Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Solvents and Chemicals
2.2. Extraction Procedure
2.3. Determination of Antioxidant Potential by Single Electron Transfer Based Assays
2.4. Determination of Antioxidant Potential by Peroxyl-Radicals Inhibition Assays Based Hydrogen Atom Transfer
2.5. Evaluation of HPLC-DPPH• Scavenging On-Line
2.6. Ultra Performance Liquid Chromatography/Electron Spray Ionisation Quadrupole Time-of-Flight Mass Spectrometry (UPLC/ESI-QTOF-MS) Analysis
2.7. Determination of α-Amylase Inhibitory Activity
2.8. Cytotoxicity Assay in Caco-2 Cells
2.9. Cellular Antioxidant Activity Assay (CAA)
2.10. Statistical Data Handling
3. Results
3.1. Total Yield, Total Phenolic Content and Radical Scavenging Capacity of Peony Extracts
3.2. Peroxyl and Hydroxyl Radicals Inhibition in ORAC, HORAC and HOSC Assays
3.3. Determination of Phytochemicals by UPLC-Q/TOF
3.4. Determination of Antioxidants by the On-Line HPLC-UV-DPPH•-Scavenging
3.5. α-Amylase Inhibitory Properties of Selected Extracts
3.6. In Vitro Cytotoxic and Cellular Antioxidant Activity (CAA) Activity of Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.L.; Wang, H.; Ho, C.T.; Li, S.M.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Venskutonis, P.R. Natural antioxidants in food systems. In Food Oxidants and Antioxidants. Chemical, Biological and Functional Properties; Grzegorz, B., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 235–301. [Google Scholar]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and biological studies of Paeoniaceae. Chem. Biodivers. 2010, 7, 805–838. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Tabassun, N.; Rasool, S. Medicinal uses and phytoconstituents of Paeonia officinalis. Int. Res. J. Pharm. 2012, 3, 85–87. [Google Scholar]
