In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Total Phenolic Content
2.3. Total Flavonoid Content
2.4. Total Anthocyanin Content
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis for the Quantification of Phenylpropanoid Content
2.6. Phenylpropanoid Extraction and HPLC Analysis
2.7. Reducing Power Assay
2.8. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Assay
2.9. Hydrogen Peroxide Radical Scavenging Activity
2.10. Superoxide Radical Scavenging Activity
2.11. Extraction Process for Screening Antibacterial Activity
2.12. Bacterial Cultivation
2.13. Screening Antibacterial Activity Using the Disc Diffusion Method
2.14. Statistical Analysis
3. Results
3.1. Total Phenolic, Flavonoid, and Anthocyanin Contents
3.2. Phenylpropanoid HPLC Analysis
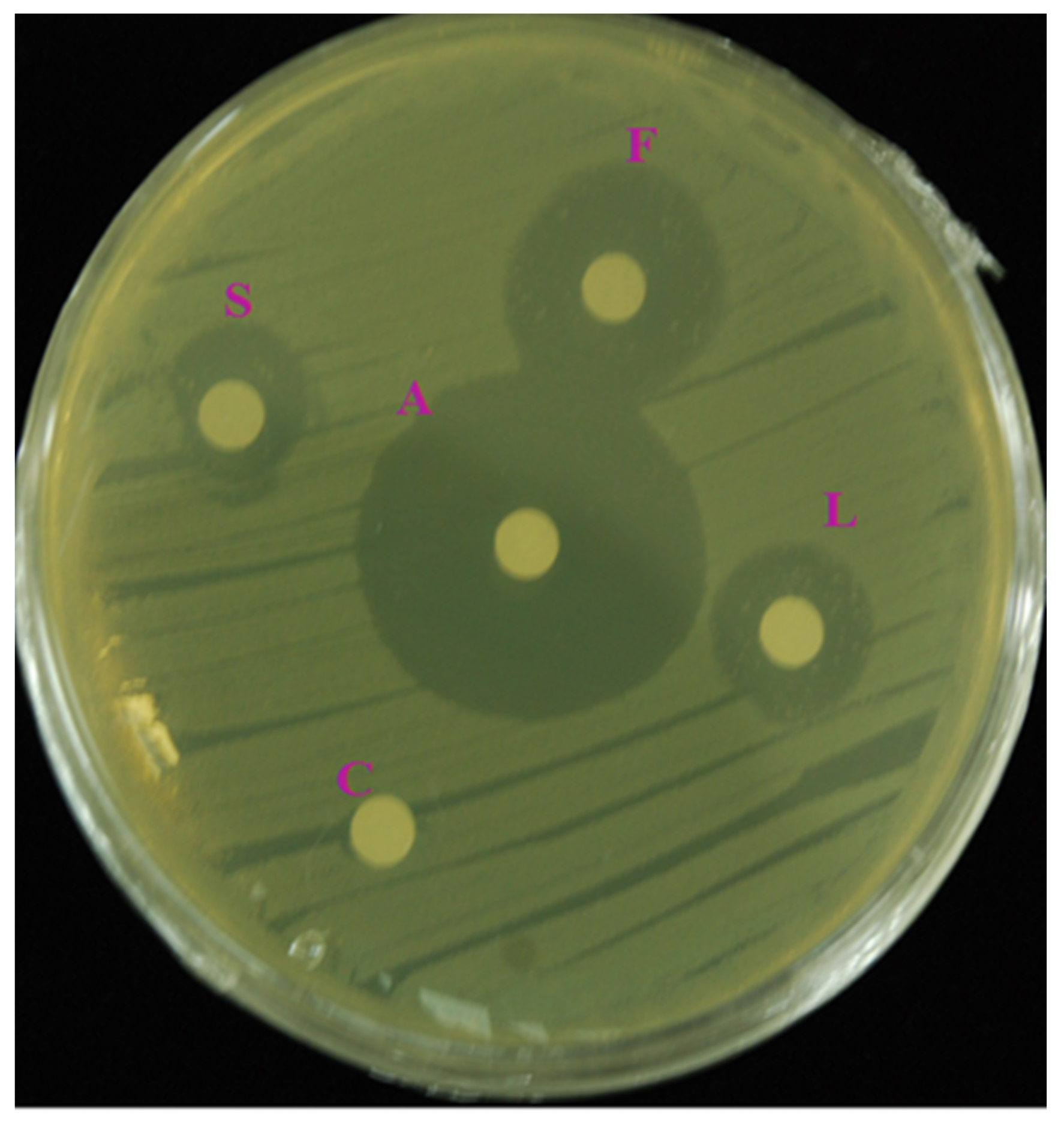
3.3. In Vitro Antibacterial Activity
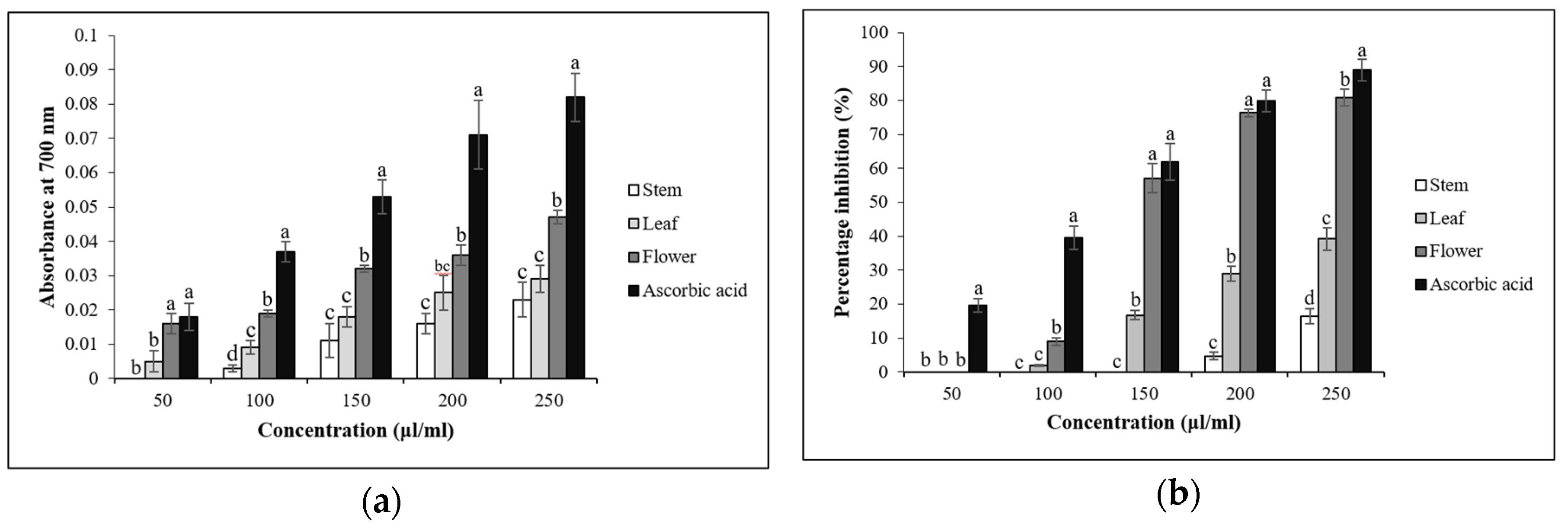
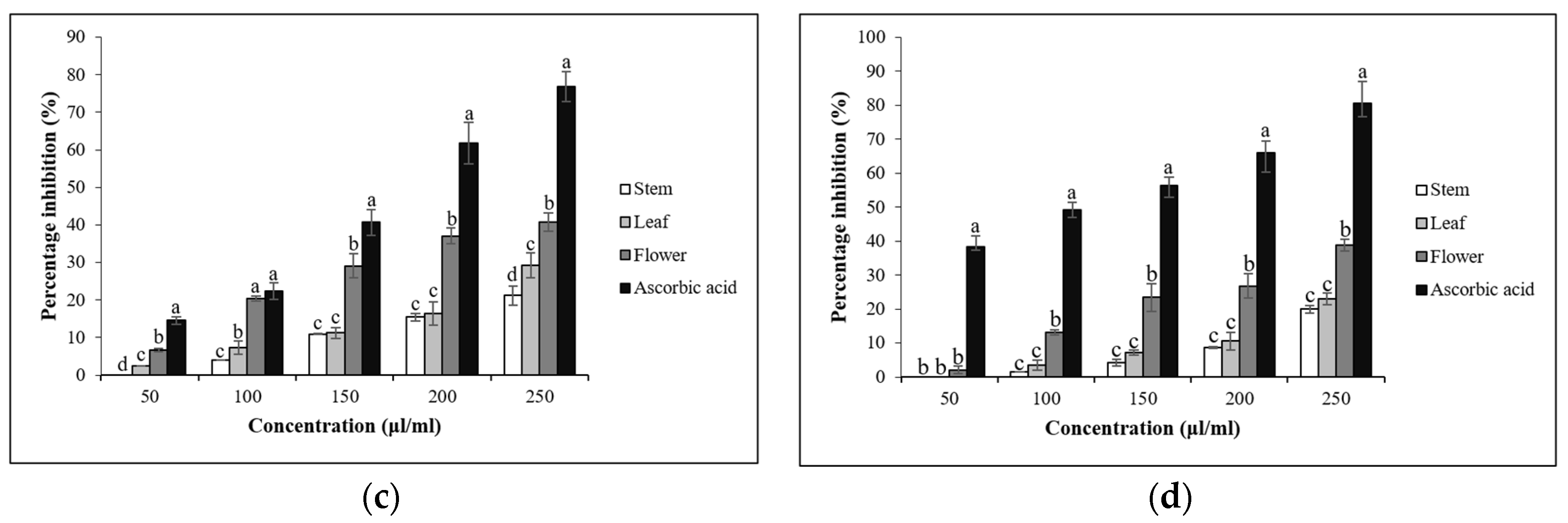
3.4. In Vitro Antioxidant Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jun, H.-J.; Chung, M.J.; Dawson, K.; Rodriguez, R.L.; Houng, S.-J.; Cho, S.-Y.; Jeun, J.; Kim, J.-Y.; Kim, K.H.; Park, K.W.; et al. Nutrigenomic analysis of hypolipidemic effects of Agastache rugosa essential oils in HepG2 cells and C57BL/6 mice. Food Sci. Biotechnol. 2010, 19, 219–227. [Google Scholar] [CrossRef]
- Kim, J. Phytotoxic and antimicrobial activities and chemical analysis of leaf essential oil from Agastache rugosa. J. Plant Biol. 2008, 51, 276–283. [Google Scholar] [CrossRef]
- Kim, M.H.; Chung, W.T.; Kim, Y.K.; Lee, J.H.; Lee, H.Y.; Hwang, B.; Park, Y.S.; Hwang, S.J.; Kim, J.H. The effect of the oil of Agastache rugosa O. Kuntze and three of its components on human cancer cell lines. J. Essent. Oil Res. 2001, 13, 214–218. [Google Scholar] [CrossRef]
- Chae, Y.; Hyun-Choong, O.; Song, J. Variability of the volatile composition of Agastache rugosa in South Korea. Acta Hortic. 2005, 675, 59–64. [Google Scholar] [CrossRef]
