Retinoprotective Effects of Abscisic Acid in Ischemic Retinopathy Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Unilateral Common Carotid Artery Occlusion (UCCAO)
2.3. Topical Administration of ABA
2.4. Optical Coherence Tomography (OCT)
2.5. Photoreceptor Labeling in Retinal Cross Section
2.6. Detection of Retinal Ganglion Cells and Glial Activation by Whole-Mount Immunolabeling
2.7. Western Blot Analysis of GFAP Expression
2.8. Apoptosis Signaling Pathway Analysis
2.9. Statistical Analysis
3. Results
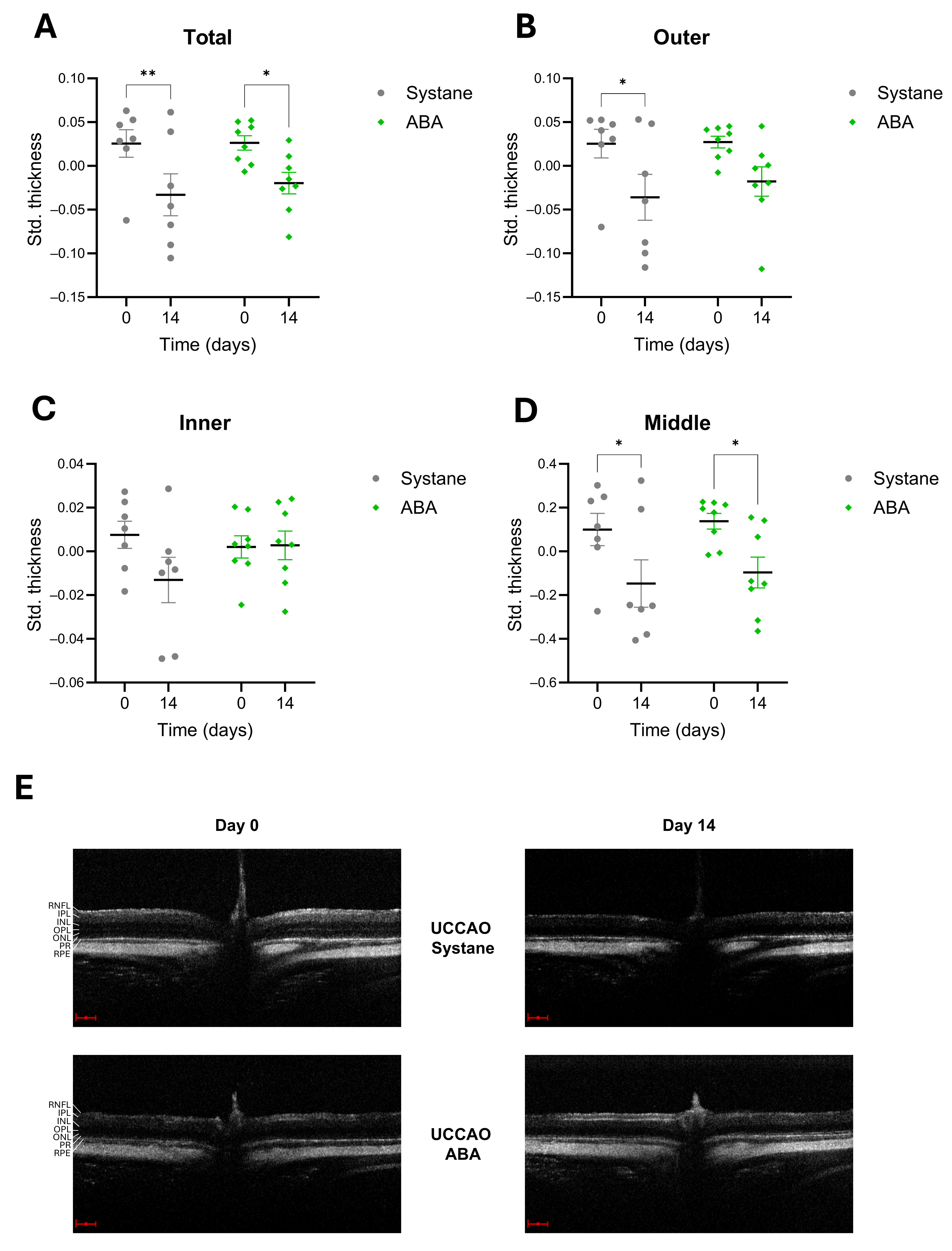
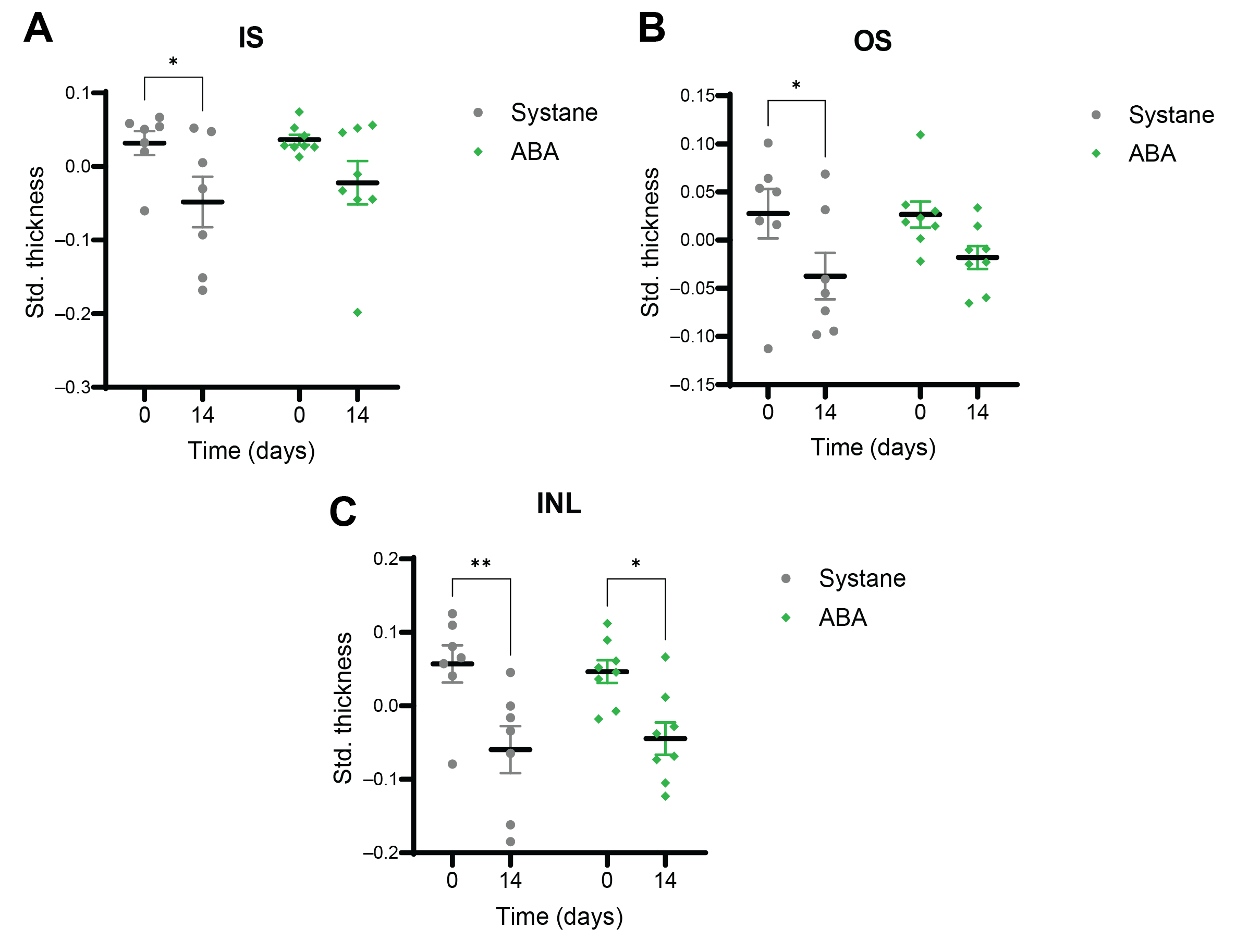
3.1. Thickness of the Retinal Layers
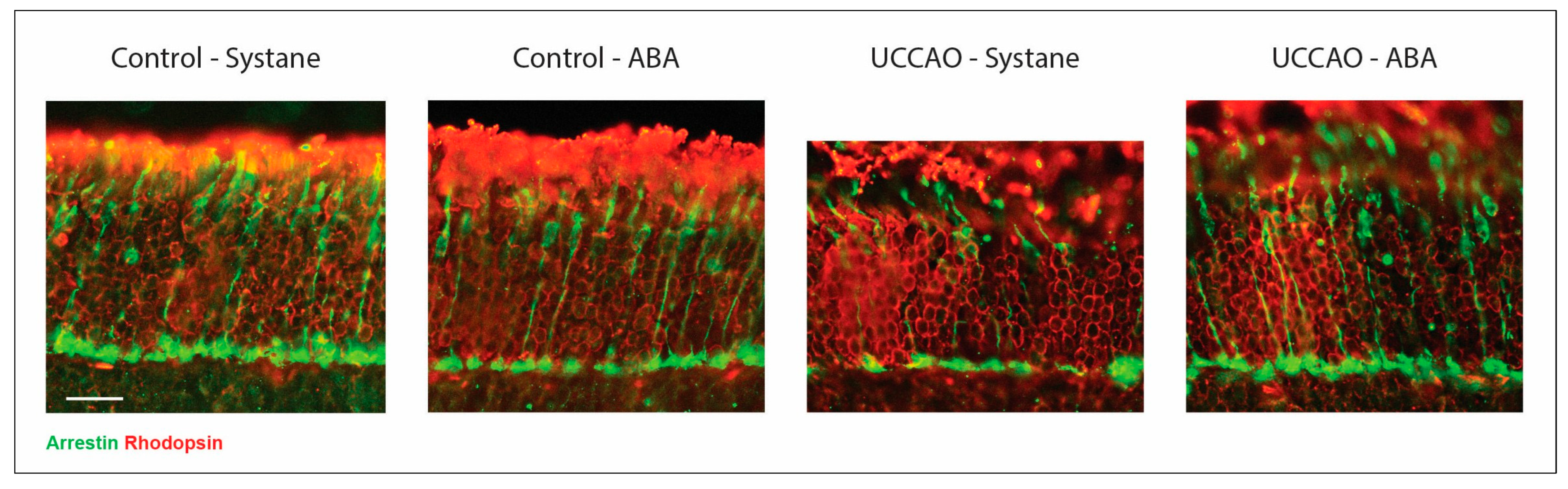
3.2. Photoreceptor Labeling on Retinal Cross Sections
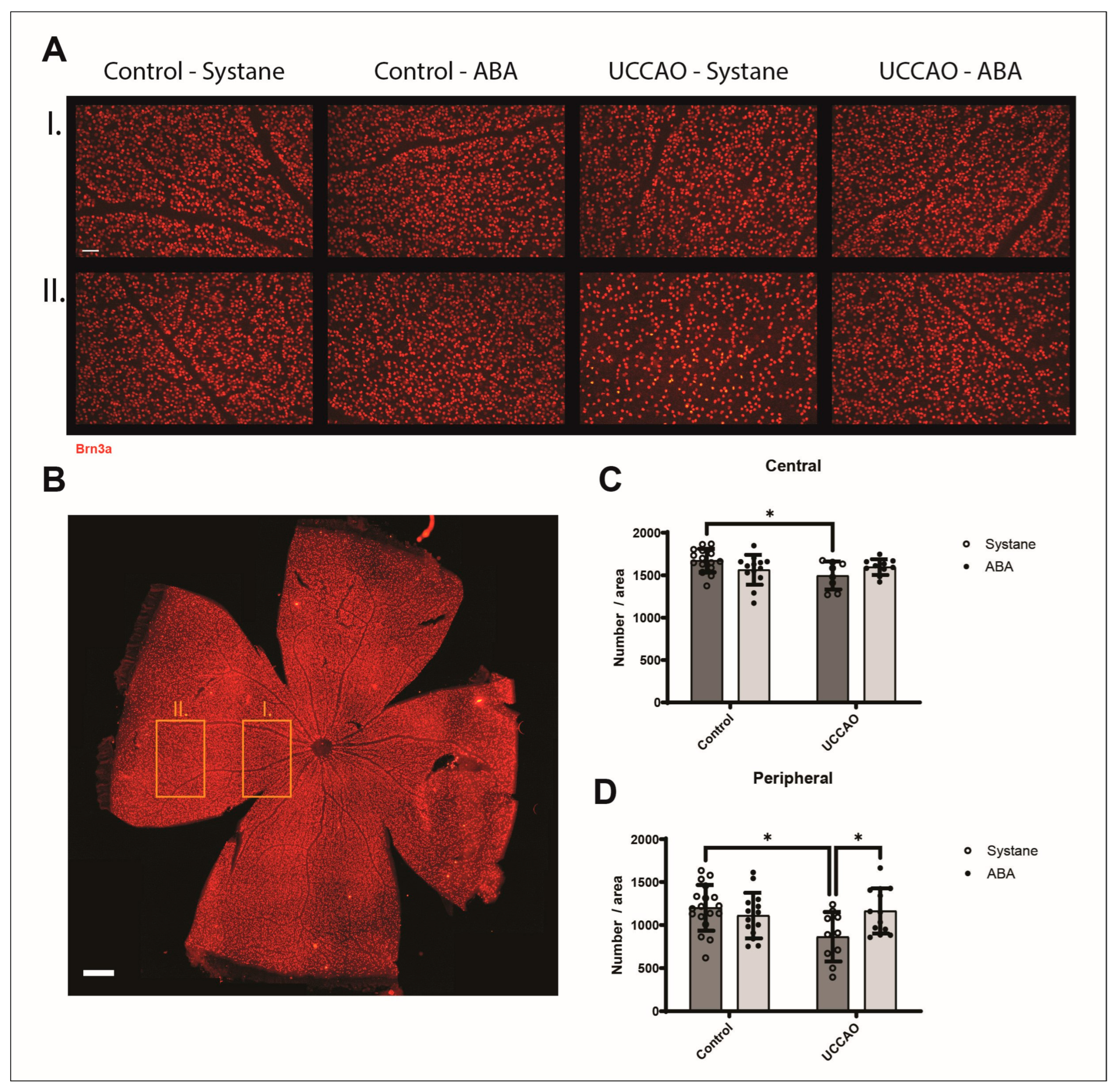
3.3. Retinal Ganglion Cells (Brn3a Labeling)
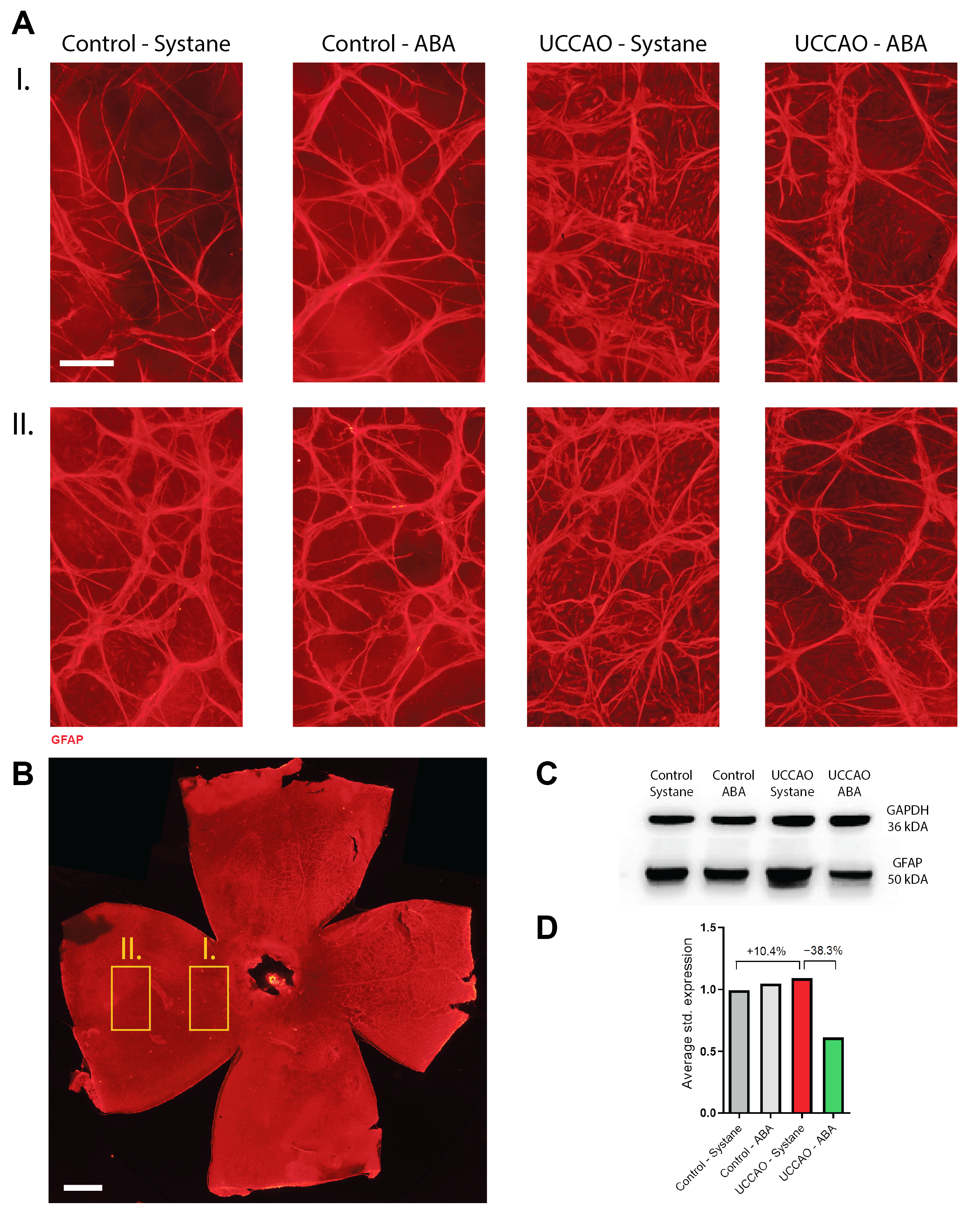
3.4. Retinal Stress (Expression of GFAP)
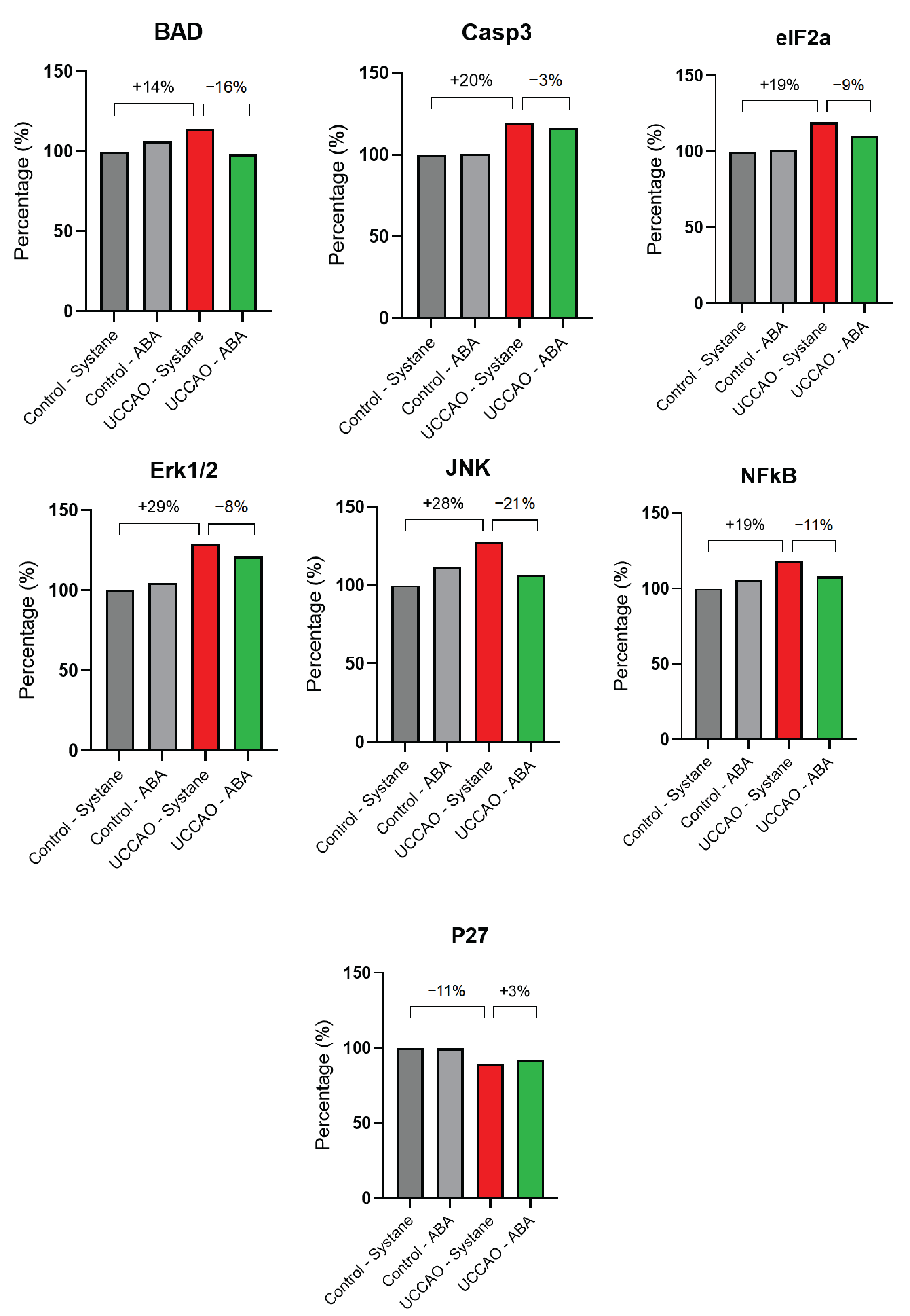
3.5. Apoptosis Signaling Pathway Array
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ADHD | Social interaction in attention-deficit/hyperactivity disorder |
| ARDS | Acute respiratory distress syndrome |
| BAD | Bcl-2-associated death promoter |
| BAX | Bcl-2-associated X protein |
| Brn3a | Brain-specific homeobox/POU domain protein 3A |
| BSA | Bovine serum albumin |
| Casp3 | Caspase-3 |
| COPD | Chronic obstructive pulmonary disease |
| DMSO | Dimethyl sulfoxide |
| eIF2a | Eukaryotic translation initiation factor 2 subunit alpha |
| Erk1/2 | Extracellular signal-related kinases 1 and 2 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GCC | Ganglion cell complex |
| GFAP | Glial fibrillary acidic protein |
| HRP | Horseradish peroxidase |
| IL-18 | Interleukin-18 |
| INL | Inner nuclear layer |
| IS | Inner segments of photoreceptors |
| JNK | Jun N-terminal kinase |
| LANC-1, LANCL-2 | Lanthionine synthetase C-like protein 1 and 2 |
| MAPK | Mitogen-activated protein kinase |
| NDS | Normal donkey serum |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitrogen monoxide |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| OCT | Optical coherence tomography |
| OS | Outer segments of photoreceptors |
| p27 | Cyclin-dependent kinase inhibitor 1B |
| p-BAD | Phospho-BAD |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PPAR γ | Peroxisome proliferator-activated receptor gamma |
| RIPA | Radioimmunoprecipitation assay |
| Std. thickness | Standardized thickness |
| SD-OCT | Spectral domain optical coherence tomography |
| SEM | Standard error of mean |
| UCCAO | Unilateral common carotid artery occlusion |
| VEGF | Vascular endothelial growth factor |
References