- Xu, S.P.; Sun, G.P.; Shen, Y.X.; Wei, W.; Peng, W.R.; Wang, H. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J. Gastroenterol. 2007, 14, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.P.; Pang, J.; Wang, X.W.; Shen, Z.Q.; Jin, M.; Li, J.W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006, 12, 4078–4081. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Wei, W.; Zhu, L.; Liu, J.X. Effects and mechanisms of paeoniflorin, a bioactive glucoside from peony root, on adjuvant arthritis in rats. J. Inflamm. Res. 2007, 56, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.O.; Ko, W.K.; Kim, J.Y.; Ro, H.S. Paeoniflorin: An antihyperlipidemic agent from Paeonia lactiflora. Fitoterapia 2004, 75, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Li, Z.; Leung, W.M.; Liu, L.; Xu, H.X.; Bian, Z.X. The analgesic effect of paeoniflorin on neonatal maternal separation-induced visceral hyperalgesia in rats. J. Pain 2008, 9, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, Y.F.; Gao, X.D.; Zhu, X.Y.; Du, M.Z.; Wang, Y.X.; Deng, R.X.; Gao, J.Y. Optimization of ultrasonic-assisted extraction of oil from the seed kernels and isolation of monoterpene glycosides from the oil residue of Paeonia lactiflora Pall. Ind. Crop Prod. 2017, 107, 260–270. [Google Scholar] [CrossRef]
- Ning, C.; Jiang, Y.; Meng, J.; Zhou, C.; Tao, J. Herbaceous peony seed oil: A rich source of unsaturated fatty acids and γ-tocopherol. Eur. J. Lipid Sci. Technol. 2015, 117, 532–542. [Google Scholar] [CrossRef]
- Lieutaghi, P. Badasson & Cie: Tradition Médicinale et Autres Usages des Plantes en Haute Provence; Actes Sud: Arles, France, 2009; p. 715. [Google Scholar]
- Dienaitė, L.; Pukalskienė, M.; Matias, A.A.; Pereira, C.V.; Pukalskas, A.; Venskutonis, P.R. Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in cell cultures. J. Funct. Foods 2018, 45, 512–522. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbas, M.M.; Kitahara, K.; Suganuma, T.; Hashimoto, F.; Tadera, K. Antioxidant and α-Amylase Inhibitory Compounds from Aerial Parts of Varthemia iphionoides Boiss. Biosci. Biotechnol. Appl. Biochem. 2006, 70, 2178–2184. