Time to Reset: The Interplay Between Circadian Rhythms and Redox Homeostasis in Skeletal Muscle Ageing and Systemic Health
Abstract
1. Introduction to Skeletal Muscle: From Structural to Metabolic and Endocrine Functions
2. Age-Associated Sarcopenia and the Role of Redox Homeostasis
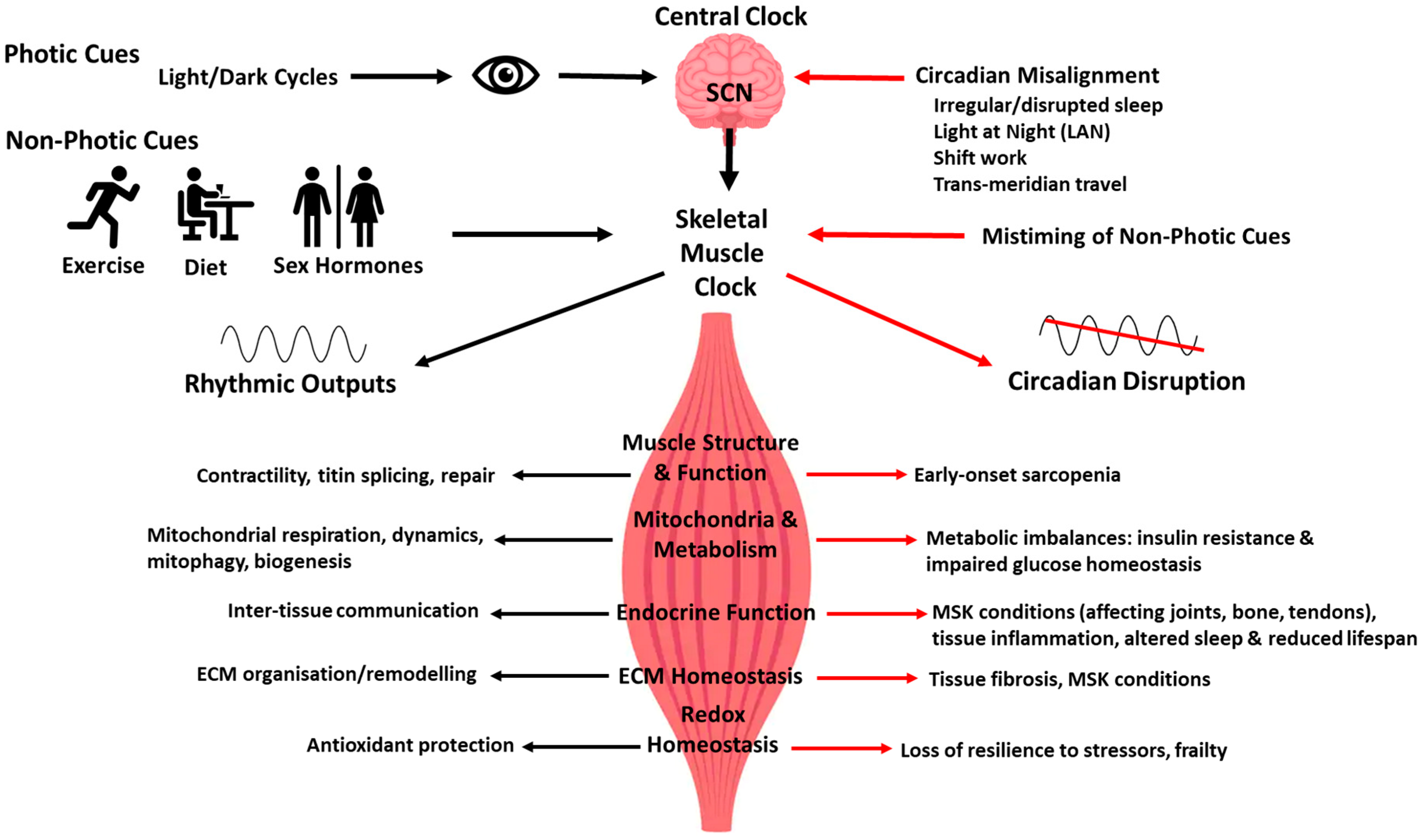
3. Introduction to the Circadian Clock Timing System
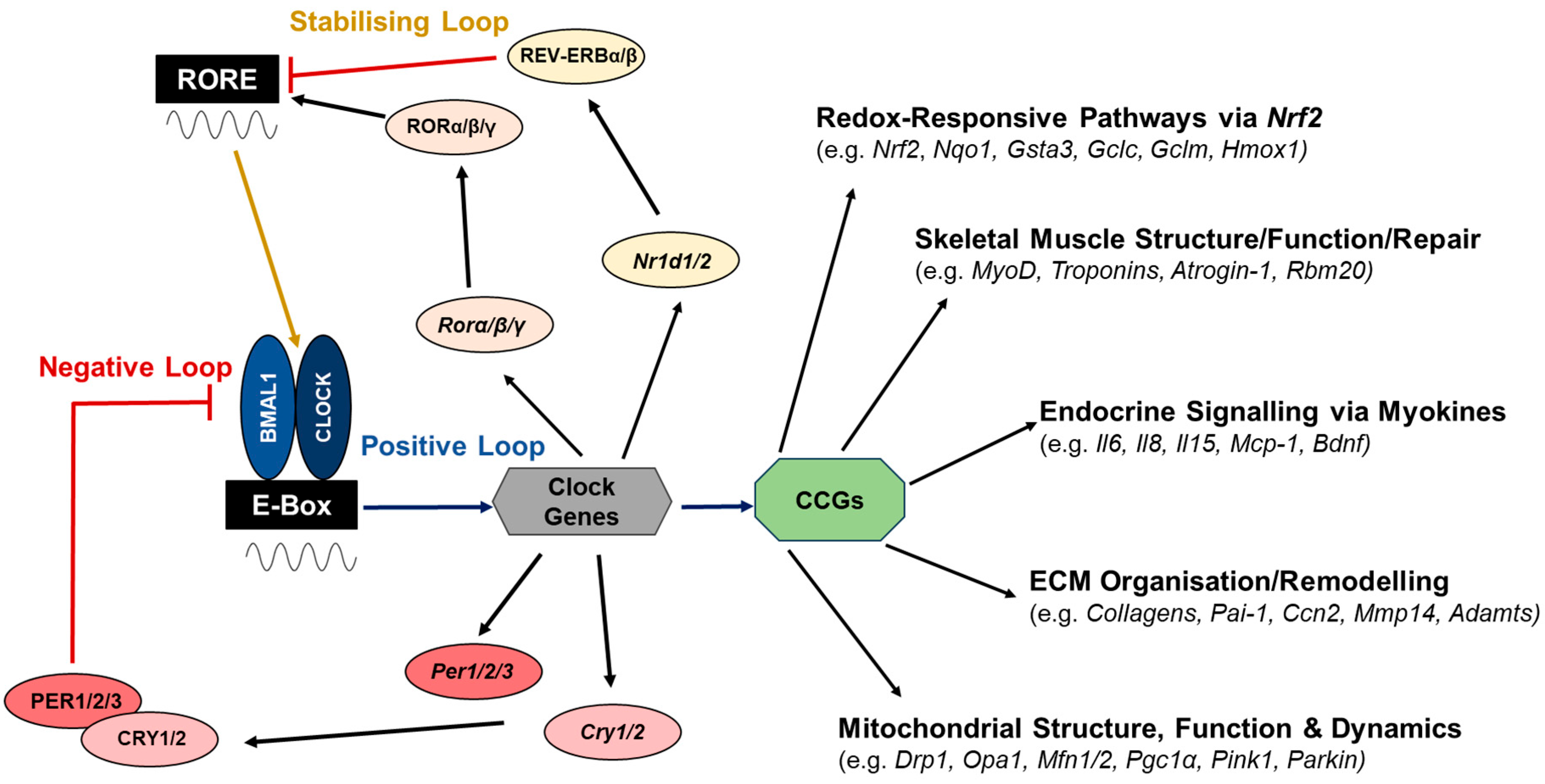
4. The Molecular Network Regulating the Clock Circuitry
5. Skeletal Muscle Clock: From Experimental Models to Human Studies
6. Circadian Regulation of Structural, Metabolic and Endocrine Functions of Skeletal Muscle with Ageing
7. Redox Homeostasis, NRF2 and Circadian Rhythms: A Bi-Directional Relationship
8. Circadian Regulation of Mitochondrial Structure, Function and Dynamics
9. Extracellular Matrix Homeostasis Regulation by the Molecular Clock
10. Chronotyping: Personalisation of Skeletal Muscle Performance
11. Time-Scheduled Exercise as a Lifestyle Intervention for Skeletal Muscle Loss with Ageing?
12. Sex-Tailored Exercise Interventions for Sarcopenia and the Role of Circadian Hormone Melatonin
13. Summary
Funding
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms modulating skeletal muscle phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. WIREs Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Fernández Costa, J.M.; Fernández-Garibay, X.; Velasco, F.; Ramón-Azcón, J. Bioengineered in vitro skeletal muscles as new tools for muscular dystrophies preclinical studies. J. Tissue Eng. 2021, 12, 2041731420981339. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L. Extracellular matrix and ageing. Subcell. Biochem. 2018, 90, 169–190. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.D.; Shook, M.; Varacallo, M. Anatomy, skeletal muscle. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kurtz, A.; Oh, S.-J. Age related changes of the extracellular matrix and stem cell maintenance. Prev. Med. 2012, 54, S50–S56. [Google Scholar] [CrossRef] [PubMed]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Cai, L.; Shi, L.; Peng, Z.; Sun, Y.; Chen, J. Ageing of skeletal muscle extracellular matrix and mitochondria: Finding a potential link. Ann. Med. 2023, 55, 2240707. [Google Scholar] [CrossRef]
- Gutierrez-Monreal, M.A.; Harmsen, J.; Schrauwen, P.; Esser, K.A. Ticking for Metabolic Health: The Skeletal-Muscle Clocks. Obesity 2020, 28, S46–S54. [Google Scholar] [CrossRef]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Goldstein, M.S. Humoral nature of the hypoglycemic factor of muscular work. Diabetes 1961, 10, 232–234. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Fischer, C.; Keller, C.; Ostrowski, K.; Schjerling, P. Exercise and cytokines with particular focus on muscle derived IL-6. Exerc. Immunol. Rev. 2001, 7, 18–31. [Google Scholar]
- Pedersen, B.K.; Steensberg, A.; Keller, P.; Keller, C.; Fischer, C.; Hiscock, N.; Van Hall, G.; Plomgaard, P.; Febbraio, M.A. Muscle-derived interleukin-6: Lipolytic, anti-inflammatory and immune regulatory effects. Pflügers Arch. 2003, 446, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Metab. 2011, 301, E504–E510. [Google Scholar] [CrossRef]
- Shero, J.A.; Lindholm, M.E.; Sandri, M.; Stanford, K.I. Skeletal muscle as a mediator of interorgan crosstalk during exercise: Implications for aging and obesity. Circ. Res. 2025, 136, 1407–1432. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; Raschke, S.; Knebel, B.; Scheler, M.; Irmler, M.; Passlack, W.; Muller, S.; Hanisch, F.-G.; Franz, T.; Li, X. Secretome profiling of primary human skeletal muscle cells. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014, 1844, 1011–1017. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L., Jr. Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Vechetti, I.J., Jr.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Tanimura, Y. Roles of skeletal muscle-derived exosomes in organ metabolic and immunological communication. Front. Endocrinol. 2021, 12, 697204. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle releases alpha-sarcoglycan positive extracellular vesicles carrying miRNAs in the bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar]
- Kirby, T.J.; McCarthy, J.J. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic. Biol. Med. 2013, 64, 95–105. [Google Scholar] [CrossRef]
- Lananna, B.V.; Musiek, E.S. The wrinkling of time: Aging, inflammation, oxidative stress, and the circadian clock in neurodegeneration. Neurobiol. Dis. 2020, 139, 104832. [Google Scholar] [CrossRef]
- Soendenbroe, C.; Heisterberg, M.F.; Schjerling, P.; Karlsen, A.; Kjaer, M.; Andersen, J.L.; Mackey, A.L. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve 2019, 60, 453–463. [Google Scholar] [CrossRef]
- Kirkeby, S.; Garbarsch, C. Aging affects different human muscles in various ways. An image analysis of the histomorphometric characteristics of fiber types in human masseter and vastus lateralis muscles from young adults and the very old. Histol. Histopathol. 2000, 15, 61–72. [Google Scholar]
- Barnouin, Y.; McPhee, J.S.; Butler-Browne, G.; Bosutti, A.; De Vito, G.; Jones, D.A.; Narici, M.; Behin, A.; Hogrel, J.; Degens, H. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J. Cachexia. Sarcopenia Muscle 2017, 8, 647–659. [Google Scholar] [CrossRef]
- Power, G.A.; Dalton, B.H.; Rice, C.L. Human neuromuscular structure and function in old age: A brief review. J. Sport Health Sci. 2013, 2, 215–226. [Google Scholar] [CrossRef]
- D’Antona, G.; Pellegrino, M.A.; Adami, R.; Rossi, R.; Carlizzi, C.N.; Canepari, M.; Saltin, B.; Bottinelli, R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J. Physiol. 2003, 552, 499–511. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Piasecki, M.; Atherton, P. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev. 2018, 47, 123–132. [Google Scholar] [CrossRef]
- Ewald, C.Y. The matrisome during aging and longevity: A systems-level approach toward defining matreotypes promoting healthy aging. Gerontology 2020, 66, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef]
- Ren, L.; Fu, Y.; Deng, Y.; Qi, L.; Jin, L. Advanced glycation end products inhibit the expression of collagens type I and III by human gingival fibroblasts. J. Periodontol. 2009, 80, 1166–1173. [Google Scholar] [CrossRef]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Joseph, A.-M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Uruno, A.; Yagishita, Y.; Katsuoka, F.; Kitajima, Y.; Nunomiya, A.; Nagatomi, R.; Pi, J.; Biswal, S.S.; Yamamoto, M.; Uruno, C.A. Nrf2-Mediated Regulation of Skeletal Muscle Glycogen Metabolism Downloaded from. Mol. Cell. Biol. 2016, 36, 1655–1672. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.H.; Kim, K.Y.; Shim, M.S.; Choi, S.H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013, 4, e820. [Google Scholar] [CrossRef]