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Mao, J.; Deng, X.; Chen, Y.; Zhang, S.; Li, H.; Zhang, X.; Xiang, Z.; Zhu, J.; Chen, Y.; Shen, L.-J. Effect of Intravitreal Injection on Optic Nerve in Infants with Retinopathy of Prematurity: A Long-Term Follow-up Study. Br. J. Ophthalmol. 2025, bjo-2024-325830. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, J.; Zhang, X.; Liu, Y.; Li, J.; Wang, H.; Luo, X.; Liu, S.; Liu, L.; Zhang, J. Global, Regional and National Burden of Retinopathy of Prematurity in Childhood and Adolescence: A Spatiotemporal Analysis Based on the Global Burden of Disease Study 2019. BMJ Paediatr. Open 2024, 8, e002267. [Google Scholar] [CrossRef]
- Stahl, A.; Sukgen, E.A.; Wu, W.-C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of Intravitreal Aflibercept vs. Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity. J. Am. Med. Assoc. 2022, 328, 348. [Google Scholar] [CrossRef]
- Chen, S.-N.; Chen, S.-J.; Wu, T.-T.; Wu, W.-C.; Yang, C.-H.; Yang, C.-M. Refining Vitrectomy for Proliferative Diabetic Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3659–3670. [Google Scholar] [CrossRef]
- Ramli, N.; Supramaniam, G.; Samsudin, A.; Juana, A.; Zahari, M.; Choo, M.M. Ocular Surface Disease in Glaucoma: Effect of Polypharmacy and Preservatives. Optom. Vis. Sci. 2015, 92, e222–e226. [Google Scholar] [CrossRef] [PubMed]
- Fineide, F.; Lagali, N.; Adil, M.Y.; Arita, R.; Kolko, M.; Vehof, J.; Utheim, T.P. Topical Glaucoma Medications–Clinical Implications for the Ocular Surface. Ocul. Surf. 2022, 26, 19–49. [Google Scholar] [CrossRef]
- Leley, S.P.; Ciulla, T.A.; Bhatwadekar, A. Diabetic Retinopathy in the Aging Population: A Perspective of Pathogenesis and Treatment. Clin. Interv. Aging 2021, 16, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-F.; Lin, T.-Y.; Chen, Y.-H.; Lu, D.-W. Erythropoietin in Glaucoma: From Mechanism to Therapy. Int. J. Mol. Sci. 2023, 24, 2985. [Google Scholar] [CrossRef]
- Arden, G.; Sivaprasad, S. Hypoxia and Oxidative Stress in the Causation of Diabetic Retinopathy. Curr. Diabetes Rev. 2011, 7, 291–304. [Google Scholar] [CrossRef]
- Yu, Y.; Di, Y.; Li, P.; Nie, Q.; Chen, X.; Wang, A.; Ren, K. LncRNA H19 and MiR-138 Modulate Retinal Neovascularization and Associated Pathological Features in Hypoxia-Induced Disease Models. Exp. Eye Res. 2025, 258, 110512. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Dong, X.; Bai, J.; Shi, W.; Zhu, Q.; Liu, J.; Wang, Z.; Yi, L.; Yin, X.; et al. Naringin Suppresses CoCl2-Induced Ferroptosis in ARPE-19 Cells. Antioxidants 2025, 14, 236. [Google Scholar] [CrossRef]
- Dutczak, R.; Pietrucha-Dutczak, M. Effects of Selected Antioxidants on Electroretinography in Rodent Diabetic Retinopathy. Antioxidants 2024, 14, 21. [Google Scholar] [CrossRef]
- Lou, M.F.; Augusteyn, R.C. Oxidation-Induced Mixed Disulfide and Cataract Formation: A Review. Antioxidants 2025, 14, 425. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive Oxygen Species, Nox and Angiotensin II in Angiogenesis: Implications for Retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Gómez-Cadenas, A.; Arbona, V. Abscisic Acid as an Emerging Modulator of the Responses of Plants to Low Oxygen Conditions. Front. Plant Sci. 2021, 12, 661789. [Google Scholar] [CrossRef]
