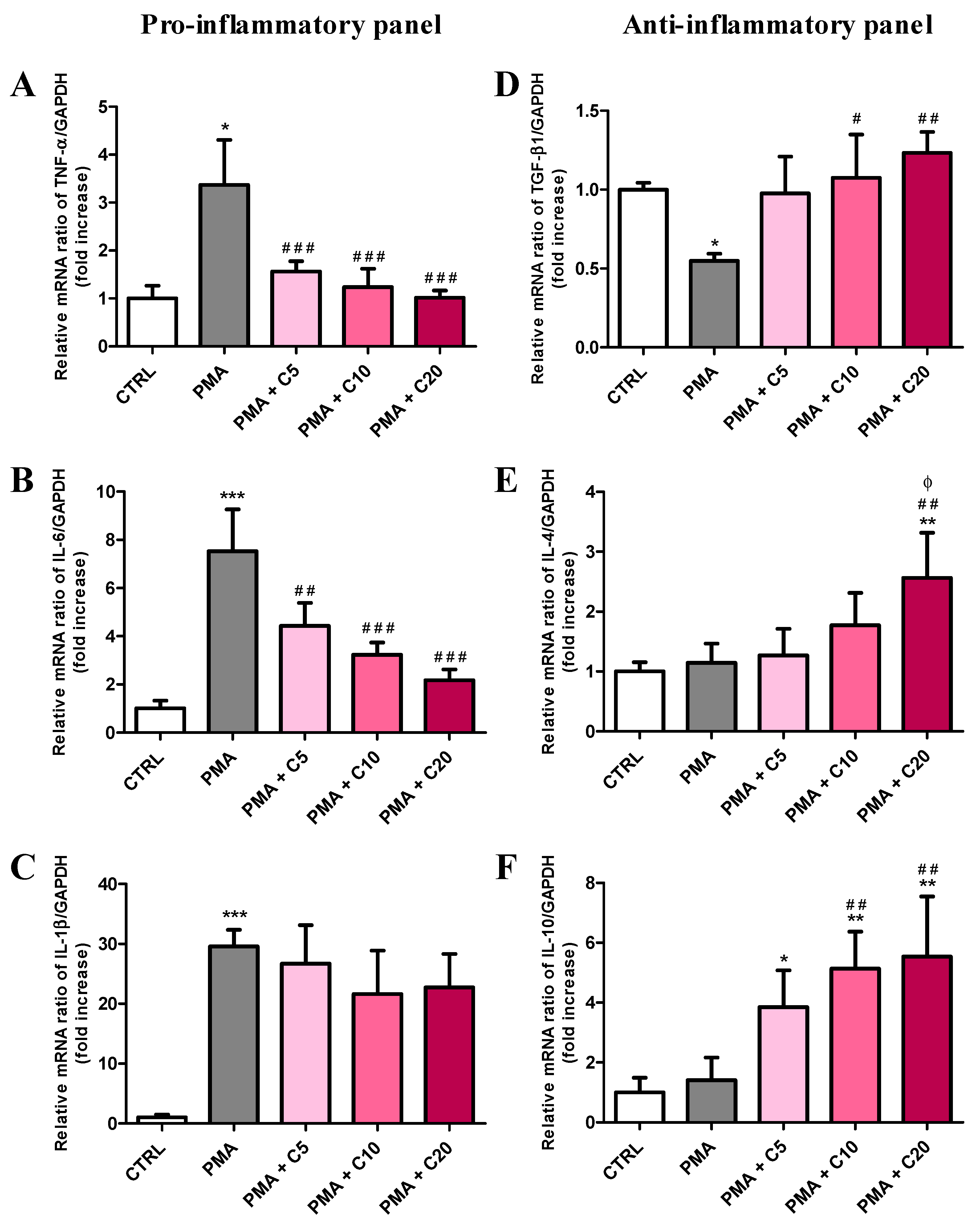
3.3. Carnosine Effects on PMA-Induced Inflammatory Response of Cultured Macrophages
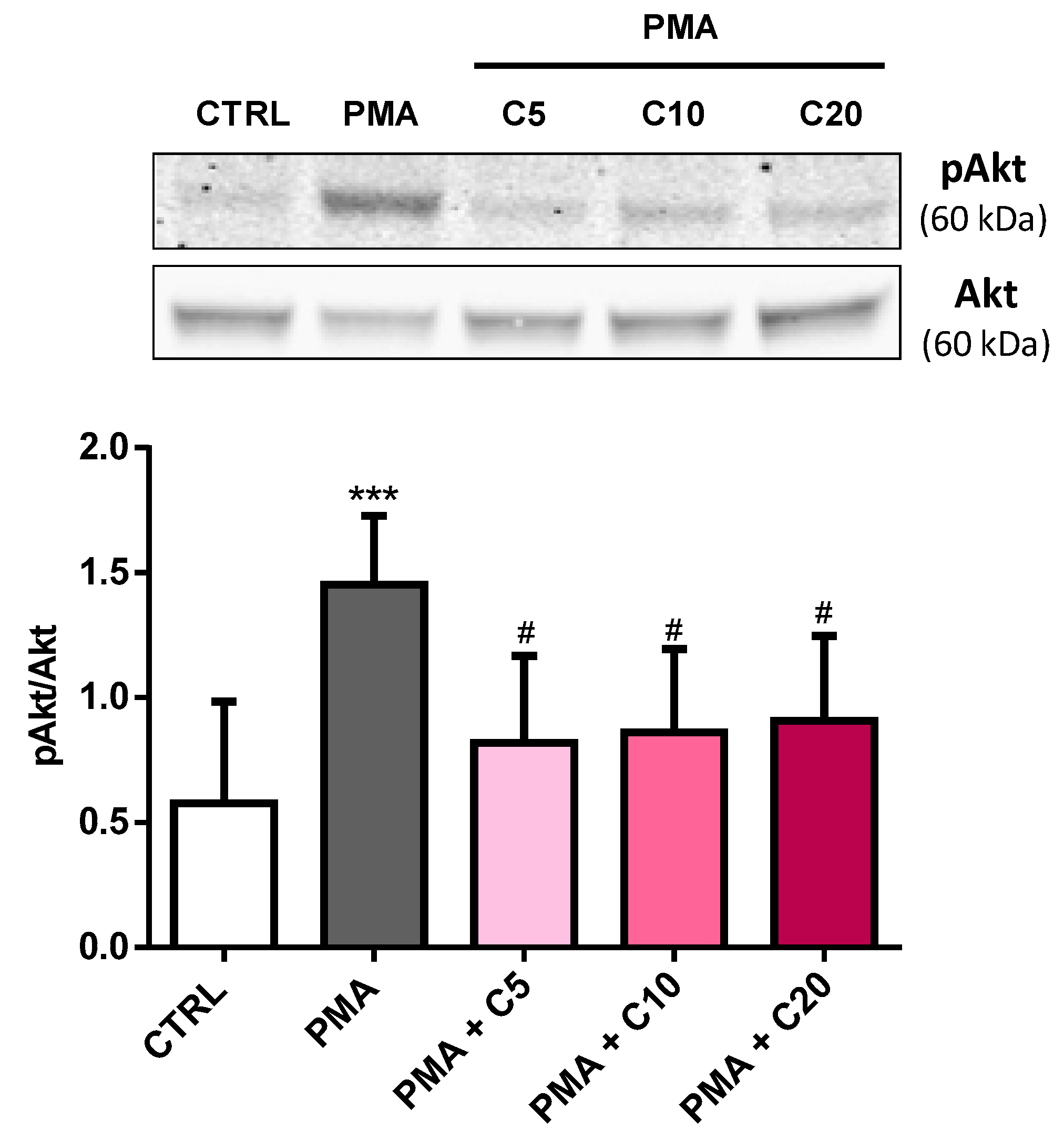
3.4. Carnosine Effects on PMA-Induced Akt Phosphorylation in Cultured Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Campos, R.P.; Siegel, J.M.; Fresta, C.G.; Caruso, G.; Da Silva, J.A.; Lunte, S.M. Indirect detection of superoxide in raw 264.7 macrophage cells using microchip electrophoresis coupled to laser-induced fluorescence. Anal. Bioanal. Chem. 2015, 407, 7003–7012. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.A.; Zhao, Z.; Turk, J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp. Diabetes Res. 2012, 2012, 703538. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, D.B.; Siegel, J.M.; Caruso, G.; Hulvey, M.K.; Lunte, S.M. Microchip electrophoresis with amperometric detection method for profiling cellular nitrosative stress markers. Analyst 2014, 139, 3265–3273. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Bagini, L.; Gorini, A.; Brunello, N.; Tascedda, F. Mitochondrial energy metabolism of rat hippocampus after treatment with the antidepressants desipramine and fluoxetine. Neuropharmacology 2017, 121, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Gorini, A.; Brunello, N.; Tascedda, F. Effect of desipramine and fluoxetine on energy metabolism of cerebral mitochondria. Neuroscience 2016, 330, 326–334. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef]
- Ullrich, V.; Bachschmid, M. Superoxide as a messenger of endothelial function. Biochem. Biophys. Res. Commun. 2000, 278, 1–8. [Google Scholar] [CrossRef]
- Afanas’ev, I.B. Signaling functions of free radicals superoxide and nitric oxide under physiological and pathological conditions. Mol. Biotechnol. 