- Chow, C.K. Vitamin E regulation of mitochondrial superoxide generation. Neurosignals 2001, 10, 112–124. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic. Biol. Med. 2013, 65, 317–323. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Tamura, Y.; Takahashi, K.; Takeda, K.; Takemasa, T.; Hatta, H. Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle. Physiol. Rep. 2019, 7, e13998. [Google Scholar] [CrossRef]
- Huang, D.-D.; Fan, S.-D.; Chen, X.-Y.; Yan, X.-L.; Zhang, X.-Z.; Ma, B.-W.; Yu, D.-Y.; Xiao, W.-Y.; Zhuang, C.-L.; Yu, Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Miller, C.J.; Gounder, S.S.; Kannan, S.; Goutam, K.; Muthusamy, V.R.; Firpo, M.A.; Symons, J.D.; Paine, R.; Hoidal, J.R.; Rajasekaran, N.S. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim. Biophys. Acta—Mol. Basis Dis. 2012, 1822, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Hong, J.; Atieno, N.; Muthusamy, V.R.; Davidson, C.J.; Abu-Rmaileh, N.; Richardson, R.S.; Gomes, A.V.; Hoidal, J.R.; Rajasekaran, N.S. Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic. Biol. Med. 2014, 71, 402–414. [Google Scholar] [CrossRef]
- Ahn, B.; Pharaoh, G.; Premkumar, P.; Huseman, K.; Ranjit, R.; Kinter, M.; Szweda, L.; Kiss, T.; Fulop, G.; Tarantini, S.; et al. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018, 17, 47–58. [Google Scholar] [CrossRef]
- Safdar, A.; deBeer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493. [Google Scholar] [CrossRef]
- Schroder, E.A.; Esser, K.A. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc. Sport Sci. Rev. 2013, 41, 224–229. [Google Scholar] [CrossRef]
- Serin, Y.; Tek, N.A. Effect of circadian rhythm on metabolic processes and the regulation of energy balance. Ann. Nutr. Metab. 2019, 74, 322–330. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Wolff, C.A.; Esser, K.A. Exercise timing and circadian rhythms. Curr. Opin. Physiol. 2019, 10, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E. Food as circadian time cue for appetitive behavior. F1000Research 2020, 9, F1000-Faculty. [Google Scholar] [CrossRef] [PubMed]
- Rey, G.; Reddy, A.B. Interplay between cellular redox oscillations and circadian clocks. Diabetes Obes. Metab. 2015, 17, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.A.; Wagner, A.L.; McGlynn, O.F.; Kharazyan, F.; Browne, J.A.; Elliott, J.A. Exercise influences circadian gene expression in equine skeletal muscle. Vet. J. 2014, 201, 39–45. [Google Scholar] [CrossRef]
- Hannibal, J. Hirota, T., Hatori, M., Panda, S., Eds.; Neuroanatomy of the Suprachiasmatic Nucleus (SCN): Multicolor Immunohistochemistry and 3D Reconstruction BT—Circadian Clocks; Springer: New York, NY, USA, 2022; pp. 85–98. ISBN 978-1-0716-2577-4. [Google Scholar]
- Ma, M.; Morrison, E. Neuroanatomy, Nucleus Suprachiasmatic. In Neuroanatomy, Nucleus Suprachiasmatic; StatPearls Publishing LLC: Treasure Island Florida USA, 2025. [Google Scholar] [PubMed]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Jewett, B.; Sharma, S. Physiology, GABA. In Physiology, GABA; StatPearls Publishing Ltd: Treasure Island Florida USA, 2023. [Google Scholar] [PubMed]
- Andrews, J.L.; Zhang, X.; Mccarthy, J.J.; Mcdearmon, E.L.; Hornberger, T.A.; Russell, B.; Campbell, K.S.; Arbogast, S.; Reid, M.B.; Walker, J.R.; et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc. Natl. Acad. Sci. USA 2010, 107, 19090–19095. [Google Scholar] [CrossRef] [PubMed]
- Mansingh, S.; Handschin, C. Time to Train: The Involvement of the Molecular Clock in Exercise Adaptation of Skeletal Muscle. Front. Physiol. 2022, 13, 902031. [Google Scholar] [CrossRef]
- Nakao, R.; Nikawa, T.; Oishi, K. The skeletal muscle circadian clock: Current insights. ChronoPhysiology Ther. 2017, 7, 47–57. [Google Scholar] [CrossRef]
- Narasimamurthy, R.; Virshup, D.M. The phosphorylation switch that regulates ticking of the circadian clock. Mol. Cell 2021, 81, 1133–1146. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef]
- Riley, L.A.; Zhang, X.; Douglas, C.M.; Mijares, J.M.; Hammers, D.W.; Wolff, C.A.; Wood, N.B.; Olafson, H.R.; Du, P.; Labeit, S. The skeletal muscle circadian clock regulates titin splicing through RBM20. Elife 2022, 11, e76478. [Google Scholar] [CrossRef]
- Kelu, J.J.; Hughes, S.M. Muscle peripheral circadian clock drives nocturnal protein degradation via raised Ror/Rev-erb balance and prevents premature sarcopenia. Proc. Natl. Acad. Sci. USA 2025, 122, e2422446122. [Google Scholar] [CrossRef]
- Kahn, R.E.; Dayanidhi, S.; Lacham-Kaplan, O.; Hawley, J.A. Molecular clocks, satellite cells, and skeletal muscle regeneration. Am. J. Physiol. Physiol. 2023, 324, C1332–C1340. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.A.; Harfmann, B.D.; Zhang, X.; Srikuea, R.; England, J.H.; Hodge, B.A.; Wen, Y.; Riley, L.A.; Yu, Q.; Christie, A.; et al. Intrinsic muscle clock is necessary for musculoskeletal health. J. Physiol. 2015, 593, 5387–5404. [Google Scholar] [CrossRef] [PubMed]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.H.; Harfmann, B.D.; Schroder, E.A.; Esser, K.A. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Ehlen, J.C.; Brager, A.J.; Baggs, J.; Pinckney, L.; Gray, C.L.; DeBruyne, J.P.; Esser, K.A.; Takahashi, J.S.; Paul, K.N. Bmal1 function in skeletal muscle regulates sleep. Elife 2017, 6, e26557. [Google Scholar] [CrossRef]
- Zylka, M.J.; Shearman, L.P.; Weaver, D.R.; Reppert, S.M. Three period homologs in mammals: Differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 1998, 20, 1103–1110. [Google Scholar] [CrossRef]
- Miller, B.H.; McDearmon, E.L.; Panda, S.; Hayes, K.R.; Zhang, J.; Andrews, J.L.; Antoch, M.P.; Walker, J.R.; Esser, K.A.; Hogenesch, J.B. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 3342–3347. [Google Scholar] [CrossRef]
- Pizarro, A.; Hayer, K.; Lahens, N.F.; Hogenesch, J.B. CircaDB: A database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013, 41, D1009-13. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Andrews, J.L.; McDearmon, E.L.; Campbell, K.S.; Barber, B.K.; Miller, B.H.; Walker, J.R.; Hogenesch, J.B.; Takahashi, J.S.; Esser, K.A. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genom. 2007, 31, 86–95. [Google Scholar] [CrossRef]
- Harfmann, B.D.; Schroder, E.A.; Esser, K.A. Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythms 2015, 30, 84–94. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Tagliazucchi, G.M.; Pallafacchina, G.; Tothova, J.; Argentini, C.; Agatea, L.; Abraham, R.; Ahdesmäki, M.; Forcato, M. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol. Metab. 2015, 4, 823–833. [Google Scholar] [CrossRef]
