In Vitro Effects of Rumex confertus Extracts on Cell Viability and Molecular Pathways in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sampling and Preparation
2.2. Biochemical Assays
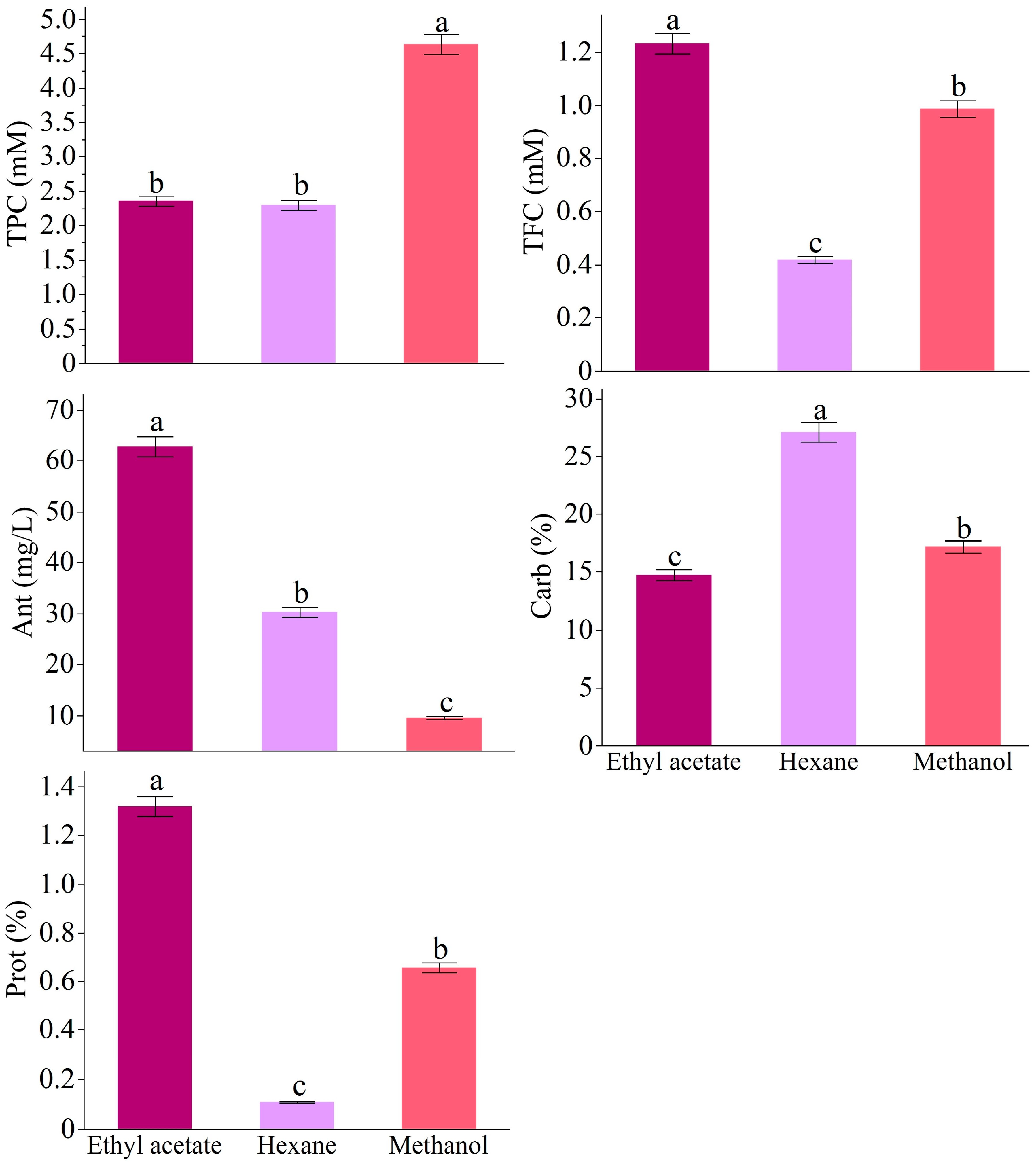
2.2.1. Total Phenolic Content (TPC)
2.2.2. Total Flavonoid Content (TFC)
2.2.3. Total Anthocyanin Content
2.2.4. Total Soluble Protein
2.2.5. Total Soluble Carbohydrates
