Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goji Berry Samples
2.2. Chemicals
2.3. Determination of Total Carbohydrate Content (TCC) of the Extracts
2.4. Determination of Total Phenol Content (TPC) of the Extracts
2.5. Determination of Total Antioxidant Capacity of Goji Berry Fruit
2.5.1. DPPH (2,2-diphenyl-1-picrylhydrazyl) Radical-Scavenging Activity
2.5.2. ABTS•+ (2,2’-Azino-bis-(3-ethyl-benzthiazoline-sulfonic Acid) Radical-Scavenging Activity
2.6. Chemical Components and Structure Analysis by Infrared Spectroscopy
2.7. Fatty-Acid Methyl Ester (FAME) Synthesis
GC-MS Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Carbohydrate and Phenol Content
3.2. Antioxidant Activity Based on DPPH• and ABTS•+
3.3. Goji Berry Fatty-Acid Profile
3.4. ATR-FT-IR Spectroscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity Potential of Polyphenolic Compounds in Human Health and their Effectiveness Against Various Food Borne and Plant Pathogens. A Review. J. Food Biosyst. Eng. 2017, 7, 1–19. [Google Scholar]
- Leontopoulos, S.V.; Skenderidis, P.; Anatolioti, V.; Kokkora, M.I.; Tsilfoglou, S.; Petrotos, K.B.; Vagelas, I. Antifungal Activity of Azadirachta indica Aqueous and Non-Aqueous Extracts on Colletotrichum gloeosporioides, Botryodiplodia theobromae and Fusarium solani. A First Approach. J. Food Biosyst. Eng. 2017, 6, 38–50. [Google Scholar]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Liang, B.; Jin, M.; Liu, H. Water-soluble polysaccharide from dried Lycium barbarum fruits: Isolation, structural features and antioxidant activity. Carbohydr. Polym. 2011, 83, 1947–1951. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Forino, M.; Tartaglione, L.; Dell’Aversano, C.; Ciminiello, P. NMR-based identification of the phenolic profile of fruits of Lycium barbarum (goji berries). Isolation and structural determination of a novel N-feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016, 194, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Zhao, X.; Shi, J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011, 125, 1365–1372. [Google Scholar] [CrossRef]
- Endes, Z.; Uslu, N.; Özcan, M.M.; Er, F. Physico-chemical properties, fatty acid composition and mineral contents of goji berry (Lycium barbarum L.) fruit. J. Agroaliment. Process. Technol. 2015, 21, 36–40. [Google Scholar]