- Perrin, L.; Loizides-Mangold, U.; Chanon, S.; Gobet, C.; Hulo, N.; Isenegger, L.; Weger, B.D.; Migliavacca, E.; Charpagne, A.; Betts, J.A. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 2018, 7, e34114. [Google Scholar] [CrossRef]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef]
- Choi, Y.I.; Park, D.K.; Chung, J.-W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J. Circadian rhythm disruption is associated with an increased risk of sarcopenia: A nationwide population-based study in Korea. Sci. Rep. 2019, 9, 12015. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanizawa, K.; Tachikawa, R.; Murase, K.; Minami, T.; Inouchi, M.; Handa, T.; Oga, T.; Hirai, T.; Chin, K. Associations of obstructive sleep apnea with truncal skeletal muscle mass and density. Sci. Rep. 2018, 8, 6550. [Google Scholar] [CrossRef]
- Nedeltcheva, A.V.; Kilkus, J.M.; Imperial, J.; Schoeller, D.A.; Penev, P.D. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann. Intern. Med. 2010, 153, 435–441. [Google Scholar] [CrossRef]
- Morrison, M.; Halson, S.L.; Weakley, J.; Hawley, J.A. Sleep, circadian biology and skeletal muscle interactions: Implications for metabolic health. Sleep Med. Rev. 2022, 66, 101700. [Google Scholar] [CrossRef]
- van Moorsel, D.; Hansen, J.; Havekes, B.; Scheer, F.A.J.L.; Jörgensen, J.A.; Hoeks, J.; Schrauwen-Hinderling, V.B.; Duez, H.; Lefebvre, P.; Schaper, N.C. Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity. Mol. Metab. 2016, 5, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Wefers, J.; Connell, N.J.; Fealy, C.E.; Andriessen, C.; de Wit, V.; van Moorsel, D.; Moonen-Kornips, E.; Jörgensen, J.A.; Hesselink, M.K.C.; Havekes, B. Day-night rhythm of skeletal muscle metabolism is disturbed in older, metabolically compromised individuals. Mol. Metab. 2020, 41, 101050. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef]
- Gao, H.; Xiong, X.; Lin, Y.; Chatterjee, S.; Ma, K. The clock regulator Bmal1 protects against muscular dystrophy. Exp. Cell Res. 2020, 397, 112348. [Google Scholar] [CrossRef]
- Chatterjee, S.; Yin, H.; Li, W.; Lee, J.; Yechoor, V.K.; Ma, K. The nuclear receptor and clock repressor Rev-erbα suppresses myogenesis. Sci. Rep. 2019, 9, 4585. [Google Scholar] [CrossRef]
- Welch, R.D.; Billon, C.; Valfort, A.-C.; Burris, T.P.; Flaveny, C.A. Pharmacological inhibition of REV-ERB stimulates differentiation, inhibits turnover and reduces fibrosis in dystrophic muscle. Sci. Rep. 2017, 7, 17142. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Gao, H.; Lin, Y.; Yechoor, V.; Ma, K. Inhibition of Rev-erbα ameliorates muscular dystrophy. Exp. Cell Res. 2021, 406, 112766. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xue, T.; Liu, S.; Geng, S.; Shi, X.; Qian, P.; He, W.; Zheng, J.; Li, Y.; Lou, J. Loss of CRY2 promotes regenerative myogenesis by enhancing PAX7 expression and satellite cell proliferation. MedComm 2023, 4, e202. [Google Scholar] [CrossRef]
- Rossi, R.; Falzarano, M.S.; Osman, H.; Armaroli, A.; Scotton, C.; Mantuano, P.; Boccanegra, B.; Cappellari, O.; Schwartz, E.; Yuryev, A. Circadian genes as exploratory biomarkers in DMD: Results from both the Mdx mouse model and patients. Front. Physiol. 2021, 12, 678974. [Google Scholar] [CrossRef]
- Betts, C.A.; Jagannath, A.; van Westering, T.L.E.; Bowerman, M.; Banerjee, S.; Meng, J.; Falzarano, M.S.; Cravo, L.; McClorey, G.; Meijboom, K.E. Dystrophin involvement in peripheral circadian SRF signalling. Life Sci. Alliance 2021, 4, e202101014. [Google Scholar] [CrossRef]
- Scotton, C.; Bovolenta, M.; Schwartz, E.; Falzarano, M.S.; Martoni, E.; Passarelli, C.; Armaroli, A.; Osman, H.; Rodolico, C.; Messina, S. Deep RNA profiling identified CLOCK and molecular clock genes as pathophysiological signatures in collagen VI myopathy. J. Cell Sci. 2016, 129, 1671–1684. [Google Scholar] [CrossRef]
- Kiperman, T.; Ma, K. Circadian clock in muscle disease etiology and therapeutic potential for duchenne muscular dystrophy. Int. J. Mol. Sci. 2024, 25, 4767. [Google Scholar] [CrossRef] [PubMed]
- Savarese, M.; Sarparanta, J.; Vihola, A.; Udd, B.; Hackman, P. Increasing role of titin mutations in neuromuscular disorders. J. Neuromuscul. Dis. 2016, 3, 293–308. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.P.; Gudiksen, A.; Solagna, F. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Perrin, L.; Loizides-Mangold, U.; Skarupelova, S.; Pulimeno, P.; Chanon, S.; Robert, M.; Bouzakri, K.; Modoux, C.; Roux-Lombard, P.; Vidal, H.; et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol. Metab. 2015, 4, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Karpac, J. Muscle directs diurnal energy homeostasis through a myokine-dependent hormone module in Drosophila. Curr. Biol. 2017, 27, 1941–1955. [Google Scholar] [CrossRef]
- de Souza Teixeira, A.A.; Biondo, L.; Silveira, L.S.; Lima, E.A.; Diniz, T.A.; Lira, F.S.; Seelaender, M.; Rosa Neto, J.C. Exercise training induces alteration of clock genes and myokines expression in tumor-bearing mice. Cell Biochem. Funct. 2023, 41, 1383–1394. [Google Scholar] [CrossRef]
- Viggars, M.R.; Berko, H.E.; Hesketh, S.J.; Wolff, C.A.; Gutierrez-Monreal, M.A.; Martin, R.A.; Jennings, I.G.; Huo, Z.; Esser, K.A. Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training. Mol. Metab. 2024, 86, 101980. [Google Scholar] [CrossRef]
- Gutierrez-Monreal, M.A.; Wolff, C.A.; Rijos, E.E.; Viggars, M.R.; Douglas, C.M.; Pagala, V.; Peng, J.; Hunt, L.C.; Ding, H.; Huo, Z. Targeted Bmal1 restoration in muscle prolongs lifespan with systemic health effects in aging model. JCI Insight 2024, 9, e174007. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Dyar, K.A.; Treebak, J.T.; Jepsen, S.L.; Ehrlich, A.M.; Ashcroft, S.P.; Trost, K.; Kunzke, T.; Prade, V.M.; Small, L. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 2022, 34, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Vallve, N.; Duglan, D.; Vaughan, M.E.; Pariollaud, M.; Handzlik, M.K.; Fan, W.; Yu, R.T.; Liddle, C.; Downes, M.; Delezie, J. Daily running enhances molecular and physiological circadian rhythms in skeletal muscle. Mol. Metab. 2022, 61, 101504. [Google Scholar] [CrossRef]
- Wolff, C.A.; Gutierrez-Monreal, M.A.; Meng, L.; Zhang, X.; Douma, L.G.; Costello, H.M.; Douglas, C.M.; Ebrahimi, E.; Pham, A.; Oliveira, A.C. Defining the age-dependent and tissue-specific circadian transcriptome in male mice. Cell Rep. 2023, 42, 111982. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Wood, Z.A.; Poole, L.B.; Karplus, P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003, 300, 650–653. [Google Scholar] [CrossRef]
