An Immune Assay to Quantify the Neutralization of Oxidation-Specific Epitopes by Human Blood Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. The Materials and Reagents
2.2. Human Plasma
2.2.1. Blood Sampling from Healthy Volunteers and COVID-19 Patients
2.2.2. Blood Sampling for the Day-to-Day Variability Study
2.3. The Standard Masking Assay
2.4. The Statistical Analysis
3. Results
3.1. Optimization of the OSE-Masking ELISA Procedure
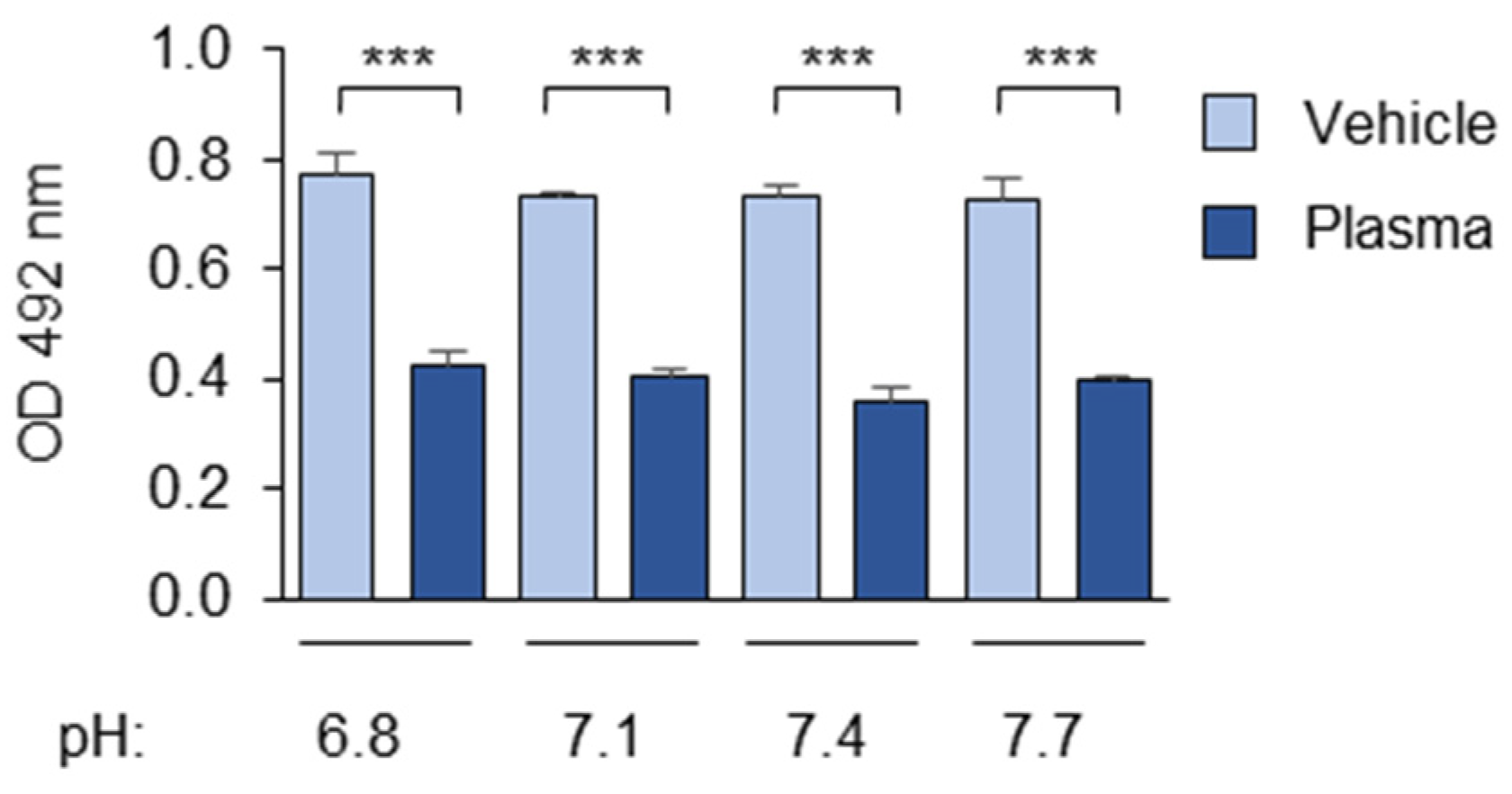
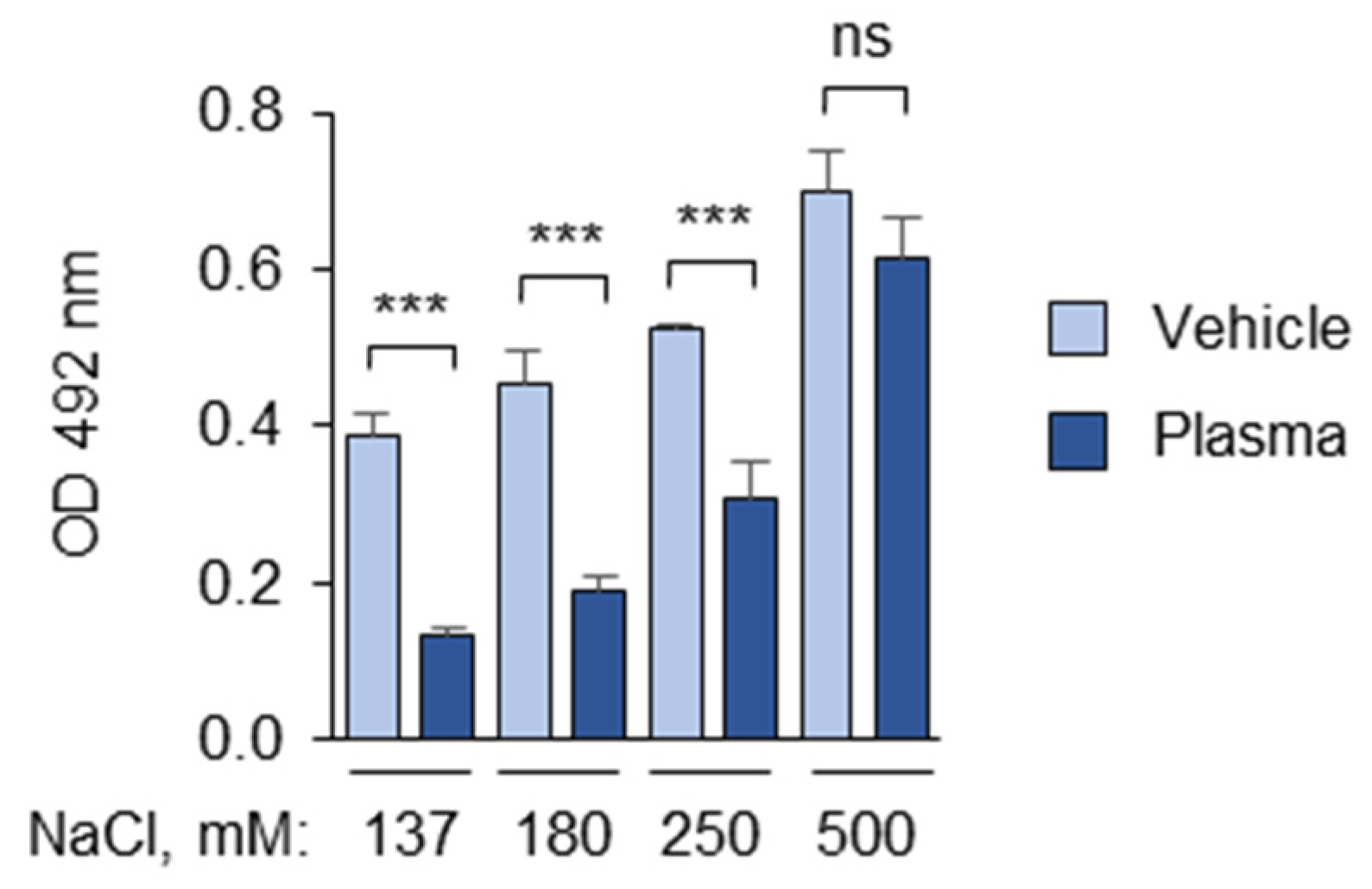
3.1.1. The Composition and pH of the Buffer
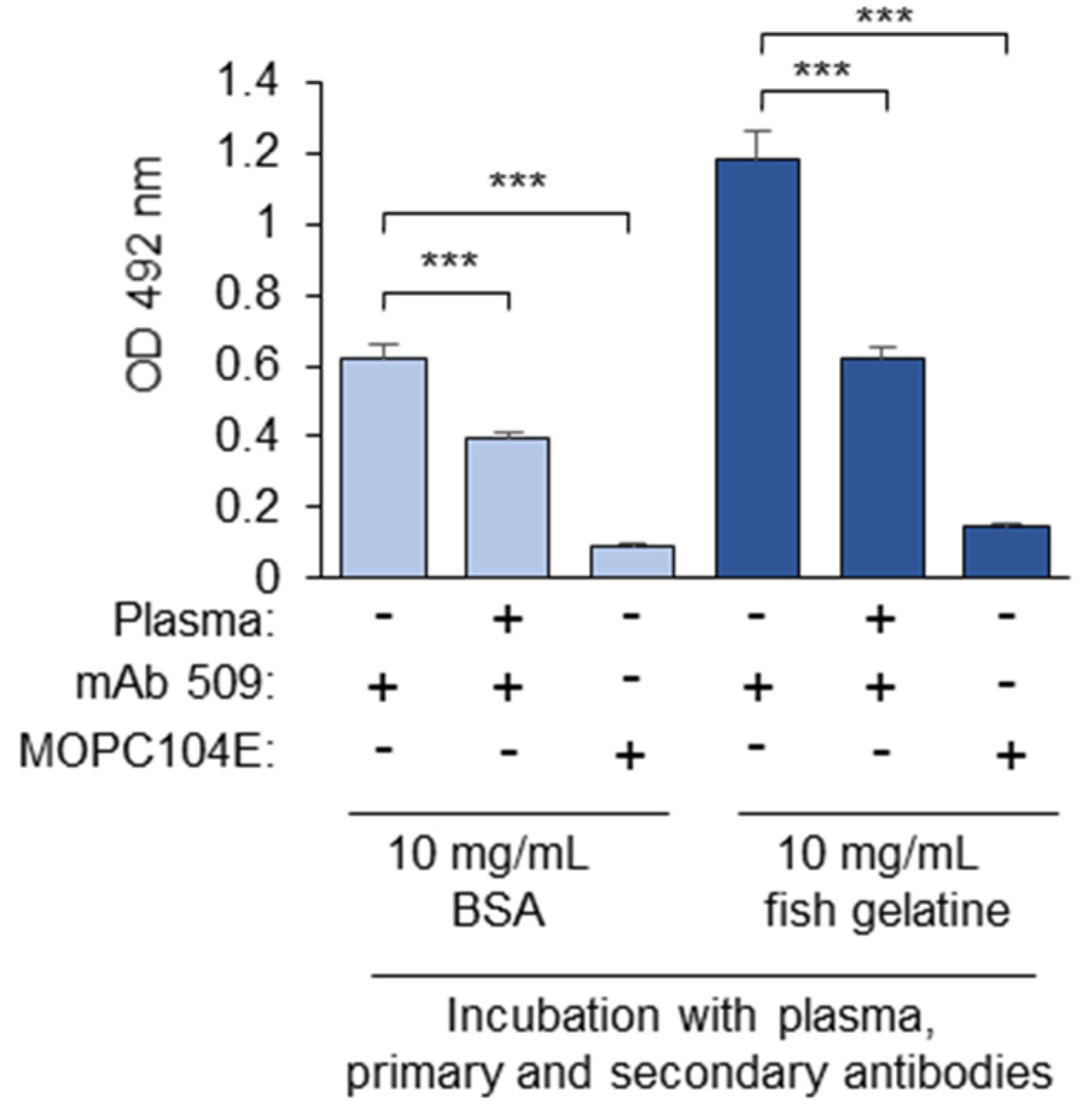
3.1.2. Blocking Proteins
3.1.3. Washing
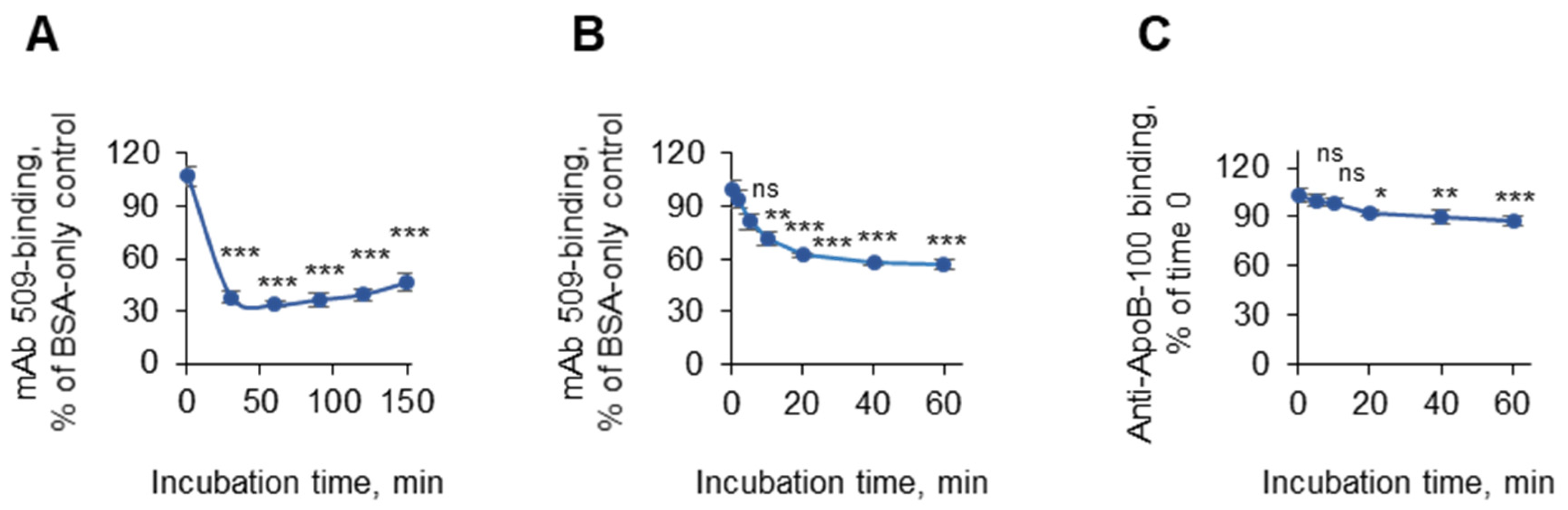
3.1.4. Incubation Times
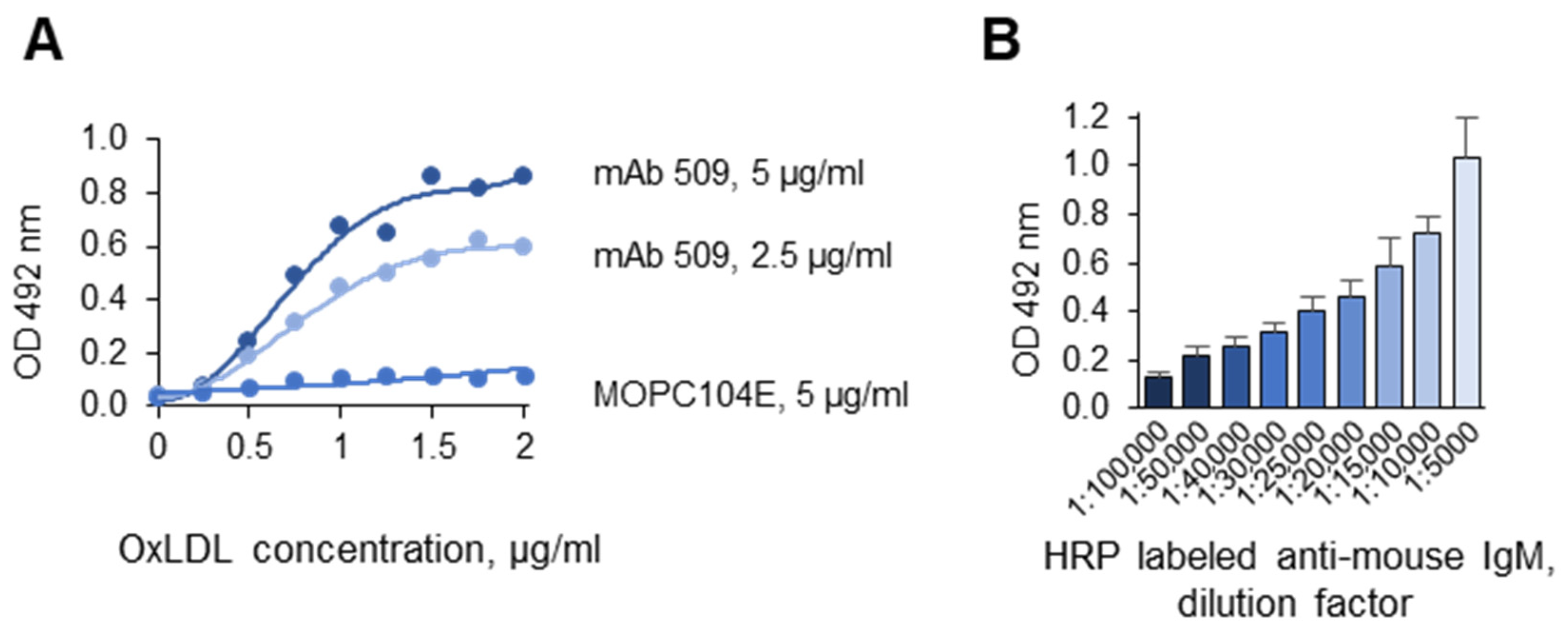
3.1.5. Optimization of the Antigen and Antibody Concentrations
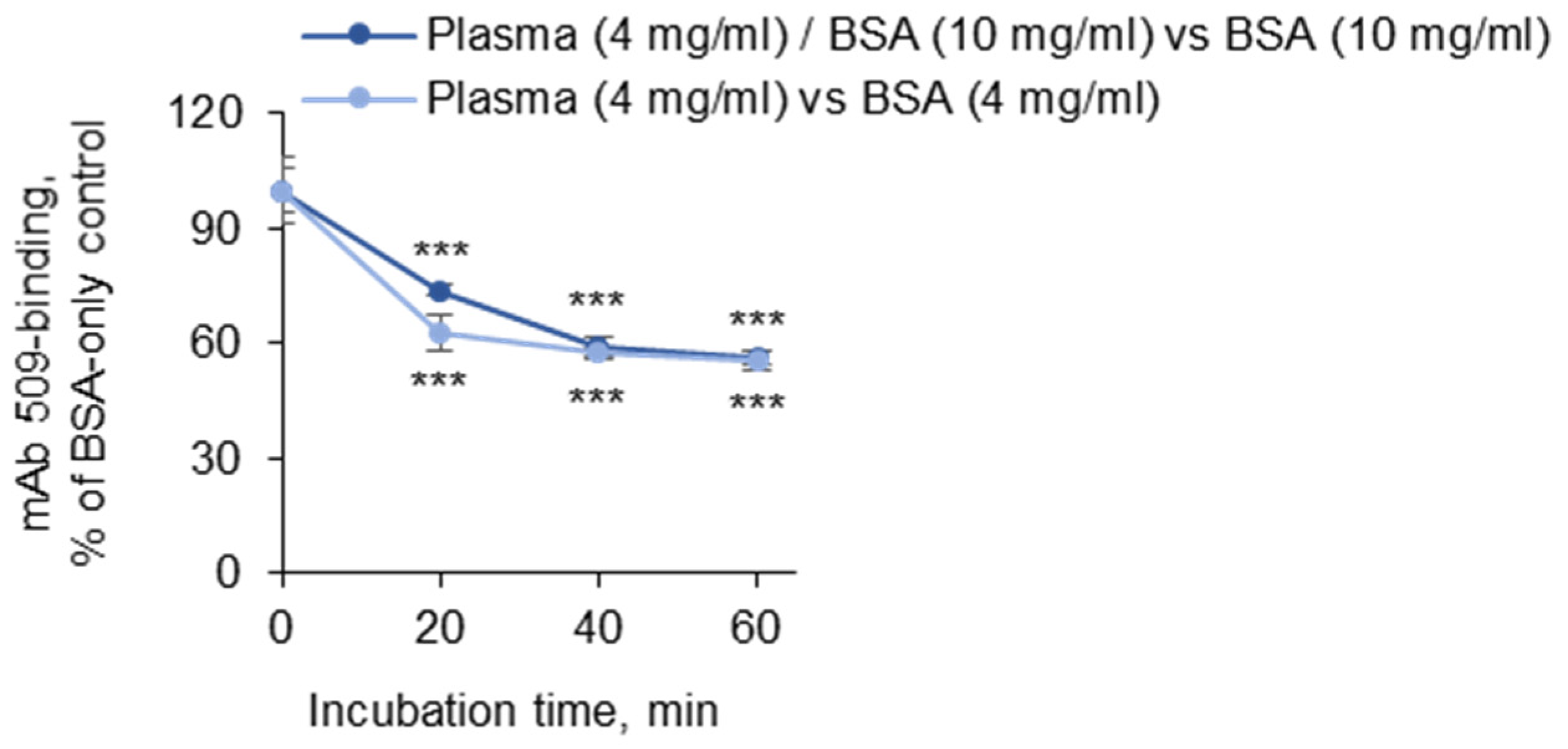
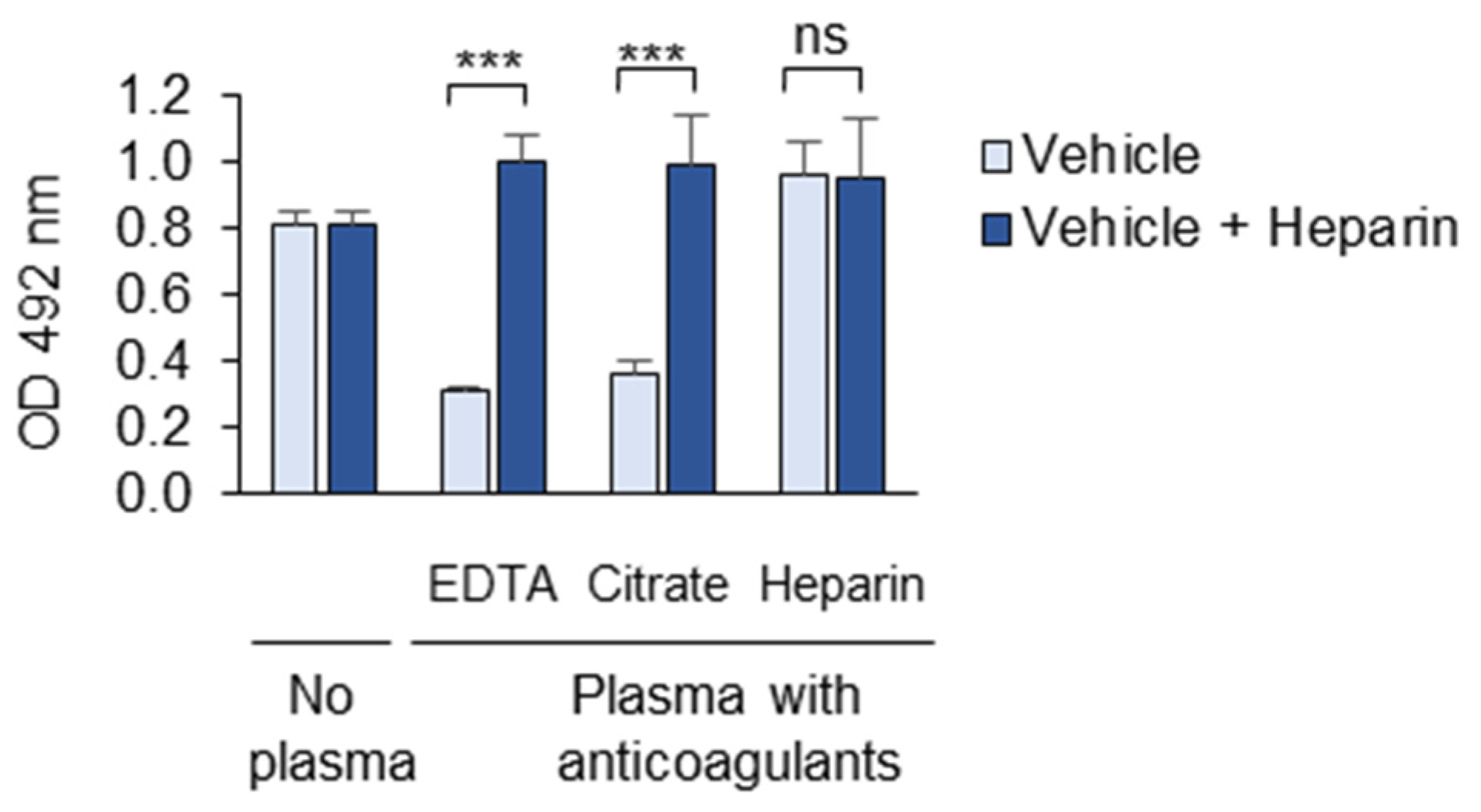
3.1.6. Anticoagulants
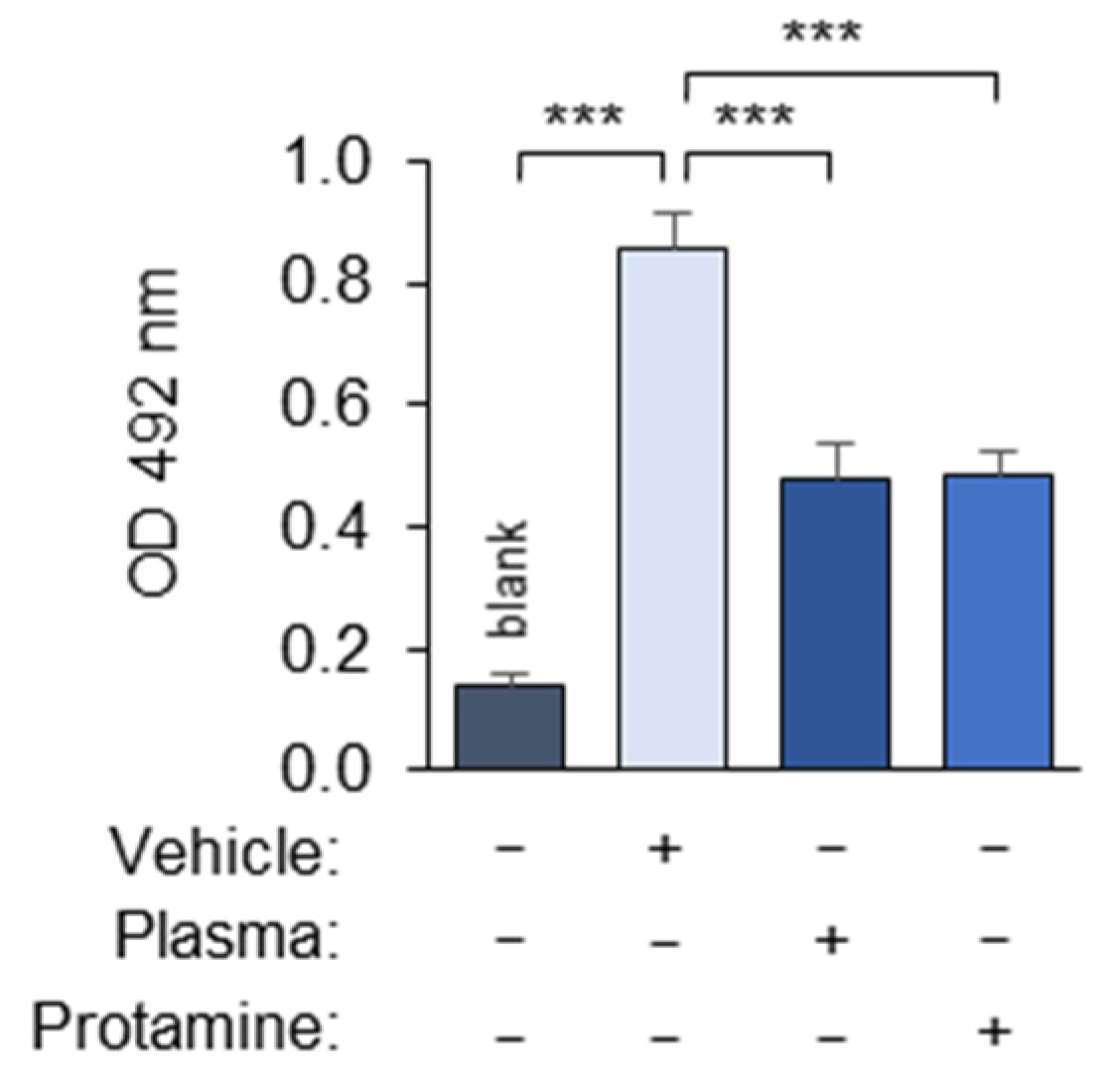
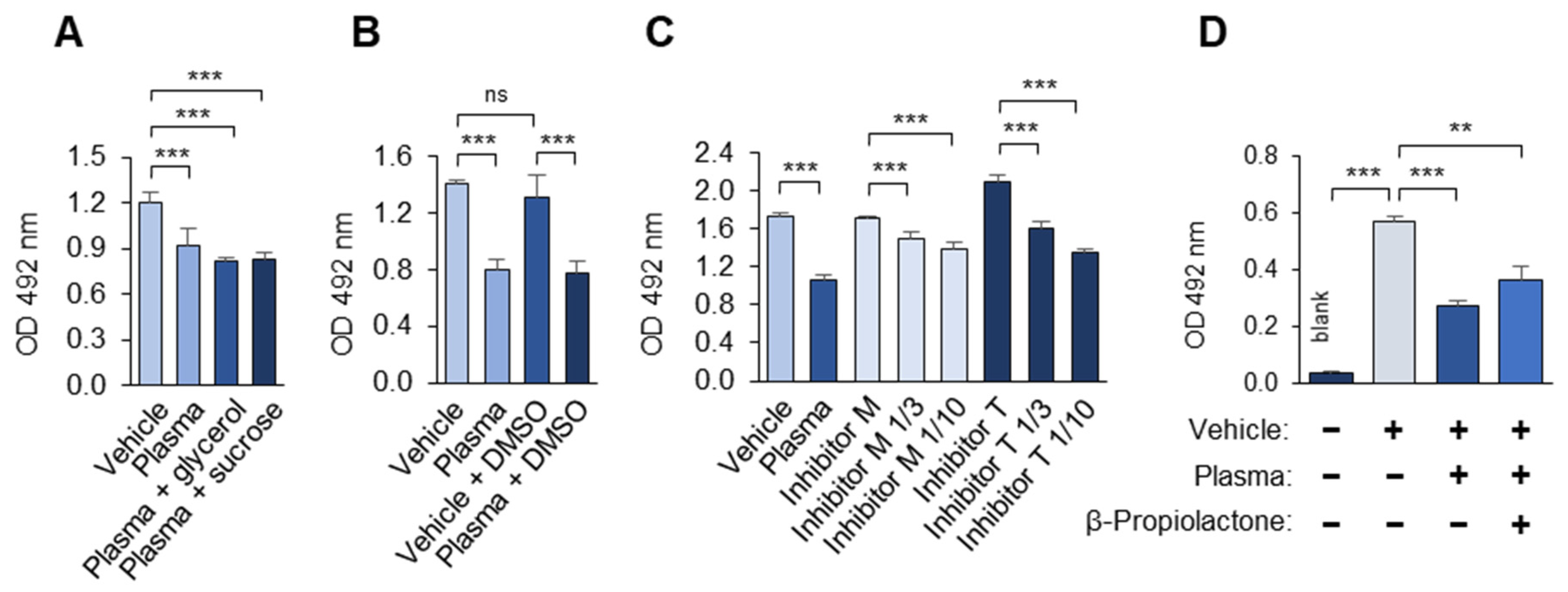
3.1.7. Polyanions and Polycations Interfere with the Masking Assay
3.1.8. Laboratory Reagents Interfere with the Masking Assay
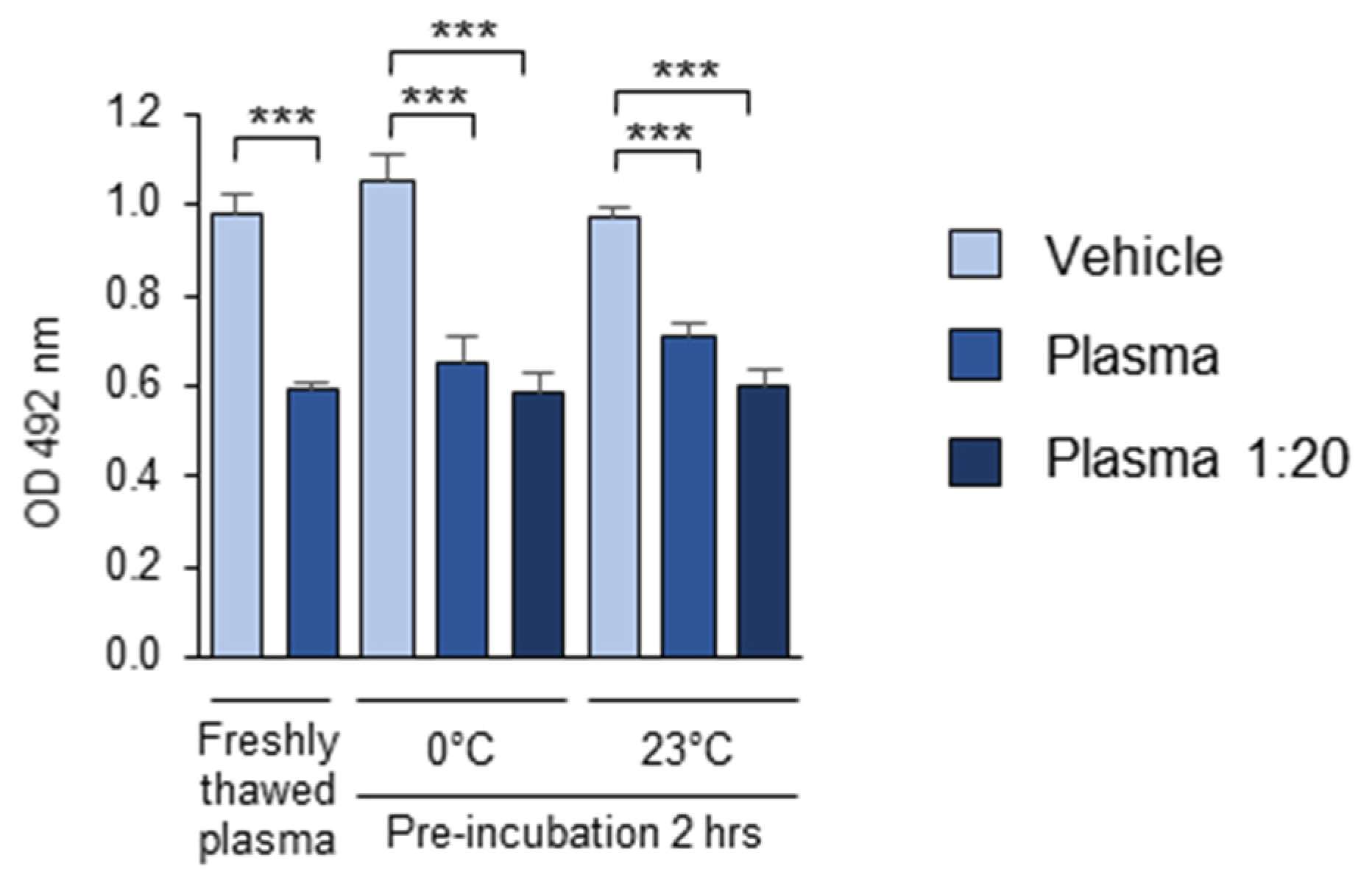
3.1.9. The Stability of the OSE-Masking Activity During the Pre-Analytical Procedures
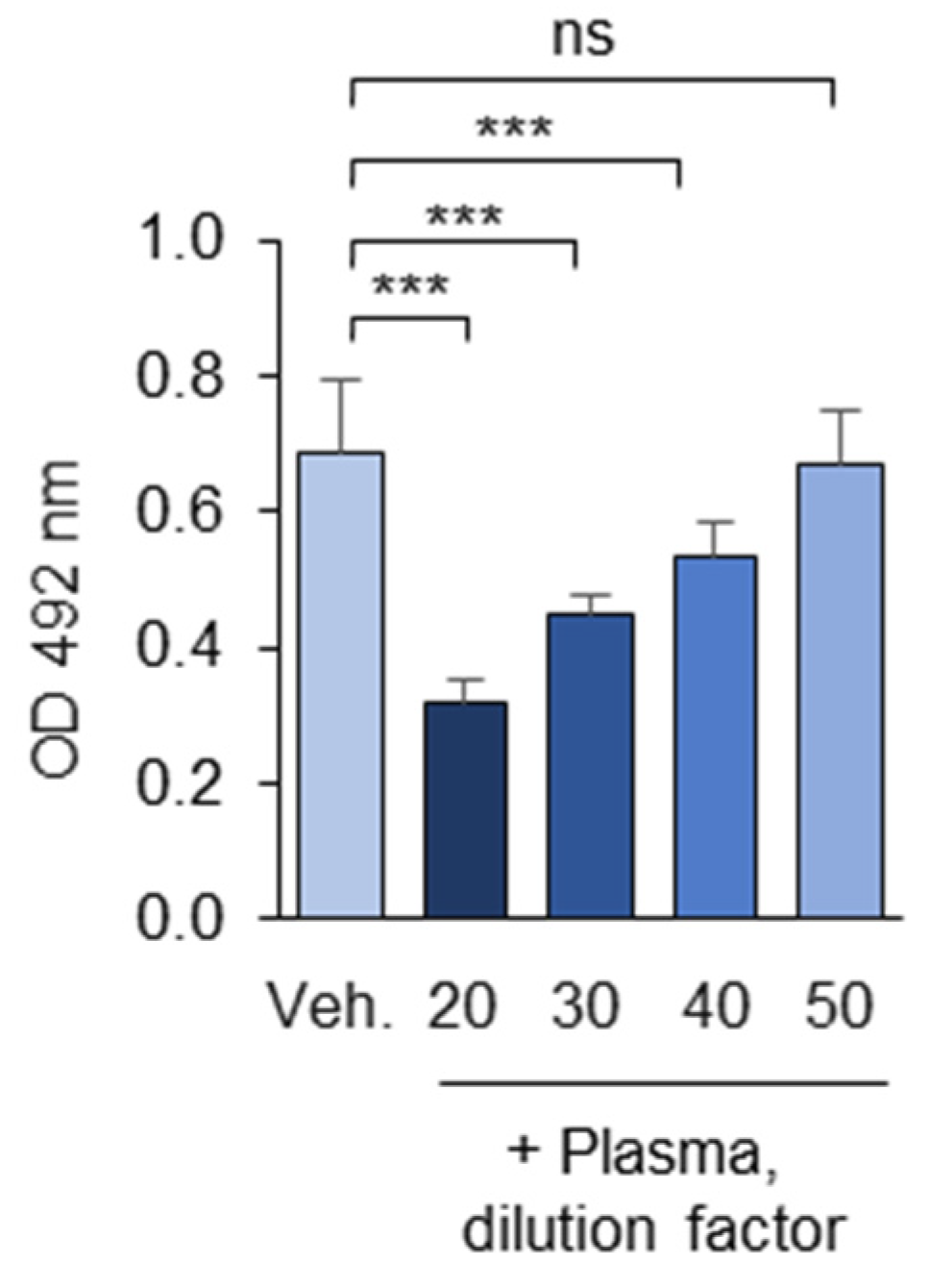
3.1.10. Optimization of the Plasma Dilution
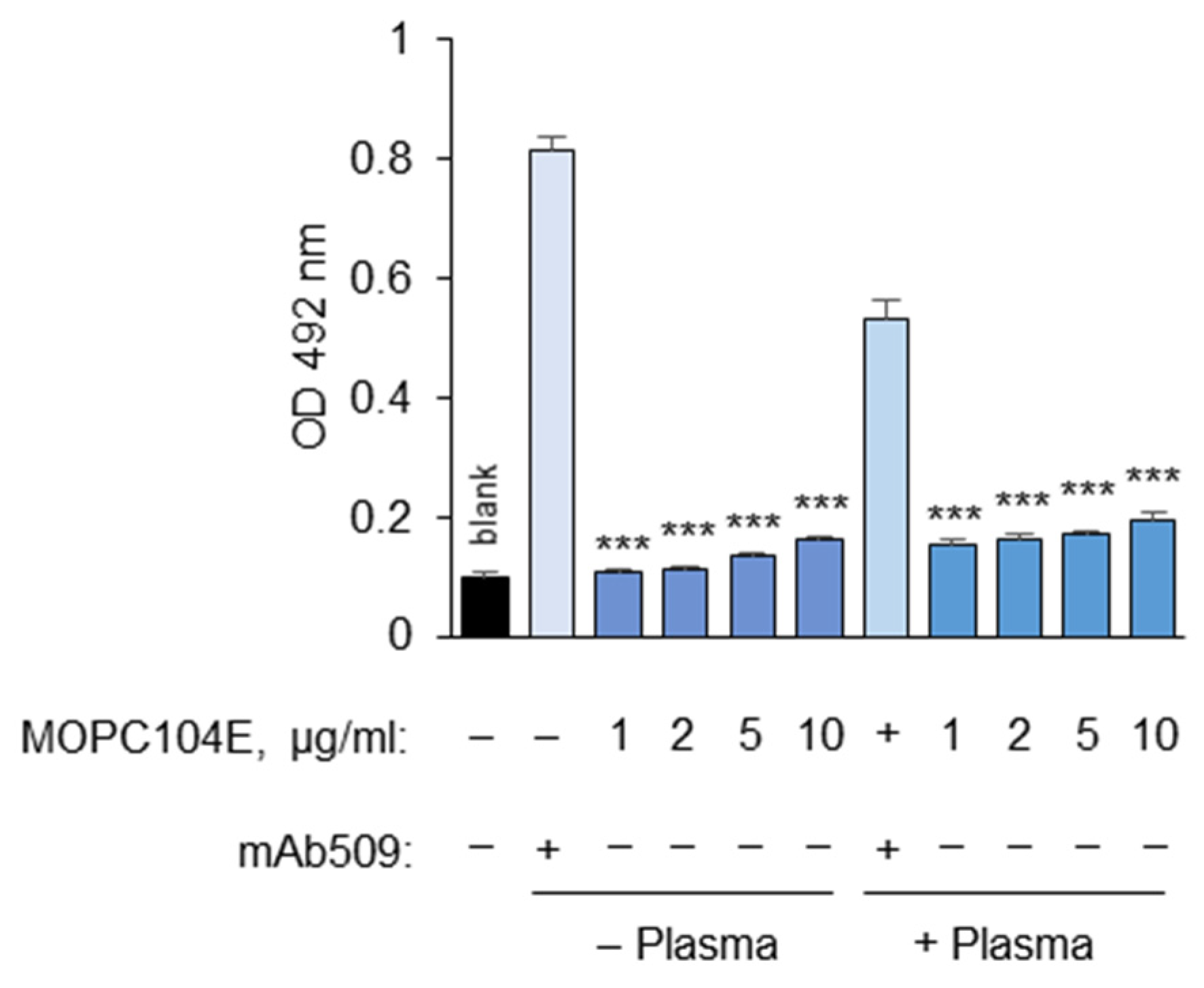
3.1.11. Determination of Non-Specific Binding
3.2. The Analytical Parameters of the OSE-Masking ELISA Procedure
3.2.1. Intra-Individual Variability in the Masking Activity
