Superoxide Dismutase Gene Family in Chili Pepper (Capsicum annuum L.): Molecular Characterization and Involvement in Redox Regulation Under Chilling Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatment
2.2. In Situ H2O2 Staining and Lipid Peroxidation Measurement
2.3. In-Gel SOD Activity Assay and Total SOD Activity Analysis
2.4. In Silico Identification and Phylogenetic Analysis of CaSOD Family
2.5. Gene Expression Analysis
2.6. Cloning and Transient Expression of CaSODs in Nicotiana Benthamiana
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physiological and Biochemical Responses of Chili Pepper Plants to Chilling Stress
3.2. Identification and Characterization of CaSODs
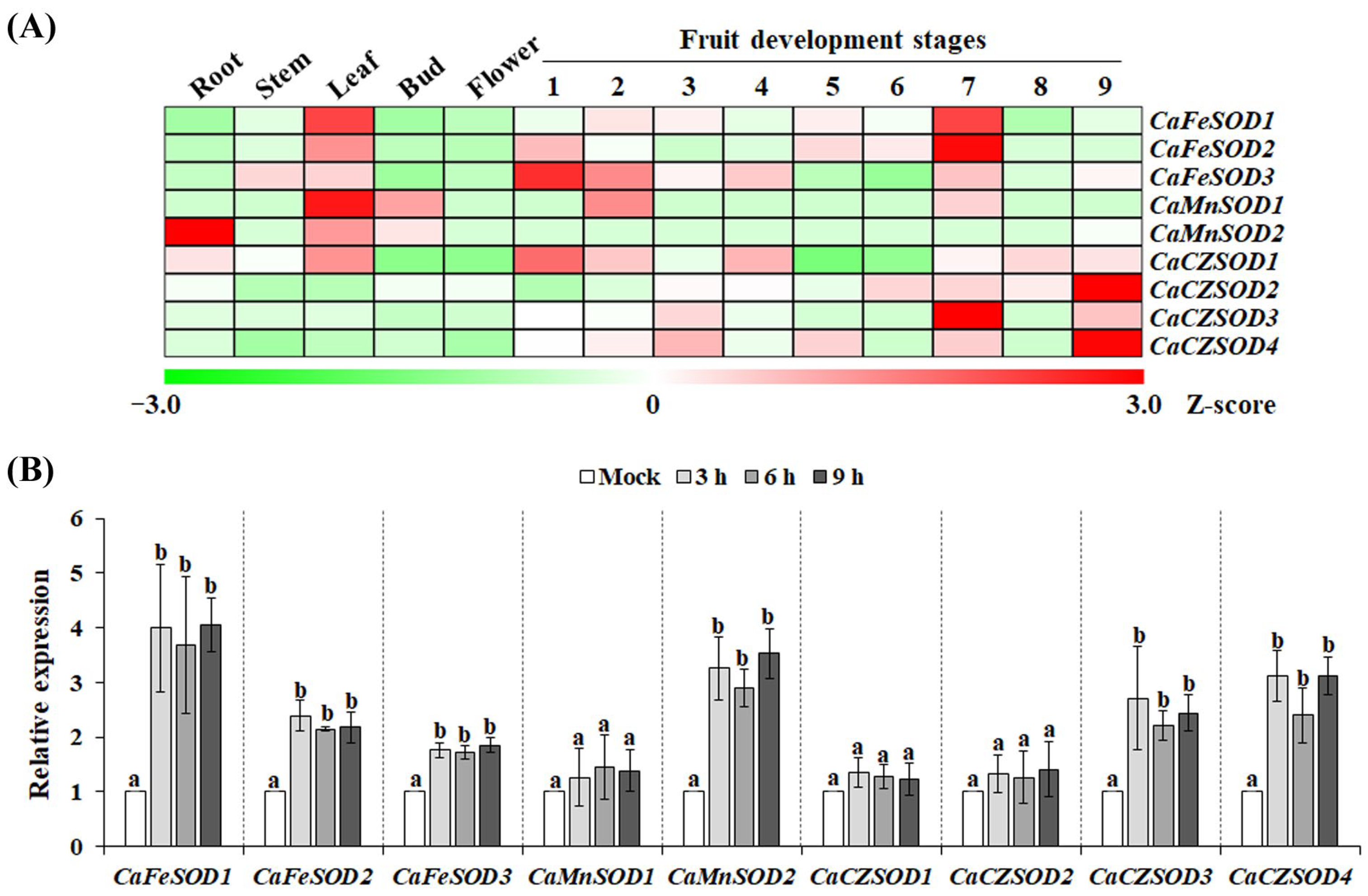
3.3. Expression Patterns of CaSODs Across Different Tissues and in Response to Chilling Stress
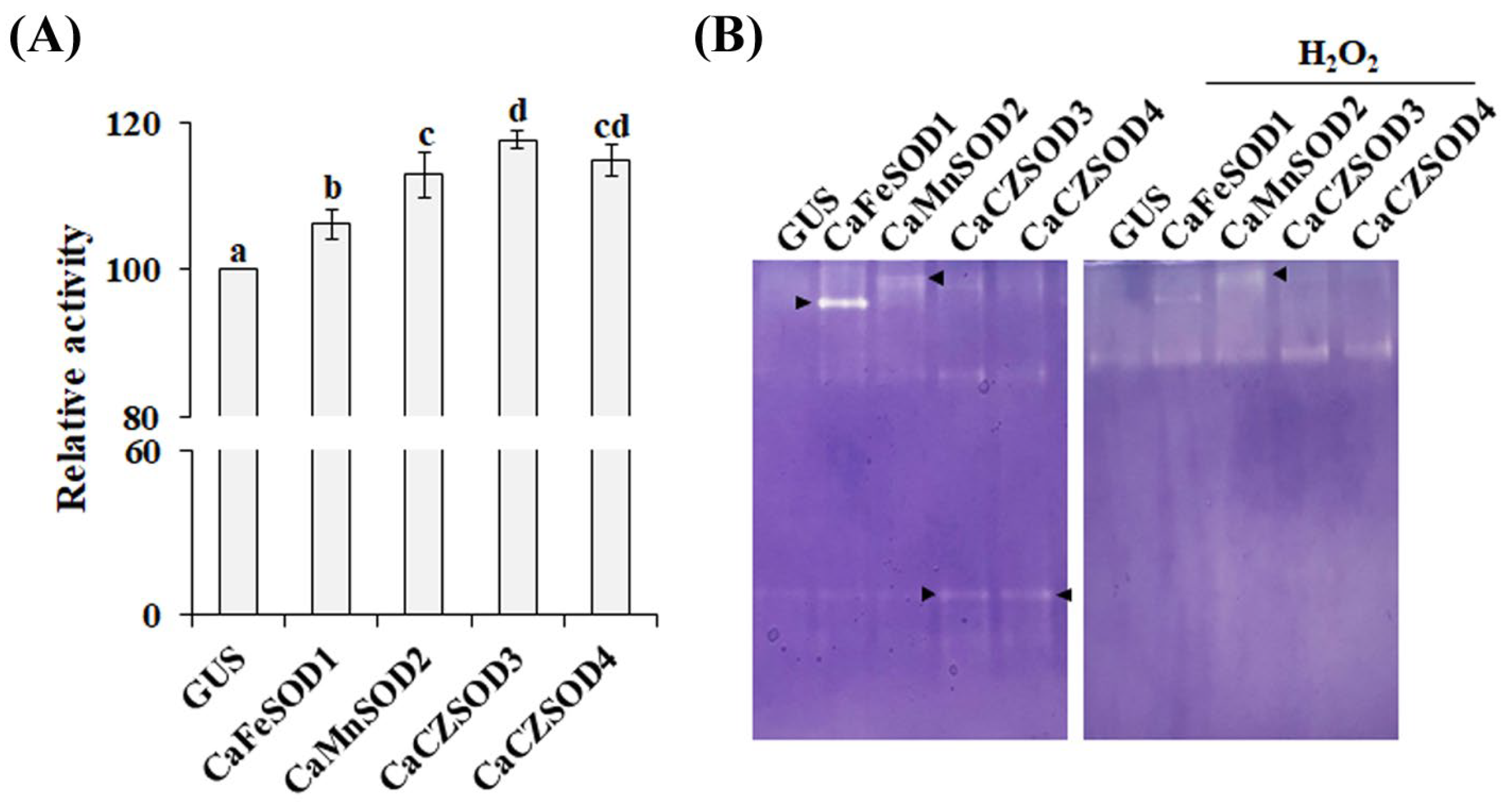
3.4. Molecular and Functional Characterization of Four Putative CaSODs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, Y.; Schafleitner, R.; Barchenger, D.W.; Lin, Y.-P.; Lee, J. Evaluation of heat stress response in pepper (Capsicum annuum L.) seedlings under controlled environmental conditions using a high-throughput 3D multispectral phenotyping. Sci. Hortic. 2025, 345, 114136. [Google Scholar] [CrossRef]
- Mandal, S.K.; Rath, S.K.; Logesh, R.; Mishra, S.K.; Devkota, H.P.; Das, N. Capsicum annuum L. and its bioactive constituents: A critical review of a traditional culinary spice in terms of its modern pharmacological potentials with toxicological issues. Phytother. Res. 2023, 37, 965–1002. [Google Scholar] [CrossRef]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Ou, L.J.; Wei, G.; Zhang, Z.Q.; Dai, X.Z.; Zou, X.X. Effects of low temperature and low irradiance on the physiological characteristics and related gene expression of different pepper species. Photosynthetica 2015, 53, 85–94. [Google Scholar] [CrossRef]
- Park, S.-Y. Elicitation of Chilling Tolerance of Pepper Seedlings Using UV-A LED. Korean J. Environ. Agric. 2020, 39, 273–279. [Google Scholar] [CrossRef]
- Guo, W.L.; Chen, R.G.; Gong, Z.H.; Yin, Y.X.; Li, D.W. Suppression subtractive hybridization analysis of genes regulated by application of exogenous abscisic acid in pepper plant (Capsicum annuum L.) leaves under chilling stress. PLoS ONE 2013, 8, e66667. [Google Scholar] [CrossRef]
