Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review
Abstract
1. Introduction
2. Carotenoids
2.1. Chemical Structure
2.2. Source of Carotenoids
2.3. Bioavailability and Bioaccessibility
2.4. Nutritional Requirements
3. Carotenoids and Hepatic Health
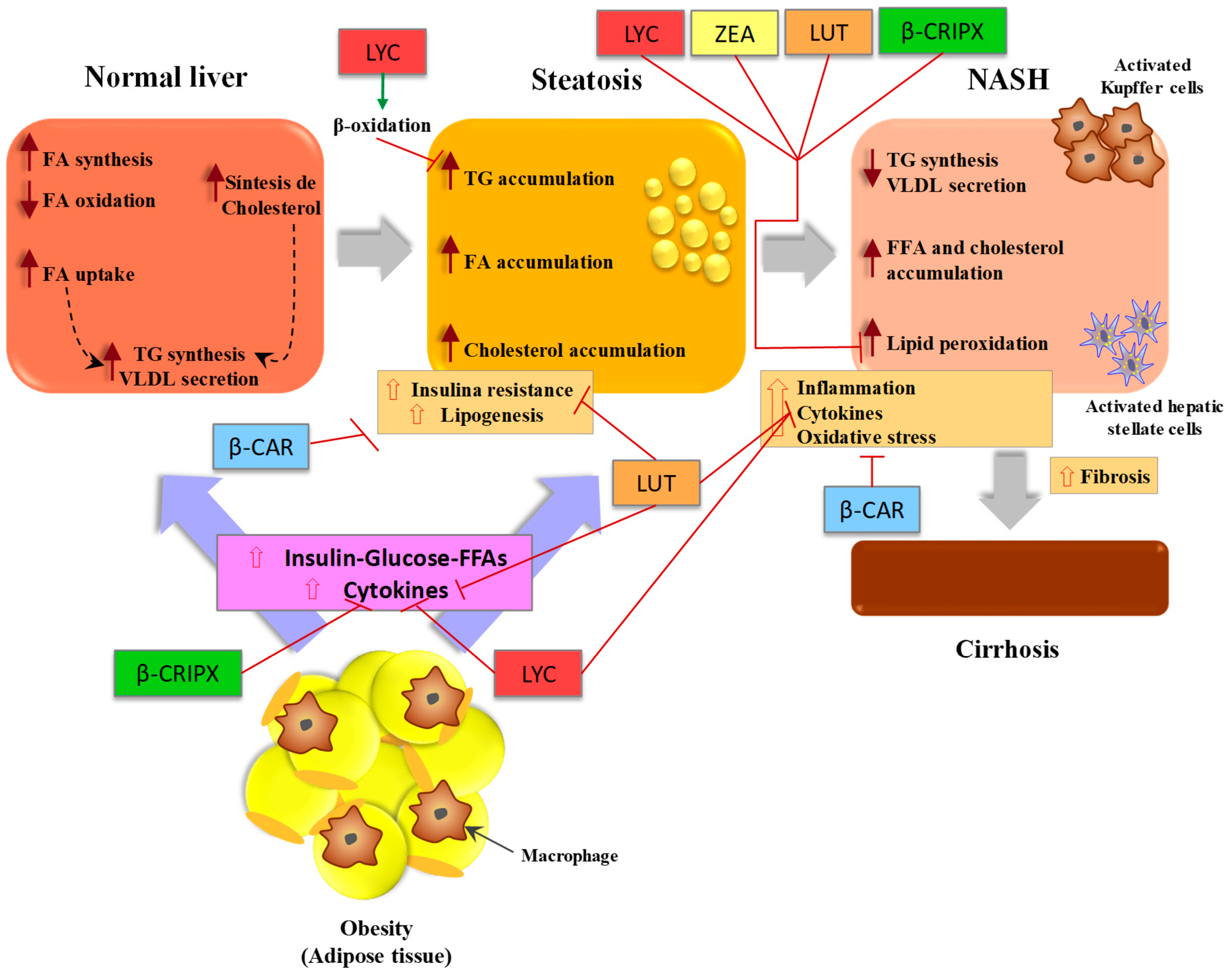
3.1. Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD)
3.2. β-Carotene
3.3. Lycopene
3.4. Lutein
3.5. β-Cryptoxanthin
3.6. Other Carotenoids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPK | Mitogen-activated protein kinase |
| CYP2E1 | Cytochrome P450 family 2 subfamily E member 1 |
| LDL | Low density lipoproteins |
| HDL | High density lipoprotein |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| GGT | Gamma-glutamyltransferase |
| UDP | Uridine diphosphate |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| NF-κB | Nuclear factor kappa B |
References
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Böhm, V. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M. Carotenoids: Liver diseases prevention. In Bioactive Foods as Dietary Interventions for Liver and Gastrointestinal Disease, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 421–436. ISBN 9780123971548. [Google Scholar]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; McClintock, C.S.; Shaw, J.E. Metabolic syndrome and serum carotenoids: Findings of a cross-sectional study in Queensland, Australia. Br. J. Nutr. 2009, 102, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Mol. Nutr. Food Res. 2012, 56, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; García-Alonso, J. Biodisponibilidad de antioxidantes en la dieta. In Antioxidantes en Alimentos y Salud, 1st ed.; Álvarez-Parilla, E., González-Aguilar, A., De la Rosa, L.A., Ayala-Zavala, J.F., Eds.; AM-Editores: Ciudad de Mexico, Mexico, 2012; pp. 257–291. ISBN 9786074372076. [Google Scholar]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in adipose tissue biology and obesity. Subcell. Biochem. 2016, 79, 377–414. [Google Scholar] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The role of carotenoids in human skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Meinke, M.C.; Darvin, M.E.; Vollert, H.; Lademann, J. Bioavailability of natural carotenoids in human skin compared to blood. Eur. J. Pharm. Biopharm. 2010, 76, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Johnson, E.J. The role of phytonutrients in skin health. Nutrients 2010, 2, 903–928. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Milic, S.; Turk Wensveen, T.; Grgic, I.; Jakopcic, I.; Stimac, D.; Orlic, L. Nonalcoholic fatty liver disease—A multisystem disease? World J. Gastroenterol. 2016, 22, 9488–9505. [Google Scholar] [CrossRef]
- Azzam, H.; Malnick, S. Non-alcoholic fatty liver disease—The heart of the matter. World J. Hepatol. 2015, 7, 1369–1376. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Zara, V. Antioxidant dietary approach in treatment of fatty liver: New insights and updates. World J. Gastroenterol. 2017, 23, 4146–4157. [Google Scholar] [CrossRef]
- Murillo, A.G.; DiMarco, D.M.; Fernandez, M.L. The potential of non-provitamin A carotenoids for the prevention and treatment of non-alcoholic fatty liver disease. Biology 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar] [PubMed]
- Christensen, K.; Lawler, T.; Mares, J. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003–2014. Nutrients 2019, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Guo, M.H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180–10188. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, B.; Miyake, T.; Yamamoto, S.; Furukawa, S.; Hiasa, Y. Usefulness of Beta-cryptoxanthin for nonalcoholic fatty liver diseases. J. Food Nutr. Disord. 2016, 5, 3. [Google Scholar]
- Elvira-Torales, L.I.; Martín-Pozuelo, G.; González-Barrio, R.; Navarro-González, I.; Pallarés, F.J.; Santaella, M.; Periago-Castón, M.J. Ameliorative effect of spinach on non-alcoholic fatty liver disease induced in rats by a high-fat diet. Int. J. Mol. Sci. 2019, 20, 1662. [Google Scholar] [CrossRef] [PubMed]
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic fatty liver disease and insulin resistance: New insights and potential new treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arabidopsis Book 2012, 10, e0158. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Bohn, T. Bioavailability of non-provitamin A carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.J.; Yahia, E.M.; Gadea-Béjar, A.A.; Pérez-Martínez, J.D.; Ochoa-Reyes, E. Biodisponibilidad y actividad biológica de carotenoides y vitamina A. In Antioxidantes en Alimentos y Salud, 1st ed.; Álvarez-Parilla, E., González-Aguilar, A., De la Rosa, L.A., Ayala-Zavala, J.F., Eds.; AM-Editores: Ciudad de Mexico, Mexico, 2012; pp. 293–327. ISBN 9786074372076. [Google Scholar]