2007, 37, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.G.; Jordan, J. Nitric oxide and superoxide, a deadly cocktail. Ann. N. Y. Acad. Sci. 2002, 962, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Metto, E.C.; Evans, K.; Barney, P.; Culbertson, A.H.; Gunasekara, D.B.; Caruso, G.; Hulvey, M.K.; Fracassi Da Silva, J.A.; Lunte, S.M.; Culbertson, C.T. An integrated microfluidic device for monitoring changes in nitric oxide production in single t-lymphocyte (jurkat) cells. Anal. Chem. 2013, 85, 10188–10195. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, D.; Weiss, D.; Lassegue, B.; Clempus, R.E.; Szocs, K.; Sorescu, G.P.; Valppu, L.; Quinn, M.T.; Lambeth, J.D.; Vega, J.D.; et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 2002, 105, 1429–1435. [Google Scholar] [CrossRef]
- Mander, P.; Brown, G.C. Activation of microglial nadph oxidase is synergistic with glial inos expression in inducing neuronal death: A dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflamm. 2005, 2, 20. [Google Scholar] [CrossRef]
- Siegel, J.M.; Schilly, K.M.; Wijesinghe, M.B.; Caruso, G.; Fresta, C.G.; Lunte, S.M. Optimization of a microchip electrophoresis method with electrochemical detection for the determination of nitrite in macrophage cells as an indicator of nitric oxide production. Anal. Methods 2019, 11, 148–156. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Malagoli, D.; Mandrioli, M.; Tascedda, F.; Ottaviani, E. Circulating phagocytes: The ancient and conserved interface between immune and neuroendocrine function. Biol. Rev. 2017, 92, 369–377. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef]
- Roman, A.; Kreiner, G.; Nalepa, I. Macrophages and depression—A misalliance or well-arranged marriage? Pharm. Rep. 2013, 65, 1663–1672. [Google Scholar] [CrossRef]
- Lucherini, O.M.; Lopalco, G.; Cantarini, L.; Emmi, G.; Lopalco, A.; Venerito, V.; Vitale, A.; Iannone, F. Critical regulation of th17 cell differentiation by serum amyloid-a signalling in behcet’s disease. Immunol. Lett. 2018, 201, 38–44. [Google Scholar] [CrossRef]
- Lopalco, G.; Lucherini, O.M.; Lopalco, A.; Venerito, V.; Fabiani, C.; Frediani, B.; Galeazzi, M.; Lapadula, G.; Cantarini, L.; Iannone, F. Cytokine signatures in mucocutaneous and ocular behcet’s disease. Front. Immunol. 2017, 8, 200. [Google Scholar] [CrossRef]