- Chung, I.; Nagella, P.; Kim, Y.A.S.; Ahmad, A. Composition of the essential oil and petroleum ether extract of Lycium chinense Miller fruits and antioxidant activity of its several extracts. J. Med. Plants Res. 2011, 5, 5973–5981. [Google Scholar]
- Cossignani, L.; Blasi, F.; Simonetti, M.S.; Montesano, D. Fatty Acids and Phytosterols to Discriminate Geographic Origin of Lycium barbarum Berry. Food Anal. Methods 2017, 11, 1180–1188. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2016, 40, e12522. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A. Goji Berry (Lycium barbarum): Composition and Health Effects—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 67–75. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar]
- Kerasioti, E.; Kiskini, A.; Veskoukis, A.; Jamurtas, A.; Tsitsimpikou, C.; Tsatsakis, A.M.; Koutedakis, Y.; Stagos, D.; Kouretas, D.; Karathanos, V. Effect of a special carbohydrate-protein cake on oxidative stress markers after exhaustive cycling in humans. Food Chem. Toxicol. 2012, 50, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids; Oily Press: Bridgwater, UK, 2003; ISBN 0953194957. [Google Scholar]
- Jing, Z. Dong Analysis on the main active components of Lycium barbarum fruits and related environmental factors. J. Med. Plants Res. 2012, 6, 2276–2283. [Google Scholar]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ionică, E.M.; Nour, V.; Trandafir, I. Polyphenols content and antioxidant capacity of goji fruits (Lycium chinense). South West. J. Hortic. Biol. Environ. 2012, 3, 121–129. [Google Scholar]
- Kosar, M.; Altintas, A.; Kirimer, N.; Baser, K.H.C. Determination of the free radicals scavenging activity of Lycium extracts. Chem. Nat. Compd. 2003, 39, 439–442. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Barontini, M.; Bernini, R.; Carastro, I.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by in vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Bounous, G. Goji berry fruit (Lycium spp.): Antioxidant compound fingerprint and bioactivity evaluation. J. Funct. Foods 2015, 18, 1070–1085. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsaga, C.; Giavasis, I.; Hadjichristodoulou, C.; Leontopoulos, S.; Petrotos, K.; Tsakalof, A. Assessment of Antimicrobial Properties of Aqueous UAE Extracts of Goji Berry Fruit and Pomegranate Fruit Peels in vitro. In Proceedings of the International Conference on Food and Biosystems Engineering, Rhodes Island, Greece, 1–4 June 2017. [Google Scholar]
