Relationships Between H2S and OT/OTR Systems in Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedures
2.2. Clinical Parameters
2.3. Sulfide Measurements
2.4. Isolation of PBMC
2.5. Tissue Processing
2.6. Immunohistochemistry
2.7. Western Blotting
2.8. Statistics
3. Results
3.1. Patient Demographics and Clinical Parameters
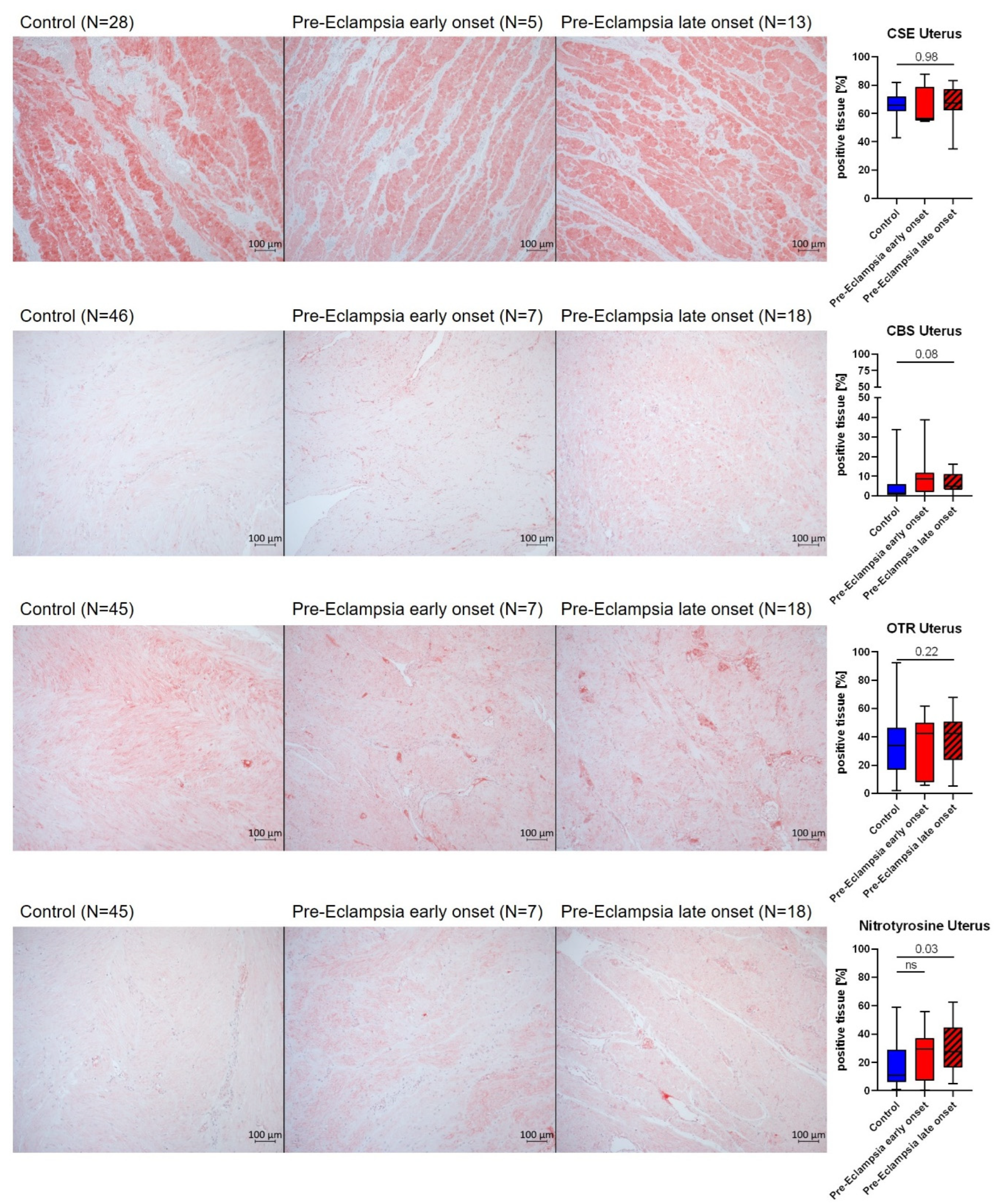
3.2. Biological Correlates of the Hydrogen Sulfide (H2S) and Oxytocin (OT) Systems
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine-aminotransferase |
| ANOVA | Analysis of variance |
| APGAR | Appearance Pulse Grimace Activity Respiratory Effort |
| ASS | Acetyl-salicylic acid |
| AST | Aspartate-aminotransferase |
| CBS | Cystathionine-β-synthase |
| CO | Carbon Monooxide |
| CRP | C-reactive protein |
| CSE | Cystathionine-γ-lyase |
| eGFR | Estimated glomerular filtration rate |
| ePE | Early onset pre-eclampsia (<34 weeks) |
| GC/MS | Gas chromatography/Mass spectrometry |
| HO-1 | Heme-oxygenase 1 |
| H2S | Hydrogen sulfide |
| IHC | Immunohistochemistry |
| IUGR | Intra-uterine growth restriction |
| KW-test | Kruskal–Wallis test |
| lPE | Late onset pre-eclampsia (>34 weeks) |
| MW-test | Mann–Whitney test |
| NaHS | Sodium hydrogen sulfide |
| NO | Nitric oxide |
| Nrf2 | Nuclear response factor 2 |
| OT | Oxytocin |
| OTR | Oxytocin receptor |
| owANOVA | One-way analysis of variance |
| PBMC | Peripheral blood mononuclear cells |
| PE | Pre-eclampsia |
| SGA | Small for gestational age |
| Vit. | Vitamin |
| WB | Western blot |
References
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; Da Silva Costa, F.; Nicolaides, K.; et al. Pre-Eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.J. Why Is Placentation Abnormal in Preeclampsia? Am. J. Obstet. Gynecol. 2015, 213, S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Staff, A.C. Preeclampsia, Biomarkers, Syncytiotrophoblast Stress, and Placental Capacity. Am. J. Obstet. Gynecol. 2015, 213, S9.e1–S9.e4. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rahman, M.; Zhang, X.; Acevedo, C.H.; Nijjar, S.; Rushton, I.; Bussolati, B.; John, J.S. Induction of Placental Heme Oxygenase-1 Is Protective Against TNF -Induced Cytotoxicity and Promotes Vessel Relaxation. Mol. Med. 2000, 6, 391–409. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Van Steenwinckel, J.; Gunn, A.J.; Bennet, L.; Korzeniewski, S.J.; Gressens, P.; Dean, J.M. Chronic Inflammation Offers Hints About Viable Therapeutic Targets for Preeclampsia and Potentially Related Offspring Sequelae. Int. J. Mol. Sci. 2024, 25, 12999. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In ncbi StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Freire, V.A.F.; Melo, A.D.D.; Santos, H.D.L.; Barros-Pinheiro, M. Evaluation of Oxidative Stress Markers in Subtypes of Preeclampsia: A Systematic Review and Meta-Analysis. Placenta 2023, 132, 55–67. [Google Scholar] [CrossRef] [PubMed]
- McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. Jt. Credit. Mech. 2021, 10, 3484. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Alster, P.; Lundeberg, T.; Uvnäs-Moberg, K. Oxytocin Causes a Long-Term Decrease of Blood Pressure in Female and Male Rats. Physiol. Behav. 1996, 60, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a Physiologic Vasorelaxant: Hypertension in Mice with Deletion of Cystathionine γ-Lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. Jt. Credit. Mech. 2019, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Langesæter, E.; Rosseland, L.A.; Stubhaug, A. Haemodynamic Effects of Oxytocin in Women with Severe Preeclampsia. Int. J. Obstet. Anesth. 2011, 20, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Merz, T.; Lukaschewski, B.; Wigger, D.; Rupprecht, A.; Wepler, M.; Gröger, M.; Hartmann, C.; Whiteman, M.; Szabo, C.; Wang, R.; et al. Interaction of the Hydrogen Sulfide System with the Oxytocin System in the Injured Mouse Heart. Intensive Care Med. Exp. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Wigger, D.C.; Gröger, N.; Lesse, A.; Krause, S.; Merz, T.; Gündel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine γ-Lyase Expression in Mice. Oxidative Med. Cell. Longev. 2020, 2020, 4309605. [Google Scholar] [CrossRef] [PubMed]
- Merz, T.; Denoix, N.; Wigger, D.; Waller, C.; Wepler, M.; Vettorazzi, S.; Tuckermann, J.; Radermacher, P.; McCook, O. The Role of Glucocorticoid Receptor and Oxytocin Receptor in the Septic Heart in a Clinically Relevant, Resuscitated Porcine Model With Underlying Atherosclerosis. Front. Endocrinol. 2020, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, B.; Merz, T.; Denoix, N.; Szabo, C.; Calzia, E.; Radermacher, P.; McCook, O. ΔMST and the Regulation of Cardiac CSE and OTR Expression in Trauma and Hemorrhage. Antioxidants 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, S.C.; Yang, H.; Lv, C.; Jia, S.; Liu, X.; Wang, X.; Meng, D.; Qin, D.; Zhu, H.; et al. Therapeutic Potential of Oxytocin in Atherosclerotic Cardiovascular Disease: Mechanisms and Signaling Pathways. Front. Neurosci. 2019, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, D.J.; Calvert, J.W.; Butler, J.; Lefer, D.J. The Cardioprotective Actions of Hydrogen Sulfide in Acute Myocardial Infarction and Heart Failure. Scientifica 2014, 2014, 768607. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Chen, Z.; Zhao, H.; Xu, C.; Liu, W.; Sun, Q.; He, P.; Gu, H.; Ni, X. Endogenous Hydrogen Sulfide Contributes to Uterine Quiescence during Pregnancy. Reproduction 2017, 153, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-C.; Lee, J.-E.; Kang, S.S.; Yang, H.-S.; Kim, S.S.; An, B.-S. The Regulation of Oxytocin and Oxytocin Receptor in Human Placenta According to Gestational Age. J. Mol. Endocrinol. 2017, 59, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Maigaard, S.; Forman, A.; Andersson, K.-E. Differential Effects of Angiotensin, Vasopressin and Oxytocin on Various Smooth Muscle Tissues within the Human Uteroplacental Unit. Acta Physiol. Scand. 