- Lee, S.Y.; Xu, H.; Kim, Y.K.; Park, S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbiol. Biotechnol. 2008, 24, 969–972. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and nematicidal activity of essential oil of Agastache rugosa against Meloidogyne incognita. Molecules 2013, 18, 4170–4180. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-J.; Choi, J.-H.; Oh, S.-R.; Lee, H.-K.; Park, J.-H.; Lee, K.-Y.; Kim, J.-J.; Jeong, T.-S.; Oh, G.T. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001, 495, 142–147. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, M.-J.; Kwon, H.-D.; Park, I.-H. Antimicrobial activity and components of extracts from Agastache rugosa during growth period. J. Food Sci. Nutr. 2001, 6, 10–15. [Google Scholar]
- Shin, S.; Kang, C.A. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Lett. Appl. Microbiol. 2003, 36, 111–115. [Google Scholar] [CrossRef]
- Min, B.S.; Hattori, M.; Lee, H.K.; Kim, Y.H. Inhibitory constituents against HIV-1 protease from Agastache rugosa. Arch. Pharm. Res. 1999, 22, 75–77. [Google Scholar] [CrossRef]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J. Agric. Food Chem. 2012, 60, 5945–5951. [Google Scholar] [PubMed]
- Oh, H.M.; Kang, Y.J.; Lee, Y.S.; Park, M.K.; Kim, S.H.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264. 7 cells from hydrogen peroxide-induced injury. J. Ethnopharmacol. 2006, 103, 229–235. [Google Scholar] [CrossRef]
- Wilson, L.A.; Senechal, N.P.; Widrlechner, M.P. Headspace analysis of the volatile oils of Agastache. J. Agric. Food Chem. 1992, 40, 1362–1366. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.J. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996, 1, 171–178. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Thomas, C. Oxygen Radicals and the Disease Process; CRC Press: Amsterdam, The Netherlands, 1998; pp. 1–282. [Google Scholar]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Shetty, A.; Magadum, S.; Managanvi, K. Vegetables as sources of antioxidants. J. Food Nutr. Disord. 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Hudson, B.J. Food Antioxidants; Elsevier Science Publishers: London, UK, 2012; pp. 1–317. [Google Scholar]