- Von Elbe, J.H.; Schwartz, S.J. Colorantes. In Química de Los Alimentos, 2nd ed.; Fennema, O.R., Ed.; ACRIBIA: Zaragoza, Spain, 2000; pp. 773–850. ISBN 8420009148. [Google Scholar]
- Rühl, R. Non-pro-vitamin A and pro-vitamin A carotenoids in atopy development. Int. Arch. Allergy Immunol. 2013, 161, 99–115. [Google Scholar] [CrossRef]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef]
- Khachik, F.; Sprangler, C.J.; Smith, J.C.; Canfield, L.M.; Steck, A.; Pfander, H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 1997, 69, 1873–1881. [Google Scholar]
- Carlsen, M.H.; Karlsen, A.; Lillegaard, I.T.; Gran, J.M.; Drevon, C.A.; Blomhoff, R.; Andersen, L.F. Relative validity of fruit and vegetable intake estimated from an FFQ, using carotenoid and flavonoid biomarkers and the method of triads. Br. J. Nutr. 2011, 105, 1530–1538. [Google Scholar] [CrossRef]
- Baldrick, F.R.; Woodside, J.V.; Elborn, J.S.; Young, I.S.; McKinley, M.C. Biomarkers of fruit and vegetable intake in human intervention studies: A systematic review. Crit. Rev. Food Sci. Nutr. 2011, 51, 795–815. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids in Food. In Carotenoids: Nutrition and Health, 4th ed.; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; pp. 45–66. [Google Scholar]
- Shete, V.; Quadro, L. Mammalian metabolism of β-carotene: Gaps in knowledge. Nutrients 2013, 5, 4849–4868. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M.; Godoy, H.T.; Amaya-Farfan, J. Updated brazilian database on food carotenoids: Factors affecting carotenoids composition. J. Food Compos. Anal. 2008, 21, 445–463. [Google Scholar] [CrossRef]
- Latief, U.; Ahmad, R. Role of dietary carotenoids in different etiologies of chronic liver diseases. In Descriptive Food Science, 1st ed.; Valero Díaz, A., García-Gimeno, R.M., Eds.; IntechOpen: London, UK, 2018; pp. 93–112. ISBN 9781789845952. [Google Scholar]
- O’Neill, M.E.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; Southon, S. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Šivel, M.; Kráčmar, S.; Fišera, M.; Klejdus, B.; Kubáň, V. Lutein content in marigold flower (Tagetes erecta L.) concentrates used for production of food supplements. Czech J. Food Sci. 2014, 32, 521–525. [Google Scholar] [CrossRef]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive database of carotenoid contents in Ibero-American foods. A valuable tool in the context of functional foods and the establishment of recommended intakes of bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef]
- Giuffrida, D.; Torre, G.; Dugo, P.; Dugo, G. Determination of the carotenoid profile in peach fruits, juice and jam. Fruits 2012, 68, 39–44. [Google Scholar] [CrossRef][Green Version]
- Mercadante, A.Z. Carotenoids in foods: Sources and stability during processing and storage. In Food Colorants: Chemical and Functional Properties, 1st ed.; Socaciu, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 213. ISBN 9780849393570. [Google Scholar]
- Yahia, E.M.; Ornelas-Paz, J.J. Chemistry, stability, and biological actions of carotenoids. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability, 1st ed.; de la Rosa, L.A., Álvarez-Parrilla, E., González-Aguilar, G.A., Eds.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 177–222. ISBN 978-0-813-80320-3. [Google Scholar]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Marze, S. Bioaccessibility of lipophilic micro-constituents from a lipid emulsion. Food Funct. 2015, 6, 3218–3227. [Google Scholar] [CrossRef]
- Van Het Hof, K.H.; West, C.E.; Weststrate, J.A.; Hautvast, J.G. Dietary factors that affect the bioavailability of carotenoids. J. Nutr. 2000, 130, 503–506. [Google Scholar] [CrossRef]
- Donhowe, E.G.; Kong, F. Beta-carotene: Digestion, microencapsulation, and in vitro bioavailability. Food Bioprocess Technol. 2014, 7, 338–354. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Colle, I.J.; Lemmens, L.; Knockaert, G.; Van Loey, A.; Hendrickx, M. Carotene Degradation and Isomerization during Thermal Processing: A Review on the Kinetic Aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 1844–1855. [Google Scholar] [CrossRef]
- Lemmens, L.; Colle, I.; Van Buggenhout, S.; Palmero, P.; Van Loey, A.; Hendrickx, M. Carotenoid bioaccessibility in fruit- and vegetable-based food products as affected by product (micro)structural characteristics and the presence of lipids: A review. Trends Food Sci. Technol. 2014, 38, 125–135. [Google Scholar] [CrossRef]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Priyadarshani, A.M.B. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1710–1717. [Google Scholar] [CrossRef]
- Bernhardt, S.; Schlich, E. Impact of different cooking methods on food quality: Retention of lipophilic vitamins in fresh and frozen vegetables. J. Food Eng. 2006, 77, 327–333. [Google Scholar] [CrossRef]