- Gulewitsch, W.; Amiradžibi, S. Ueber das carnosin, eine neue organische base des fleischextractes. Ber. Der Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kwon, H.Y.; Kwon, O.B.; Kang, J.H. Hydrogen peroxide-mediated cu,zn-superoxide dismutase fragmentation: Protection by carnosine, homocarnosine and anserine. Biochim. Biophys. Acta 1999, 1472, 651–657. [Google Scholar] [CrossRef]
- Parker, C.J., Jr.; Ring, E. A comparative study of the effect of carnosine on myofibrillar-atpase activity of vertebrate and invertebrate muscles. Comp. Biochem. Physiol. 1970, 37, 413–419. [Google Scholar] [CrossRef]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as atp-grasp domain-containing protein 1 (atpgd1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef]
- Kalyankar, G.D.; Meister, A. Enzymatic synthesis of carnosine and related beta-alanyl and gamma-aminobutyryl peptides. J. Biol. Chem. 1959, 234, 3210–3218. [Google Scholar]
- Winnick, R.E.; Winnick, T. Carnosineanserine synthetase of muscle. I. Preparation and properties of soluble enzyme from chick muscle. Biochim. Biophys. Acta 1959, 31, 47–55. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Gariballa, S.E.; Sinclair, A.J. Carnosine: Physiological properties and therapeutic potential. Age Ageing 2000, 29, 207–210. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Preston, J.E.; Himsworth, D.T.; Worthington, V.C.; Keown, M.; Michaelis, J.; Lawrence, J.; Mateen, A.; Allende, L.; Eagles, P.A.; et al. Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann. N. Y. Acad. Sci. 1998, 854, 37–53. [Google Scholar] [CrossRef]
- Zhang, X.; Song, L.; Cheng, X.; Yang, Y.; Luan, B.; Jia, L.; Xu, F.; Zhang, Z. Carnosine pretreatment protects against hypoxia-ischemia brain damage in the neonatal rat model. Eur. J. Pharm. 2011, 667, 202–207. [Google Scholar] [CrossRef]