- Kratsch, H.A.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Ji, H.S.; Bang, S.G.; Ahn, M.A.; Kim, G.; Kim, E.; Eom, S.H.; Hyun, T.K. Molecular Cloning and Functional Characterization of Heat Stress-Responsive Superoxide Dismutases in Garlic (Allium sativum L.). Antioxidants 2021, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Sei, S.C.; Su, Y.H.; Chiang, C.M. Overexpression of the Arabidopsis and winter squash superoxide dismutase genes enhances chilling tolerance via ABA-sensitive transcriptional regulation in transgenic Arabidopsis. Plant Signal. Behav. 2019, 14, 1685728. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Zhang, L.; Qiu, H.; Liu, C.; Wang, A.; Deng, F.; Zhu, J. A Cu/Zn superoxide dismutase gene from Saussurea involucrata Kar. et Kir., SiCSD, enhances drought, cold and oxidative stress in transgenic tobacco. Can. J. Plant Sci. 2017, 97, 816–826. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Li, X.; Xu, J.J.; Wang, L. Identification and characterization of a PutCu/Zn-SOD gene from Puccinellia tenuiflora (Turcz.) Scribn. et Merr. Plant Growth Regul. 2016, 79, 55–64. [Google Scholar] [CrossRef]
- Wang, T.; Song, H.; Zhang, B.; Lu, Q.; Liu, Z.; Zhang, S.; Guo, R.; Wang, C.; Zhao, Z.; Liu, J.; et al. Genome-wide identification, characterization, and expression analysis of superoxide dismutase (SOD) genes in foxtail millet (Setaria italica L.). 3 Biotech 2018, 8, 486. [Google Scholar] [CrossRef]
- Hyun, T.K. The Superoxide Dismutase Family in Balloon Flower (Platycodon grandiflorus): Phylogenetic Relationships, Structural Characteristics, and Expression Patterns. Curr. Issues Mol. Biol. 2025, 47, 351. [Google Scholar] [CrossRef]
- Zameer, R.; Fatima, K.; Azeem, F.; ALgwaiz, H.I.M.; Sadaqat, M.; Rasheed, A.; Batool, R.; Shah, A.N.; Zaynab, M.; Shah, A.A.; et al. Genome-Wide Characterization of Superoxide Dismutase (SOD) Genes in Daucus carota: Novel Insights Into Structure, Expression, and Binding Interaction With Hydrogen Peroxide (H2O2) Under Abiotic Stress Condition. Front. Plant Sci. 2022, 13, 870241. [Google Scholar] [CrossRef]