- Fernandez-Garcia, E.; Carvajal-Lerida, I.; Jaren-Galan, M.; Garrido-Fernandez, J.; Perez-Galvez, A.; Hornero-Mendez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Honda, M.; Maeda, H.; Fukaya, T.; Goto, M. Effects of Z-isomerization on the bioavailability and functionality of carotenoids: A review. In Descriptive Food Science, 1st ed.; Valero Díaz, A., García-Gimeno, R.M., Eds.; IntechOpen: London, UK, 2018; pp. 141–159. ISBN 9781789845952. [Google Scholar]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Schwartz, S.J. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef]
- Coral-Hinostroza, G.N.; Ytrestøyl, T.; Ruyter, B.; Bjerkeng, B. Plasma appearance of unesterified astaxanthin geometrical E/Z and optical R/S isomers in men given single doses of a mixture of optical 3 and 3′R/S isomers of astaxanthin fatty acyl diesters. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 139, 99–110. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Goto, M. Improved carotenoid processing with sustainable solvents utilizing Z-isomerization-induced alteration in physicochemical properties: A review and future directions. Molecules 2019, 7, 2149. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef]
- Yeum, K.J.; Russell, R.M. Carotenoid bioavailability and bioconversion. Annu. Rev. Nutr. 2002, 22, 483–504. [Google Scholar] [CrossRef]
- Goltz, S.R.; Campbell, W.W.; Chitchumroonchokchai, C.; Failla, M.L.; Ferruzzi, M.G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol. Nutr. Food Res. 2012, 56, 866–877. [Google Scholar] [CrossRef]
- Roodenburg, A.J.; Leenen, R.; Hof, K.H.; Weststrate, J.A.; Tijburg, L.B. Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. Am. J. Clin. Nutr. 2000, 71, 1187–1193. [Google Scholar] [CrossRef]
- Periago, M.J.; Bravo, S.; García-Alonso, F.J.; Rincón, F. Detection of key factors affecting lycopene in vitro accessibility. J. Agric. Food Chem. 2013, 61, 3859–3867. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Raju, M.; Prakash, M.K.; Baskaran, V. Phospholipid, oleic acid micelles and dietary olive oil influence the lutein absorption and activity of antioxidant enzymes in rats. Lipids 2009, 44, 799–806. [Google Scholar] [CrossRef]
- Victoria-Campos, C.I.; Ornelas-Paz, J.; De, J.; Yahia, E.M.; Failla, M.L. Effect of the interaction of heat-processing style and fat type on the micellarization of lipid-soluble pigments from green and red pungent peppers (Capsicum annuum). J. Agric. Food Chem. 2013, 61, 3642–3653. [Google Scholar] [CrossRef]
- Hoffmann, J.; Linseisen, J.; Riedl, J.; Wolfram, G. Dietary fiber reduces the antioxidative effect of a carotenoid and alpha-tocopherol mixture on LDL oxidation ex vivo in humans. Eur. J. Nutr. 1999, 38, 278–285. [Google Scholar] [CrossRef]
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018, 58, 1–36. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Jansen, M.C.; Van Kappel, A.L.; Ocké, M.C.; Van ‘t Veer, P.; Boshuizen, H.C.; Riboli, E.; Bueno-de-Mesquita, H.B. Plasma carotenoid levels in Dutch men and women, and the relation with vegetable and fruit consumption. Eur. J. Clin. Nutr. 2004, 58, 1386–1395. [Google Scholar] [CrossRef]
- Brevik, A.; Andersen, L.F.; Karlsen, A.; Trygg, K.U.; Blomhoff, R.; Drevon, C.A. Six carotenoids in plasma used to assess recommended intake of fruits and vegetables in a controlled feeding study. Eur. J. Clin. Nutr. 2004, 58, 1166–1173. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Martínez-Cuesta, M.C. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites--a position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef]
- Riboli, E.; Péquignot, G.; Repetto, F.; Axerio, M.; Raymond, L.; Boffetta, P.; Tuyns, A.J. A comparative study of smoking, drinking and dietary habits in population samples in France, Italy, Spain and Switzerland. I. Study design and dietary habits. Rev. Epidemiol. Sante Publique 1988, 36, 151–165. [Google Scholar]
- Olmedilla, B.; Granado, F.; Southon, S.; Wright, A.J.; Blanco, I.; Gil-Martinez, E.; Thurnham, D.I. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br. J. Nutr. 2001, 85, 227–238. [Google Scholar] [CrossRef]
- Elia, M.; Stratton, R.J. Geographical inequalities in nutrient status and risk of malnutrition among English people aged 65 y and older. Nutrition 2005, 21, 1100–1106. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Ribot, J. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Stice, C.P.; Xia, H.; Wang, X.D. Tomato lycopene prevention of alcoholic fatty liver disease and hepatocellular carcinoma development. Chronic Dis. Transl. Med. 2018, 4, 211–224. [Google Scholar] [CrossRef]