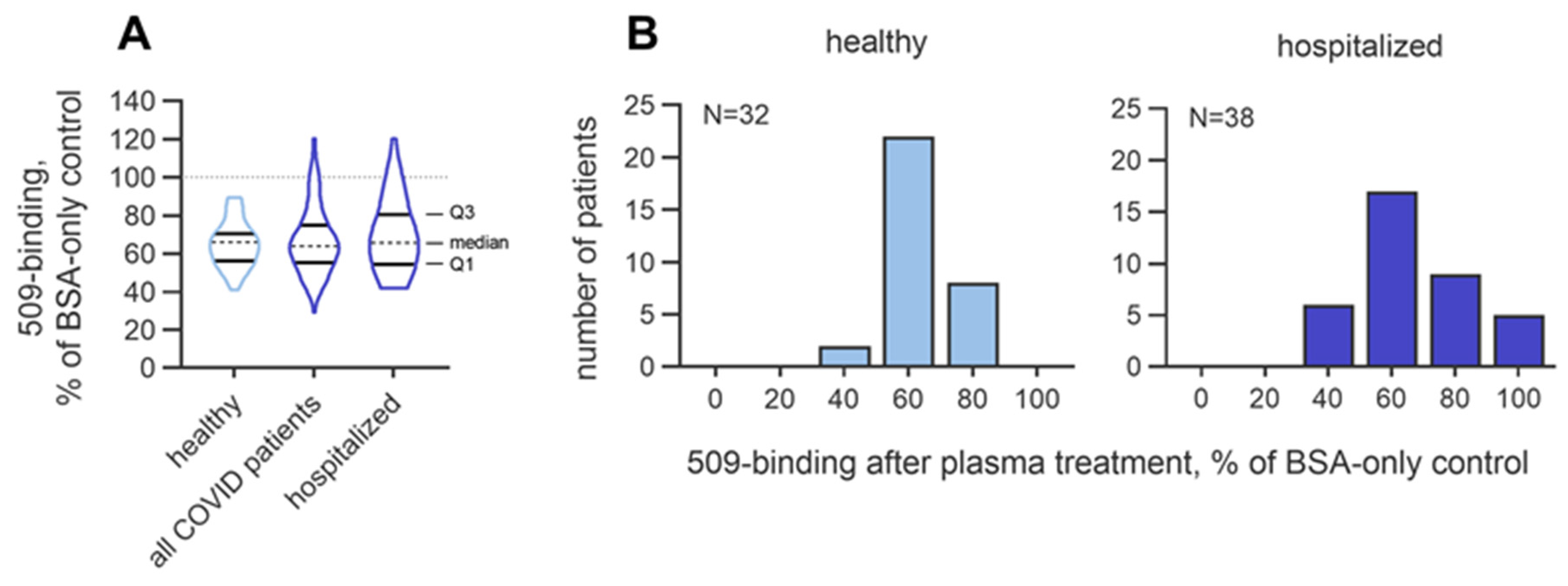
3.2.2. Inter-Individual Variability in the Masking Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| apoB | Apolipoprotein B-100 |
| BSA | Bovine serum albumin |
| C1q | Complement C1q subcomponent |
| CFH | Complement factor H |
| CRP | C-reactive protein |
| DAMPs | Damage-associated molecular patterns |
| FcR | Fc-receptor |
| IgM | Immunoglobulins of the M class |
| Lp-PLA2 | Lipoprotein-associated phospholipase A2 |
| Lp(a) | Lipoprotein (a) |
| LPPs | Lipid peroxidation products |
| mAb | Monoclonal antibody |
| MDA | Malondialdehyde |
| OSE | Oxidation-specific epitope |
| OxLDL | Oxidized low-density lipoprotein |
| OxPAPC | Oxidized palmitoyl-arachidonoyl-phosphatidylcholine |
| OxPC | Oxidized phosphatidylcholine |
| OxPLs | Oxidized phospholipids |
| OxPE | Oxidized phosphatidylethanolamine |
| PBS | Phosphate-buffered saline |
| PBSE | Phosphate-buffered saline supplemented with EDTA |
References
- Binder, C.J.; Papac-Milicevic, N.; Witztum, J.L. Innate sensing of oxidation-specific epitopes in health and disease. Nat. Rev. Immunol. 2016, 16, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Hörkkö, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef] [PubMed]
- Scipione, C.A.; Sayegh, S.E.; Romagnuolo, R.; Tsimikas, S.; Marcovina, S.M.; Boffa, M.B.; Koschinsky, M.L. Mechanistic insights into Lp(a)-induced IL-8 expression: A role for oxidized phospholipid modification of apo(a). J. Lipid Res. 2015, 56, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef] [PubMed]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Protty, M.B.; Jenkins, P.V.; Collins, P.W.; O’Donnell, V.B. The role of procoagulant phospholipids on the surface of circulating blood cells in thrombosis and haemostasis. Open Biol. 2022, 12, 210318. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Hung, M.-Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Seidman, J.S.; Zhao, P.; Troutman, T.D.; Spann, N.J.; Que, X.; Zhou, F.; Liao, Z.; Pasillas, M.; Yang, X.; et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 2020, 31, 189–206.e8. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Hasanally, D.; Que, X.; Hung, M.-Y.; Stamenkovic, A.; Chan, D.; Chaudhary, R.; Margulets, V.; Edel, A.L.; Hoshijima, M.; et al. Reduction of myocardial ischaemia-reperfusion injury by inactivating oxidized phospholipids. Cardiovasc. Res. 2019, 115, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, E.; Que, X.; Wang, S.; Yamaguchi, F.; Weinstein, R.S.; Tsimikas, S.; Manolagas, S.C.; Witztum, J.L.; Jilka, R.L. Oxidation-specific epitopes restrain bone formation. Nat. Commun. 2018, 9, 2193. [Google Scholar] [CrossRef] [PubMed]
- Zhivaki, D.; Kagan, J.C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 2022, 22, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Jokesch, P.; Oskolkova, O.; Fedorova, M.; Gesslbauer, B.; Bochkov, V. Contribution of individual phospholipase A2 enzymes to the cleavage of oxidized phospholipids in human blood plasma. J. Lipid Res. 2025, 66, 100742. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-Y.; Fogelstrand, L.; Hartvigsen, K.; Hansen, L.F.; Woelkers, D.; Shaw, P.X.; Choi, J.; Perkmann, T.; Bäckhed, F.; Miller, Y.I.; et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Investig. 2009, 119, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Hutchinson, M.A.; Gearhart, P.J.; Maul, R.W. Antibodies in action: The role of humoral immunity in the fight against atherosclerosis. Immun. Ageing 2022, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Thorp, E.; Cui, D.; Schrijvers, D.M.; Kuriakose, G.; Tabas, I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of Apoe−/− mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Malik, T.H.; Ehrenstein, M.R.; Boyle, J.J.; Botto, M.; Haskard, D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009, 120, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-K.; Binder, C.J.; Torzewski, M.; Witztum, J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2002, 99, 13043–13048. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- Jokesch, P.; Holzer, L.; Jantscher, L.; Guttzeit, S.; Übelhart, R.; Oskolkova, O.; Bochkov, V.; Gesslbauer, B. Identification of plasma proteins binding oxidized phospholipids using pull-down proteomics and OxLDL masking assay. J. Lipid Res. 2025, 66, 100704. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, V.; Schoenenberger, A.W.; Oskolkova, O.; Toth, U.; Stöckl, J.; Majdic, O.; Daci, A.; Resink, T.J.; Erne, P.; Philippova, M. Novel immune assay for quantification of plasma protective capacity against oxidized phospholipids. Biomark. Med. 2016, 10, 797–810. [Google Scholar] [CrossRef] [PubMed]