1986, 128, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xu, T.; Ding, H.; Li, H.; Yang, Y.; He, Y.; Tang, J.; Liu, Y.; Chen, X.; Chen, J.; et al. DNA Methylation-reprogrammed Oxytocin Receptor Underlies Insensitivity to Oxytocin in Pre-eclamptic Placental Vasculature. J. Cell. Mol. Medi. 2019, 23, 4118–4126. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.F.; et al. Dysregulation of Hydrogen Sulfide Producing Enzyme Cystathionine γ-Lyase Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Palei, A.C.; Pinto-Souza, C.C.; Cavalli, R.; Dias-Junior, C.A.; Sandrim, V. Circulating Levels of Hydrogen Sulphide Negatively Correlate to Nitrite Levels in Gestational Hypertensive and Preeclamptic Pregnant Women. Clin. Exp. Pharma. Physio. 2021, 48, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, K.M.; Bos, E.M.; Rajakumar, A.; Ris-Stalpers, C.; Van Pampus, M.G.; Timmer, A.; Erwich, J.J.H.M.; Faas, M.M.; Van Goor, H.; Lely, A.T. Hydrogen Sulfide Producing Enzymes in Pregnancy and Preeclampsia. Placenta 2012, 33, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Herrera, E.A.; Niu, Y.; Kingdom, J.; Giussani, D.A.; Burton, G.J. Reduced Cystathionine γ-Lyase and Increased miR-21 Expression Are Associated with Increased Vascular Resistance in Growth-Restricted Pregnancies. Am. J. Pathol. 2013, 182, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins Gestational Hypertension and Preeclampsia—Clinical Management Guidelines for Obstetrician–Gynecologists; ACOG Practice Bulletin: Washington, DC, USA, 2020; Volume 135, pp. e237–e260. [Google Scholar]
- McCook, O.; Radermacher, P.; Volani, C.; Asfar, P.; Ignatius, A.; Kemmler, J.; Möller, P.; Szabó, C.; Whiteman, M.; Wood, M.E.; et al. H2S during Circulatory Shock: Some Unresolved Questions. Nitric Oxide 2014, 41, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Chen, B.; Dilday, B.; Palmer, H.; Hosoi, H.; Singh, S.; Wu, C.; Li, X.; Thompson, J.; Parham, D.; et al. Strong Immunostaining for Myogenin in Rhabdomyosarcoma Is Significantly Associated with Tumors of the Alveolar Subclass. Am. J. Pathol. 2000, 156, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Merz, T.; Wepler, M.; Nußbaum, B.; Vogt, J.; Calzia, E.; Wang, R.; Szabo, C.; Radermacher, P.; McCook, O. Cystathionine-γ-Lyase Expression Is Associated with Mitochondrial Respiration during Sepsis-Induced Acute Kidney Injury in Swine with Atherosclerosis. Intensive Care Med. Exp. 2018, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Denoix, N.; Merz, T.; Unmuth, S.; Hoffmann, A.; Nespoli, E.; Scheuerle, A.; Huber-Lang, M.; Gündel, H.; Waller, C.; Radermacher, P.; et al. Cerebral Immunohistochemical Characterization of the H2S and the Oxytocin Systems in a Porcine Model of Acute Subdural Hematoma. Front. Neurol. 2020, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Münz, F.; Datzmann, T.; Hoffmann, A.; Gröger, M.; Mathieu, R.; Mayer, S.; Zink, F.; Gässler, H.; Wolfschmitt, E.-M.; Hogg, M.; et al. The Effect of Targeted Hyperoxemia on Brain Immunohistochemistry after Long-Term, Resuscitated Porcine Acute Subdural Hematoma and Hemorrhagic Shock. Int. J. Mol. Sci. 2024, 25, 6574. [Google Scholar] [CrossRef] [PubMed]