- Albrecht, T.; Schilperoort, M.; Zhang, S.; Braun, J.D.; Qiu, J.; Rodriguez, A.; Pastene, D.O.; Kramer, B.K.; Koppel, H.; Baelde, H.; et al. Carnosine attenuates the development of both type 2 diabetes and diabetic nephropathy in btbr ob/ob mice. Sci. Rep. 2017, 7, 44492. [Google Scholar] [CrossRef]
- Bermudez, M.L.; Skelton, M.R.; Genter, M.B. Intranasal carnosine attenuates transcriptomic alterations and improves mitochondrial function in the thy1-asyn mouse model of parkinson’s disease. Mol. Genet. Metab. 2018, 125, 305–313. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. Beta-alanyl-l-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef]
- Caruso, G.; Caraci, F.; Jolivet, R.B. Pivotal role of carnosine in the modulation of brain cells activity: Multimodal mechanism of action and therapeutic potential in neurodegenerative disorders. Prog. Neurobiol. 2019, 175, 35–53. [Google Scholar] [CrossRef]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Onufriev, M.V.; Potanova, G.I.; Silaeva, S.A.; Nikolaev, A. Carnosine as a stimulator of cytotoxic and phagocytic function of peritoneal macrophages. Biokhimiia 1992, 57, 1352–1359. [Google Scholar]
- Caruso, G.; Fresta, C.G.; Martinez-Becerra, F.; Antonio, L.; Johnson, R.T.; De Campos, R.P.S.; Siegel, J.M.; Wijesinghe, M.B.; Lazzarino, G.; Lunte, S.M. Carnosine modulates nitric oxide in stimulated murine raw 264.7 macrophages. Mol. Cell. Biochem. 2017, 431, 197–210. [Google Scholar] [CrossRef]
- Fresta, C.G.; Chakraborty, A.; Wijesinghe, M.B.; Amorini, A.M.; Lazzarino, G.; Lazzarino, G.; Tavazzi, B.; Lunte, S.M.; Caraci, F.; Dhar, P.; et al. Non-toxic engineered carbon nanodiamond concentrations induce oxidative/nitrosative stress, imbalance of energy metabolism, and mitochondrial dysfunction in microglial and alveolar basal epithelial cells. Cell Death Dis. 2018, 9, 245. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine prevents abeta-induced oxidative stress and inflammation in microglial cells: A key role of tgf-beta1. Cells 2019, 8, 64. [Google Scholar] [CrossRef]