- Jackson, M.J.; Pollock, N.; Staunton, C.A.; Stretton, C.; Vasilaki, A.; McArdle, A. Oxidative stress in skeletal muscle: Unraveling the potential beneficial and deleterious roles of reactive oxygen species. Oxidative Stress 2020, 2020, 713–733. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Milev, N.B.; Reddy, A.B. Circadian redox oscillations and metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437. [Google Scholar] [CrossRef]
- O’Neill, J.S.; Van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.Y.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Wang, T.A.; Yu, Y.V.; Govindaiah, G.; Ye, X.; Artinian, L.; Coleman, T.P.; Sweedler, J.V.; Cox, C.L.; Gillette, M.U. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science 2012, 337, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef]
- Lai, A.G.; Doherty, C.J.; Mueller-Roeber, B.; Kay, S.A.; Schippers, J.H.M.; Dijkwel, P.P. Circadian Clock-Associated 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl. Acad. Sci. USA 2012, 109, 17129–17134. [Google Scholar] [CrossRef]
- Krishnan, N.; Davis, A.J.; Giebultowicz, J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008, 374, 299–303. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Biol. Chem. 2003, 278, 21592–21600. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Z.; Wang, X.-J.; Jiang, T.; Huang, Z.; Fang, D.; Zhang, D.D. Direct interaction between Nrf2 and p21Cip1/WAF1 upregulates the Nrf2-mediated antioxidant response. Mol. Cell 2009, 34, 663–673. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K.I. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathionemediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, D.; Jin, T.; Cai, D.J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic Antioxidant Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hur, E.; Ryoo, I.; Jung, K.-A.; Kwak, J.; Kwak, M.-K. Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic β-cells. Toxicol. Appl. Pharmacol. 2012, 264, 431–438. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef]
- Rutter, J.; Reick, M.; Wu, L.C.; McKnight, S.L. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 2001, 293, 510–514. [Google Scholar] [CrossRef]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in mus musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef]
- Dirksen, R.T. Sarcoplasmic reticulum–mitochondrial through-space coupling in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 389–395. [Google Scholar] [CrossRef]
- Cooper, G.M. The Mechanism of Oxidative Phosphorylation. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland Massachusetts USA, 2000; pp. 396–402. [Google Scholar]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef]
- Mezhnina, V.; Ebeigbe, O.P.; Poe, A.; Kondratov, R.V. Circadian control of mitochondria in reactive oxygen species homeostasis. Antioxid. Redox Signal. 2022, 37, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.-Y.; Yu, W.; Sena, L.A.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 2013, 342, 1243417. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Patel, V.R.; Eckel-Mahan, K.L.; Peleg, S.; Forne, I.; Ladurner, A.G.; Baldi, P.; Imhof, A.; Sassone-Corsi, P. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 3339–3344. [Google Scholar] [CrossRef]
- Cela, O.; Scrima, R.; Pazienza, V.; Merla, G.; Benegiamo, G.; Augello, B.; Fugetto, S.; Menga, M.; Rubino, R.; Fuhr, L. Clock genes-dependent acetylation of complex I sets rhythmic activity of mitochondrial OxPhos. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 596–606. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Atger, F.; Dayon, L.; Galindo, A.N.; Wang, J.; Martin, E.; Da Silva, L.; Montoliu, I.; Collino, S.; Martin, F.-P. Circadian and feeding rhythms orchestrate the diurnal liver acetylome. Cell Rep. 2017, 20, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef]
- Oliva-Ramírez, J.; Moreno-Altamirano, M.M.B.; Pineda-Olvera, B.; Cauich-Sánchez, P.; Sánchez-García, F.J. Crosstalk between circadian rhythmicity, mitochondrial dynamics and macrophage bactericidal activity. Immunology 2014, 143, 490–497. [Google Scholar] [CrossRef]
- Aguilar-López, B.A.; Moreno-Altamirano, M.M.B.; Dockrell, H.M.; Duchen, M.R.; Sánchez-García, F.J. Mitochondria: An integrative hub coordinating circadian rhythms, metabolism, the microbiome, and immunity. Front. Cell Dev. Biol. 2020, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- De Goede, P.; Wefers, J.; Brombacher, E.C.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J.D. Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 2007, 447, 477–481. [Google Scholar] [CrossRef]
- Pacelli, C.; Rotundo, G.; Lecce, L.; Menga, M.; Bidollari, E.; Scrima, R.; Cela, O.; Piccoli, C.; Cocco, T.; Vescovi, A.L. Parkin mutation affects clock gene-dependent energy metabolism. Int. J. Mol. Sci. 2019, 20, 2772. [Google Scholar] [CrossRef]
- Ulgherait, M.; Chen, A.; McAllister, S.F.; Kim, H.X.; Delventhal, R.; Wayne, C.R.; Garcia, C.J.; Recinos, Y.; Oliva, M.; Canman, J.C. Circadian regulation of mitochondrial uncoupling and lifespan. Nat. Commun. 2020, 11, 1927. [Google Scholar] [CrossRef]
- Kim, J.; Sun, W. Circadian coordination: Understanding interplay between circadian clock and mitochondria. Anim. Cells Syst. 2024, 28, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Casas, Y.; Fernández-Martínez, J.; Martín-Estebané, M.; Aranda-Martínez, P.; López-Rodríguez, A.; Esquivel-Ruiz, S.; Yang, Y.; Escames, G.; Acuña-Castroviejo, D. Melatonin and Exercise Restore Myogenesis and Mitochondrial Dynamics Deficits Associated With Sarcopenia in iMS-Bmal1−/− Mice. J. Pineal Res. 2025, 77, e70049. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.D.; Kriebs, A.; Vaughan, M.; Duglan, D.; Fan, W.; Henriksson, E.; Huber, A.-L.; Papp, S.J.; Nguyen, M.; Afetian, M. CRY1/2 selectively repress PPARδ and limit exercise capacity. Cell Metab. 2017, 26, 243–255. [Google Scholar] [CrossRef]
- Mansingh, S.; Maier, G.; Delezie, J.; Westermark, P.O.; Ritz, D.; Duchemin, W.; Santos, G.; Karrer-Cardel, B.; Steurer, S.A.; Albrecht, U. More than the clock: Distinct regulation of muscle function and metabolism by PER2 and RORα. J. Physiol. 2024, 602, 6373–6402. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Altıntaş, A.; Smith, J.A.B.; Sardon-Puig, L.; Zhang, X.; Basse, A.L.; Laker, R.C.; Gao, H.; Liu, Z.; Dollet, L. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci. Adv. 2021, 7, eabi9654. [Google Scholar] [CrossRef]