- European Commission. CORDIS—EU Research Results, BIOmarkers of Robustness of Metabolic Homeostasis for Nutrigenomics-Derived Health CLAIMS Made on Food (BIOCLAIMS). Available online: https://cordis.europa.eu/project/id/244995 (accessed on 1 March 2010).
- Winklhofer-Roob, B.M.; Faustmann, G.; Walter, N.; Hafner-Giessauf, H.; Sattler, M.C.; Zelzer, S.; Meinitzer, A.; Tiran, B.; Roob, J.M. Between and within subject variability of biomarkers of antioxidant status in the BIOCLAIMS cohort. Aktuelle Ernährungsmedizin 2017, 42, 241–272. [Google Scholar]
- Palmieri, M.; Kim, H.-N.; Gomez-Acevedo, H.; Que, X.; Tsimikas, S.; Jilka, R.L.; Manolagas, S.C.; Witztum, J.L.; Ambrogini, E. A neutralizing antibody targeting oxidized phospholipids promotes bone anabolism in chow-fed young adult mice. J. Bone Miner. Res. 2021, 36, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, C.M.; Yeudall, S.; Pavelec, C.M.; Merk, D.; Greulich, J.; Manjegowda, M.; Raghavan, S.S.; Bochkis, I.M.; Scott, M.M.; Perez-Reyes, E.; et al. Targeting oxidized phospholipids by AAV-based gene therapy in mice with established hepatic steatosis prevents progression to fibrosis. Sci. Adv. 2022, 8, eabn0050. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Grievink, H.W.; Gal, P.; Ozsvar Kozma, M.; Klaassen, E.S.; Kuiper, J.; Burggraaf, J.; Binder, C.J.; Moerland, M. The effect of a 13-valent conjugate pneumococcal vaccine on circulating antibodies against oxidized LDL and phosphorylcholine in man, a randomized placebo-controlled clinical