- Blasi, F.; Montesano, D.; Simonetti, M.S.; Cossignani, L. A Simple and Rapid Extraction Method to Evaluate the Fatty Acid Composition and Nutritional Value of Goji Berry Lipid. Food Anal. Methods 2016, 64, 970–979. [Google Scholar] [CrossRef]
- World Health Organization. Fats and Fatty Acids in Human Nutrition; World Health Organization: Geneva, Switzerland, 2008; Volume 91, ISBN 9789251067338. [Google Scholar]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Zeng, X. Nutritional, phytochemical characterization and antioxidant capacity of Ningxia Wolfberry (Lycium barbarum L.). J. Chem. Soc. Pak. 2014, 36, 1079. [Google Scholar]
- He, K.-H.; Zou, X.-L.; Liu, X.; Zeng, H.-Y. Determination of canthaxanthin and astaxanthin in egg yolks by reversed phase high performance liquid chromatography with diode array detection. J. Sichuan Univ. 2012, 43, 113–117. [Google Scholar]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.; Fung, M.L.; Xu, M.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes 2013, 3, e81. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-Q.; Fan, H.-X.; He, R.-R.; Xiao, J.; Tsoi, B.; Lan, K.-H.; Kurihara, H.; So, K.-F.; Yao, X.-S.; Gao, H. Lycibarbarspermidines A-O, New Dicaffeoylspermidine Derivatives from Wolfberry, with Activities against Alzheimer’s Disease and Oxidation. J. Agric. Food Chem. 2016, 64, 2223–2237. [Google Scholar] [CrossRef] [PubMed]
- Fellah, A.; Anjukandi, P.; Waterland, M.R.; Williams, M.A.K. Determining the degree of methylesterification of pectin by ATR/FT-IR: Methodology optimisation and comparison with theoretical calculations. Carbohydr. Polym. 2009, 78, 847–853. [Google Scholar] [CrossRef]
- Teli, M.D.; Waghmare, N.G. Synthesis of superabsorbent from carbohydrate waste. Carbohydr. Polym. 2009, 78, 492–496. [Google Scholar] [CrossRef]
| Parameters | L. chinense Mill. | L. barbarum L. | L. barbarum L. | L. barbarum L. | ||||
|---|---|---|---|---|---|---|---|---|
| June 2016 Collection | August 2016 Collection | October 2016 Collection | June 2016 Collection | August 2016 Collection | October 2016 Collection | Origin China | Origin Mongolia | |
| TCC * | 395 4.1 | 440 a,b,c,d 5.2 | 329 2.7 | 452 3.8 | 490 a 6.8 | 370 4.3 | 459 a,b,c,d 3.8 | 434 b,c,d 4.3 |
| TPC * | 8.5 0.4 | 8.9 b 0.7 | 7.4 0.6 | 9.7 0.2 | 10.1 a,b 0.4 | 6.9 0.3 | 9.9 a,b 0.6 | 10.9 a,b 0.4 |
| IC50 of DPPH• * | 1085 2.9 μg/mL | 950 a 4.7 μg/mL | 1254 5.1 μg/mL | 894 6.4 μg/mL | 830 b,c,d 5.4 μg/mL | 1150 7.1 μg/mL | 795 b,c,d,e 2.4 μg/mL | 784 c,d,e 3.6 μg/mL |