- Solanas, G.; Peixoto, F.O.; Perdiguero, E.; Jardí, M.; Ruiz-Bonilla, V.; Datta, D.; Symeonidi, A.; Castellanos, A.; Welz, P.-S.; Caballero, J.M. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 2017, 170, 678–692. [Google Scholar] [CrossRef]
- Dudek, M.; Angelucci, C.; Pathiranage, D.; Wang, P.; Mallikarjun, V.; Lawless, C.; Swift, J.; Kadler, K.E.; Boot-Handford, R.P.; Hoyland, J.A. Circadian time series proteomics reveals daily dynamics in cartilage physiology. Osteoarthr. Cartil. 2021, 29, 739–749. [Google Scholar] [CrossRef]
- Chang, J.; Garva, R.; Pickard, A.; Yeung, C.Y.C.; Mallikarjun, V.; Swift, J.; Holmes, D.F.; Calverley, B.; Lu, Y.; Adamson, A.; et al. Circadian control of the secretory pathway maintains collagen homeostasis. Nat. Cell Biol. 2020, 22, 74–86. [Google Scholar] [CrossRef]
- Garva, R.; Yeung, C.-Y.C.; Pickard, A.; Lu, Y.; Mallikarjun, V.; Swift, J.; Taylor, S.H.; Rai, J.; Eyre, D.R.; Chaturvedi, M. Mmp14-controlled extracellular matrix homeostasis is required for circadian rhythm. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cunningham, P.S.; Meijer, P.; Nazgiewicz, A.; Anderson, S.G.; Borthwick, L.A.; Bagnall, J.; Kitchen, G.B.; Lodyga, M.; Begley, N.; Venkateswaran, R.V.; et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development. Proc. Natl. Acad. Sci. USA 2020, 117, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Hahn, K.; Duraisamy, S.K.; Salathe, M.A.; Huang, S.K.; Burris, T.P.; Sundar, I.K. Rev-erbα agonists suppresses TGFβ1-induced fibroblast-to-myofibroblast transition and pro-fibrotic phenotype in human lung fibroblasts. Biochem. Biophys. Res. Commun. 2023, 669, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Gongora, R.; Sosulski, M.L.; Luo, F.; Sanchez, C.G. Regulation of transforming growth factor-beta1 (TGF-β1)-induced pro-fibrotic activities by circadian clock gene BMAL1. Respir. Res. 2016, 17, 4. [Google Scholar] [PubMed]
- Wang, Q.; Sundar, I.K.; Lucas, J.H.; Park, J.-G.; Nogales, A.; Martinez-Sobrido, L.; Rahman, I. Circadian clock molecule REV-ERBα regulates lung fibrotic progression through collagen stabilization. Nat. Commun. 2023, 14, 1295. [Google Scholar] [CrossRef]
- Ingle, K.A.; Kain, V.; Goel, M.; Prabhu, S.D.; Young, M.E.; Halade, G.V. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am. J. Physiol. Circ. Physiol. 2015, 309, H1827–H1836. [Google Scholar] [CrossRef]
- Mia, S.; Kane, M.S.; Latimer, M.N.; Reitz, C.J.; Sonkar, R.; Benavides, G.A.; Smith, S.R.; Frank, S.J.; Martino, T.A.; Zhang, J. Differential effects of REV-ERBα/β agonism on cardiac gene expression, metabolism, and contractile function in a mouse model of circadian disruption. Am. J. Physiol. Circ. Physiol. 2020, 318, H1487–H1508. [Google Scholar] [CrossRef]
- Liang, Q.; Xu, H.; Liu, M.; Qian, L.; Yan, J.; Yang, G.; Chen, L. Postnatal deletion of Bmal1 in cardiomyocyte promotes pressure overload induced cardiac remodeling in mice. J. Am. Heart Assoc. 2022, 11, e025021. [Google Scholar] [CrossRef]
- Durgan, D.J.; Pulinilkunnil, T.; Villegas-Montoya, C.; Garvey, M.E.; Frangogiannis, N.G.; Michael, L.H.; Chow, C.-W.; Dyck, J.R.B.; Young, M.E. Ischemia/reperfusion tolerance is time-of-day–dependent: Mediation by the cardiomyocyte circadian clock. Circ. Res. 2010, 106, 546–550. [Google Scholar] [CrossRef]
- Yoshida, Y.; Matsunaga, N.; Nakao, T.; Hamamura, K.; Kondo, H.; Ide, T.; Tsutsui, H.; Tsuruta, A.; Kurogi, M.; Nakaya, M. Alteration of circadian machinery in monocytes underlies chronic kidney disease-associated cardiac inflammation and fibrosis. Nat. Commun. 2021, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, S.; Xing, J.; Yu, W.; Rao, T.; Zhou, X.; Ruan, Y.; Li, S.; Xia, Y.; Song, T. BMAL1 inhibits renal fibrosis and renal interstitial inflammation by targeting the ERK1/2/ELK-1/Egr-1 axis. Int. Immunopharmacol. 2023, 125, 111140. [Google Scholar] [CrossRef]
- Chen, W.-D.; Yeh, J.-K.; Peng, M.-T.; Shie, S.-S.; Lin, S.-L.; Yang, C.-H.; Chen, T.-H.; Hung, K.-C.; Wang, C.-C.; Hsieh, I.-C. Circadian CLOCK mediates activation of transforming growth factor-β signaling and renal fibrosis through cyclooxygenase 2. Am. J. Pathol. 2015, 185, 3152–3163. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, C.; Liang, Q.; Zheng, F.; Guan, Y.; Yang, G.; Chen, L. Postnatal deletion of Bmal1 in mice protects against obstructive renal fibrosis via suppressing Gli2 transcription. FASEB J. 2021, 35, e21530. [Google Scholar] [CrossRef]
- Crouchet, E.; Dachraoui, M.; Jühling, F.; Roehlen, N.; Oudot, M.A.; Durand, S.C.; Ponsolles, C.; Gadenne, C.; Meiss-Heydmann, L.; Moehlin, J. Targeting the liver clock improves fibrosis by restoring TGF-β signaling. J. Hepatol. 2025, 82, 120–133. [Google Scholar] [CrossRef]
- Jokl, E.; Llewellyn, J.; Simpson, K.; Adegboye, O.; Pritchett, J.; Zeef, L.; Donaldson, I.; Athwal, V.S.; Purssell, H.; Street, O. Circadian disruption primes myofibroblasts for accelerated activation as a mechanism underpinning fibrotic progression in non-alcoholic fatty liver disease. Cells 2023, 12, 1582. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Han, Z.; Yang, P.; Zhu, L.; Hua, Z.; Zhang, J. Loss of clock gene mPer2 promotes liver fibrosis induced by carbon tetrachloride. Hepatol. Res. 2010, 40, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Lin, L.; Pei, W.; Li, Y. Rev-erbα: The circadian guardian against NLRP3-driven liver fibrosis. Mol. Med. Rep. 2025, 32, 270. [Google Scholar]
- Chen, P.; Kakan, X.; Wang, S.; Dong, W.; Jia, A.; Cai, C.; Zhang, J. Deletion of clock gene Per2 exacerbates cholestatic liver injury and fibrosis in mice. Exp. Toxicol. Pathol. 2013, 65, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, L.; Ju, D.; Lu, Z.; Wang, C.; Guo, X.; Zhao, H.; Shen, S.; Cheng, Z.; Shen, J. The pancreatic clock is a key determinant of pancreatic fibrosis progression and exocrine dysfunction. Sci. Transl. Med. 2022, 14, eabn3586. [Google Scholar] [CrossRef]
- Hunter, A.L.; Pelekanou, C.E.; Barron, N.J.; Northeast, R.C.; Grudzien, M.; Adamson, A.D.; Downton, P.; Cornfield, T.; Cunningham, P.S.; Billaud, J.-N. Adipocyte NR1D1 dictates adipose tissue expansion during obesity. Elife 2021, 10, e63324. [Google Scholar] [CrossRef]
- Xiong, X.; Lin, Y.; Lee, J.; Paul, A.; Yechoor, V.; Figueiro, M.; Ma, K. Chronic circadian shift leads to adipose tissue inflammation and fibrosis. Mol. Cell. Endocrinol. 2021, 521, 111110. [Google Scholar] [CrossRef]
- Gossan, N.; Zeef, L.; Hensman, J.; Hughes, A.; Bateman, J.F.; Rowley, L.; Little, C.B.; Piggins, H.D.; Rattray, M.; Boot-Handford, R.P. The circadian clock in murine chondrocytes regulates genes controlling key aspects of cartilage homeostasis. Arthritis Rheum. 2013, 65, 2334–2345. [Google Scholar] [CrossRef]