- Momose, I.; Terashima, M.; Nakashima, Y.; Sakamoto, M.; Ishino, H.; Nabika, T.; Hosokawa, Y.; Tanigawa, Y. Phorbol ester synergistically increases interferon regulatory factor-1 and inducible nitric oxide synthase induction in interferon-gamma-treated raw 264.7 cells. Biochim. Biophys. Acta 2000, 1498, 19–31. [Google Scholar] [CrossRef]
- Zhao, K.; Huang, Z.; Lu, H.; Zhou, J.; Wei, T. Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in raw264.7 macrophages. Biosci. Rep. 2010, 30, 233–241. [Google Scholar] [CrossRef]
- Idelman, G.; Smith, D.L.; Zucker, S.D. Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of nadph oxidase. Redox Biol. 2015, 5, 398–408. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Siegel, J.M.; Wijesinghe, M.B.; Lunte, S.M. Microchip electrophoresis with laser-induced fluorescence detection for the determination of the ratio of nitric oxide to superoxide production in macrophages during inflammation. Anal. Bioanal. Chem. 2017, 409, 4529–4538. [Google Scholar] [CrossRef]
- Rajaram, M.V.; Ganesan, L.P.; Parsa, K.V.; Butchar, J.P.; Gunn, J.S.; Tridandapani, S. Akt/protein kinase b modulates macrophage inflammatory response to francisella infection and confers a survival advantage in mice. J. Immunol. 2006, 177, 6317–6324. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Fazzina, G.; Vagnozzi, R.; Signoretti, S.; Donzelli, S.; Di Stasio, E.; Giardina, B.; Tavazzi, B. Single-sample preparation for simultaneous cellular redox and energy state determination. Anal. Biochem. 2003, 322, 51–59. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Muzii, L.; Amorini, A.M.; Longo, S.; Di Stasio, E.; Caruso, G.; D’Urso, S.; Puglia, I.; Pisani, G.; et al. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum. Reprod. 2018, 33, 1817–1828. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water-and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 20 June 2019).
- Available online: https://www.qiagen.com/it/shop/pcr/real-time-pcr-enzymes-and-kits/two-step-qrt-pcr/quantitect-primer-assays/ (accessed on 20 June 2019).