- García-Andrade, M.; González-Laredo, R.; Rocha-Guzmán, N.; Gallegos-Infante, J.; Rosales-Castro, M.; Medina-Torres, L. Mesquite leaves (Prosopis laevigata), a natural resource with antioxidant capacity and cardioprotection potential. Ind. Crops Prod. 2013, 44, 336–342. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.-Y.; Kim, S.-J.; Valan Arasu, M.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Sutharut, J.; Sudarat, J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int. Food Res. J. 2012, 19, 215–221. [Google Scholar]
- Park, C.H.; Yeo, H.J.; Park, Y.J.; Morgan, A.; Valan Arasu, M.; Al-Dhabi, N.A.; Park, S.U. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 2017, 22, 374. [Google Scholar] [CrossRef] [PubMed]
- Rejiniemon, T.S.; Hussain, R.R.; Rajamani, B. In-vitro functional properties of Lactobacillus plantarum isolated from fermented ragi malt. South Indian J. Biol. 2015, 1, 15–23. [Google Scholar]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Al-Dhabi, N.A.; Agastian, P.; Ignacimuthu, S. Antioxidant and free radical scavenging effects of β-amyrin isolated from S. cochinchinensis Moore. leaves. Ind. Crops Prod. 2014, 61, 510–516. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Emi, N.; Ignacimuthu, S. Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from Southern Western Ghats. South Indian J. Biol. 2015, 1, 7–14. [Google Scholar] [CrossRef]
- Korkina, L. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-J.; Lee, J.-H.; Gu, M.J.; Han, J.-H.; Cho, W.-K.; Ma, J.Y. Agastache rugosa Kuntze extract, containing the active component rosmarinic acid, prevents atherosclerosis through up-regulation of the cyclin-dependent kinase inhibitors p21WAF1/CIP1 and p27KIP1. J. Funct. Foods 2017, 30, 30–38. [Google Scholar] [CrossRef]
- Desta, K.T.; Kim, G.S.; Kim, Y.H.; Lee, W.S.; Lee, S.J.; Jin, J.S.; Abd El-Aty, A.; Shin, H.C.; Shim, J.H.; Shin, S.C. The polyphenolic profiles and antioxidant effects of Agastache rugosa Kuntze (Banga) flower, leaf, stem and root. Biomed. Chromatogr. 2016, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, S.; Matkowski, A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Torel, J.; Cillard, J.; Cillard, P. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry 1986, 25, 383–385. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Park, Y.-K.; Lee, G.-D. The nitrite scavenging and electron donating ability of phenolic compounds. Korean J. Food Sci. Technol. 1996, 28, 232–239. [Google Scholar]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure− activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma boninense. J. Agric. Sci. 2009, 1, 15–20. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Herald, P.; Davidson, P. Antibacterial activity of selected hydroxycinnamic acids. J. Food Sci. 1983, 48, 1378–1379. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Baskar, T.B.; Kim, J.K.; Park, S.U. Metabolic Profiling and Chemical-Based Antioxidant Assays of Green and Red Lettuce (Lactuca sativa). Nat. Prod. Commun. 2018, 13, 315–322. [Google Scholar] [CrossRef]
- Pratt, D. Natural antioxidants from plant material. In Phenolic Compounds in Food and Their Effects on Health II; Huang, M.T., Ho, C.T., Lee, C.Y., Eds.; ACS Symposium Series; ACS Publications: New York, NY, USA, 1992; Volume 507, pp. 54–71. [Google Scholar]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar]
- Azarbani, F.; Saki, Z.; Zareei, A.; Mohammadi, A. Phenolic contents, antibacterial and antioxidant activities of flower, leaf and stem extracts of ferulago angulata (schlecht) boiss. Int. J. Pharm. Pharm. Sci. 2014, 6, 123–125. [Google Scholar]
- Bettaieb, I.; Bourgou, S.; Wannes, W.A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef] [PubMed]
- Torey, A.; Sasidharan, S.; Latha, L.Y.; Sudhakaran, S.; Ramanathan, S. Antioxidant activity and total phenolic content of methanol extracts of Ixora coccinea. Pharm. Biol. 2010, 48, 1119–1123. [Google Scholar] [CrossRef]
- Noumedem, J.A.; Mihasan, M.; Lacmata, S.T.; Stefan, M.; Kuiate, J.R.; Kuete, V. Antibacterial activities of the methanol extracts of ten Cameroonian vegetables against Gram-negative multidrug-resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 26. [Google Scholar] [CrossRef]