- Zang, Y.; Chen, J.; Li, R.; Shang, S.; Tang, X. Genome-wide analysis of the superoxide dismutase (SOD) gene family in Zostera marina and expression profile analysis under temperature stress. Peer J. 2020, 8, e9063. [Google Scholar] [CrossRef]
- Li, G.; Hu, F.; Zhang, Y.; Zhao, Y.; Wang, H.; Chen, T.; Cheng, X.; Cai, Y. Comparative genomic analysis of superoxide dismutase (SOD) genes in three Rosaceae species and expression analysis in Pyrus bretschneideri. Physiol. Mol. Biol. Plants 2021, 27, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ding, Q.; Wei, B. Genome-wide identification of superoxide dismutase gene families and their expression patterns under low-temperature, salt and osmotic stresses in watermelon and melon. 3 Biotech 2021, 11, 194. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–281. [Google Scholar] [PubMed]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef]
- Ahn, M.-A.; Son, G.H.; Hyun, T.K. Histone deacetylase family in balloon flower (Platycodon grandiflorus): Genome-wide identification and expression analysis under waterlogging stress. J. Plant Biotechnol. 2023, 50, 232–238. [Google Scholar] [CrossRef]
- Ji, H.S.; Hyun, T.K. Physiological and sucrose metabolic responses to waterlogging stress in balloon flower (Platycodon grandiflorus (Jacq.) A. DC). Physiol. Mol. Biol. Plants 2023, 29, 591–600. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Iqbal, A.; Qadri, R.; Shi, P.; Wang, Y.; Wu, Y.; Fan, H.; Wu, G. Correlation analysis of cold-related gene expression with physiological and biochemical indicators under cold stress in oil palm. PLoS ONE 2019, 14, e0225768. [Google Scholar] [CrossRef]
- Zhang, X.; Teixeira da Silva, J.A.; Niu, M.; Li, M.; He, C.; Zhao, J.; Zeng, S.; Duan, J.; Ma, G. Physiological and transcriptomic analyses reveal a response mechanism to cold stress in Santalum album L. leaves. Sci. Rep. 2017, 7, 42165. [Google Scholar] [CrossRef]
- Gülen, H.; Çetinkaya, C.; Kadıoğlu, M.; Kesici, M.; Cansev, A.; Eriş, A. Peroxidase Activity and Lipid Peroxidation in Strawberry (Fragaria X ananassa) Plants Under Low Temperature. J. Biol. Environ. Sci. 2008, 2, 95–100. [Google Scholar]