- Olds, C.L.; Glennon, E.K.K.; Luckhart, S. Abscisic Acid: New Perspectives on an Ancient Universal Stress Signaling Molecule. Microbes Infect. 2018, 20, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Le Page-Degivry, M.T.; Bidard, J.N.; Rouvier, E.; Bulard, C.; Lazdunski, M. Presence of Abscisic Acid, a Phytohormone, in the Mammalian Brain. Proc. Natl. Acad. Sci. USA 1986, 83, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Humma, Z.; Magnone, M.; Zocchi, E.; Sturla, L. Role of Abscisic Acid in the Whole-Body Regulation of Glucose Uptake and Metabolism. Nutrients 2024, 17, 13. [Google Scholar] [CrossRef]
- Meseguer-Beltrán, M.; Sánchez-Sarasúa, S.; Kerekes, N.; Landry, M.; Real-López, M.; Sánchez-Pérez, A.M. Abscisic Acid Rescues Behavior in Adult Female Mice in Attention Deficit Disorder with Hyperactivity Model of Dopamine Depletion by Regulating Microglia and Increasing Vesicular GABA Transporter Expression. J. Neuroimmune Pharmacol. 2025, 20, 39. [Google Scholar] [CrossRef]
- Soti, M.; Ilaghi, M.; Ranjbar, H.; Kohlmeier, K.A.; Sabzalizadeh, M.; Shabani, M. Abscisic Acid Enhances Motor and Cognitive Function in the 3-Acetylpyridine Mouse Model of Cerebellar Ataxia. J. Neurosci. Res. 2025, 103, e70055. [Google Scholar] [CrossRef] [PubMed]
- Shabani, M.; Naderi, R. Phytohormone Abscisic Acid Elicits Positive Effects on Harmaline-induced Cognitive and Motor Disturbances in a Rat Model of Essential Tremor. Brain Behav. 2022, 12, e2564. [Google Scholar] [CrossRef]
- Kooshki, R.; Anaeigoudari, A.; Abbasnejad, M.; Askari-Zahabi, K.; Esmaeili-Mahani, S. Abscisic Acid Interplays with PPARγ Receptors and Ameliorates Diabetes-Induced Cognitive Deficits in Rats. Avicenna J. Phytomed 2021, 11, 247–257. [Google Scholar]
- Spinelli, S.; Magnone, M.; Guida, L.; Sturla, L.; Zocchi, E. The ABA/LANCL Hormone/Receptor System in the Control of Glycemia, of Cardiomyocyte Energy Metabolism, and in Neuroprotection: A New Ally in the Treatment of Diabetes Mellitus? Int. J. Mol. Sci. 2023, 24, 1199. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Wu, Q.-Y.; Li, S.; Hu, K.-B.; Liu, H.-L.; Wang, H.-Y.; Long, Z.-Y.; Lu, X.-M.; Wang, Y.-T. The Ameliorative Effects and Mechanisms of Abscisic Acid on Learning and Memory. Neuropharmacology 2023, 224, 109365. [Google Scholar] [CrossRef]
- Han, T.; Xu, Y.; Liu, H.; Sun, L.; Cheng, X.; Shen, Y.; Wei, J. Function and Mechanism of Abscisic Acid on Microglia-Induced Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 4920. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Marquez, C.; Meseguer-Beltran, M.; Sanchez-Sarasua, S.; Sanchez-Perez, A.M. Abscisic Acid, an Evolutionary Conserved Hormone: Biosynthesis, Therapeutic and Diagnostic Applications in Mammals. Biochem. Pharmacol. 2024, 229, 116521. [Google Scholar] [CrossRef]