- Lucarini, M.; Lanzi, S.; D’Evoli, L.; Aguzzi, A.; Lombardi-Boccia, G. Intake of vitamin A and carotenoids from the Italian population--results of an Italian total diet study. Int. J. Vitam. Nutr. Res. 2006, 76, 103–109. [Google Scholar] [CrossRef]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Barnhart, S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef]
- Blumberg, J.; Block, G. The Alpha-Tocopherol, Beta-Carotene cancer prevention study in Finland. Nutr. Rev. 1994, 52, 242–245. [Google Scholar] [CrossRef]
- Haider, C.; Ferk, F.; Bojaxhi, E.; Martano, G.; Stutz, H.; Bresgen, N.; Eckl, P. Effects of β-carotene and its cleavage products in primary pneumocyte type II cells. Antioxidants 2017, 6, 37. [Google Scholar] [CrossRef]
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W., Jr. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Cano, M.P.; De Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Martín, A. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, prostaglandin E2 and monocyte chemotactic protein-1 in healthy humans. J. Nutr. Biochem. 2006, 17, 183–189. [Google Scholar] [CrossRef]
- Heath, E.; Seren, S.; Sahin, K.; Kucuk, O. The role of tomato lycopene in the treatment of prostate cancer. In Tomatoes, Lycopene and Human Health: Preventing Chronic Diseases, 1st ed.; Rao, A.V., Ed.; Caledonian Science Press: Scotland, UK, 2007; pp. 127–140. ISBN 9780955356506. [Google Scholar]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef]
- Gyamfi, D.; Patel, V. Liver metabolism: Biochemical and molecular regulations. In Nutrition, Diet Therapy, and the Liver, 1st ed.; Preedy, V.R., Lakshman, R., Srirajaskanthan, R., Watson, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 3–15. ISBN 9781138111790. [Google Scholar]
- Cabré Gelada, E.; Peña Quintana, L.; Virgili Casas, N. Nutrición en las enfermedades hepatobiliares. In Tratado de Nutrición: Nutrición y Enfermedad, 3rd ed.; Gil, A., Ed.; Editorial Médica Panamericana: Madrid, Spain, 2017; pp. 865–906. ISBN 9788491101949. [Google Scholar]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Singhal, S.; Baker, S.S.; Baker, R.D.; Zhu, L. Role of paraoxonase 1 as an antioxidant in nonalcoholic steatohepatitis. In The Liver, 1st ed.; Patel, V.B., Rajendram, R., Preedy, V.R., Eds.; Academic Press: London, UK, 2018; pp. 15–20. ISBN 9780128039519. [Google Scholar]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Calzadilla Bertot, L.; Adams, L.A. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef]
- Mencin, A.A.; Lavine, J.E. Nonalcoholic fatty liver disease in children. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 151–157. [Google Scholar] [CrossRef]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Zhu, L. Antioxidant mechanisms in nonalcoholic fatty liver disease. Curr. Drug Targets 2015, 16, 1301–1314. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef]
- Shiota, G.; Tsuchiya, H. Pathophysiology of NASH: Insulin resistance, free fatty acids and oxidative stress. J. Clin. Biochem. Nutr. 2006, 38, 127–132. [Google Scholar] [CrossRef]
- Ota, T.; Takamura, T.; Kurita, S.; Matsuzawa, N.; Kita, Y.; Uno, M.; Nakanuma, Y. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology 2007, 132, 282–293. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725. [Google Scholar] [CrossRef]
- Karadeniz, G.; Acikgoz, S.; Tekin, I.O.; Tascýlar, O.; Gun, B.D.; Cömert, M. Oxidized low-density-lipoprotein accumulation is associated with liver fibrosis in experimental cholestasis. Clinics 2008, 63, 531–540. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Rozman, D. Nonalcoholic Fatty liver disease: Focus on lipoprotein and lipid deregulation. J. Lipids 2011, 2011, 783976. [Google Scholar] [CrossRef]
- Werman, M.J.; Ben-Amotz, A.; Mokady, S. Availability and antiperoxidative effects of beta-carotene from Dunaliella bardawil in alcohol-drinking rats. J. Nutr. Biochem. 1999, 10, 449–454. [Google Scholar] [CrossRef]
- Chen, H.; Tappel, A. Protection by multiple antioxidants against lipid peroxidation in rat liver homogenate. Lipids 1996, 31, 47–50. [Google Scholar] [CrossRef]
- Whittaker, P.; Wamer, W.G.; Chanderbhan, R.F.; Dunkel, V.C. Effects of alpha-tocopherol and beta-carotene on hepatic lipid peroxidation and blood lipids in rats with dietary iron overload. Nutr. Cancer 1996, 25, 119–128. [Google Scholar] [CrossRef]
- Senoo, H.; Yoshikawa, K.; Morii, M.; Miura, M.; Imai, K.; Mezaki, Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol. Int. 2010, 34, 1247–1272. [Google Scholar] [CrossRef]
- Xiao, M.L.; Chen, G.D.; Zeng, F.F.; Qiu, R.; Shi, W.Q.; Lin, J.S.; Chen, Y.M. Higher serum carotenoids associated with improvement of non-alcoholic fatty liver disease in adults: A prospective study. Eur. J. Nutr. 2019, 58, 721–730. [Google Scholar] [CrossRef]
