The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ultrasonic Assisted Extraction (UAE) and Kinetic Modeling
2.4. Spectrophotometric Measurements
2.4.1. Quantification of Chlorophylls and Carotenoids
2.4.2. Total Phenolic Content
2.4.3. Antioxidant Activity Determination
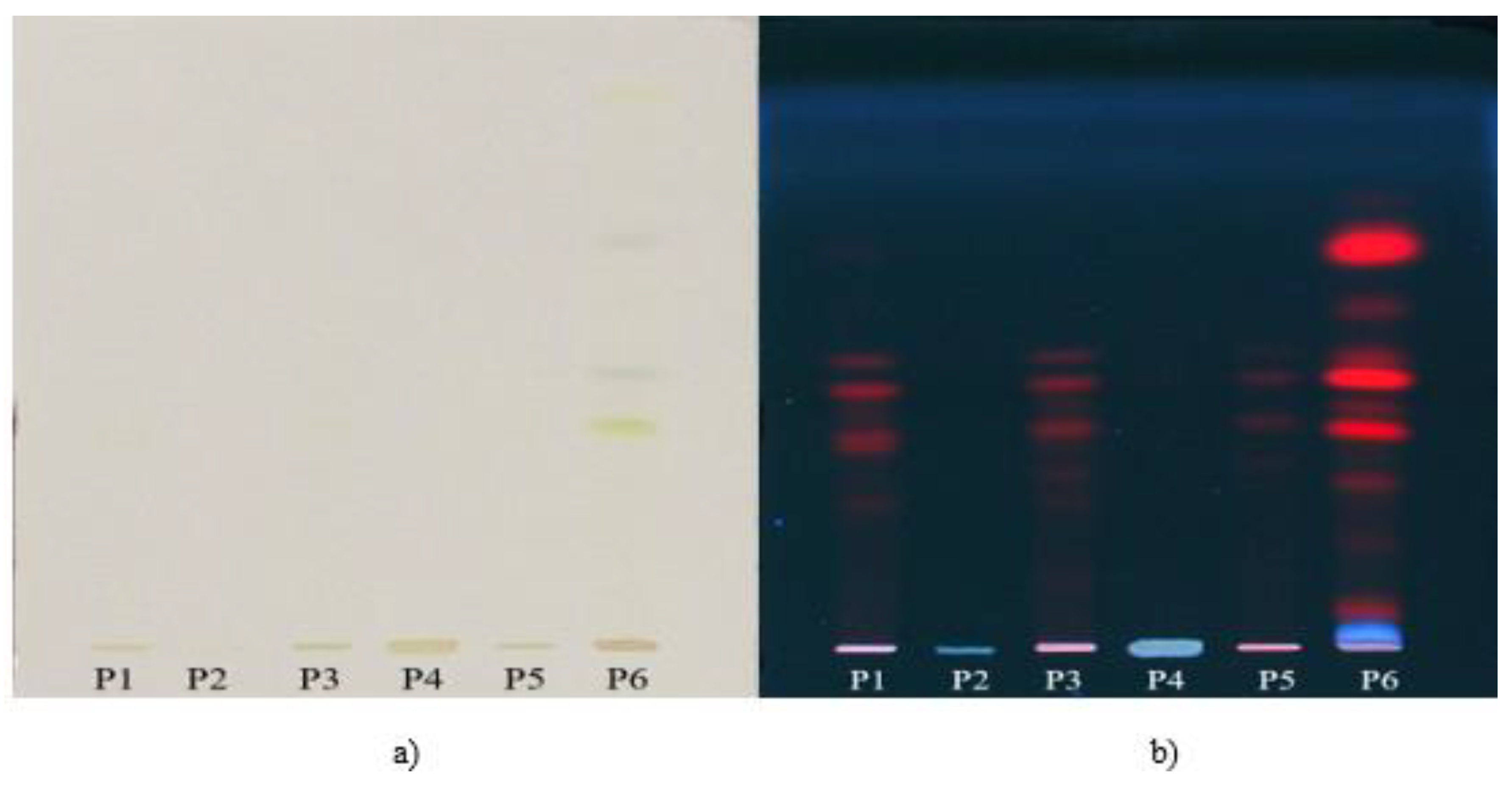
2.5. TLC Analysis
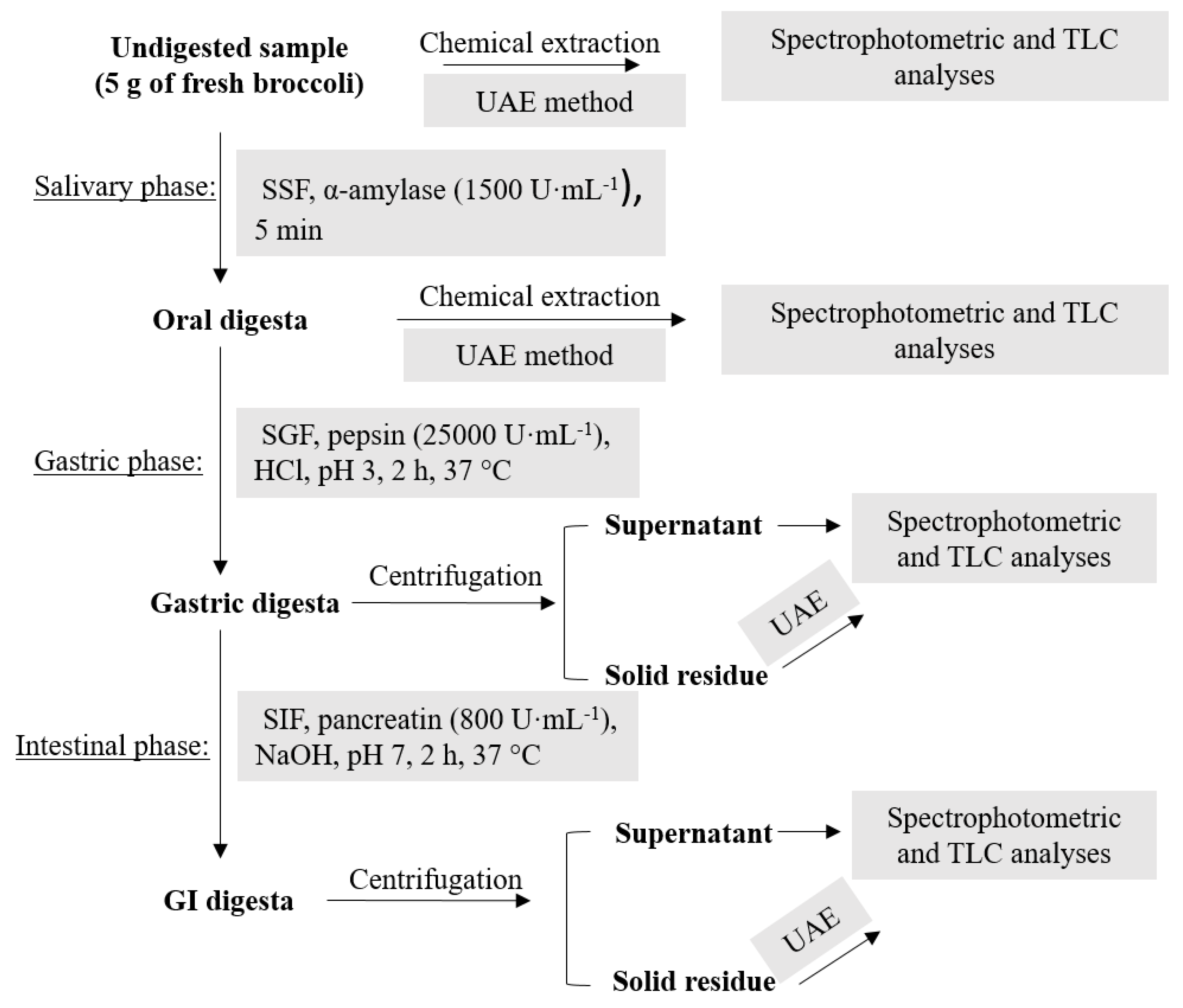
2.6. In Vitro Simulated GI Digestion
2.7. Statistics
3. Results and Discussion
3.1. UAE of Chlorophylls and Carotenoids from Brassica Oleracea
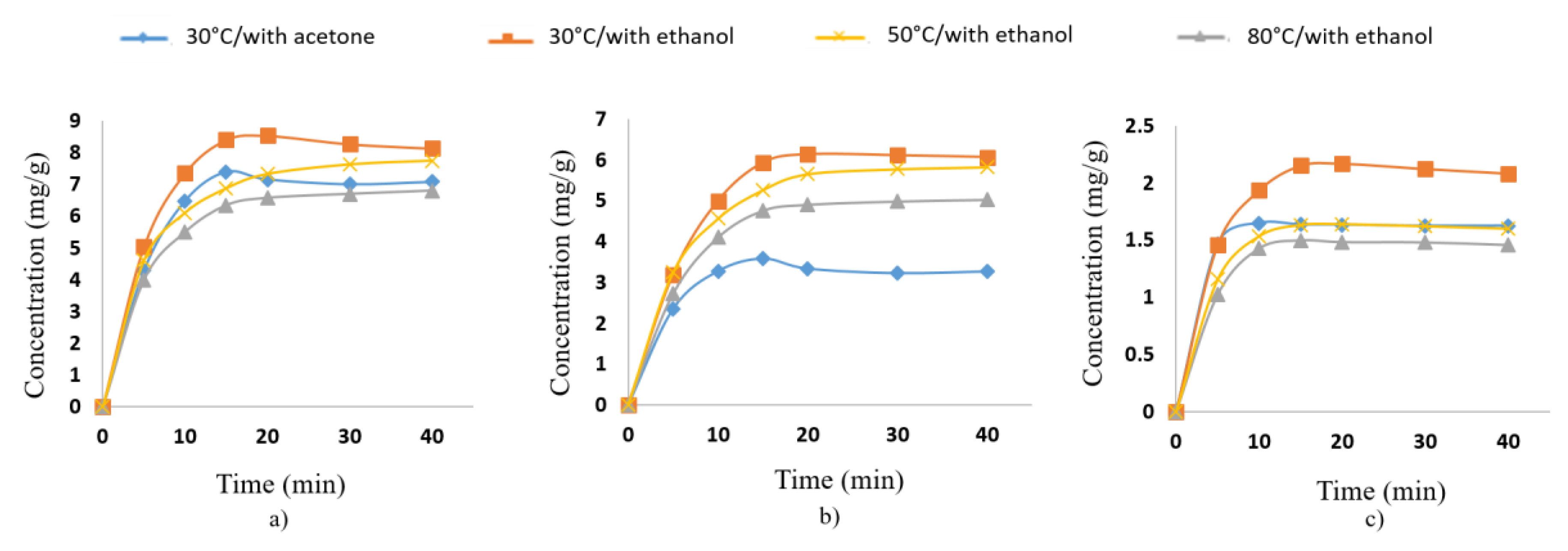
3.1.1. Optimization of UAE
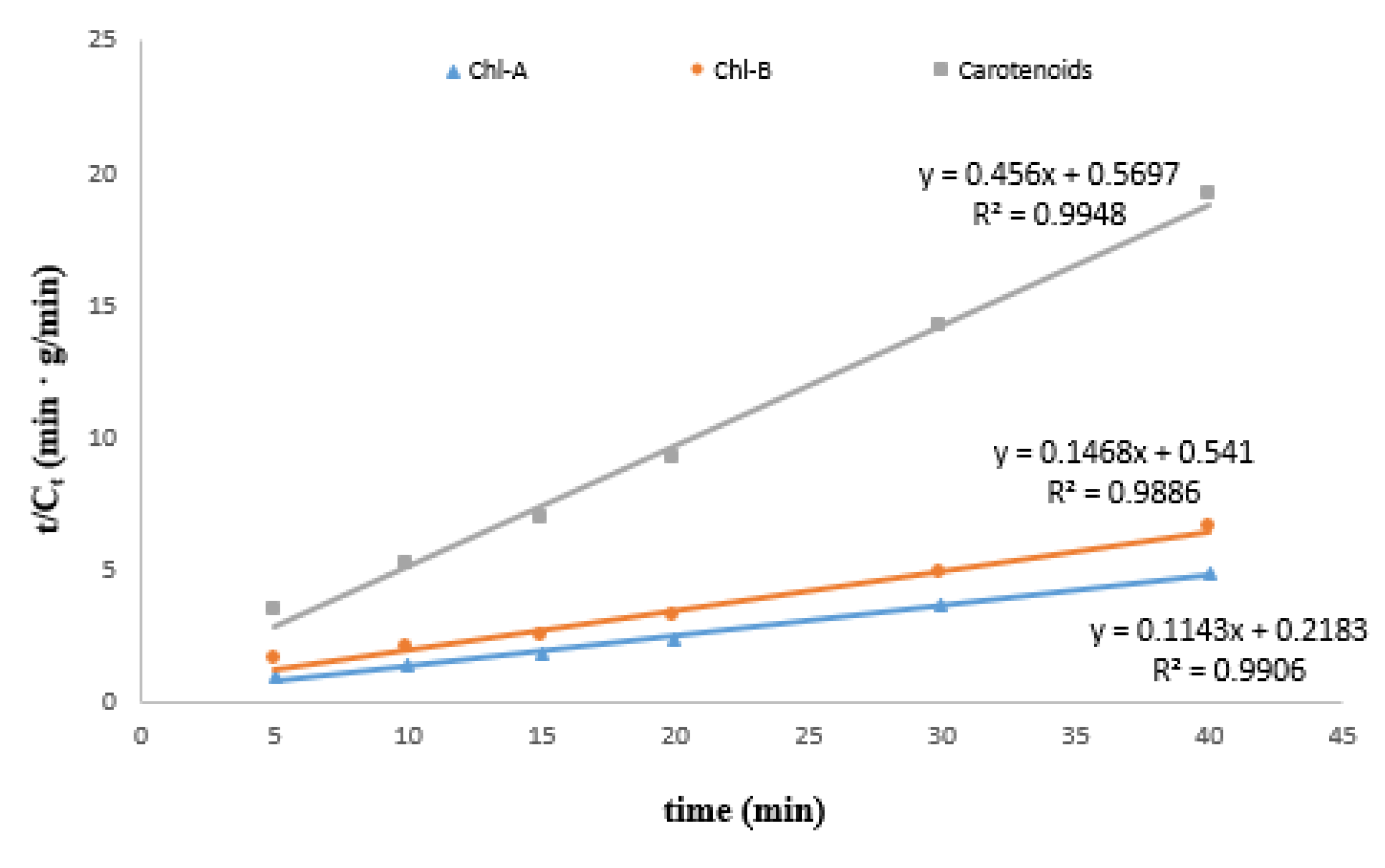
3.1.2. Kinetic Modeling
3.2. In Vitro Simulated GI Digestion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality 1234. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Fernández-León, M.; Fernández-León, A.; Lozano, M.; Ayuso, M.; González-Gómez, D. Identification, quantification and comparison of the principal bioactive compounds and external quality parameters of two broccoli cultivars. J. Funct. Foods 2012, 4, 465–473. [Google Scholar] [CrossRef]
- Gunathilakea, K.D.P.P.; Ranaweerab, K.K.D.S.; Rupasinghec, H.P.V. Change of phenolics, carotenoids, and antioxidant capacity following simulated gastrointestinal digestion and dialysis of selected edible green leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012, 75, 588–602. [Google Scholar] [CrossRef]
- Cilla, A.; Gonzalez-Sarrías, A.; Tomás-Barberán, F.A.; Espin, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Goula, A.M. Ultrasound-assisted extraction of pomegranate seed oil—Kinetic modeling. J. Food Eng. 2013, 117, 492–498. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fernández-León, M.; Lozano, M.; Ayuso, M.; Fernández-León, A.; González-Gómez, D. Fast and accurate alternative UV-chemometric method for the determination of chlorophyll A and B in broccoli (Brassica oleracea Italica) and cabbage (Brassica oleracea Sabauda) plants. J. Food Compos. Anal. 2010, 23, 809–813. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Wang, L.-J.; Li, D.; Jiao, S.-S.; Chen, X.D.; Mao, Z.-H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Qu, W.; Pan, Z.; Ma, H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010, 99, 16–23. [Google Scholar] [CrossRef]
- Courraud, J.; Berger, J.; Cristol, J.-P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2008, 27, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In vitro digestion of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 40, 400–407. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
| Volume of Constituents (mL) | ||||||