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The CaCo-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on CaCo-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arrįez-Romįn, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- He, C.; Peng, B.; Dan, Y.; Peng, Y.; Xiao, P. Chemical taxonomy of tree peony species from China based on root cortex metabolic fingerprinting. Phytochemistry 2014, 107, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, H.; Wang, L.; Shu, Q.; Zheng, Y.; Xu, Y.; Zhang, J.; Yang, R.; Ge, Y. Flavonoid composition and antioxidant activity of tree peony (Paeonia section Moutan) yellow flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Kim, C.Y.; Kim, H.J.; Park, J.H.; Ahn, M.J. Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovate. J. Med. Food 2015, 18, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Tabassum, N. Effect of 70% ethanolic extract of roots of Paeonia officinalis Linn. on hepatotoxicity. J. Acute Med. 2013, 3, 45–49. [Google Scholar] [CrossRef]
- Ahmad, F.; Tabassum, N. Preliminary phytochemical, acute oral toxicity and antihepatotoxic study of roots of Paeonia officinalis Linn. Asian Pac. J. Trop. Biomed. 2013, 3, 64–68. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartind, P.; Meltone, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, A.C.; Biasoto, A.C.T.; Schwember, A.R.; Granato, D.; Rasera, G.B.; Franchin, M.; Rosalen, P.L.; Alencar, S.M.; Shahidi, F. Should we ban total phenolics and antioxidant screening methods? The link between antioxidant potential and activation of NF-κB using phenolic compounds from grape by-products. Food Chem. 2019, 290, 229–238. [Google Scholar] [CrossRef]
- Kleinrichert, K.; Alappat, B. Comparative analysis of antioxidant and anti-amyloidogenic properties of various polyphenol rich phytoceutical extracts. Antioxidants 2019, 8, 13. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.N.; Khang, D.T.; Tuyen, P.T.; Minh, L.T.; Anh, L.H.; Quan, N.V.; Ha, P.T.T.; Quan, N.T.; Toan, N.P.; Elzaawely, A.A.; et al. Phenolic compounds and antioxidant activity of Phalaenopsis orchid hybrids. Antioxidants 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the Probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Moore, J.; Yin, J.J.; Yu, L.L. Novel fluorometric assay for hydroxyl radical scavenging capacity (HOSC) estimation. J. Agric. Food Chem. 2006, 54, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Soare, L.C.; Ferde, M.; Stefanov, S.; Denkova, Z.; Nicolova, R.; Denev, P.; Ungureanu, C. Antioxidant and antimicrobial properties of some plant extracts. Rev. Chim. 2012, 63, 432–434. [Google Scholar]