trial. Biology 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Navab, M.; Hough, G.; Grijalva, V.; Mukherjee, P.; Fogelman, H.R.; Hwang, L.H.; Faull, K.F.; Lusis, A.J.; Reddy, S.T.; et al. Tg6F ameliorates the increase in oxidized phospholipids in the jejunum of mice fed unsaturated LysoPC or WD. J. Lipid Res. 2016, 57, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Li, Y.; Zhang, C.-O.; Ke, Y.; Promnares, K.; Birukova, A.A.; Eggerman, T.L.; Bocharov, A.V.; Birukov, K.G. Amphipathic helical peptide L37pA protects against lung vascular endothelial dysfunction caused by truncated oxidized phospholipids via antagonism with CD36 receptor. Am. J. Respir. Cell Mol. Biol. 2024, 70, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Hellauer, K.; Oskolkova, O.V.; Gesslbauer, B.; Bochkov, V. Pharmacological heat-shock protein inducers and chemical chaperones inhibit upregulation of interleukin-8 by oxidized phospholipids. Inflammopharmacology 2023, 31, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Antibodies against Phosphorylcholine—Implications for Chronic Inflammatory Diseases. Metabolites 2023, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, D.; Tellis, C.; Tselepis, A.D. Oxidized phospholipids and lipoprotein-associated phospholipase A2 (Lp-PLA2) in atherosclerotic cardiovascular disease: An update. Biofactors 2022, 48, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Hörkkö, S.; Miller, E.; Steinbrecher, U.P.; Powell, H.C.; Curtiss, L.K.; Witztum, J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Investig. 