- Zhang, L.; Du, F.H.; Kun, K.X.; Yan, Y. Abscisic Acid Improves Non-Alcoholic Fatty Liver Disease in Mice Through the AMPK/NRF2/KEAP1 Signaling Axis. Biochem. Biophys. Res. Commun. 2025, 747, 151291. [Google Scholar] [CrossRef]
- Hontecillas, R.; Bassaganya-Riera, J. Expression of PPAR γ in Intestinal Epithelial Cells Is Dispensable for the Prevention of Colitis by Dietary Abscisic Acid. ESPEN J. 2012, 7, e189–e195. [Google Scholar] [CrossRef]
- Guri, A.J.; Hontecillas, R.; Bassaganya-Riera, J. Abscisic Acid Ameliorates Experimental IBD by Downregulating Cellular Adhesion Molecule Expression and Suppressing Immune Cell Infiltration. Clin. Nutr. 2010, 29, 824–831. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Xie, Q.; Zhang, H.; Fei, G.; Wu, H. Abscisic Acid Suppresses the Activation of NLRP3 Inflammasome and Oxidative Stress in Murine Allergic Airway Inflammation. Phytother. Res. 2021, 35, 3298–3309. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zou, H.; Xiao, X.; Wu, H.; Zhu, Y.; Li, J.; Liu, X.; Shen, Q. Abscisic Acid Inhibited Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress by Regulating the PPAR-γ Signaling Pathway in ARDS Mice. Phytother. Res. 2021, 35, 7027–7038. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Kim, N.; Ju, Y.-J.; Gee, M.S.; Lee, D.; Lee, J.K. Phytohormone Abscisic Acid Improves Memory Impairment and Reduces Neuroinflammation in 5xFAD Mice by Upregulation of LanC-Like Protein 2. Int. J. Mol. Sci. 2020, 21, 8425. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Turker, M.; Tuluce, Y. Abcisic Acid and Gibberellic Acid Cause Increased Lipid Peroxidation and Fluctuated Antioxidant Defense Systems of Various Tissues in Rats. J. Hazard. Mater. 2007, 148, 623–629. [Google Scholar] [CrossRef]
- Chaqour, J.; Lee, S.; Ravichandra, A.; Chaqour, B. Abscisic Acid–an Anti-Angiogenic Phytohormone That Modulates the Phenotypical Plasticity of Endothelial Cells and Macrophages. J. Cell Sci. 2018, 131, jcs210492. [Google Scholar] [CrossRef]
- Bosnyak, I.; Farkas, N.; Molitor, D.; Meresz, B.; Patko, E.; Atlasz, T.; Vaczy, A.; Reglodi, D. Optimization of an Ischemic Retinopathy Mouse Model and the Consequences of Hypoxia in a Time-Dependent Manner. Int. J. Mol. Sci. 2024, 25, 8008. [Google Scholar] [CrossRef]
- Kvarik, T.; Reglodi, D.; Werling, D.; Vaczy, A.; Kovari, P.; Szabo, E.; Kovacs, K.; Hashimoto, H.; Ertl, T.; Gyarmati, J.; et al. The Protective Effects of Endogenous PACAP in Oxygen-Induced Retinopathy. J. Mol. Neurosci. 2021, 71, 2546–2557. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of Real-Time PCR Gene Expression Data from Independent Biological Replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef]