- Yang, N.; Williams, J.; Pekovic-Vaughan, V.; Wang, P.; Olabi, S.; McConnell, J.; Gossan, N.; Hughes, A.; Cheung, J.; Streuli, C.H.; et al. Cellular mechano-environment regulates the mammary circadian clock. Nat. Commun. 2017, 8, 14287. [Google Scholar] [CrossRef]
- Ding, S.-L.; Zhang, T.-W.; Zhang, Q.-C.; Ding, W.; Li, Z.-F.; Han, G.-J.; Bai, J.-S.; Li, X.-L.; Dong, J.; Wang, H.-R. Excessive mechanical strain accelerates intervertebral disc degeneration by disrupting intrinsic circadian rhythm. Exp. Mol. Med. 2021, 53, 1911–1923. [Google Scholar] [CrossRef]
- Dudek, M.; Gossan, N.; Yang, N.; Im, H.-J.; Ruckshanthi, J.P.D.; Yoshitane, H.; Li, X.; Jin, D.; Wang, P.; Boudiffa, M. The chondrocyte clock gene Bmal1 controls cartilage homeostasis and integrity. J. Clin. Investig. 2016, 126, 365–376. [Google Scholar] [CrossRef]
- Dudek, M.; Morris, H.; Rogers, N.; Pathiranage, D.R.J.; Raj, S.S.; Chan, D.; Kadler, K.E.; Hoyland, J.; Meng, Q.-J. The clock transcription factor BMAL1 is a key regulator of extracellular matrix homeostasis and cell fate in the intervertebral disc. Matrix Biol. 2023, 122, 1–9. [Google Scholar] [CrossRef]
- Lastella, M.; Miller, D.J.; Quilelli, M.; Roberts, S.; Aisbett, B.; Condo, D. The impact of chronotype on the sleep and training responses of elite female Australian footballers. Clocks & Sleep 2021, 3, 528–535. [Google Scholar] [CrossRef]
- Facer-Childs, E.; Brandstaetter, R. The impact of circadian phenotype and time since awakening on diurnal performance in athletes. Curr. Biol. 2015, 25, 518–522. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; Münch, M.; Kruger, R. Chronotype differences in body composition, dietary intake and eating behavior outcomes: A scoping systematic review. Adv. Nutr. 2022, 13, 2357–2405. [Google Scholar] [CrossRef] [PubMed]
- Facer-Childs, E.R.; Boiling, S.; Balanos, G.M. The effects of time of day and chronotype on cognitive and physical performance in healthy volunteers. Sport. Med. 2018, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Murray, G.; Herlihy, M.; Weiss, C.; King, J.; Hutchinson, E.; Albert, N.; Ingram, K.K. Circadian effects on performance and effort in collegiate swimmers. J. Circadian Rhythms 2018, 16, 8. [Google Scholar] [CrossRef]
- Kunorozva, L.; Roden, L.C.; Rae, D.E. Perception of effort in morning-type cyclists is lower when exercising in the morning. J. Sports Sci. 2014, 32, 917–925. [Google Scholar] [CrossRef]
- Lang, C.; Richardson, C.; Short, M.A.; Gradisar, M. Low-intensity scheduled morning exercise for adolescents with a late chronotype: A novel treatment to advance circadian phase? Sleep Adv. 2022, 3, zpac021. [Google Scholar] [CrossRef]
- Facer-Childs, E.R.; Middleton, B.; Skene, D.J.; Bagshaw, A.P. Resetting the late timing of ‘night owls’ has a positive impact on mental health and performance. Sleep Med. 2019, 60, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Kern, P.A.; Bush, H.M.; McQuerry, K.J.; Black, W.S.; Clasey, J.L.; Pendergast, J.S. Circadian rhythm phase shifts caused by timed exercise vary with chronotype. JCI Insight 2020, 5, e134270. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B.H. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, J.-H.; Seo, D.-Y.; Han, J.; Jung, S.-J.; Kwak, H.-B. Effects of acute exercise on mitochondrial function, dynamics, and mitophagy in rat cardiac and skeletal muscles. Int. Neurourol. J. 2019, 23, S22–S31. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian, J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]
- Ostrom, E.L.; Traustadóttir, T. Aerobic exercise training partially reverses the impairment of Nrf2 activation in older humans. Free Radic. Biol. Med. 2020, 160, 418–432. [Google Scholar] [CrossRef]
- Martinez-Canton, M.; Galvan-Alvarez, V.; Martin-Rincon, M.; Calbet, J.A.L.; Gallego-Selles, A. Unlocking peak performance: The role of Nrf2 in enhancing exercise outcomes and training adaptation in humans. Free Radic. Biol. Med. 2024, 224, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Kritikaki, E.; Asterling, R.; Ward, L.; Padget, K.; Barreiro, E.; Simoes, D.C.M. Exercise training-induced extracellular matrix protein adaptation in locomotor muscles: A systematic review. Cells 2021, 10, 1022. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef]
- Gao, L.; Kumar, V.; Vellichirammal, N.N.; Park, S.-Y.; Rudebush, T.L.; Yu, L.; Son, W.-M.; Pekas, E.J.; Wafi, A.M.; Hong, J.; et al. Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle. J. Physiol. 2020, 598, 5427–5451. [Google Scholar] [CrossRef]
- Oh, S.; Komine, S.; Warabi, E.; Akiyama, K.; Ishii, A.; Ishige, K.; Mizokami, Y.; Kuga, K.; Horie, M.; Miwa, Y. Nuclear factor (erythroid derived 2)-like 2 activation increases exercise endurance capacity via redox modulation in skeletal muscles. Sci. Rep. 2017, 7, 12902. [Google Scholar] [CrossRef]
- Onoki, T.; Izumi, Y.; Takahashi, M.; Murakami, S.; Matsumaru, D.; Ohta, N.; Wati, S.M.; Hatanaka, N.; Katsuoka, F.; Okutsu, M. Skeletal muscle-specific Keap1 disruption modulates fatty acid utilization and enhances exercise capacity in female mice. Redox Biol. 2021, 43, 101966. [Google Scholar] [CrossRef]
- Basti, A.; Yalçin, M.; Herms, D.; Hesse, J.; Aboumanify, O.; Li, Y.; Aretz, Z.; Garmshausen, J.; El-Athman, R.; Hastermann, M. Diurnal variations in the expression of core-clock genes correlate with resting muscle properties and predict fluctuations in exercise performance across the day. BMJ Open Sport Exerc. Med. 2021, 7, e000876. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jia, J.; Yu, J.; Miao, S.; Zhang, Y. The impact of aerobic exercise timing on BMAL1 protein expression and antioxidant responses in skeletal muscle of mice. Free Radic. Res. 2024, 58, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, K.; Tanabe, Y.; Tanji, F.; Ohnishi, T.; Takahashi, H. Diurnal variations in muscle and liver glycogen differ depending on the timing of exercise. J. Physiol. Sci. 2021, 71, 35. [Google Scholar] [CrossRef] [PubMed]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Björnholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef]
- Savikj, M.; Stocks, B.; Sato, S.; Caidahl, K.; Krook, A.; Deshmukh, A.S.; Zierath, J.R.; Wallberg-Henriksson, H. Exercise timing influences multi-tissue metabolome and skeletal muscle proteome profiles in type 2 diabetic patients–A randomized crossover trial. Metabolism 2022, 135, 155268. [Google Scholar] [CrossRef]