- Caraci, F.; Gulisano, W.; Guida, C.A.; Impellizzeri, A.A.; Drago, F.; Puzzo, D.; Palmeri, A. A key role for tgf-beta1 in hippocampal synaptic plasticity and memory. Sci. Rep. 2015, 5, 11252. [Google Scholar] [CrossRef]
- Xia, C.; Bai, X.; Hou, X.; Gou, X.; Wang, Y.; Zeng, H.; Huang, M.; Jin, J. Cryptotanshinone reverses cisplatin resistance of human lung carcinoma a549 cells through down-regulating nrf2 pathway. Cell. Physiol. Biochem. 2015, 37, 816–824. [Google Scholar] [CrossRef]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug. Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Van Der Heijden, J.; Bosman, E.S.; Reynolds, L.A.; Finlay, B.B. Direct measurement of oxidative and nitrosative stress dynamics in salmonella inside macrophages. Proc. Natl. Acad. Sci. USA 2015, 112, 560–565. [Google Scholar] [CrossRef]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef]
- Fresta, C.G.; Hogard, M.L.; Caruso, G.; Melo Costa, E.E.; Lazzarino, G.; Lunte, S.M. Monitoring carnosine uptake by raw 264.7 macrophage cells using microchip electrophoresis with fluorescence detection. Anal. Methods 2017, 9, 402–408. [Google Scholar] [CrossRef]
- Munzel, T.; Harrison, D.G. Increased superoxide in heart failure: A biochemical baroreflex gone awry. Circulation 1999, 100, 216–218. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Fukai, T.; Folz, R.J.; Landmesser, U.; Harrison, D.G. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc. Res. 2002, 55, 239–249. [Google Scholar] [CrossRef]
- Klebanov, G.I.; Teselkin Yu, O.; Babenkova, I.V.; Popov, I.N.; Levin, G.; Tyulina, O.V.; Boldyrev, A.A.; Vladimirov Yu, A. Evidence for a direct interaction of superoxide anion radical with carnosine. Biochem. Mol. Biol. Int. 1997, 43, 99–106. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of l-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Vera-Aviles, M.; Vantana, E.; Kardinasari, E.; Koh, N.L.; Latunde-Dada, G.O. Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals 2018, 11, 111. [Google Scholar] [CrossRef]
- Szczesniak, D.; Budzen, S.; Kopec, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily carnosine and anserine supplementation alters verbal episodic memory and resting state network connectivity in healthy elderly adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/carnosine supplementation suppresses the expression of the inflammatory chemokine ccl24 in peripheral blood mononuclear cells from elderly people. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef]
- Alpsoy, L.; Akcayoglu, G.; Sahin, H. Anti-oxidative and anti-genotoxic effects of carnosine on human lymphocyte culture. Hum. Exp. Toxicol. 2011, 30, 1979–1985. [Google Scholar] [CrossRef]
- Aydin, A.F.; Kusku-Kiraz, Z.; Dogru-Abbasoglu, S.; Uysal, M. Effect of carnosine treatment on oxidative stress in serum, apob-containing lipoproteins fraction and erythrocytes of aged rats. Pharm. Rep. 2010, 62, 733–739. [Google Scholar] [CrossRef]
- Tsai, S.J.; Kuo, W.W.; Liu, W.H.; Yin, M.C. Antioxidative and anti-inflammatory protection from carnosine in the striatum of mptp-treated mice. J. Agric. Food Chem. 2010, 58, 11510–11516. [Google Scholar] [CrossRef]
- Rodriguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.; Lopez-Villegas, E.O.; Sanchez-Garcia, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef]
- Saffarzadeh, M. Neutrophil extracellular traps as a drug target to counteract chronic and acute inflammation. Curr. Pharm. Biotechnol. 2018, 19, 1196–1202. [Google Scholar] [CrossRef]