- Xu, C.; Wang, Y.; Yang, H.; Tang, Y.; Liu, B.; Hu, X.; Hu, Z. Cold acclimation alleviates photosynthetic inhibition and oxidative damage induced by cold stress in citrus seedlings. Plant Signal. Behav. 2023, 18, 2285169. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Alburquerque, N.; Martínez, D.; Carrera, E.; García-Bruntón, J.; Barba-Espín, G. Interplay among Antioxidant System, Hormone Profile and Carbohydrate Metabolism during Bud Dormancy Breaking in a High-Chill Peach Variety. Antioxidants 2021, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Dubey, K.; Arora, K.; Dalal, M.; Rai, G.K.; Mishra, D.; Chaturvedi, K.K.; Rai, A.; Kumar, S.N.; Singh, B.; et al. Characterizing the putative mitogen-activated protein kinase (MAPK) and their protective role in oxidative stress tolerance and carbon assimilation in wheat under terminal heat stress. Biotechnol. Rep. 2021, 29, e00597. [Google Scholar] [CrossRef]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Mondal, K.; Malhotra, S.P.; Jain, V.; Singh, R. Oxidative stress and antioxidant systems in Guava (Psidium guajava L.) fruits during ripening. Physiol. Mol. Biol. Plants 2009, 15, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Rashid, A.; Manzoor, M.A.; Li, L.; Sun, W.; Riaz, M.W.; Li, D.; Zhuge, Q. Genome-Wide Evolution and Comparative Analysis of Superoxide Dismutase Gene Family in Cucurbitaceae and Expression Analysis of Lagenaria siceraria Under Multiple Abiotic Stresses. Front. Genet. 2022, 12, 784878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, D.; Guo, J.; Wang, Q.; Zhang, K.; Wang, X.; Shao, L.; Luo, C.; Xia, Y.; Zhang, J. Identification and Analysis of the Superoxide Dismutase (SOD) Gene Family and Potential Roles in High-Temperature Stress Response of Herbaceous Peony (Paeonia lactiflora Pall.). Antioxidants 2024, 13, 1128. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Siemiatkowska, B.; Toleco, M.R.; Jing, Y.; Strotmann, V.; Zhang, J.; Stahl, Y.; Fernie, A.R. A Highly Efficient Agrobacterium-Mediated Method for Transient Gene Expression and Functional Studies in Multiple Plant Species. Plant Commun. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Thagun, C.; Kodama, Y. Agroinfiltration technique for elucidating the functions of strawberry genes in Fragaria vesca. Sci. Rep. 2025, 15, 20392. [Google Scholar] [CrossRef]