|---|---|---|---|---|---|---|
| Simulated Digestion Fluid | KCl (37.3 g/L) | KH2PO4 (68 g/L) | NaHCO3 (84 g/L) | NaCl (117 g/L) | MgCl2(H2O)6 (30.5 g/L) | (NH4)2CO3 (48 g/L) |
| SSF (pH = 7) | 15.1 | 3.7 | 6.8 | __ | 0.5 | 0.06 |
| SGF (pH = 3) | 6.9 | 0.9 | 12.5 | 11.8 | 0.4 | 0.5 |
| SIF (pH = 7) | 6.8 | 0.8 | 42.5 | 9.6 | 1.1 | __ |
| Compound | B = 1/Ce | A = 1/h | Ce = 1/B (mg/g) | h = 1/A | k = h/(Ce)2 (g/mg·min) | R2 |
|---|---|---|---|---|---|---|
| Chl-A | 0.1143 | 0.2183 | 8.7490 | 4.5809 | 0.0598 | 0.9906 |
| Chl-B | 0.1468 | 0.5410 | 6.8120 | 1.8484 | 0.0398 | 0.9886 |
| Carotenoids | 0.4560 | 0.5697 | 2.1930 | 1.7553 | 0.3650 | 0.9948 |
| Digestion Phase | Concentration | Antioxidant Activity (µmol/mL) | ||
|---|---|---|---|---|
| Carotenoids (mg/mL) | Chlorophylls (mg/mL) | Polyphenols (µg/mL) | ||
| Initial | 6.11 ± 0.98 | 18.21 ± 1.21 | 136.44 ± 9.85 | 1.05 ± 0.03 |
| Gastric | 1.34 ± 0.12 | 7.49 ± 0.84 | 126.36 ± 5.16 | 0.43 ± 0.01 |
| Intestinal | 1.34 ± 0.14 | 5.09 ± 0.62 | 69.24 ± 4.25 | 0.20 ± 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212. https://doi.org/10.3390/antiox8070212
Scrob T, Hosu A, Cimpoiu C. The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants. 2019; 8(7):212. https://doi.org/10.3390/antiox8070212
Chicago/Turabian StyleScrob, Teodora, Anamaria Hosu, and Claudia Cimpoiu. 2019. "The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content" Antioxidants 8, no. 7: 212. https://doi.org/10.3390/antiox8070212
APA StyleScrob, T., Hosu, A., & Cimpoiu, C. (2019). The Influence of in Vitro Gastrointestinal Digestion of Brassica oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants, 8(7), 212. https://doi.org/10.3390/antiox8070212