- Koronyo, Y.; Rentsendorj, A.; Mirzaei, N.; Regis, G.C.; Sheyn, J.; Shi, H.; Barron, E.; Cook-Wiens, G.; Rodriguez, A.R.; Medeiros, R.; et al. Retinal Pathological Features and Proteome Signatures of Alzheimer’s Disease. Acta Neuropathol. 2023, 145, 409–438. [Google Scholar] [CrossRef]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Patko, E.; Vaczy, A.; Molitor, D.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Retinoprotective Effects of PACAP Eye Drops in Microbead-Induced Glaucoma Model in Rats. Int. J. Mol. Sci. 2021, 22, 8825. [Google Scholar] [CrossRef]
- Wang, X.; Xu, C.; Bian, C.; Ge, P.; Lei, J.; Wang, J.; Xiao, T.; Fan, Y.; Gu, Q.; Li, H.-Y.; et al. M2 Microglia-Derived Exosomes Promote Vascular Remodeling in Diabetic Retinopathy. J. Nanobiotechnology 2024, 22, 56. [Google Scholar] [CrossRef]
- Baliño, P.; Gómez-Cadenas, A.; López-Malo, D.; Romero, F.J.; Muriach, M. Is There A Role for Abscisic Acid, A Proven Anti-Inflammatory Agent, in the Treatment of Ischemic Retinopathies? Antioxidants 2019, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, K.; Lee, D.; Negishi, K.; Kurihara, T. Unilateral Common Carotid Artery Occlusion in Adult Mice with Streptozotocin Comorbidity Leads to Early Retinal Inflammation. Int. J. Mol. Sci. 2025, 26, 4385. [Google Scholar] [CrossRef]
- Zaitoun, I.S.; Shahi, P.K.; Suscha, A.; Chan, K.; McLellan, G.J.; Pattnaik, B.R.; Sorenson, C.M.; Sheibani, N. Hypoxic–Ischemic Injury Causes Functional and Structural Neurovascular Degeneration in the Juvenile Mouse Retina. Sci. Rep. 2021, 11, 12670. [Google Scholar] [CrossRef] [PubMed]
- Yashin, K.; Bonsanto, M.M.; Achkasova, K.; Zolotova, A.; Wael, A.-M.; Kiseleva, E.; Moiseev, A.; Medyanik, I.; Kravets, L.; Huber, R.; et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives. Diagnostics 2022, 12, 335. [Google Scholar] [CrossRef]
- Kurihara, T. Roles of Hypoxia Response in Retinal Development and Pathophysiology. Keio J. Med. 2017, 67, 1–9. [Google Scholar] [CrossRef]
- Palmhof, M.; Frank, V.; Rappard, P.; Kortenhorn, E.; Demuth, J.; Biert, N.; Stute, G.; Dick, H.B.; Joachim, S.C. From Ganglion Cell to Photoreceptor Layer: Timeline of Deterioration in a Rat Ischemia/Reperfusion Model. Front. Cell Neurosci. 2019, 13, 174. [Google Scholar] [CrossRef]
- Ebner, L.J.A.; Samardzija, M.; Storti, F.; Todorova, V.; Karademir, D.; Behr, J.; Simpson, F.; Thiersch, M.; Grimm, C. Transcriptomic Analysis of the Mouse Retina after Acute and Chronic Normobaric and Hypobaric Hypoxia. Sci. Rep. 2021, 11, 16666. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Lin, C.-M.; Shanmugam, S.; Hager, H.; Cao, M.; Liu, X.; Dreffs, A.; Habash, A.; Abcouwer, S.F. Diabetes Renders Photoreceptors Susceptible to Retinal Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2024, 65, 46. [Google Scholar] [CrossRef]