- Mahalakshmi, N.; Dhanasekaran, S.; Ravi, C.; Lingathurai, S. In-vitro antimicrobial activities of Pongamia glabra and Phyllanthus niruri. South Indian J. Biol. 2016, 2, 236–244. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Dhar, M.; Dhar, M.; Dhawan, B.; Mehrotra, B. Screening of Indian plants for biological activity: Part II. Indian J. Exp. Biol. 1969, 7, 250–262. [Google Scholar]
- Thaker, A.; Anjaria, J. Antimicrobial and infected wound healing response of some traditional drugs. Indian J. Pharmacol. 1986, 18, 171–174. [Google Scholar]
- Vlachos, V.; Critchley, A.; Von Holy, A. Antimicrobial activity of extracts from selected Southern African marine macroalgae. S. Afr. J. Sci. 1997, 93, 328–332. [Google Scholar]
- Akerele, O. Nature’s medicinal bounty: Don’t throw it away. World Health Forum 1993, 14, 390–395. [Google Scholar] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Assob, J.C.; Kamga, H.L.; Nsagha, D.S.; Njunda, A.L.; Nde, P.F.; Asongalem, E.A.; Njouendou, A.J.; Sandjon, B.; Penlap, V.B. Antimicrobial and toxicological activities of five medicinal plant species from Cameroon Traditional Medicine. BMC Complement. Altern. Med. 2011, 11, 70. [Google Scholar] [CrossRef]
- Yuan, X.; Chapman, R.L.; Wu, Z. Analytical methods for heavy metals in herbal medicines. Phytochem. Anal. 2011, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al-Dhabi, N.; Valan Arasu, M.; Rejiniemon, T.S. In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evid. Based Complement. Altern. Med. 2015, 2015, 164261. [Google Scholar] [CrossRef]
- Maruo, V.; Bernardi, M.; Spinosa, H. Toxicological evaluations of long-term consumption of Solanum lycocarpum St. Hill fruits in male and female adult rats. Phytomedicine 2003, 10, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Wintola, O.; Sunmonu, T.; Afolayan, A. Toxicological evaluation of aqueous extract of Aloe ferox Mill. in loperamide-induced constipated rats. Hum. Exp. Toxicol. 2011, 30, 425–431. [Google Scholar] [CrossRef] [PubMed]
| Organ | Total Phenolics (mg GAE/g dw) | Total Flavonoid (mg RE/g dw) | Total Anthocynin (mg CGE/g dw) |
|---|---|---|---|
| Flower | 24.53 ± 1.01 a | 18.52 ± 1.23 a | 0.22 ± 0.09 a |
| Leaf | 17.57 ± 0.91 b | 16.91 ± 0.23 b | 0.10 ± 0.02 b |
| Stem | 7.65 ± 1.21 c | 8.17 ± 0.47 c | 0.01 ± 0.00 b |