- Burri, B.J. Lycopene and human health. In Phytochemicals in Nutrition and Health, 1st ed.; Meskin, M.S., Bidlack, W.R., Davies, A.J., Omaye, S.T., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 157–172. ISBN 9781587160837. [Google Scholar]
- Sarni, R.O.; Suano de Souza, F.I.; Ramalho, R.A.; Schoeps Dde, O.; Kochi, C.; Catherino, P.; Colugnati, F.B. Serum retinol and total carotene concentrations in obese pre-school children. Med. Sci. Monit. 2005, 11, CR510–CR514. [Google Scholar]
- Seif El-Din, S.H.; El-Lakkany, N.M.; El-Naggar, A.A.; Hammam, O.A.; Abd El-Latif, H.A.; Ain-Shoka, A.A.; Ebeid, F.A. Effects of rosuvastatin and/or β-carotene on non-alcoholic fatty liver in rats. Res. Pharm. Sci. 2015, 10, 275–287. [Google Scholar]
- Baybutt, R.C.; Molteni, A. Dietary beta-carotene protects lung and liver parenchyma of rats treated with monocrotaline. Toxicology 1999, 137, 69–80. [Google Scholar] [CrossRef]
- Patel, V.; Sail, S. β-carotene protects the physiological antioxidants against aflatoxin-B1 induced carcinogenesis in albino rats. Pak. J. Biol. Sci. 2006, 9, 1104–1111. [Google Scholar]
- Kheir Eldin, A.A.; Motawi, T.M.; Sadik, N.A. Effect of some natural antioxidants on aflatoxin B1-induced hepatic toxicity. EXCLI J. 2008, 7, 119–131. [Google Scholar]
- Harari, A.; Harats, D.; Marko, D.; Cohen, H.; Barshack, I.; Kamari, Y.; Shaish, A. A 9-cis beta-carotene-enriched diet inhibits atherogenesis and fatty liver formation in LDL receptor knockout mice. J. Nutr. 2008, 138, 1923–1930. [Google Scholar] [CrossRef]
- Ozturk, F.; Gul, M.; Ates, B.; Ozturk, I.C.; Cetin, A.; Vardi, N.; Yilmaz, I. Protective effect of apricot (Prunus armeniaca L.) on hepatic steatosis and damage induced by carbon tetrachloride in Wistar rats. Br. J. Nutr. 2009, 102, 1767–1775. [Google Scholar] [CrossRef]
- Liu, Q.; Bengmark, S.; Qu, S. Nutrigenomics therapy of hepatisis C virus induced-hepatosteatosis. BMC Gastroenterol. 2010, 10, 49. [Google Scholar] [CrossRef]
- Yadav, D.; Hertan, H.I.; Schweitzer, P.; Norkus, E.P.; Pitchumoni, C.S. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am. J. Gastroenterol. 2002, 97, 2634–2639. [Google Scholar] [CrossRef]
- Tainaka, T.; Shimada, Y.; Kuroyanagi, J.; Zang, L.; Oka, T.; Nishimura, Y.; Tanaka, T. Transcriptome analysis of anti-fatty liver action by Campari tomato using a zebrafish diet-induced obesity model. Nutr. Metab. 2011, 8, 88. [Google Scholar] [CrossRef]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.C.; Fung, M.L.; Xu, A.M.; Tipoe, G.L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes 2013, 3, e81. [Google Scholar] [CrossRef]
- Villaça Chaves, G.; Pereira, S.E.; Saboya, C.J.; Ramalho, A. Non-alcoholic fatty liver disease and its relationship with the nutritional status of vitamin A in individuals with class III obesity. Obes. Surg. 2008, 18, 378–385. [Google Scholar] [CrossRef]
- Lan, Q.Y.; Zhang, Y.J.; Liao, G.C.; Zhou, R.F.; Zhou, Z.G.; Chen, Y.M.; Zhu, H.L. The Association between Dietary Vitamin A and Carotenes and the Risk of Primary Liver Cancer: A Case–Control Study. Nutrients 2016, 8, 624. [Google Scholar] [CrossRef]
- Wang, L.; Ding, C.; Zeng, F.; Zhu, H. Low Levels of Serum β-Carotene and β-Carotene/Retinol Ratio Are Associated with Histological Severity in Nonalcoholic Fatty Liver Disease Patients. Ann. Nutr. Metab. 2019, 74, 156–164. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Aust, O.; Tronnier, H.; Sies, H. Lycopene-rich products and dietary photoprotection. Photochem. Photobiol. Sci. 2006, 5, 238–242. [Google Scholar] [CrossRef]
- Shivashangari, K.S.; Ravikumar, V.; Vinodhkumar, R.; Sheriff, S.A.; Devaki, T. Hepatoprotective potential of lycopene on D-galactosamine/lipopolysaccharide induced hepatitis in rats. Pharmacologyonline 2006, 2, 151–170. [Google Scholar]
- Sheriff, S.A.; Devaki, T. Lycopene stabilizes lipoprotein levels during D-galactosamine/lipopolysaccharide induced hepatitis in experimental rats. Asian Pac. J. Trop. Biomed. 2012, 2, 975–980. [Google Scholar] [CrossRef]
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674. [Google Scholar] [CrossRef]
- Bahcecioglu, I.H.; Kuzu, N.; Metin, K.; Ozercan, I.H.; Ustündag, B.; Sahin, K.; Kucuk, O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010, 2010, 262179. [Google Scholar] [CrossRef]
- Bernal, C.; Martín-Pozuelo, G.; Lozano, A.B.; Sevilla, A.; García-Alonso, J.; Canovas, M.; Periago, M.J. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem. 2013, 24, 1870–1881. [Google Scholar] [CrossRef]
- Ip, B.; Wang, X.D. Non-alcoholic steatohepatitis and hepatocellular carcinoma: Implications for lycopene intervention. Nutrients 2013, 6, 124–162. [Google Scholar] [CrossRef]
- Piña-Zentella, R.M.; Rosado, J.L.; Gallegos-Corona, M.A.; Madrigal-Pérez, L.A.; García, O.P.; Ramos-Gomez, M. Lycopene improves diet-mediated recuperation in rat model of nonalcoholic fatty liver disease. J. Med. Food 2016, 19, 607–614. [Google Scholar] [CrossRef]