- Sato, S.; Basse, A.L.; Schönke, M.; Chen, S.; Samad, M.; Altıntaş, A.; Laker, R.C.; Dalbram, E.; Barrès, R.; Baldi, P. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 2019, 30, 92–110. [Google Scholar] [CrossRef]
- Hughes, A.T.L.; Samuels, R.E.; Baño-Otálora, B.; Belle, M.D.C.; Wegner, S.; Guilding, C.; Northeast, R.C.; Loudon, A.S.I.; Gigg, J.; Piggins, H.D. Timed daily exercise remodels circadian rhythms in mice. Commun. Biol. 2021, 4, 761. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Dandavate, V.; Ezagouri, S.; Manella, G.; Zwighaft, Z.; Sobel, J.; Kuperman, Y.; Golik, M.; Auerbach, A.; Itkin, M. Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl. Acad. Sci. USA 2021, 118, e2101115118. [Google Scholar] [CrossRef]
- Pourabdi, R.; Shahidi, F.; Tabandeh, M.R.; Salehpour, M. Aerobic exercise timing affects mitochondrial dynamics and insulin resistance by regulating the circadian clock protein expression and NAD+-SIRT1-PPARα-MFN2 pathway in the skeletal muscle of high-fat-diet-induced diabetes mice. J. Physiol. Biochem. 2025, 81, 199–214. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal. 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sport. Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef]
- Lok, R.; Zerbini, G.; Gordijn, M.C.M.; Beersma, D.G.M.; Hut, R.A. Gold, silver or bronze: Circadian variation strongly affects performance in Olympic athletes. Sci. Rep. 2020, 10, 16088. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, J.; Sedliak, M.; Valadés, D.; Muñoz, A.; Buffet-García, J.; García-Oviedo, R.; Rodríguez-Aragón, M.; Pérez-López, A.; López-Samanes, Á. Impact of time-of-day and chronotype on neuromuscular performance in semi-professional female volleyball players. Chronobiol. Int. 2022, 39, 1006–1014. [Google Scholar] [CrossRef]
- López-Samanes, Á.; Moreno-Pérez, D.; Maté-Muñoz, J.L.; Domínguez, R.; Pallarés, J.G.; Mora-Rodriguez, R.; Ortega, J.F. Circadian rhythm effect on physical tennis performance in trained male players. J. Sports Sci. 2017, 35, 2121–2128. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Trabelsi, K.; Padulo, J.; Turki, M.; El Abed, K.; Hoekelmann, A.; Hakim, A. Temporal specificity of training: Intra-day effects on biochemical responses and Olympic-Weightlifting performances. J. Sports Sci. 2015, 33, 358–368. [Google Scholar] [CrossRef] [PubMed]
- West, D.J.; Cook, C.J.; Beaven, M.C.; Kilduff, L.P. The influence of the time of day on core temperature and lower body power output in elite rugby union sevens players. J. Strength Cond. Res. 2014, 28, 1524–1528. [Google Scholar] [CrossRef]
- Henst, R.H.P.; Jaspers, R.T.; Roden, L.C.; Rae, D.E. A chronotype comparison of South African and Dutch marathon runners: The role of scheduled race start times and effects on performance. Chronobiol. Int. 2015, 32, 858–868. [Google Scholar] [CrossRef]
- Ayala, V.; Martínez-Bebia, M.; Latorre, J.A.; Gimenez-Blasi, N.; Jimenez-Casquet, M.J.; Conde-Pipo, J.; Bach-Faig, A.; Mariscal-Arcas, M. Influence of circadian rhythms on sports performance. Chronobiol. Int. 2021, 38, 1522–1536. [Google Scholar] [PubMed]
- Landi, F.; Marzetti, E.; Martone, A.M.; Bernabei, R.; Onder, G. Exercise as a remedy for sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 25–31. [Google Scholar] [CrossRef]
- Meignié, A.; Toussaint, J.-F.; Antero, J. Dealing with menstrual cycle in sport: Stop finding excuses to exclude women from research. Eur. J. Appl. Physiol. 2022, 122, 2489–2490. [Google Scholar] [CrossRef]
- Justice, M.J. Sex matters in preclinical research. Dis. Model. Mech. 2024, 17, dmm050759. [Google Scholar] [CrossRef]
- Paul, R.W.; Sonnier, J.H.; Johnson, E.E.; Hall, A.T.; Osman, A.; Connors, G.M.; Freedman, K.B.; Bishop, M.E. Inequalities in the evaluation of male versus female athletes in sports medicine research: A systematic review. Am. J. Sports Med. 2023, 51, 3335–3342. [Google Scholar] [PubMed]
- Ose, B.M.; Eisenhauer, J.; Roepe, I.; Herda, A.A.; Vopat, B.G.; Vopat, L.M. Where are all the female participants in sports and exercise medicine research? A decade later. Am. J. Sports Med. 2025, 53, 2022–2028. [Google Scholar] [CrossRef]
- Ellison, T.M.; Flagstaff, I.; Johnson, A.E. Sexual dimorphisms in anterior cruciate ligament injury: A current concepts review. Orthop. J. Sport. Med. 2021, 9, 23259671211025304. [Google Scholar]
- Emmert, M.E.; Emmert, A.S.; Goh, Q.; Cornwall, R. Sexual dimorphisms in skeletal muscle: Current concepts and research horizons. J. Appl. Physiol. 2024, 137, 274–299. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Harmer, A.R.; Ruell, P.A.; Hunter, S.K.; McKenna, M.J.; Thom, J.M.; Chisholm, D.J.; Flack, J.R. Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+-ATPase activity. J. Physiol. 2014, 592, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Esbjörnsson, M.E.; Dahlström, M.S.; Gierup, J.W.; Jansson, E.C. Muscle fiber size in healthy children and adults in relation to sex and fiber types. Muscle Nerve 2021, 63, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Moesgaard, L.; Jessen, S.; Mackey, A.L.; Hostrup, M. Myonuclear addition is associated with sex-specific fiber hypertrophy and occurs in relation to fiber perimeter not cross-sectional area. J. Appl. Physiol. 2022, 133, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.; Boldrin, L.; Morgan, J.E. The satellite cell in male and female, developing and adult mouse muscle: Distinct stem cells for growth and regeneration. PLoS ONE 2012, 7, e37950. [Google Scholar] [CrossRef] [PubMed]
- Srinivas-Shankar, U.; Roberts, S.A.; Connolly, M.J.; O’Connell, M.D.L.; Adams, J.E.; Oldham, J.A.; Wu, F.C.W. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2010, 95, 639–650. [Google Scholar] [CrossRef]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef]
- Ribas, V.; Drew, B.G.; Zhou, Z.; Phun, J.; Kalajian, N.Y.; Soleymani, T.; Daraei, P.; Widjaja, K.; Wanagat, J.; de Aguiar Vallim, T.Q. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 2016, 8, 334ra54. [Google Scholar] [CrossRef]
- Fernández-Martínez, J.; Ramírez-Casas, Y.; Yang, Y.; Aranda-Martínez, P.; Martínez-Ruiz, L.; Escames, G.; Acuña-Castroviejo, D. From Chronodisruption to Sarcopenia: The Therapeutic Potential of Melatonin. Biomolecules 2023, 13, 1779. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Manfredi, M.; Ribas-Serna, J.; Garcia-Moreno, H.; Calvo, J.R. Melatonin administrated immediately before an intense exercise reverses oxidative stress, improves immunological defenses and lipid metabolism in football players. Physiol. Behav. 2012, 105, 1099–1103. [Google Scholar] [CrossRef]