- Gröger, M.; Hogg, M.; Abdelsalam, E.; Kress, S.; Hoffmann, A.; Stahl, B.; Saub, V.; Denoix, N.; McCook, O.; Calzia, E.; et al. Effects of Sodium Thiosulfate During Resuscitation From Trauma-and-Hemorrhage in Cystathionine Gamma Lyase (CSE) Knockout Mice. Shock 2022, 57, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gröger, M.; Hogg, M.; Abdelsalam, E.; Kress, S.; Hoffmann, A.; Stahl, B.; Calzia, E.; Wachter, U.; Vogt, J.A.; Wang, R.; et al. Effects of Sodium Thiosulfate During Resuscitation From Trauma-and-Hemorrhage in Cystathionine-γ-Lyase Knockout Mice With Diabetes Type 1. Front. Med. 2022, 9, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Szeto, A.; Sun-Suslow, N.; Mendez, A.J.; Hernandez, R.I.; Wagner, K.V.; McCabe, P.M. Regulation of the Macrophage Oxytocin Receptor in Response to Inflammation. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E183–E189. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Banerjee, S.; Kumar, A.; Biswas, U. Association between Serum Levels of Nitric Oxide and Hydrogen Sulfide in Pre-Eclampsia. Biochem. Anal Biochem. 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Olson, K.R. A Practical Look at the Chemistry and Biology of Hydrogen Sulfide. Antioxid. Redox Signal. 2012, 17, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Acılmıs, Y.G.; Dikensoy, E.; Kutlar, A.I.; Balat, O.; Cebesoy, F.B.; Ozturk, E.; Cicek, H.; Pence, S. Homocysteine, Folic Acid and Vitamin B12 Levels in Maternal and Umbilical Cord Plasma and Homocysteine Levels in Placenta in Pregnant Women with Pre-Eclampsia: Homocysteine Metabolism in Pre-Eclampsia. J. Obstet. Gynaecol. Res. 2011, 37, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cotter, A.M.; Molloy, A.M.; Scott, J.M.; Daly, S.F. Elevated Plasma Homocysteine in Early Pregnancy: A Risk Factor for the Development of Severe Preeclampsia. Am. J. Obstet. Gynecol. 2001, 185, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Hultberg, B.; Brattström, L.; Isaksson, A. Decreased Serum Homocysteine in Pregnancy. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 377–379. [Google Scholar] [PubMed]
- Powers, R.W.; Evans, R.W.; Majors, A.K.; Ojimba, J.I.; Ness, R.B.; Crombleholme, W.R.; Roberts, J.M. Plasma Homocysteine Concentration Is Increased in Preeclampsia and Is Associated with Evidence of Endothelial Activation. Am. J. Obstet. Gynecol. 1998, 179, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- De León Bautista, M.P.; Romero-Valdovinos, M.; Zavaleta-Villa, B.; Martínez-Flores, A.; Olivo-Díaz, A. Association of Cystathionine β-Synthase Gene Polymorphisms With Preeclampsia. Clin. Appl. Thromb. Hemost. 