| IC50 of ABTS•+ * | 385 2.9 μg/mL | 220 a,b 6.1 μg/mL | 407 4.9 μg/mL | 241 7.7 μg/mL | 195 a,b 3.5 μg/mL | 397 4.2 μg/mL | 198 a,b 2.8 μg/mL | 192 a,b 3.6 μg/mL |
| Fatty Acids | Fatty Acids (%) | L. barbarum L. | L. chinense Mill. | L. barbarum L. Ningxia Origin | L. barbarum L. Mongolia Origin | ||||
|---|---|---|---|---|---|---|---|---|---|
| LbC1 | LbC2 | LbC3 | LcC1 | LcC2 | LcC3 | ||||
| Palmitic acid | C16:0 | 21.79 0.0 | 21.55 0.1 | 19.38 0.6 | 21.59 0.0 | 21.02 0.2 | 24.67 0.0 | 18.96 0.0 | 15.08 0.0 |
| Palmitoleic acid | C16:1 n−9 | 1.90 0.0 | 3.07 0.0 | 1.66 0.0 | 1.78 0.0 | 1.51 0.0 | 1.80 0.1 | 1.01 0.0 | 1.00 0.0 |
| Palmitoleic acid | C16:1 n−7 | ND | 1.80 0.0 | 0.54 0.1 | 0.55 0.1 | 0.66 0.1 | 0.58 0.0 | 1.16 0.1 | 1.17 0.1 |
| Stearic acid | C18:0 | 4.78 0.2 | 4.05 0.0 | 3.69 0.1 | 3.43 0.0 | 3.75 0.0 | 4.20 0.0 | 2.61 0.0 | 2.69 0.1 |
| Oleic acid | C18:1 n−9 | 16.71 0.1 | 17.32 0.1 | 19.29 0.0 | 20.08 0.1 | 20.66 0.1 | 22.39 0.1 | 20.07 0.1 | 19.61 0.4 |
| Linoleic acid | C18:2 n−6 | 42.64 0.4 | 39.69 0.3 | 43.96 0.1 | 40.71 0.0 | 38.65 0.4 | 36.96 0.1 | 37.89 0.1 | 42.2 0.1 |
| Arachidic acid | C20:0 | 1.59 0.0 | 1.03 0.5 | 3.03 0.0 | 1.14 0.1 | 0.99 0.0 | 1.15 0.0 | 1.86 0.0 | 2.03 0.0 |
| α-Linolenic acid | C18:3 n−3 | 7.99 0.0 | 8.85 0.3 | 7.15 0.5 | 8.52 0.0 | 7.47 0.1 | 6.27 0.0 | 6.46 0.0 | 5.39 0.2 |
| Gondoic acid | C20:1 n−11 | ND | 1.41 0.0 | 1.30 0.1 | 0.89 0.0 | 0.91 0.2 | 0.74 0.2 | 3.95 0.2 | 4.05 0.0 |
| Behenic acid | C22 | 2.60 0.0 | ND | ND | 0.56 0.0 | 2.88 0.2 | 0.48 0.1 | 6.03 0.0 | 6.78 0.1 |
| Lignoceric acid | C24 | ND | 1.23 0.0 | ND | 0.75 0.1 | 1.50 0.1 | 0.76 0.0 | ND | ND |
| ΣSFA | 30.76 | 27.86 | 26.10 | 27.47 | 30.14 | 31.26 | 29.46 | 26.58 | |
| ΣMUFA | 18.61 | 23.60 | 22.79 | 23.30 | 23.74 | 25.51 | 26.19 | 25.83 | |
| ΣPUFA | 50.63 | 48.54 | 51.11 | 49.23 | 46.12 | 43.23 | 44.35 | 47.59 | |
| PUFA/MUFA | 2.72 | 2.05 | 2.24 | 2.11 | 1.94 | 1.69 | 1.69 | 1.84 | |
| PUFA/SFA | 1.64 | 1.74 | 1.95 | 1.79 | 1.53 | 1.38 | 1.50 | 1.79 | |
| n6/n3 | 5.34 | 4.48 | 6.15 | 4.78 | 5.17 | 5.89 | 5.87 | 7.83 | |
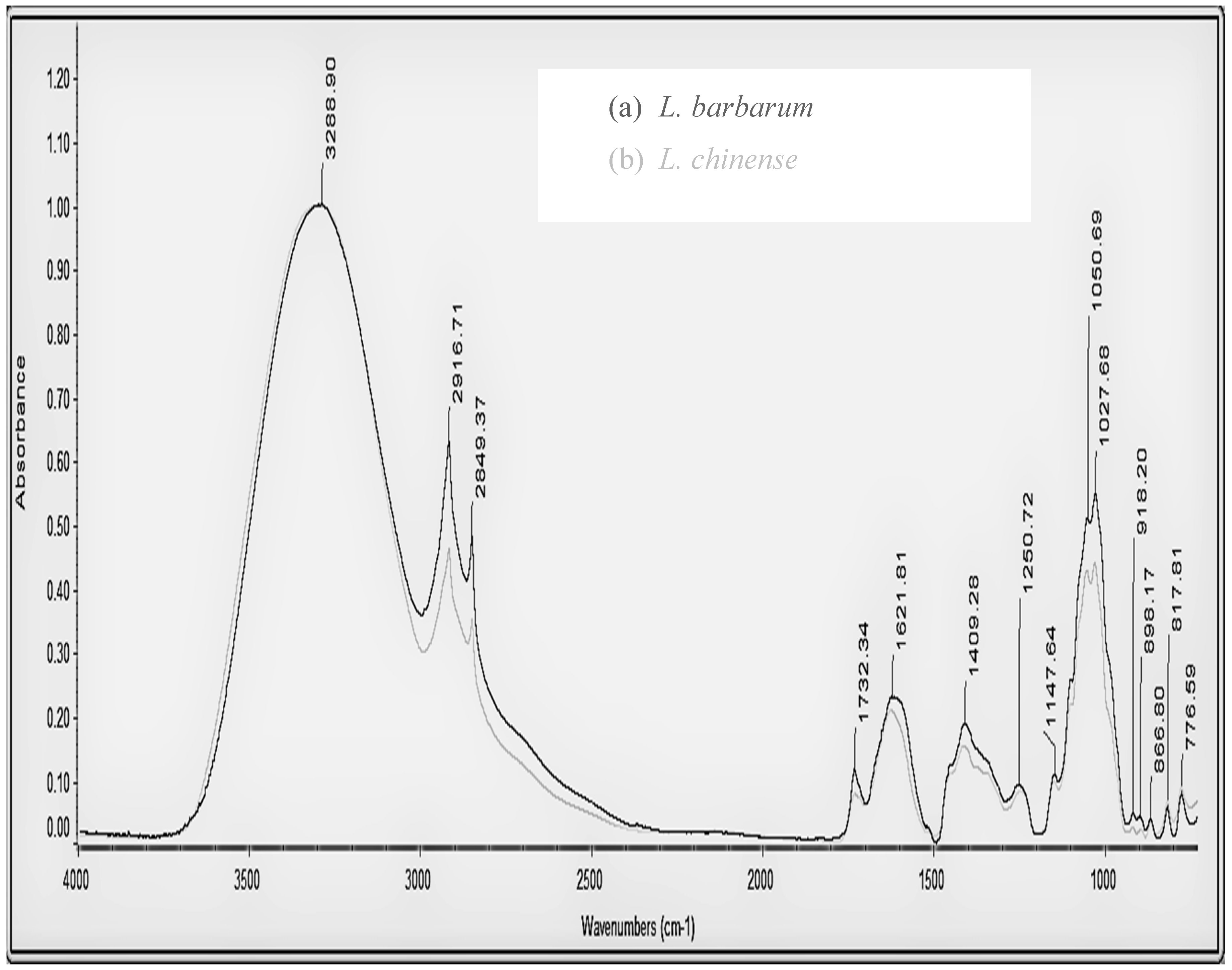
| L. barbarum Absorption (cm−1) | Peak Intensity | L. chinense Absorption (cm−1) | Peak Intensity | Functional Group | Structural Characteristic |
|---|---|---|---|---|---|
| 3288.9 | 1 | 3296.7 | 1 | hydroxyl group (–OH) amino group (–NH2) | stretching vibration of O–H stretching vibration of N–H |