- Kujawska, M.; Ewertowska, M.; Adamska, T.; Sadowski, C.; Ignatowicz, E.; Jodynis-Liebert, J. Antioxidant effect of lycopene-enriched tomato paste on N-nitrosodiethylamine-induced oxidative stress in rats. J. Physiol. Biochem. 2014, 70, 981–990. [Google Scholar] [CrossRef]
- Ip, B.C.; Liu, C.; Ausman, L.M.; Von Lintig, J.; Wang, X.D. Lycopene attenuated hepatic tumorigenesis via differential mechanisms depending on carotenoid cleavage enzyme in mice. Cancer Prev. Res. 2014, 7, 1219–1227. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Alonso, J.; Periago, M.J. Bioactive compounds of tomato: Cancer chemopreventive effects and influence on the transcriptome in hepatocytes. J. Funct. Foods 2018, 42, 271–280. [Google Scholar] [CrossRef]
- Martín-Pozuelo, G.; Navarro-González, I.; González-Barrio, R.; Santaella, M.; García-Alonso, J.; Hidalgo, N.; Periago, M.J. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur. J. Nutr. 2015, 54, 933–944. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; Da Silva, T.P.; De Araujo, G.R.; Araujo, C.M.; Da Silva, R.C.; Lima, W.G.; Costa, D.C. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2017, 263, 7–17. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; Da Silva, R.C.; Júnior, J.V.R.; Figueiredo, V.P.; Talvani, A.; Cangussú, S.D.; Costa, D.C. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorg. Med. Chem. 2017, 25, 1057–1065. [Google Scholar] [CrossRef]
- Yefsah-Idres, A.; Benazzoug, Y.; Otman, A.; Latour, A.; Middendorp, S.; Janel, N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016, 7, 2862–2869. [Google Scholar] [CrossRef]
- Gupta, P.; Bhatia, N.; Bansal, M.P.; Koul, A. Lycopene modulates cellular proliferation, glycolysis and hepatic ultrastructure during hepatocellular carcinoma. World J. Hepatol. 2016, 8, 1222–1233. [Google Scholar] [CrossRef]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods. 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Navarro-González, I.; González-Barrio, R.; Martín-Pozuelo, G.; Doménech, G.; Seva, J.; Periago-Castón, M. Tomato juice supplementation influences the gene expression related to steatosis in rats. Nutrients 2018, 10, 1215. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Pang, R.; Tao, J.Y.; Zhang, S.L.; Zhao, L.; Yue, X.; Wang, Y.F.; Wu, J.G. In vitro antiviral activity of lutein against hepatitis B virus. Phytother. Res. 2010, 24, 1627–1630. [Google Scholar] [CrossRef]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Firdous, A.P.; Preethi, K.C.; Kuttan, R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride-and ethanol-induced hepatic damage. J. Pharm. Pharmacol. 2013, 62, 1054–1060. [Google Scholar] [CrossRef]
- Qiu, X.; Gao, D.H.; Xiang, X.; Xiong, Y.F.; Zhu, T.S.; Liu, L.G.; Hao, L.P. Ameliorative effects of lutein on non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2015, 21, 8061–8072. [Google Scholar] [CrossRef]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; DiMarco, D.M.; Missimer, A.; Hu, S.; Fernandez, M.L. Compared with powdered lutein, a lutein nanoemulsion increases plasma and liver lutein, protects against hepatic steatosis, and affects lipoprotein metabolism in guinea pigs. J. Nutr. 2016, 146, 1961–1969. [Google Scholar] [CrossRef]
- Haegele, A.D.; Gillette, C.; O’Neill, C.; Wolfe, P.; Heimendinger, J.; Sedlacek, S.; Thompson, H.J. Plasma xanthophyll carotenoids correlate inversely with indices of oxidative DNA damage and lipid peroxidation. Cancer Epidemiol. Biomark. Prev. 2000, 9, 421–425. [Google Scholar]
- Katsuura, S.; Imamura, T.; Bando, N.; Yamanishi, R. Beta-Carotene and beta-cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol. Nutr. Food Res. 2009, 53, 1396–1405. [Google Scholar] [CrossRef]
- Takayanagi, K. Prevention of adiposity by the oral administration of β-cryptoxanthin. Front. Neurol. 2011, 2, 67. [Google Scholar] [CrossRef]
- Kobori, M.; Ni, Y.; Takahashi, Y.; Watanabe, N.; Sugiura, M.; Ogawa, K.; Ota, T. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLoS ONE 2014, 9, e98294. [Google Scholar] [CrossRef]
- Ni, Y.; Nagashimada, M.; Zhan, L.; Nagata, N.; Kobori, M.; Sugiura, M.; Ota, T. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology 2015, 156, 987–999. [Google Scholar] [CrossRef]
- Murakoshi, M.; Nishino, H.; Satomi, Y.; Takayasu, J.; Hasegawa, T.; Tokuda, H.; Iwasaki, R. Potent preventive action of α-carotene against carcinogenesis: Spontaneous liver carcinogénesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by α-carotene than by β-carotene. Cancer Res. 1992, 52, 6583–6587. [Google Scholar]