- Shi, Y.H.; Zhu, S.; Ge, Y.W.; Toume, K.; Wang, Z.; Batkhuu, J.; Komatsu, K. Characterization and quantification of monoterpenoids in different types of peony root and the related Paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 581–592. [Google Scholar] [CrossRef]
- He, C.; Peng, Y.; Xiao, W.; Liu, H.; Xiao, P.G. Determination of chemical variability of phenolic and monoterpene glycosides in the seeds of Paeonia species using HPLC and profiling analysis. Food Chem. 2013, 38, 2108–2114. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Xu, Y.F.; Zhu, X.Y.; Xu, X.F.; Chang, S.; Deng, R.X. Three new monoterpene glycosides from oil peony seed cake. Ind. Crop Prod. 2018, 111, 371–378. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Gao, J.Y.; Du, M.Z.; Zhang, K.; Zhang, J.L.; Xue, N.C.; Yan, M.; Qu, C.X.; Deng, R.X. HPLC-DAD analysis of 15 monoterpene glycosides in oil peony seed cakes sourced from different cultivation areas in China. Ind. Crop Prod. 2018, 118, 259–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Gao, J.Y.; Wang, X.S.; Yan, M.; Xue, N.C.; Qu, C.X.; Deng, R.X. Paeonia veitchii seeds as a promising high potential by-product: Proximate composition, phytochemical components, bioactivity evaluation and potential applications. Ind. Crop Prod. 2018, 125, 248–260. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chiang, S.Y.; Li, Y.Z.; Chen, M.F.; Chen, Y.S.; Wu, J.Y.; Liu, Y.W. Anti-tumor effect of Radix Paeoniae Rubra extract on mice bladder tumors using intravesical therapy. Oncol. Lett. 2016, 12, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Lee, Y.R.; Chiang, S.Y.; Li, Y.Z.; Chen, Y.S.; Hsu, C.D.; Liu, Y.W. Cortex Moutan induces bladder cancer cell death via apoptosis and retards tumor growth in mouse bladders. Evid. Based Complement. Alternat. Med. 2013, 2013, 207279. [Google Scholar] [CrossRef] [PubMed]