- Shen, Y.; Tian, Y.; Yang, J.; Shi, X.; Ouyang, L.; Gao, J.; Lu, J. Dual effects of carnosine on energy metabolism of cultured cortical astrocytes under normal and ischemic conditions. Regul. Pept. 2014, 192, 45–52. [Google Scholar] [CrossRef]
- Macedo, L.W.; Cararo, J.H.; Maravai, S.G.; Goncalves, C.L.; Oliveira, G.M.; Kist, L.W.; Guerra Martinez, C.; Kurtenbach, E.; Bogo, M.R.; Hipkiss, A.R.; et al. Acute carnosine administration increases respiratory chain complexes and citric acid cycle enzyme activities in cerebral cortex of young rats. Mol. Neurobiol. 2016, 53, 5582–5590. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, Y.; Bao, Y.; Xu, H.; Cheng, J.; Wang, B.; Shen, Y.; Chen, Z.; Lyu, J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to ogd/recovery. Brain. Res. Bull. 2016, 124, 76–84. [Google Scholar] [CrossRef]
- Schinetti, M.L.; Lazzarino, G. Inhibition of phorbol ester-stimulated chemiluminescence and superoxide production in human neutrophils by fructose 1, 6-diphosphate. Biochem. Pharm. 1986, 35, 1762–1764. [Google Scholar] [CrossRef]
- Hipkiss, A.R.; Cartwright, S.P.; Bromley, C.; Gross, S.R.; Bill, R.M. Carnosine: Can understanding its actions on energy metabolism and protein homeostasis inform its therapeutic potential? Chem. Cent J. 2013, 7, 38. [Google Scholar] [CrossRef]
- Cassetta, L.; Cassol, E.; Poli, G. Macrophage polarization in health and disease. Sci. World J. 2011, 11, 2391–2402. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Bukhari, A.; Ikutani, M.; Oya, T.; Kanatani, Y.; Tsuneyama, K.; Nagai, Y.; Takatsu, K.; Urakaze, M.; et al. Regulatory mechanisms for adipose tissue m1 and m2 macrophages in diet-induced obese mice. Diabetes 2009, 58, 2574–2582. [Google Scholar] [CrossRef]
- Caraci, F.; Spampinato, S.F.; Morgese, M.G.; Tascedda, F.; Salluzzo, M.G.; Giambirtone, M.C.; Caruso, G.; Munafo, A.; Torrisi, S.A.; Leggio, G.M.; et al. Neurobiological links between depression and ad: The role of tgf-beta1 signaling as a new pharmacological target. Pharm. Res. 2018, 130, 374–384. [Google Scholar] [CrossRef]
- Chen, J.H.; Ke, K.F.; Lu, J.H.; Qiu, Y.H.; Peng, Y.P. Protection of tgf-beta1 against neuroinflammation and neurodegeneration in abeta1-42-induced alzheimer’s disease model rats. PLoS ONE 2015, 10, e0116549. [Google Scholar]
- Fisichella, V.; Giurdanella, G.; Platania, C.B.; Romano, G.L.; Leggio, G.M.; Salomone, S.; Drago, F.; Caraci, F.; Bucolo, C. Tgf-beta1 prevents rat retinal insult induced by amyloid-beta (1-42) oligomers. Eur. J. Pharm. 2016, 787, 72–77. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Fisichella, V.; Fidilio, A.; Geraci, F.; Lazzara, F.; Leggio, G.M.; Salomone, S.; Drago, F.; Pignatello, R.; Caraci, F.; et al. Topical ocular delivery of tgf-beta1 to the back of the eye: Implications in age-related neurodegenerative diseases. Int. J. Mol. Sci. 2017, 18, 2076. [Google Scholar] [CrossRef]
- Shull, M.M.; Ormsby, I.; Kier, A.B.; Pawlowski, S.; Diebold, R.J.; Yin, M.; Allen, R.; Sidman, C.; Proetzel, G.; Calvin, D.; et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992, 359, 693–699. [Google Scholar] [CrossRef]
- Kulkarni, A.B.; Karlsson, S. Inflammation and tgf beta 1: Lessons from the tgf beta 1 null mouse. Res. Immunol. 1997, 148, 453–456. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Cai, W.; Qiu, L. Hypaphorine attenuates lipopolysaccharide-induced endothelial inflammation via regulation of tlr4 and ppar-γ dependent on pi3k/akt/mtor signal pathway. Int. J. Mol. Sci. 2017, 18, 844. [Google Scholar] [CrossRef]