1996, 98, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Papac-Milicevic, N.; Busch, C.J.-L.; Binder, C.J. Malondialdehyde epitopes as targets of immunity and the implications for atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Sottero, B.; Rossin, D.; Staurenghi, E.; Gamba, P.; Poli, G.; Testa, G. Omics analysis of oxysterols to better understand their pathophysiological role. Free Radic. Biol. Med. 2019, 144, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Penkov, S.; Fedorova, M. Membrane epilipidome—Lipid modifications, their dynamics, and functional significance. Cold Spring Harb. Perspect. Biol. 2024, 16, a041417. [Google Scholar] [CrossRef] [PubMed]
- Parchem, K.; Letsiou, S.; Petan, T.; Oskolkova, O.; Medina, I.; Kuda, O.; O’Donnell, V.B.; Nicolaou, A.; Fedorova, M.; Bochkov, V.; et al. Oxylipin profiling for clinical research: Current status and future perspectives. Prog. Lipid Res. 2024, 95, 101276. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Nepachalovich, P.; Garcia-Del Rio, D.F.; Lange, M.; Ni, Z.; Baroni, M.; Cruciani, G.; Goracci, L.; Blüher, M.; Fedorova, M. Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma. Nat. Commun. 2022, 13, 6547. [Google Scholar] [CrossRef] [PubMed]
- Deroissart, J.; Binder, C.J.; Porsch, F. Role of antibodies and their specificities in atherosclerotic cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
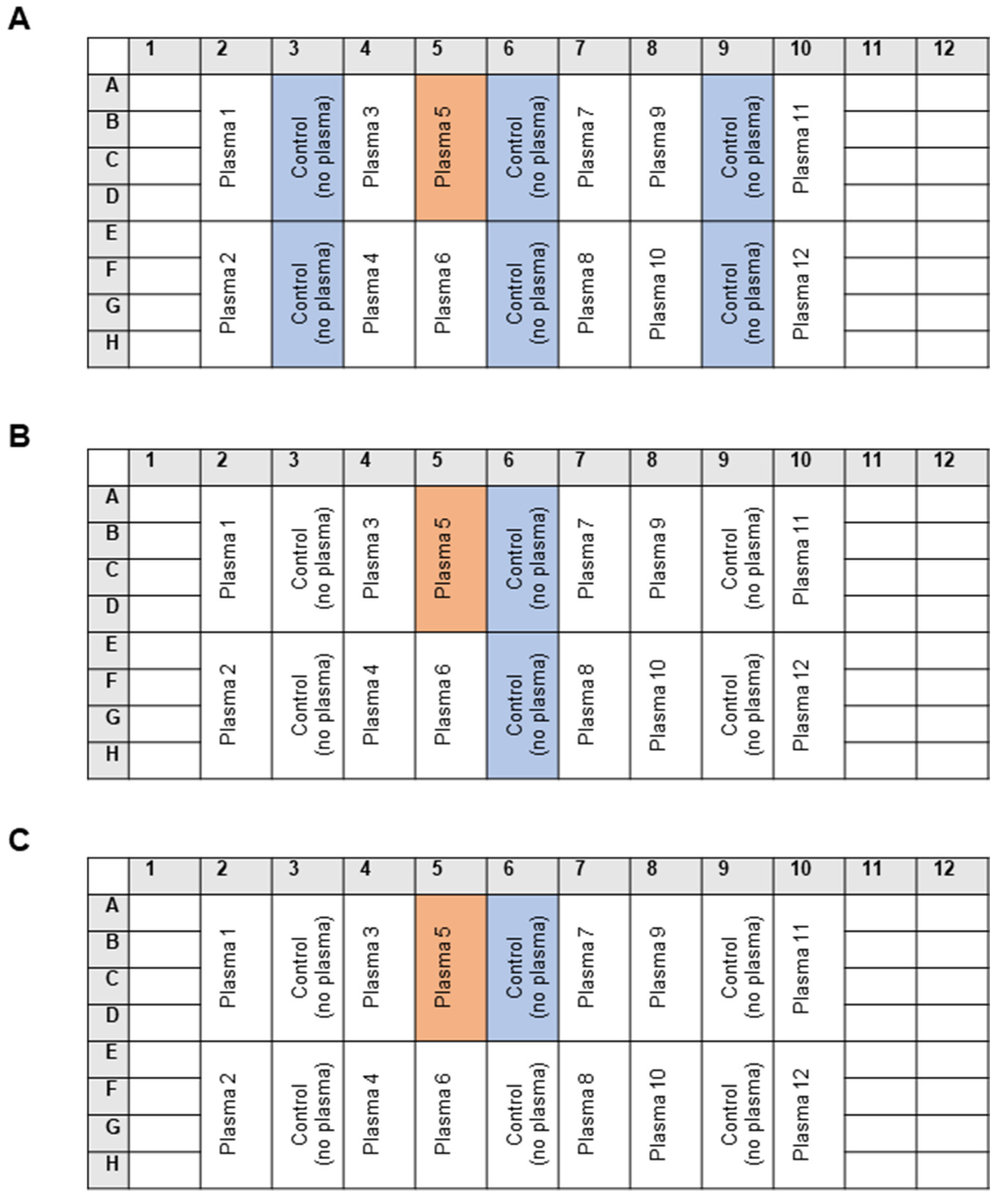
| Masking Activity of Pooled Plasma Normalized to | |||
|---|---|---|---|