- Vujosevic, S.; Alovisi, C.; Piccoli, G.; Brambilla, M.; Torti, E.; Marenzi, E.; Leporati, F.; Luzi, L.; Nucci, P. Severity of Disorganization of Retinal Layers and Visual Function Impairment in Diabetic Retinopathy. Ophthalmol. Retina 2024, 8, 880–888. [Google Scholar] [CrossRef]
- Datlinger, F.; Wassermann, L.; Reumueller, A.; Hajdu, D.; Steiner, I.; Salas, M.; Drexler, W.; Pircher, M.; Schmidt-Erfurth, U.; Pollreisz, A. Assessment of Detailed Photoreceptor Structure and Retinal Sensitivity in Diabetic Macular Ischemia Using Adaptive Optics-OCT and Microperimetry. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fan, C.; Hu, X.; Ban, X.; Wan, H.; He, Y.; Zhang, Q.; Xiong, K. Regulated Cell Death of Retinal Ganglion Cells in Glaucoma: Molecular Insights and Therapeutic Potentials. Cell Mol. Neurobiol. 2023, 43, 3161–3178. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.M.; Bumbaru, S.M.; Fakhouri, L.S.; Zhang, D.-Q. Long–Term Impairment of Retinal Ganglion Cell Function After Oxygen–Induced Retinopathy. Cells 2025, 14, 512. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, J.; Gao, J. Extracellular Vesicles from Adipose-derived Mesenchymal Stem Cells Prevent High Glucose-induced Retinal Ganglion Cell Pyroptosis through a MicroRNA-26a-5p-dependent Mechanism. J. Diabetes Investig. 2025, 16, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, H.; Fang, F.; Liu, L.; Sun, Y.; Hu, Y. Longitudinal Morphological and Functional Assessment of RGC Neurodegeneration After Optic Nerve Crush in Mouse. Front. Cell Neurosci. 2020, 14, 109. [Google Scholar] [CrossRef]
- Ng, D.S.; Chiang, P.P.; Tan, G.; Cheung, C.G.; Cheng, C.; Cheung, C.Y.; Wong, T.Y.; Lamoureux, E.L.; Ikram, M.K. Retinal Ganglion Cell Neuronal Damage in Diabetes and Diabetic Retinopathy. Clin. Exp. Ophthalmol. 2016, 44, 243–250. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Zangwill, L.M.; Anderson, D.R.; Liebmann, J.M.; Girkin, C.A.; Harwerth, R.S.; Fredette, M.-J.; Weinreb, R.N. Estimating the Rate of Retinal Ganglion Cell Loss in Glaucoma. Am. J. Ophthalmol. 2012, 154, 814–824.e1. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Lisboa, R.; Weinreb, R.N.; Liebmann, J.M.; Girkin, C.; Zangwill, L.M. Retinal Ganglion Cell Count Estimates Associated with Early Development of Visual Field Defects in Glaucoma. Ophthalmology 2013, 120, 736–744. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, T.; Kong, X.; Wei, J. Neuroprotective Effect of Abscisic Acid on MPTP-Induced Parkinson’s Disease in Mice. Mol. Nutr. Food Res. 2025, 69, e70111. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Guida, L.; Vigliarolo, T.; Passalacqua, M.; Begani, G.; Magnone, M.; Sturla, L.; Benzi, A.; Ameri, P.; Lazzarini, E.; et al. The ABA-LANCL1/2 Hormone-Receptors System Protects H9c2 Cardiomyocytes from Hypoxia-Induced Mitochondrial Injury via an AMPK- and NO-Mediated Mechanism. Cells 2022, 11, 2888. [Google Scholar] [CrossRef]