2018, 24 (Suppl. 9), 285S–293S. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Bilska, A.; Mittal, T.K.; Stangret, A.; Wejman, J.; Szewczyk, G.; Pyzlak, M.; Zamlynski, J. Myometrial Contractility Influences Oxytocin Receptor (OXTR) Expression in Term Trophoblast Cells Obtained from the Maternal Surface of the Human Placenta. BMC Pregnancy Childbirth 2015, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Denoix, N.; McCook, O.; Ecker, S.; Wang, R.; Waller, C.; Radermacher, P.; Merz, T. The Interaction of the Endogenous Hydrogen Sulfide and Oxytocin Systems in Fluid Regulation and the Cardiovascular System. Antioxidants 2020, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Palei, A.C.T.; Metzger, I.F.; Gomes, V.A.; Cavalli, R.C.; Tanus-Santos, J.E. Nitric Oxide Formation Is Inversely Related to Serum Levels of Antiangiogenic Factors Soluble Fms-Like Tyrosine Kinase-1 and Soluble Endogline in Preeclampsia. Hypertension 2008, 52, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, G.; Jahan, S.; Ain, Q.U.; Ullah, A.; Afsar, T.; Almajwal, A.; Alam, I.; Razak, S. Placental Endothelial Nitric Oxide Synthase Expression and Role of Oxidative Stress in Susceptibility to Preeclampsia in Pakistani Women. Molec. Gen. Gen. Med. 2020, 8, e1019. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, J.N.S.; Minami, C.K.; Peraçoli, M.T.S.; Romão-Veiga, M.; Ribeiro-Vasques, V.R.; Peraçoli, J.C.; Palei, A.C.T.; Cavalli, R.C.; Nunes, P.R.; Luizon, M.R.; et al. Plasma eNOS Concentration in Healthy Pregnancy and in Hypertensive Disorders of Pregnancy: Evidence of Reduced Concentrations in Pre-Eclampsia from Two Independent Studies. Diseases 2023, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative Stress: Normal Pregnancy versus Preeclampsia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Kodliwadmath, M.V.; Kodliwadmath, S.M. Lipid Peroxidation and Antioxidant Activity in Complicated Pregnancies. Clin. Exp. Obst. Gyn. 2009, 36, 110–112. [Google Scholar]
- Siddiqui, I.A.; Jaleel, A.; Tamimi, W.; Al Kadri, H.M.F. Role of Oxidative Stress in the Pathogenesis of Preeclampsia. Arch. Gynecol. Obs. 2010, 282, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-Sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Hoque, M.; Bains, S.; Green, C.J.; Motterlini, R. Haem and Nitric Oxide: Synergism in the Modulation of the Endothelial Haem Oxygenase-1 Pathway. Biochem. J. 2003, 372, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Green, C.J.; Foresti, R. Regulation of Heme Oxygenase-1 by Redox Signals Involving Nitric Oxide. Antioxid. Redox Signal. 2002, 4, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.; Foresti, R.; Bains, S.K.; Hoque, M.; Green, C.J.; Motterlini, R. Induction of Heme Oxygenase 1 by Nitrosative Stress. J. Biol. Chem. 2002, 277, 40666–40674. [Google Scholar] [CrossRef] [PubMed]