- Almosnid, N.M.; Gao, Y.; He, C.; Park, H.S.; Altman, E. In vitro antitumor effects of two novel oligostilbenes, cis- and trans-suffruticosol D, isolated from Paeonia suffruticosa seeds. Int. J. Oncol. 2015, 48, 646–656. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Shen, C. Phenolic compounds and antioxidant capacities of 10 common edible flowers from China. J. Food Sci. 2014, 79, 517–525. [Google Scholar] [CrossRef]
- Huang, W.; Mao, S.; Zhang, L.; Lu, B.; Zheng, L.; Zhou, F.; Li, M. Phenolic compounds, antioxidant potential and antiproliferative potential of 10 common edible flowers from China assessed using a simulated in vitro digestion–dialysis process combined with cellular assays. J. Sci. Food Agric. 2017, 97, 4760–4769. [Google Scholar] [CrossRef]
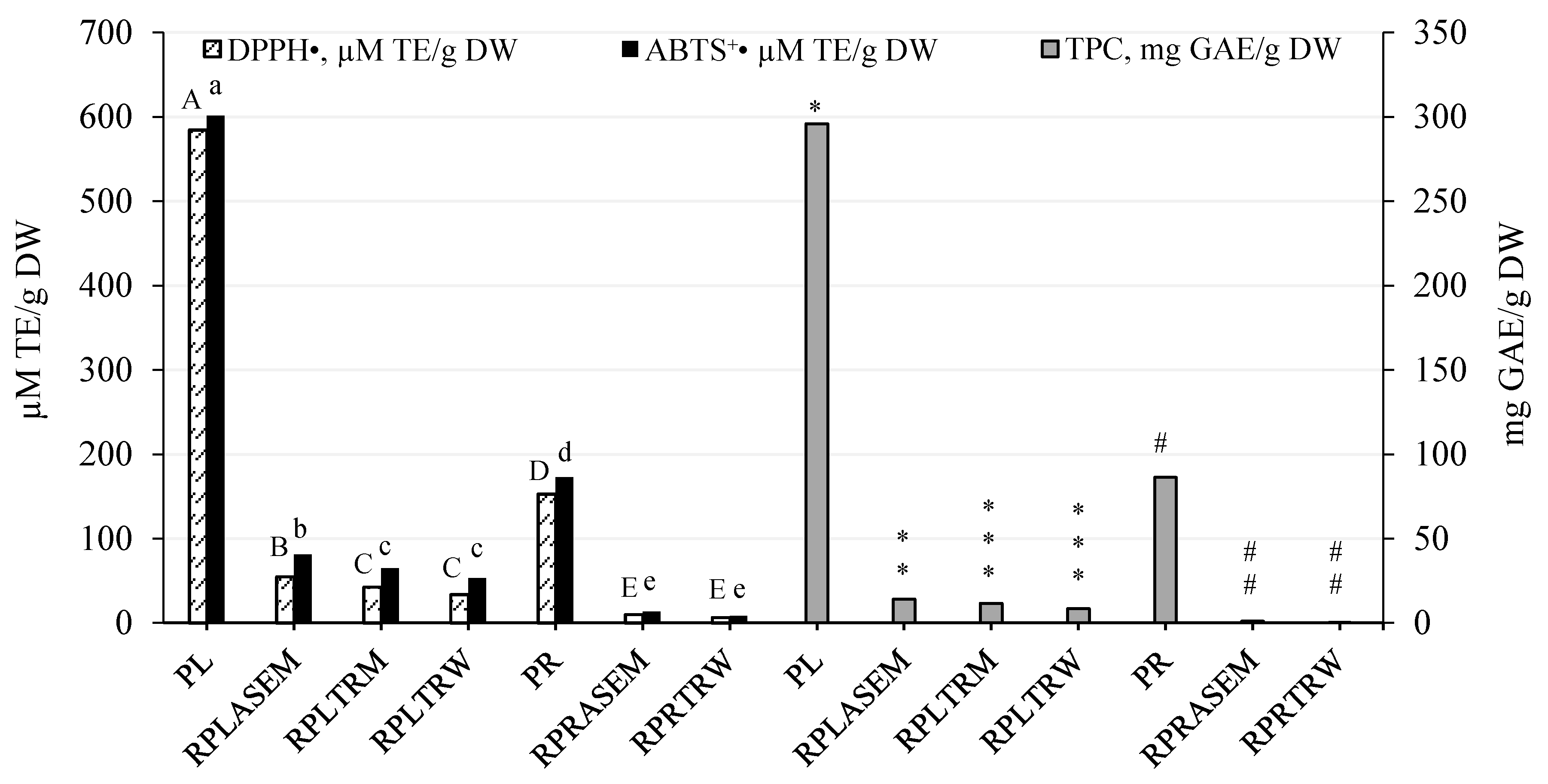

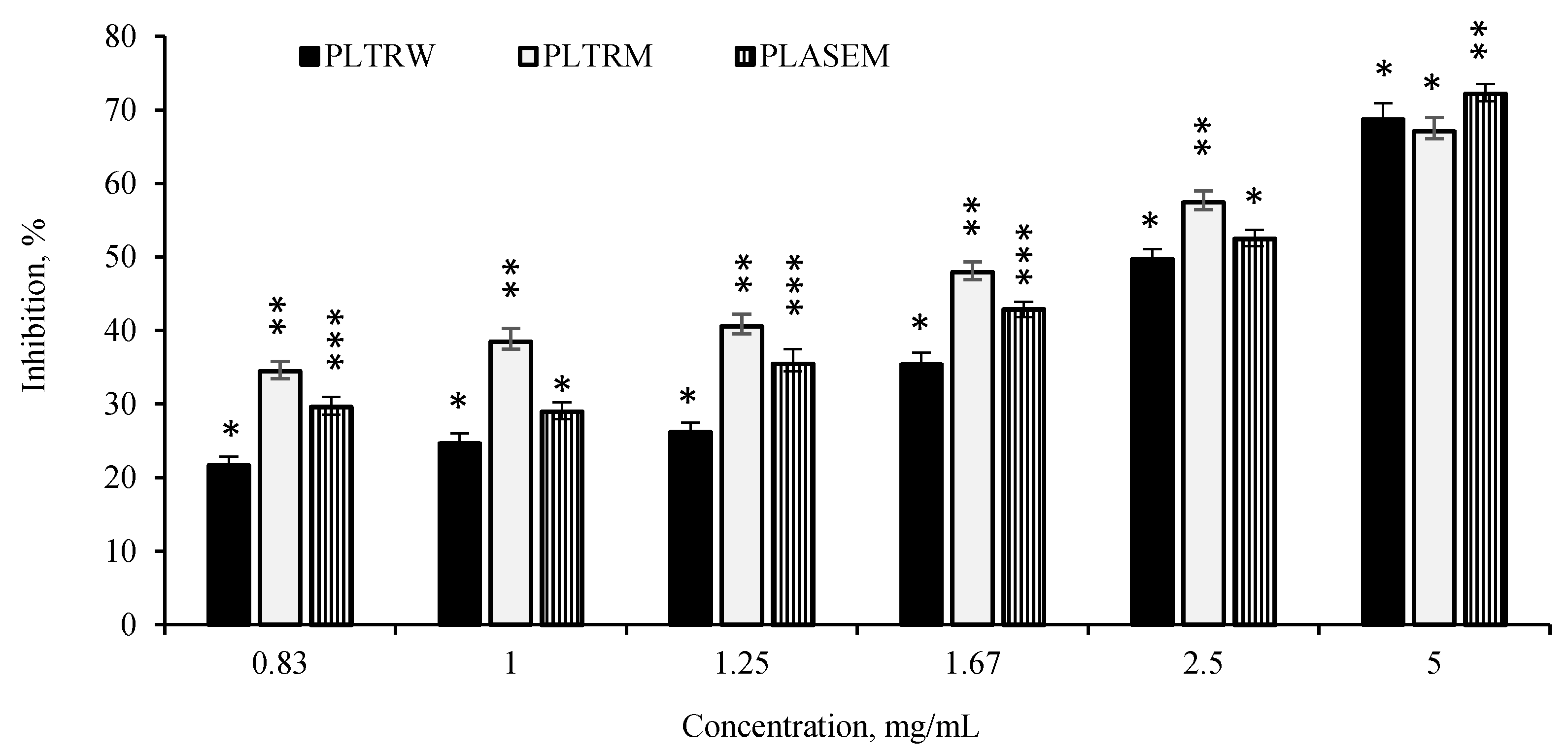
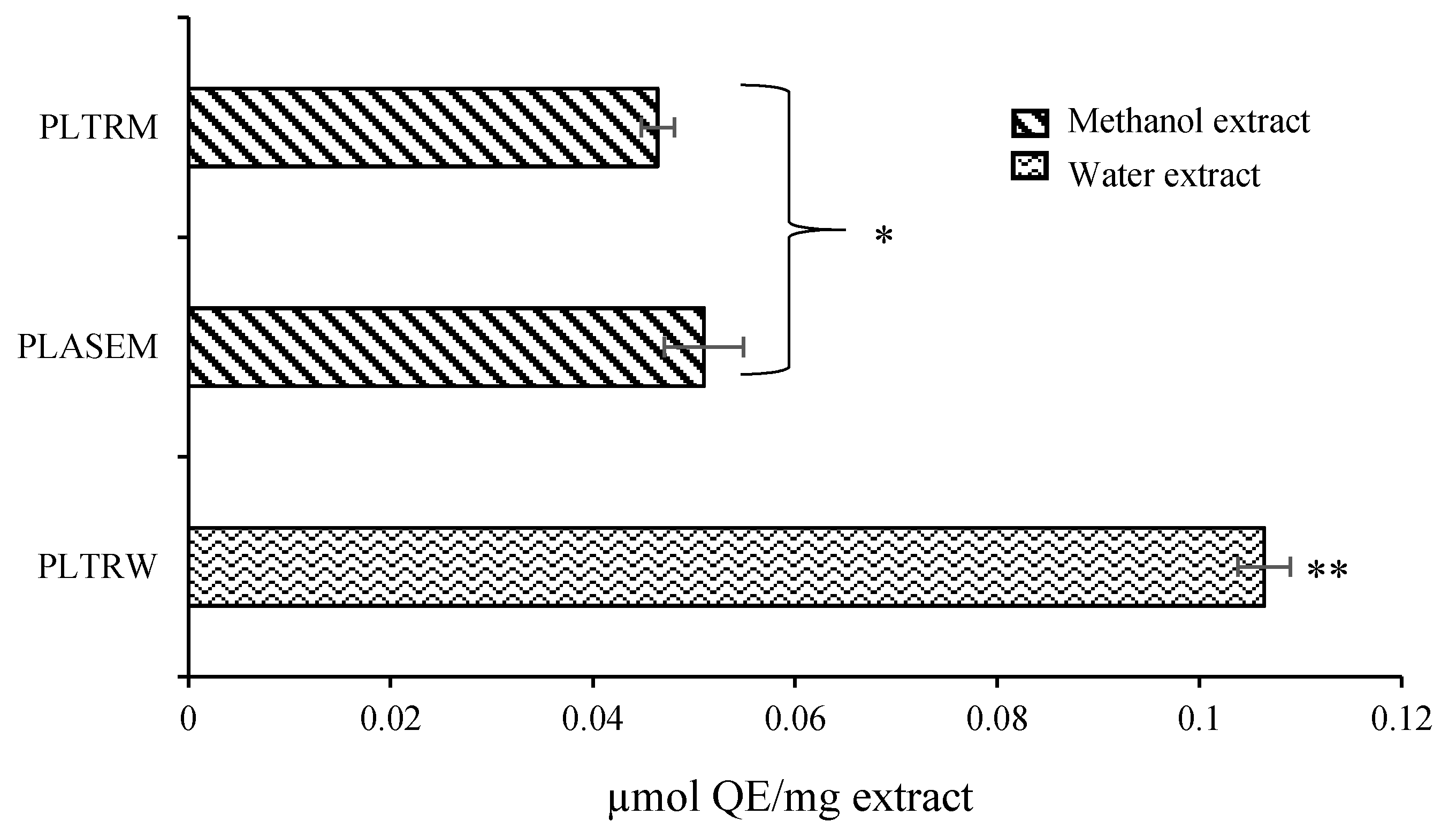
| Samples | Yield, % | TPC, mg GAE/g | DPPH•, μM TE/g | ABTS•+, μM TE/g | |||
| DWE | DWP | DWE | DWP | DWE | DWP | ||
| PLASEM | 47.6 ± 1.1 a | 516.4 ± 14.53 a | 245.6 ± 6.91 a | 2424 ± 23.66 a | 1153 ± 11.25 a | 4524 ± 26.75 a | 2151 ± 12.72 a |
| PRASEM | 23.9 ± 0.6 b | 247.3 ± 8.79 b | 59.1 ± 2.1 b | 567.7 ± 11.38 b | 135.6 ± 2.72 b | 935.7 ± 4.51 b | 223.5 ± 1.08 b |
| PLTRM | 43.5 ± 0.8 c | 601.1 ± 10.51 c | 285.9 ± 4.99 c | 2553 ± 28.40 c | 1110 ± 12.34 c | 4610 ± 18.70 c | 2004 ± 8.13 c |
| PLTRW | 33.2 ± 0.2 d | 430.6 ± 9.49 d | 187.2 ± 4.13 d | 2138 ± 13.41 d | 710.7 ± 4.46 d | 4231 ± 14.07 d | 1406 ± 6.76 d |
| PRTRW | 19.2 ± 0.5 e | 215.7 ± 3.05 e | 41.4 ± 0.6 e | 343.4 ± 6.06 e | 65.94 ± 1.16 e | 886.0 ± 7.36 e | 170.1 ± 1.41 e |
| ORAC, μM TE/g | HOSC, μM TE/g | HORAC, µmol CAE/g | |||||
| PLASEM | 1433 ± 6.93 a | 681.4 ± 3.48 a | 1957 ± 7.47 a | 931.0 ± 9.53 a | 1891 ± 13.06 a | 899.4 ± 5.45 a | |
| PLTRM | 1257 ± 10.50 b | 546.6 ± 2.59 b | 2012 ± 10.22 b | 874.6 ± 6.61 b | 1758 ± 7.56 b | 764.1 ± 8.69 b | |
| PLTRW | 1232 ± 7.61 c | 409.6 ± 1.34 c | 2010 ± 8.02 b | 668.2 ± 4.15 c | 1566 ± 8.45 c | 520.3 ± 3.16 b | |