- Chamberlain, S.M.; Hall, J.D.; Patel, J.; Lee, J.R.; Marcus, D.M.; Sridhar, S.; Bartoli, M. Protective effects of the carotenoid zeaxanthin in experimental nonalcoholic steatohepatitis. Dig. Dis. Sci. 2009, 54, 1460–1464. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, C.; Liu, J.; Liu, Z.M.; Ling, W.H.; Chen, Y.M. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci. Rep. 2015, 5, 12951. [Google Scholar] [CrossRef]
| Food | Lutein | Zeaxanthin | β-Cryptoxanthin | α-Carotene | β-Carotene | Lycopene |
|---|---|---|---|---|---|---|
| Avocado | 0.21–0.36 | 0.01 | 0.02–0.03 | 0.02–0.03 | 0.05–0.08 | – |
| Banana | 0.09–0.19 | – | n.d.–0.01 | 0.06–0.16 | 0.04–0.13 | n.d.–0.25 |
| Peach | – | – | – | – | – | 0.01 |
| Guava | – | – | 0.02–0.12 | n.d. | 0.10–2.67 | 0.77–1.82 |
| Fig | 0.08 | – | 0.01 | 0.02 | 0.04 | 0.32 |
| Kiwi | – | – | – | – | <0.02 | <0.01 |
| Mandarin Orange | – | – | 0.63–1.06 | n.d. | 0.11–0.32 | – |
| Mango | – | – | 0.02–0.32 | n.d. | 0.11–1.20 | <0.01–0.72 |
| Apple | 0.02 | n.d. | n.d. | n.d. | 0.019 | n.d. |
| Passion fruit | – | – | 0.18 | – | 0.36–0.78 | – |
| Orange | – | – | 0.07–0.14 | n.d. | 0.17–0.48 | n.d. |
| Peach | – | 0.02–0.04 | 0.004–0.02 | – | 0.14–0.26 | – |
| Papaya | 0.09–0.32 | – | n.d.–1.03 | n.d. | 0–08–0.66 | n.d.–7.56 |
| Pineapple | – | – | 0.07–0.12 | n.d. | 0.14–0.35 | 0.27–0.61 |
| Watermelon | – | – | n.d. | n.d. | 0.31–0.78 | 4.77–13.52 |
| Grapefruit | – | – | – | – | – | 0.75 |
| Tangerine | 0.17 | il | 0.43 | 0.03 | 0.26 | – |
| Grape | 0.01 | n.d. | n.d. | n.d. | 0.02 | n.d. |
| Plum | 0.08–0.09 | n.d. | n.d. | n.d. | 0.09–0.14 | n.d. |
| Apricot | 0.12–0.19 | n.d.–0.04 | – | n.d.–0.04 | 0.59–3.80 | 0.05 |
| Chard | 3.60 | 0.01 | n.d. | n.d. | 2.90 | n.d. |
| Artichoke | 0.59–0.63 | – | – | – | 0.27–0.37 | – |
| Broccoli | 0.71–3.30 | – | n.d. | n.d. | 0.29–1.75 | n.d. |
| Pumpkin | 0.63 | – | 0.06 | – | 0.49 | 0.50 |
| Sweet Potato | 0.05 | – | – | – | 7.83 | – |
| Peas | 1.91 | il | n.d. | n.d. | 0.52 | n.d. |
| Red Pepper | 0.25–8.51 | 0.59–1.35 | 0.25–0.45 | n.d.–0.29 | 1.44–2.39 | – |
| Jalapeño Pepper | 0.84 | – | – | 0.01–0.17 | 0.38–8.58 | – |
| Spinach | 5.93–7.90 | il | n.d. | n.d. | 3.10–4.81 | n.d. |
| Lettuce | 1.00–4.78 | – | – | – | 0.87–2.96 | – |
| Corn | 0.41 | 0.22 | n.d. | n.d. | n.d. | n.d. |
| Cucumber | 0.46–0.84 | il | n.d. | n.d. | 0.11–0.27 | n.d. |
| Red chili | n.d. | – | – | – | 6.53–15.40 | – |
| Cabbage | 0.45 | il | n.d. | n.d. | 0.41 | n.d. |
| Tomato | 0.05–0.21 | il | n.d. | n.d. | 0.32–1.50 | 0.85–12.70 |
| Carrot | 0.25–0.51 | il | n.d. | 2.84–4.96 | 4.35–8.84 | n.d. |
| Kale | 4.80–11.47 | – | – | – | 1.02–7.38 | – |
| Parsley | 6.40–10.65 | il | n.d. | n.d. | 4.44–4.68 | n.d. |
| Coriander | 6.00–14.80 | – | – | 2.90–11.30 | 4.80–8.40 | – |
| Sample (N), Country | Woman/Man (Age) | Dietary Intake (mg/day) | |||||
|---|---|---|---|---|---|---|---|
| α-car | β-car | β-cryp | Lut/ Zea | Lyco | Total | ||
| EUROPE | |||||||
| N = 1968, Italy | W, M (> 1) | 0.15 | 2.6 | 0.17 | 4.01 | 7.38 | 14.31 |
| N=75, France | W, M (25–45) | [0.74] | [5.84] | [0.45] | [2.50] | [4.75] | 14.28 |
| N = 65, North Ireland | W, M (25–45) | 1.04 | 5.55 | 0.99 | 1.59 | 5.01 | 14.18 |
| N = 71, United Kingdom | W, M (25–45) | [1.04] | [5.55] | [0.99] | [1.59] | [5.01] | 14.18 |
| N = 73, Ireland | W, M (25–45) | 1.23 | 5.16 | 0.78 | 1.56 | 4.43 | 13.16 |
| N = 72, Netherlands | W, M (25–45) | 0.68 | 4.35 | 0.97 | 2.01 | 4.86 | 12.87 |
| N = 159, Sweden | W (56–75) | 1.03 | 3.47 | 0.46 | 2.64 | 2.15 | 9.75 |
| N = 3000, Spain | W, M (18–64) | 0.27 | 1.46 | 0.32 | 1.24 | 3.06 | 6.35 |
| OCEANIA | |||||||
| N = 91, Australia | W (18–70) | [2.0] | [6.87] | [2.28] | [5.05] | 16.2 | |
| AMERICA | |||||||
| N = 459, Costa Rica | 115 W (59±10) 344 M (56±11) | 0.73 0.45 | 4.67 3.41 | 0.55 0.38 | 2.89 2.41 | 5.77 5.45 | 14.61 12.10 |
| N = 402, USA (Afro-American) | 155 M (34–84) 247 W (34–84) | [0.33] [0.25] | [2.21] [2.21] | [0.11] [0.13] | [1.85) [1.93] | [3.16] [2.60] | 7.66 7.12 |
| N = 50, Dominican Republic | W, M (50–90) | 0.7 | 2.7 | 0.22 | 1.33 | 1.46 | 6.41 |
| USA | W, M (≥ 20) | 0.4 | 1.9 | 0.2 | 1.4 | 1.4 | 5.3 |
| N = 55,950, Brazil | W, M (≥ 10) | 0.16 | 0.92 | 0.16 | 0.83 | 0.83 | 2.9 |
| Agent | Model | Main Results | Reference |
|---|---|---|---|
| β-carotene | Rat: carcinogenesis induced by AFB1 | ↑ Antioxidantes enzymes (GSH-Px, catalase, GST) and vitamin C ↓ Risk of toxicity due to AFB1 | [120] |
| Alga Dunaliella bardawil (rich in (9Z)-β-carotene) | Mouse: fed high-fat diet, LDL receptor knockout mouse | ↓ Plasma cholesterol and atherogenesis (VLDL y LDL) ↓ Accumulation of fat and liver inflammation ↓ Levels of hepatic inflammatory genes (VCAM-1, IL-1α, MCP-1, INF-γ) | [122] |
| Apricot (rich in β-carotene) | Rat: Hepatic steatosis and damage induced by CCL4 | ↓ Liver MDA ↑ Levels of total GSH, catalase, SOD and GSH-Px ↓ Oxidative stress ↓ Hepatic steatosis and liver damage | [124] |