- He, C.; Liu, C.; Ren, C.; Zhao, H.; Zhang, X. Immunological Landscape of Retinal Ischemia-Reperfusion Injury: Insights into Resident and Peripheral Immune Cell Responses. Aging Dis. 2024, 16, 115–136. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- El Tabaa, M.M.; El Tabaa, M.M.; Mohsen, M.; Abo-alazm, H.M.; Abd Elaziz, D.M.; Akram, M.; Eldeeb, E.M.; Nadar, Z.A.; Fahmy, O.M.; Mansy, M.A.; et al. Reduced NF-ΚB/NLRP3/IL-18 Signaling Increases the Protective Effect of L-Glutamine against LPS-Induced Retinal Inflammation in Mice: Utilization of Network Pharmacology and Experimental Validation. Eur. J. Pharmacol. 2025, 1002, 177840. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Guri, A.J.; Lu, P.; Climent, M.; Carbo, A.; Sobral, B.W.; Horne, W.T.; Lewis, S.N.; Bevan, D.R.; Hontecillas, R. Abscisic Acid Regulates Inflammation via Ligand-Binding Domain-Independent Activation of Peroxisome Proliferator-Activated Receptor γ. J. Biol. Chem. 2011, 286, 2504–2516. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Liu, Y.; Payne, G.; Lutz, R.J.; Chittenden, T. Growth Factors Inactivate the Cell Death Promoter BAD by Phosphorylation of Its BH3 Domain on Ser155. J. Biol. Chem. 2000, 275, 25046–25051. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Andreeva, K.; Cooper, N.G.F. Ischemia-Reperfusion Injury of the Retina Is Linked to Necroptosis via the ERK1/2-RIP3 Pathway. Mol. Vis. 2014, 20, 1374–1387. [Google Scholar]
- Hayashi, A.; Koroma, B.M.; Imai, K.; de Juan, E. Increase of Protein Tyrosine Phosphorylation in Rat Retina after Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2146–2156. [Google Scholar]
- Ohi, N.; Nishikawa, Y.; Tokairin, T.; Yamamoto, Y.; Doi, Y.; Omori, Y.; Enomoto, K. Maintenance of Bad Phosphorylation Prevents Apoptosis of Rat Hepatic Sinusoidal Endothelial Cells In Vitro and In Vivo. Am. J. Pathol. 2006, 168, 1097–1106. [Google Scholar] [CrossRef]
- Condorelli, F.; Salomoni, P.; Cotteret, S.; Cesi, V.; Srinivasula, S.M.; Alnemri, E.S.; Calabretta, B. Caspase Cleavage Enhances the Apoptosis-Inducing Effects of BAD. Mol. Cell Biol. 2001, 21, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Jiao, S.; Duan, K.; Wang, Y.-X.; Petralia, R.S.; Li, Z. The BAD-BAX-Caspase-3 Cascade Modulates Synaptic Vesicle Pools via Autophagy. J. Neurosci. 2021, 41, 1174–1190. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Sugiura, R.; Satoh, R.; Takasaki, T. ERK: A Double-Edged Sword in Cancer. ERK-Dependent Apoptosis as a Potential Therapeutic Strategy for Cancer. Cells 2021, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Uppala, J.K.; Ghosh, C.; Sathe, L.; Dey, M. Phosphorylation of Translation Initiation Factor EIF 2α at Ser51 Depends on Site- and Context-specific Information. FEBS Lett. 2018, 592, 3116–3125. [Google Scholar] [CrossRef]