| Peak No. | Compound | Molecular Formula | tR (min) | m/z, [M − H]− | PLASEM | PRASEM | PLTRM | PLTRW | PRTRW | MS Fragments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid a | C7H12O6 | 0.5 | 191.0563 | + | − | + | + | + | - |
| 2 | Dihexose c | C12H21O11 | 0.6 | 341.1097 | + | + | + | + | + | 191.0564; 149.0459; 89.0246 |
| 3 | Galloyl-hexoside b,d | C13H15O10 | 0.8 | 331.0671 | + | − | + | + | + | 169.0140 |
| 4 | Gallic acid a | C7H5O5 | 0.9 | 169.0144 | + | + | + | + | + | - |
| 5 | Digallic acid b,d | C14H9O9 | 1.9 | 321.0253 | − | − | − | + | − | 169.0138; 125.0240 |
| 6 | Methyl galate b,d | C8H7O5 | 2.8 | 183.0302 | + | + | + | + | − | 168.0060; 140.0112; 124.0166 |
| 7 | Tri-galloyl-hexoside d | C27H23O18 | 4.3 | 635.0889 | + | − | − | − | − | 483.0838; 465.0641; 169.0175 |
| 8 | Unidentified | C21H31O13 | 4.5 | 491.1768 | + | − | − | − | + | - |
| 9 | Paeoniflorin derivative b,d | C24H29O13 | 5.3 | 525.1617 | + | + | + | + | + | 479.1508; 449.1448; 357.1191; 327.1086; 283.0818; 165.0556; 121.0294 |
| 10 | Tetra-galloyl-hexoside b,d | C34H27O22 | 5.9 | 787.1006 | + | − | + | + | − | 617.0793; 456.0683; 169.0139 |
| 11 | Quercetin dihexoside c,d | C27H29O16 | 6.1 | 609.1460 | + | − | + | + | − | 463.0883; 301.0325; |
| 12 | Quercetin-galloyl-hexoside b,d | C28H23O16 | 6.2 | 615.0993 | + | − | + | + | − | 463.0884; 301.0324; 169.0132 |
| 13 | Quercetin pentoside b,d | C20H17O11 | 7.5 | 433.0779 | + | − | + | + | + | 301.0340 |
| 14 | Methyl digallate b,d | C15H11O9 | 7.6 | 335.0410 | + | + | + | − | + | 183.0301; 124.0170 |
| 15 | Penta-galloyl-hexoside b,d | C41H31O26 | 7.8 | 939.1122 | + | + | + | + | - | 769.0901; 617.0795; 447.0569; 169.0132 |
| 16 | Isorhamnetin-galloyl-hexosyde b,d | C29H25O16 | 8.2 | 629.1149 | + | + | + | + | + | 477.1046; 315.0568; 169.0141 |
| 17 | Dihydroxybenzoic acetate-digallate derivative b,d | C24H17O15 | 8.4 | 545.0580 | + | − | − | − | − | 469.0489; 393.0466; 169.0135 |
| 18 | Paeoniflorin derivative b,d | C24H29O13 | 8.4 | 525.1615 | − | − | + | + | − | 479.1358; 449.1440; 357.1088; 327.1075; 283.0714; 165.0544; 121.0281 |