| Meansof 3 × 8 Control Wellsin Columns 3, 6 and 9 | Meansof 1 × 8 Control Wellsin Column 9 | Meansof 4 Vicinal Control Wellsin Column 9 | |
| Mean of 12 plates | 0.58 | 0.62 | 0.64 |
| SD | 0.06 | 0.06 | 0.06 |
| CV, % | 10.9 | 10.4 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelic, M.; Jokesch, P.; Oskolkova, O.; Faustmann, G.; Winklhofer-Roob, B.M.; Ullrich, B.; Krauss, J.; Übelhart, R.; Gesslbauer, B.; Bochkov, V. An Immune Assay to Quantify the Neutralization of Oxidation-Specific Epitopes by Human Blood Plasma. Antioxidants 2025, 14, 903. https://doi.org/10.3390/antiox14080903
Jelic M, Jokesch P, Oskolkova O, Faustmann G, Winklhofer-Roob BM, Ullrich B, Krauss J, Übelhart R, Gesslbauer B, Bochkov V. An Immune Assay to Quantify the Neutralization of Oxidation-Specific Epitopes by Human Blood Plasma. Antioxidants. 2025; 14(8):903. https://doi.org/10.3390/antiox14080903
Chicago/Turabian StyleJelic, Marija, Philipp Jokesch, Olga Oskolkova, Gernot Faustmann, Brigitte M. Winklhofer-Roob, Bernd Ullrich, Jürgen Krauss, Rudolf Übelhart, Bernd Gesslbauer, and Valery Bochkov. 2025. "An Immune Assay to Quantify the Neutralization of Oxidation-Specific Epitopes by Human Blood Plasma" Antioxidants 14, no. 8: 903. https://doi.org/10.3390/antiox14080903
APA StyleJelic, M., Jokesch, P., Oskolkova, O., Faustmann, G., Winklhofer-Roob, B. M., Ullrich, B., Krauss, J., Übelhart, R., Gesslbauer, B., & Bochkov, V. (2025). An Immune Assay to Quantify the Neutralization of Oxidation-Specific Epitopes by Human Blood Plasma. Antioxidants, 14(8), 903. https://doi.org/10.3390/antiox14080903