- Faria, V.S.; Manchado-Gobatto, F.B.; Scariot, P.P.M.; Zagatto, A.M.; Beck, W.R. Melatonin Potentiates Exercise-Induced Increases in Skeletal Muscle PGC-1 α and Optimizes Glycogen Replenishment. Front. Physiol. 2022, 13, 803126. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.K.A.; Fernández-Ortiz, M.; Diaz-Casado, M.E.; Rusanova, I.; Rahim, I.; Escames, G.; López, L.C.; Mokhtar, D.M.; Acuña-Castroviejo, D. The protective effect of melatonin against age-associated, sarcopenia-dependent tubular aggregate formation, lactate depletion, and mitochondrial changes. J. Gerontol. Ser. A 2018, 73, 1330–1338. [Google Scholar]
- Song, Y.; Choi, G.; Jang, L.; Kim, S.-W.; Jung, K.-H.; Park, H. Circadian rhythm gene expression and daily melatonin levels vary in athletes and sedentary males. Biol. Rhythm Res. 2018, 49, 237–245. [Google Scholar] [CrossRef]
- Fieldsend, T.W.; O’Neill, C.R.; Shrivastava, A.; Ogden, H.E.; Dand, N.; Hughes, S.M. Sexual dimorphism in human muscle ageing. MedRxiv 2025, 2001–2025. [Google Scholar] [CrossRef]
- Kasai, T.; Ishiguro, N.; Matsui, Y.; Harada, A.; Takemura, M.; Yuki, A.; Kato, Y.; Otsuka, R.; Ando, F.; Shimokata, H. Sex-and age-related differences in mid-thigh composition and muscle quality determined by computed tomography in middle-aged and elderly J apanese. Geriatr. Gerontol. Int. 2015, 15, 700–706. [Google Scholar] [CrossRef]
- Callahan, D.M.; Bedrin, N.G.; Subramanian, M.; Berking, J.; Ades, P.A.; Toth, M.J.; Miller, M.S. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: Relationship to single-fiber function. J. Appl. Physiol. 2014, 116, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Della Peruta, C.; Lozanoska-Ochser, B.; Renzini, A.; Moresi, V.; Sanchez Riera, C.; Bouché, M.; Coletti, D. Sex differences in inflammation and muscle wasting in aging and disease. Int. J. Mol. Sci. 2023, 24, 4651. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Van Kranenburg, J.; Nilwik, R.; van Loon, L.J.C. Elderly men and women benefit equally from prolonged resistance-type exercise training. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 769–779. [Google Scholar] [CrossRef]
- Desvergne, A.; Ugarte, N.; Petropoulos, I.; Friguet, B. Circadian modulation of proteasome activities and removal of carbonylated proteins. Free Radic. Biol. Med. 2014, 75, S18. [Google Scholar]
- Desvergne, A.; Ugarte, N.; Radjei, S.; Gareil, M.; Petropoulos, I.; Friguet, B. Circadian modulation of proteasome activity and accumulation of oxidized protein in human embryonic kidney HEK 293 cells and primary dermal fibroblasts. Free Radic. Biol. Med. 2016, 94, 195–207. [Google Scholar] [CrossRef]
- Klichko, V.I.; Chow, E.S.; Kotwica-Rolinska, J.; Orr, W.C.; Giebultowicz, J.M.; Radyuk, S.N. Aging alters circadian regulation of redox in Drosophila. Front. Genet. 2015, 6, 83. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Wang, Y.; Lu, J.; Lu, Y.; Wang, P.; Li, L.; Yan, W.; Cai, H.; Leigh, W.H.; et al. Achilles tendinopathy treatment via circadian rhythm regulation. J. Adv. Res. 2025, 75, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiang, J.; Zhou, M.; Huang, R.; Zhang, J.; Cui, Y.; Jiang, X.; Li, Y.; Zhou, R.; Xin, H. Dietary timing enhances exercise by modulating fat-muscle crosstalk via adipocyte AMPKα2 signaling. Cell Metab. 2025, 37, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Palmese, F.; Druda, Y.; Del Toro, R.; Bedogni, G.; Domenicali, M.; Silvani, A. The role of the circadian timing system in sarcopenia in old age: A scoping review. Eur. Geriatr. Med. 2025, 16, 447–460. [Google Scholar] [CrossRef]
- Bouchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S. Adverse metabolic response to regular exercise: Is it a rare or common occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef]
- Bherer, L.; Juneau, M. Exerkines: Mediators of the Health Benefits of Exercise. 2023. Available online: https://observatoireprevention.org/en/2023/10/30/exerkines-mediators-of-the-health-benefits-of-exercise/ (accessed on 31 July 2025).
- Aoyama, S.; Shibata, S. Time-of-day-dependent physiological responses to meal and exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Entrainment of the mouse circadian clock: Effects of stress, exercise, and nutrition. Free Radic. Biol. Med. 2018, 119, 129–138. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A. Insulin/IGF-1 drives PERIOD synthesis to entrain circadian rhythms with feeding time. Cell 2019, 177, 896–909. [Google Scholar] [CrossRef]
- Lak, M.; Bagheri, R.; Ghobadi, H.; Campbell, B.; Wong, A.; Shahrbaf, A.; Shariatzadeh, M.; Dutheil, F. Timing matters? The effects of two different timing of high protein diets on body composition, muscular performance, and biochemical markers in resistance-trained males. Front. Nutr. 2024, 11, 1397090. [Google Scholar] [CrossRef]
- Kreider, R.B.; Campbell, B. Protein for exercise and recovery. Phys. Sportsmed. 2009, 37, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Kaya, Y.; Stenbäck, V.; Sharma, R.; Sodum, N.; Mutt, S.J.; Gagnon, D.D.; Tulppo, M.; Järvelin, M.; Herzig, K. Effect of Aerobic Exercise and Time-Restricted Feeding on Metabolic Markers and Circadian Rhythm in Mice Fed with the High-Fat Diet. Mol. Nutr. Food Res. 2024, 68, 2300465. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef] [PubMed]
- Rogeri, P.S.; Zanella, R., Jr.; Martins, G.L.; Garcia, M.D.A.; Leite, G.; Lugaresi, R.; Gasparini, S.O.; Sperandio, G.A.; Ferreira, L.H.B.; Souza-Junior, T.P. Strategies to prevent sarcopenia in the aging process: Role of protein intake and exercise. Nutrients 2021, 14, 52. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M. International society of sports nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutton, E.; Pekovic-Vaughan, V. Time to Reset: The Interplay Between Circadian Rhythms and Redox Homeostasis in Skeletal Muscle Ageing and Systemic Health. Antioxidants 2025, 14, 1132. https://doi.org/10.3390/antiox14091132
Sutton E, Pekovic-Vaughan V. Time to Reset: The Interplay Between Circadian Rhythms and Redox Homeostasis in Skeletal Muscle Ageing and Systemic Health. Antioxidants. 2025; 14(9):1132. https://doi.org/10.3390/antiox14091132
Chicago/Turabian StyleSutton, Elizabeth, and Vanja Pekovic-Vaughan. 2025. "Time to Reset: The Interplay Between Circadian Rhythms and Redox Homeostasis in Skeletal Muscle Ageing and Systemic Health" Antioxidants 14, no. 9: 1132. https://doi.org/10.3390/antiox14091132
APA StyleSutton, E., & Pekovic-Vaughan, V. (2025). Time to Reset: The Interplay Between Circadian Rhythms and Redox Homeostasis in Skeletal Muscle Ageing and Systemic Health. Antioxidants, 14(9), 1132. https://doi.org/10.3390/antiox14091132