| 19 | Dihydroxybenzoic acetate-digallate derivative b,d | C24H17O15 | 8.7 | 545.0581 | + | + | + | + | − | 469.0407; 393.0461; 169.0139 |
| 20 | Dihydroxybenzoic acetate-digallate derivative b,d | C24H17O15 | 9.3 | 545.0582 | + | - | + | + | − | 469.0381; 393.0468; 169.0140 |
| 21 | Hexa-galloyl-hexoside b,d | C48H35O30 | 9.3 | 1091.1236 | + | − | + | + | − | 939.1101; 769.0895; 617.0811; 169.0143 |
| 22 | Unidentified | C15H25O26 | 9.9 | 621.0637 | + | + | + | + | + | - |
| 23 | Unidentified | C31H21O19 | 10.2 | 697.0695 | + | − | − | + | − | - |
| Sample | Quinic Acid | Gallic Acid | Quercetin Dihexoside * | |||
|---|---|---|---|---|---|---|
| DWP | DWE | DWP | DWE | DWP | DWE | |
| PLASEM | 3.61 ± 0.12 a | 7.58 ± 0.99 a | 2.59 ± 0.08 a | 5.45 ± 0.09 a | 0.50 ± 0.001 a | 1.04 ± 0.05 a |
| PRASEM | nd | nd | 0.11 ± 0.001 b | 0.44 ± 0.001 b | nd | nd |
| PLTRM | 1.52 ± 0.09 b | 3.50 ± 0.31 b | 0.22 ± 0.001 b,c | 0.51 ± 0.002 b | 0.66 ± 0.003 b | 1.52 ± 0.02 b |
| PLTRW | 1.83 ± 0.10 c | 5.51 ± 0.32 c | 4.10 ± 0.25 d | 12.33 ± 0.87 c | 0.55 ± 0.001 c | 1.64 ± 0.01 b |
| PRTRW | 0.41 ± 0.001 d | 2.14 ± 0.12 b | 0.34 ± 0.002 b,c | 1.75 ± 0.44 d | nd | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants 2019, 8, 249. https://doi.org/10.3390/antiox8080249
Dienaitė L, Pukalskienė M, Pukalskas A, Pereira CV, Matias AA, Venskutonis PR. Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants. 2019; 8(8):249. https://doi.org/10.3390/antiox8080249
Chicago/Turabian StyleDienaitė, Lijana, Milda Pukalskienė, Audrius Pukalskas, Carolina V. Pereira, Ana A. Matias, and Petras Rimantas Venskutonis. 2019. "Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities" Antioxidants 8, no. 8: 249. https://doi.org/10.3390/antiox8080249
APA StyleDienaitė, L., Pukalskienė, M., Pukalskas, A., Pereira, C. V., Matias, A. A., & Venskutonis, P. R. (2019). Isolation of Strong Antioxidants from Paeonia Officinalis Roots and Leaves and Evaluation of Their Bioactivities. Antioxidants, 8(8), 249. https://doi.org/10.3390/antiox8080249