- Sandrim, V.C.; Caldeira-Dias, M.; Bettiol, H.; Barbieri, M.A.; Cardoso, V.C.; Cavalli, R.C. Circulating Heme Oxygenase-1: Not a Predictor of Preeclampsia but Highly Expressed in Pregnant Women Who Subsequently Develop Severe Preeclampsia. Oxidative Med. Cell. Longev. 2018, 2018, 6035868. [Google Scholar] [CrossRef] [PubMed]
- Yoshiki, N.; Kubota, T.; Aso, T. Expression and Localization of Heme Oxygenase in Human Placental Villi. Biochem. Biophys. Res. Commun. 2000, 276, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Levytska, K.; Kingdom, J.; Baczyk, D.; Drewlo, S. Heme Oxygenase-1 in Placental Development and Pathology. Placenta 2013, 34, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.; Robson, S.C.; Myatt, L.; Bulmer, J.N.; Lyall, F. Heme Oxygenase Expression in Human Placenta and Placental Bed: Reduced Expression of Placenta Endothelial HO-2 in Preeclampsia and Fetal Growth Restriction. FASEB J. 2001, 15, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C.; Lim, E.; Knoeller, S.; Knackstedt, M.; Hertwig, K.; Hagen, E.; Klapp, B.F.; Arck, P.C. Heme Oxygenases in Pregnancy II: HO-2 Is Downregulated in Human Pathologic Pregnancies. Am. J. Rep. Immunol. 2003, 50, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Kaitu’u-Lino, T.J.; Onda, K.; Beard, S.; Hastie, R.; Binder, N.K.; Cluver, C.; Tuohey, L.; Whitehead, C.; Brownfoot, F.; et al. Heme Oxygenase-1 Is Not Decreased in Preeclamptic Placenta and Does Not Negatively Regulate Placental Soluble Fms-Like Tyrosine Kinase-1 or Soluble Endoglin Secretion. Hypertension 2015, 66, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, B.E.; Lash, G.E.; Smith, G.N.; Marks, G.S.; Nakatsu, K.; Graham, C.H.; Brien, J.F. Heme Oxygenase Expression in Selected Regions of Term Human Placenta. Exp. Biol. Med. 2003, 228, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Li, C.; Cui, L.; Ma, J.; Hui, Y. The Non-Canonical Effects of Heme Oxygenase-1, a Classical Fighter against Oxidative Stress. Redox Biol. 2021, 47, 102170. [Google Scholar] [CrossRef] [PubMed]





| Control Group | PE (Early Onset) | PE (Late Onset) | p-Value | |
|---|---|---|---|---|
| Patient age [y] | N = 49 33 [30; 37] | N = 8 35 [32; 36] | N = 36 33 [28; 36] | 0.10 (owANOVA) |
| Patient height [cm] | N = 49 168 [163; 172] | N = 8 164.5 [161; 166] | N = 36 166 [162;170] | 0.56 (owANOVA) |
| Patient weight [kg] | N = 49 81 [72; 96] | N = 8 87 [78; 105] | N = 35 95 [81; 103] | 0.12 (owANOVA) |
| Vaginal deliveries | 0/51 | 0/8 | 14/36 (39%) | n.a. |
| Induction of labor | 1/51 | 0/8 | 20/36 (56%) | n.a. |
| Gestational age at parturition [d] | N = 51 272 [267; 274] | N = 8 207 [194; 222] a,b | N = 36 258 [250; 266] a,b | <0.0001 (KW-test) |
| Blood pressure at admission [mmHg] | N = 51 systolic 122 [117; 130] diastolic 76 [70; 84] | N = 8 systolic 169 [145; 190] a,b diastolic 100 [90; 110] a | N = 33 systolic 150 [144; 163] a,b diastolic 100 [93; 107] a | Group: <0.0001 (Two-way ANOVA) |
| Blood glucose 1 h after 50 g oGTT [mg/dL] | N = 32 115 [100; 128] | N = 4 141.5 [120; 217] | N = 25 109 [97; 127] | 0.10 (KW-test) |