- Kadry, M.O.; Ali, H.M. Downregulation of hif1-alpha, smad-2, akt, and bax gene expression in sodium nitrite-induced lung injury via some antioxidants. J. Biochem. Mol. Toxicol. 2017, 31, e21909. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, L.; Wu, X.; Liu, G.; Peng, Y.; Xin, X.; Jiao, B.; Kong, X. Carnosine inhibits the proliferation of human gastric carcinoma cells by retarding akt/mtor/p70s6k signaling. J. Cancer 2014, 5, 382–389. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, J.; Liu, Y.; Xu, M.; Huang, Y.; Zhang, J.; Cao, P.; Lyu, J.; Shen, Y. Profiling and targeting of cellular mitochondrial bioenergetics: Inhibition of human gastric cancer cell growth by carnosine. Acta Pharmacol. Sin. 2019, 40, 938–948. [Google Scholar] [CrossRef]
- Ramirez-Camacho, I.; Flores-Herrera, O.; Zazueta, C. The relevance of the supramolecular arrangements of the respiratory chain complexes in human diseases and aging. Mitochondrion 2019, 47, 266–272. [Google Scholar] [CrossRef]

| Official Name # | Official Symbol | Alternative Titles/Symbols | Detected Transcript | Amplicon Length | Cat. No. † |
|---|---|---|---|---|---|
| nitric oxide synthase 2, inducible | Nos2 | iNOS; Nos-2; Nos2a; i-NOS; NOS-II; MAC-NOS | NM_010927 | 118 bp | QT00100275 |
| NADPH oxidase 1 | Nox1 | MOX1; NOH1; NOH-1; NOX1a; Nox-1; GP91-2; NOX1alpha | NM_172203 XM_006528515 | 180 bp | QT00140091 |
| cytochrome b-245, beta polypeptide | Cybb | Cgd; Cyd; Nox2; C88302; gp91-1; gp91phox; CGD91-phox | NM_007807 XM_006527565 | 146 bp | QT00139797 |
| interleukin 1 beta | Il1b | Il-1b; IL-1beta; IL-1β | NM_008361 XM_006498795 | 150 bp 682 bp | QT01048355 |
| tumor necrosis factor | Tnf | DIF; Tnfa; TNF-a; TNFSF2; Tnlg1f; Tnfsf1a; TNFalpha; TNF-alpha; TNF-α | NM_013693 NM_001278601 | 112 bp 112 bp | QT00104006 |
| interleukin 6 | Il6 | Il-6 | NM_031168 | 128 bp | QT00098875 |
| interleukin 4 | Il4 | Il-4; BSF-1 | NM_021283 | 132 bp | QT02418311 |
| interleukin 10 | Il10 | CSIF; Il-10 | NM_010548 | 103 bp | QT00106169 |
| transforming growth factor, beta 1 | Tgfb1 | Tgfb; Tgfb-1; TGFbeta1; TGF-beta1 | NM_011577 | 145 bp | QT00145250 |
| glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Gapd | NM_008084 XM_001003314 XM_990238 NM_001289726 | 144 bp | QT01658692 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants 2019, 8, 281. https://doi.org/10.3390/antiox8080281
Caruso G, Fresta CG, Fidilio A, O’Donnell F, Musso N, Lazzarino G, Grasso M, Amorini AM, Tascedda F, Bucolo C, et al. Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants. 2019; 8(8):281. https://doi.org/10.3390/antiox8080281
Chicago/Turabian StyleCaruso, Giuseppe, Claudia G. Fresta, Annamaria Fidilio, Fergal O’Donnell, Nicolò Musso, Giacomo Lazzarino, Margherita Grasso, Angela M. Amorini, Fabio Tascedda, Claudio Bucolo, and et al. 2019. "Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages" Antioxidants 8, no. 8: 281. https://doi.org/10.3390/antiox8080281
APA StyleCaruso, G., Fresta, C. G., Fidilio, A., O’Donnell, F., Musso, N., Lazzarino, G., Grasso, M., Amorini, A. M., Tascedda, F., Bucolo, C., Drago, F., Tavazzi, B., Lazzarino, G., Lunte, S. M., & Caraci, F. (2019). Carnosine Decreases PMA-Induced Oxidative Stress and Inflammation in Murine Macrophages. Antioxidants, 8(8), 281. https://doi.org/10.3390/antiox8080281