- Göritzer, K.; Kallolimath, S.; Strasser, R. Posttranslational modifications of heterologous proteins expressed in Nicotiana benthamiana. Plant Biotechnol. J. 2025. [Google Scholar] [CrossRef] [PubMed]


| Name | Accession Number | Chromosome Location | pI | MW (kDa) | Localization |
|---|---|---|---|---|---|
| CaFeSOD1 | ZLC03G0026460 | Chr03: 101046984-101056167 | 6.03 | 34.23 | Chloroplast |
| CaFeSOD2 | ZLC06G0026510 | Chr06: 171945626-171949651 | 8.59 | 28.19 | Chloroplast |
| CaFeSOD3 | ZLC12G0014730 | Chr12: 128908499-128917081 | 7.08 | 29.77 | Chloroplast |
| CaMnSOD1 | ZLC01G0038490 | Chr01: 273502447-273503108 | 9.73 | 21.97 | Mitochondrion |
| CaMnSOD2 | ZLC05G0019920 | Chr05: 233592291-233603550 | 8.39 | 25.53 | Mitochondrion |
| CaCZSOD1 | ZLC01G0005110 | Chr01: 9050068-9053210 | 6.54 | 32.81 | Chloroplast |
| CaCZSOD2 | ZLC01G0032140 | Chr01: 236323717-236328354 | 6.07 | 33.67 | Cytoplasm |
| CaCZSOD3 | ZLC10G0002210 | Chr10: 4241074-4245338 | 7.13 | 15.87 | Cytoplasm |
| CaCZSOD4 | ZLC11G0002710 | Chr11: 5405444-5416665 | 5.94 | 21.69 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, S.H.; Song, C.E.; Eom, S.H.; Hyun, T.K. Superoxide Dismutase Gene Family in Chili Pepper (Capsicum annuum L.): Molecular Characterization and Involvement in Redox Regulation Under Chilling Stress. Antioxidants 2025, 14, 1131. https://doi.org/10.3390/antiox14091131
Ban SH, Song CE, Eom SH, Hyun TK. Superoxide Dismutase Gene Family in Chili Pepper (Capsicum annuum L.): Molecular Characterization and Involvement in Redox Regulation Under Chilling Stress. Antioxidants. 2025; 14(9):1131. https://doi.org/10.3390/antiox14091131
Chicago/Turabian StyleBan, Seo Hyeon, Chae Eun Song, Seung Hee Eom, and Tae Kyung Hyun. 2025. "Superoxide Dismutase Gene Family in Chili Pepper (Capsicum annuum L.): Molecular Characterization and Involvement in Redox Regulation Under Chilling Stress" Antioxidants 14, no. 9: 1131. https://doi.org/10.3390/antiox14091131
APA StyleBan, S. H., Song, C. E., Eom, S. H., & Hyun, T. K. (2025). Superoxide Dismutase Gene Family in Chili Pepper (Capsicum annuum L.): Molecular Characterization and Involvement in Redox Regulation Under Chilling Stress. Antioxidants, 14(9), 1131. https://doi.org/10.3390/antiox14091131