| Total plasma protein [g/dL] | N = 48 6.7 [6.4; 7.0] | N = 8 6.0 [5.4; 6.45] a | N = 36 6.2 [5.8; 6.4] a | <0.0001 (owANOVA) |
| Protein in 24 h urine [g/dL] | n.d. | N = 8 5.02 [0.78; 8.40] b | N = 29 0.81 [0.35; 2.23] b | 0.04 (MW-test) |
| Creatinine [mg/dL] | N = 48 0.59 [0.51; 0.64] | N = 8 0.74 [0.63; 0.83] a | N = 36 0.63 [0.57; 0.76] a | 0.0006 (owANOVA) |
| AST [U/L] | N = 48 17.5 [15; 21.8] | N = 8 26.5 [16.5; 56.8] | N = 36 21.5 [18.3; 27.8] a | 0.001 (KW-test) |
| ALT [U/L] | N = 48 14 [10; 16] | N = 8 21 [18; 38.5] a | N = 35 18 [14; 22] a | <0.0001 (KW-test) |
| Thrombocytes [×109/L] | N = 51 222 [197; 263] | N = 8 237 [213; 286] | N = 36 226 [180; 260] | 0.83 (owANOVA) |
| Hemoglobin [g/dL] | N = 51 11.8 [11.2; 12.3] | N = 8 11.6 [11.0; 12.9] | N = 36 11.9 [11.0; 12.4] | 0.99 (owANOVA) |
| Folic Acid [ng/mL] | N = 51 15.3 [10.4; 18.5] | N = 7 14.0 [12.8; 26.1] | N = 30 15.3 [11.6; 17.5] | 0.11 (owANOVA) |
| Vitamin B6 [µg/L] | N = 51 19.2 [14.6; 23.5] | N = 7 21.1 [12.9; 27.0] | N = 31 20.0 [14.0; 25.1] | 0.78 (KW-test) |
| Vitamin B12 [pg/mL] | N = 49 301 [251; 386] | N = 7 360 [248; 467] | N = 30 313 [208; 370] | 0.61 (KW-test) |
| C-reactive protein [mg/dL] | N = 48 0.5 [0.5; 1.1] | N = 8 0.5 [0.5; 0.9] | N = 36 0.5 [0.5; 0.8] | 0.24 (KW-test) |
| Control Group | PE (Early Onset) | PE (Late Onset) | p-Value | |
|---|---|---|---|---|
| Birth weight [g] | 3130 [2940; 3650] | 1105 [885; 1648] a,b | 2715 [2203; 3123] a,b | <0.0001 |
| Sex (male/female) | 21/30 | 4/4 | 17/19 | n.a. |
| Apgar 1 min | 9 [8; 9] | 7 [7; 7.5] a,b | 9 [8; 9] | 0.0008 |
| Apgar 5 min | 10 [9; 10] | 8 [8; 8] a,b | 10 [9; 10] | 0.0009 |
| Apgar 10 min | 10 [10; 10] | 9 [8.25; 9.75] a,b | 10 [10; 10] | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merz, T.; Ecker, S.; Denoix, N.; McCook, O.; Kranz, S.; Wachter, U.; Rottler, E.; Papadopoulos, T.; Fusch, C.; Brucker, C.; et al. Relationships Between H2S and OT/OTR Systems in Preeclampsia. Antioxidants 2025, 14, 880. https://doi.org/10.3390/antiox14070880
Merz T, Ecker S, Denoix N, McCook O, Kranz S, Wachter U, Rottler E, Papadopoulos T, Fusch C, Brucker C, et al. Relationships Between H2S and OT/OTR Systems in Preeclampsia. Antioxidants. 2025; 14(7):880. https://doi.org/10.3390/antiox14070880
Chicago/Turabian StyleMerz, Tamara, Sarah Ecker, Nicole Denoix, Oscar McCook, Stefanie Kranz, Ulrich Wachter, Edit Rottler, Thomas Papadopoulos, Christoph Fusch, Cosima Brucker, and et al. 2025. "Relationships Between H2S and OT/OTR Systems in Preeclampsia" Antioxidants 14, no. 7: 880. https://doi.org/10.3390/antiox14070880
APA StyleMerz, T., Ecker, S., Denoix, N., McCook, O., Kranz, S., Wachter, U., Rottler, E., Papadopoulos, T., Fusch, C., Brucker, C., Triebel, J., Bertsch, T., Radermacher, P., & Waller, C. (2025). Relationships Between H2S and OT/OTR Systems in Preeclampsia. Antioxidants, 14(7), 880. https://doi.org/10.3390/antiox14070880