| 2916.71 | 0.631 | 2916.61 | 0.461 | alkyl group (–CH2–) | stretching vibration of C–H |
| 2849.37 | 0.480 | 2849.13 | 0.350 | alkyl group (–CH2 or –CH3) | stretching vibration of CH2 and CH3 |
| 1732.34 | 0.115 | 1732.26 | 0.077 | carboxyl group (–COOH), aldehyde group (–CHO) or esterfunction (–COOR) | stretching vibration of C=O |
| 1621.81 | 0.229 | 1624.74 | 0.207 | carbonyl group (–C=O or –CHO) amide group (–NH2 or –COR) amino group (–NH2) | stretching vibration of C=O bending vibration of N-H or stretching vibration of C=O bending vibration of N–H bound water |
| alkyl group (–CH2- or –CH3) | bending vibration of C–H | ||||
| 1409.28 | 0.186 | 1416.25 | 0.151 | carboxyl group (–COOH) | stretching vibration of C–O |
| 1250.72 | 0.09 | 1245.96 | 0.08 | carboxyl group (–COOH) | bending vibration of O–H |
| 1147.64 | 0.107 | 1148.14 | 0.095 | b-anomeric configuration | |
| 1050.69 1027.68 | 0.51 0.55 | 1053.72 1030.17 | 0.426 0.438 | hydroxyl group (–OH) D-glucopyranose ring | bending vibration of O–H symmetrical ring vibration |
| 918.20 | 0.04 | 918.98 | 0.024 | α-type glycosidic linkage | symmetrical ring vibration |
| 898.17 | 0.03 | 898.12 | 0.022 | b-anomeric configuration | |
| 866.80 | 0.03 | 865.69 | 0.031 | α-type glycosidic linkage | symmetrical ring vibration |
| 817.81 | 0.05 | 817.64 | 0.063 | α-D-galactopyranose | symmetrical ring vibration |
| 776.59 | 0.07 | 776.99 | 0.08 | D-glucopyranose ring | symmetrical ring vibration |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60. https://doi.org/10.3390/antiox8030060
Skenderidis P, Lampakis D, Giavasis I, Leontopoulos S, Petrotos K, Hadjichristodoulou C, Tsakalof A. Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants. 2019; 8(3):60. https://doi.org/10.3390/antiox8030060
Chicago/Turabian StyleSkenderidis, Prodromos, Dimitrios Lampakis, Ioannis Giavasis, Stefanos Leontopoulos, Konstantinos Petrotos, Christos Hadjichristodoulou, and Andreas Tsakalof. 2019. "Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits" Antioxidants 8, no. 3: 60. https://doi.org/10.3390/antiox8030060
APA StyleSkenderidis, P., Lampakis, D., Giavasis, I., Leontopoulos, S., Petrotos, K., Hadjichristodoulou, C., & Tsakalof, A. (2019). Chemical Properties, Fatty-Acid Composition, and Antioxidant Activity of Goji Berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants, 8(3), 60. https://doi.org/10.3390/antiox8030060