| Tomato “Campari” (rich in β-carotene and lycopene) | Zebrafish: Obesity induced by diet | ↓ SREBF1 in the Marn ↑ FOXO1 in the expression of genes ↓ Diet-induced obesity, dyslipidemia and hepatic steatosis | [127] |
| Lycium barbarum polysaccharides (rich in β-carotene) | Rat: NASH induced by a high-fat diet | ↑ Modulation of NF-κB and the MAPK pathway ↓ Accumulation of liver fat, inflammatory liver response, fibrosis and oxidative stress ↑ Hepatoprotective properties | [128] |
| Dietary carotenes and vitamin A | Human: patients with primary liver cancer | ↓ Risk of primary liver cancer | [129] |
| Lycopene | Rat: NASH induced by high-fat diet | ↓ Levels of CYP2E1 protein, MDA (plasma and liver) and TNF-α ↑ Hepatic GSH level ↓ Steatosis and inflammation | [138] |
| Tomato juice | Rat: hypercholesterolemic and NAFLD induced by the diet | ↓ Levels of TG in plasma and isoprostanes in urine ↑ Acummulation of lycopene in the liver ↑ Relief of amino acid depletion ↑ Recovery of the redox balance in the liver ↑ Levels of L-carnitine ↑ Protective effect of NAFLD | [139] |
| Tomato juice | Rat: NAFLD induced by a high-fat diet | ↓ Isoprostanes in urine, plasma TG and LDL ↑ Activity of mitochondrial β-oxidation and peroxisomal ↓ Steatosis | [145] |
| Lycopene | SK-Hep-1 cells: PKC pathway mediated by ROS production. Mouse: Hepatotoxicity induced by APAP overdose | ↓ Production of ROS, NADPH oxidase and MMP-2, GSSG ↑ GSH and CAT | [146] |
| Lycopene | Rat: NAFLD induced by a high-fat diet | ↓ ALT, AST, triglyceride, total cholesterol, MDA, LDL and FFA ↓ CYP2E1 and TNF-α ↑ GSH, SOD y HDL ↑ Protective effect on NAFLD | [16] |
| Lycopene | Rat: NAFLD induced by a high-fat diet | ↓ liver weight, LDL and liver total cholesterol ↑ GSH-Px, SOD and CAT en the liver | [141] |
| Lycopene | Mouse: liver injury induced by AFB1 | ↓ Acummulatio of AFB1-ADN adducts in the liver ↑ Activation of Nrf2 signaling ↑ Antioxidant potential and liver detoxification | [150] |
| Tomato juice | Rat: hypercholesterolemic and NAFLD induced by the diet | ↑ Regulation of CD36, FXR and HNF4A ↓ Regulation of APOB and LPL ↓ Synthesis of fatty acids, triglycerides and cholesterol ↑ Levels of metabolites related to the antioxidant response | [151] |
| Lutein | Guinea pig: Hepatic steatosis induced by a hypercholesterolemic diet | ↓ Hepatic free cholesterol ↓ Malondialdehyde and hepatic TNF-α ↓ Binding to the hepatic DNA of NF-κB | [154] |
| Lutein | Rat: Hepatocellular carcinoma induced by N-nitrosodiethylamine (NDEA) | ↓ ALT, AST, alkaline phosphatase in plasma and liver tissue ↑ GSH ↓ GGT ↑ UDP-glucoronyl transferase and glutathione-S-transferase | [155] |
| Lutein | Rat: NAFLD induced by a high-fat diet | ↓ Liver total cholesterol and triglycerides ↑ HDL in serum ↓ ALT in serum ↑ Hepatic insulin sensitivity ↑ Hepatic fatty acids catabolism | [156] |
| Lutein | Guinea pig: Hepatic steatosis induced by a hypercholesterolemic diet | ↓ Hepatic steatosis (evaluated histologically) ↓ Total hepatic cholesterol ↓ Plasma ALT and LDL activity | [157] |
| β-cryptoxanthin | Mouse: Obese model | ↓ Body weight and abdominal adipose tissue ↓ Triglycerides and serum total cholesterol ↓ Inflammatory citokines ↑ Lipid metabolism and energy consumption | [160] |
| β-cryptoxanthin | Mouse: NASH induced by a diet high in cholesterol and high in fat | ↓ Liver TBARS ↑ Suppresses the expression of the inducible LPS and TNF-α genes ↓ Inflammatory response (suppresses the activation of macrophages, T helper and citototoxic cells) | [161] |
| β-cryptoxanthin | Mouse: Hepatic steatosis and NASH induced by the diet high in fat and cholesterol | ↓ Total content of hepatic macrophages and T cells | [162] |
| β-cryptoxanthin | Human: Patients with NAFLD (NASH and NAFL) | ↓ GGT, LDL and serum IL-6 ↑ SOD and serum IL-10 ↑ Antioxidant and anti-inflammtory activities | [17] |
| α-carotene | Mouse: spontaneous hepatic carcinogenesis | ↓ Hepatomas | [163] |
| Zeaxanthin | Gerbil from Mongolia: NASH induced by a diet deficient in methionine and choline | ↓ Liver fibrosis ↓ Hepatic lipid hydroperoxides | [164] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. https://doi.org/10.3390/antiox8070229
Elvira-Torales LI, García-Alonso J, Periago-Castón MJ. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants. 2019; 8(7):229. https://doi.org/10.3390/antiox8070229
Chicago/Turabian StyleElvira-Torales, Laura Inés, Javier García-Alonso, and María Jesús Periago-Castón. 2019. "Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review" Antioxidants 8, no. 7: 229. https://doi.org/10.3390/antiox8070229
APA StyleElvira-Torales, L. I., García-Alonso, J., & Periago-Castón, M. J. (2019). Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants, 8(7), 229. https://doi.org/10.3390/antiox8070229