- Hiromura, K.; Pippin, J.W.; Fero, M.L.; Roberts, J.M.; Shankland, S.J. Modulation of Apoptosis by the Cyclin-Dependent Kinase Inhibitor P27Kip1. J. Clin. Investig. 1999, 103, 597–604. [Google Scholar] [CrossRef]
- Parajuli, K.; Jung, Y.; Taichman, R. Abscisic Acid Signaling through LANCL2 and PPARγ Induces Activation of P38MAPK Resulting in Dormancy of Prostate Cancer Metastatic Cells. Oncol. Rep. 2024, 51, 39. [Google Scholar] [CrossRef]
- Hoang, Q.T.M.; Nguyen, V.K.; Oberacher, H.; Fuchs, D.; Hernandez-Vargas, E.A.; Borucki, K.; Waldburg, N.; Wippermann, J.; Schreiber, J.; Bruder, D.; et al. Serum Concentration of the Phytohormone Abscisic Acid Is Associated with Immune-Regulatory Mediators and Is a Potential Biomarker of Disease Severity in Chronic Obstructive Pulmonary Disease. Front. Med. 2021, 8, 676058. [Google Scholar] [CrossRef]
- Wang, L.; Shen, J.; Liu, W.; Li, W.; Tang, W.; Zha, B.; Wu, H.; Liu, X.; Shen, Q. Abscisic Acid for Acute Respiratory Distress Syndrome Therapy by Suppressing Alveolar Macrophage Pyroptosis via Upregulating Acyloxyacyl Hydrolase Expression. Eur. J. Pharmacol. 2024, 977, 176672. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, Y.; Ye, H.; Zhu, W.; Chen, L.; Mao, Y. Abscisic-acid-induced Cellular Apoptosis and Differentiation in Glioma via the Retinoid Acid Signaling Pathway. Int. J. Cancer 2016, 138, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Hontecillas, R.; Leber, A.; Einerhand, A.; Carbo, A.; Bruzzone, S.; Tubau-Juni, N.; Philipson, N.; Zoccoli-Rodriguez, V.; Sturla, L.; et al. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Preti, P.S.; Tenore, G.; Novellino, E. Abscisic Acid Treatment in Patients with Prediabetes. Nutrients 2020, 12, 2931. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosnyak, I.; Nagy, A.; Molitor, D.; Meresz, B.; Szabo, E.; Reglodi, D.; Atlasz, T.; Vaczy, A. Retinoprotective Effects of Abscisic Acid in Ischemic Retinopathy Mouse Model. Antioxidants 2025, 14, 1133. https://doi.org/10.3390/antiox14091133
Bosnyak I, Nagy A, Molitor D, Meresz B, Szabo E, Reglodi D, Atlasz T, Vaczy A. Retinoprotective Effects of Abscisic Acid in Ischemic Retinopathy Mouse Model. Antioxidants. 2025; 14(9):1133. https://doi.org/10.3390/antiox14091133
Chicago/Turabian StyleBosnyak, Inez, Agnes Nagy, Dorottya Molitor, Balazs Meresz, Edina Szabo, Dora Reglodi, Tamas Atlasz, and Alexandra Vaczy. 2025. "Retinoprotective Effects of Abscisic Acid in Ischemic Retinopathy Mouse Model" Antioxidants 14, no. 9: 1133. https://doi.org/10.3390/antiox14091133
APA StyleBosnyak, I., Nagy, A., Molitor, D., Meresz, B., Szabo, E., Reglodi, D., Atlasz, T., & Vaczy, A. (2025). Retinoprotective Effects of Abscisic Acid in Ischemic Retinopathy Mouse Model. Antioxidants, 14(9), 1133. https://doi.org/10.3390/antiox14091133