| Organ | Phenolic Compounds (mg/g dw) | ||||||
|---|---|---|---|---|---|---|---|
| Catechin | Chlorogenic Acid | Caffeic Acid | Ferulic Acid | Rutin | Kaempferol | Total | |
| Flower | 0.014 ± 0.005 b | 0.011 ± 0.006 b | 0.267 ± 0.001 b | 0.815 ± 0.001 b | 0.061 ± 0.009 ab | 0.137 ± 0.007 a | 1.305 ± 0.024 b |
| Leaf | 0.148 ± 0.005 a | 0.027 ± 0.021 b | 0.327 ± 0.030 a | 0.830 ± 0.001 a | 0.081 ± 0.012 a | 0.157 ± 0.021 a | 1.570 ± 0.064 a |
| Stem | 0.004 ± 0.003 c | 0.103 ± 0.010 a | 0.272 ± 0.003 b | ND | 0.053 ± 0.013 b | ND | 0.433 ± 0.022 c |
| Bacterial Strain | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Flower | Leaf | Stem | Streptomycin | |
| E.coli (KF918342) | 18.3 ± 0.5 b | 14.0 ± 1.0 c | 11.0 ± 1.0 d | 27.6 ± 0.6 a |
| Staphylococcus hemolyticus | 16.6 ± 0.5 b | 12.0 ± 1.0 c | 8.0 ± 1.0 d | 26.3 ± 0.6 a |
| Aeromonas hydrophila | 17.0 ± 1.0 b | 14.6 ± 0.5 c | 7.6 ± 0.5 d | 27.0 ± 0.0 a |
| E.coli (ATCC 35150) | 16.3 ± 1.5 b | 13.6 ± 1.5 c | 10.0 ± 1.0 d | 28.3 ± 0.6 a |
| Cronobacter sakazakii | 15.0 ± 1.0 b | 12.0 ± 1.7 c | 9.3 ± 0.5 d | 25.6 ± 0.6 a |
| Aeromonas salmonicida | 16.6 ± 0.5 b | 9.3 ± 0.5 c | 8.0 ± 1.0 c | 27.0 ± 1.0 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Yeo, H.J.; Baskar, T.B.; Park, Y.E.; Park, J.S.; Lee, S.Y.; Park, S.U. In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint. Antioxidants 2019, 8, 75. https://doi.org/10.3390/antiox8030075
Park CH, Yeo HJ, Baskar TB, Park YE, Park JS, Lee SY, Park SU. In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint. Antioxidants. 2019; 8(3):75. https://doi.org/10.3390/antiox8030075
Chicago/Turabian StylePark, Chang Ha, Hyeon Ji Yeo, Thanislas Bastin Baskar, Ye Eun Park, Jong Seok Park, Sook Young Lee, and Sang Un Park. 2019. "In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint" Antioxidants 8, no. 3: 75. https://doi.org/10.3390/antiox8030075
APA StylePark, C. H., Yeo, H. J., Baskar, T. B., Park, Y. E., Park, J. S., Lee, S. Y., & Park, S. U. (2019). In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint. Antioxidants, 8(3), 75. https://doi.org/10.3390/antiox8030075