2.2.6. Antioxidant Activity Determination
DPPH Radical Scavenging Activity
Copper (II) Ion Reducing Antioxidant Capacity (CUPRAC)
ABTS Free Radical Scavenging Activity
Ferric (III) Reducing Antioxidant Power (FRAP)
2.3. Cell Culture and In Vitro Experiments
2.3.1. Cell Culture Conditions and MTT Assay
2.3.2. IC50 Determination
2.3.3. Total RNA Extraction, Purification, and cDNA Synthesis with Real-Time qPCR
2.4. Metabolic Responses of Cancer Cells
2.5. Flow Cytometer Analysis
2.5.1. Cell Cycle Analysis
2.5.2. Apoptosis Analysis
2.6. Experimental Design and Statistics
3. Results and Discussion
3.1. Biochemical Composition
3.2. Antioxidant Capacities
3.3. Cytotoxicity Results
3.4. IC50 Determination
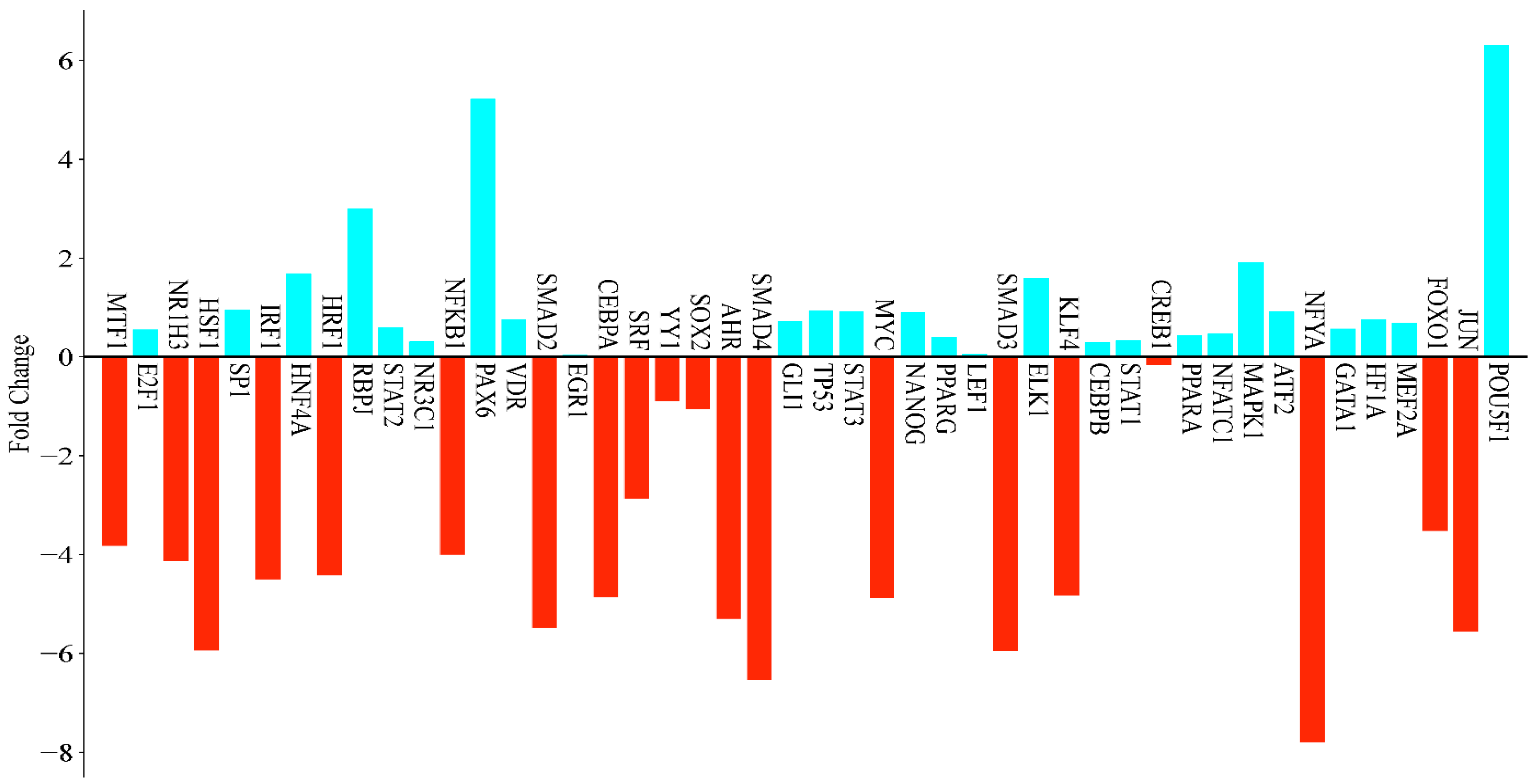
3.5. Real-Time qPCR Analysis
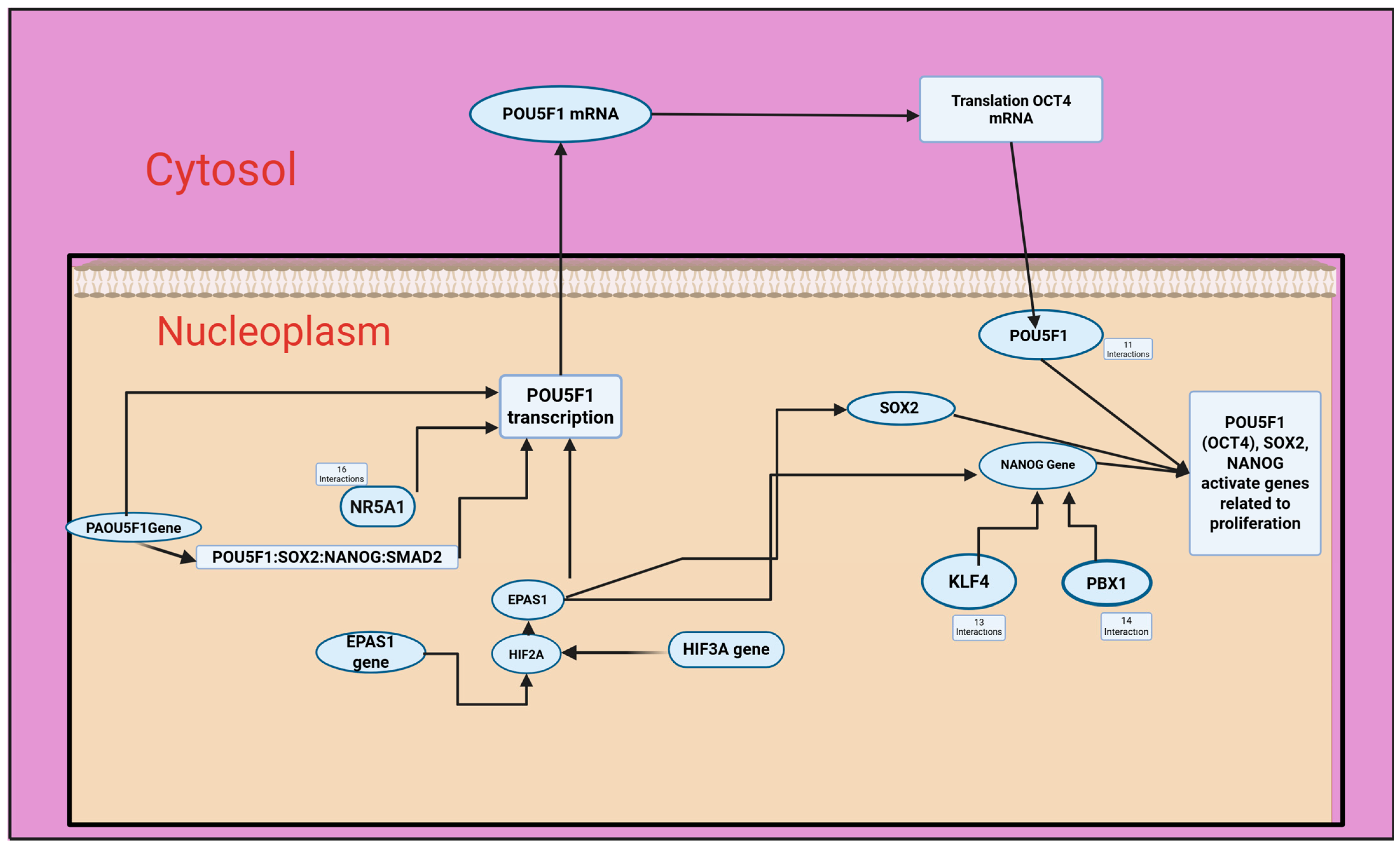
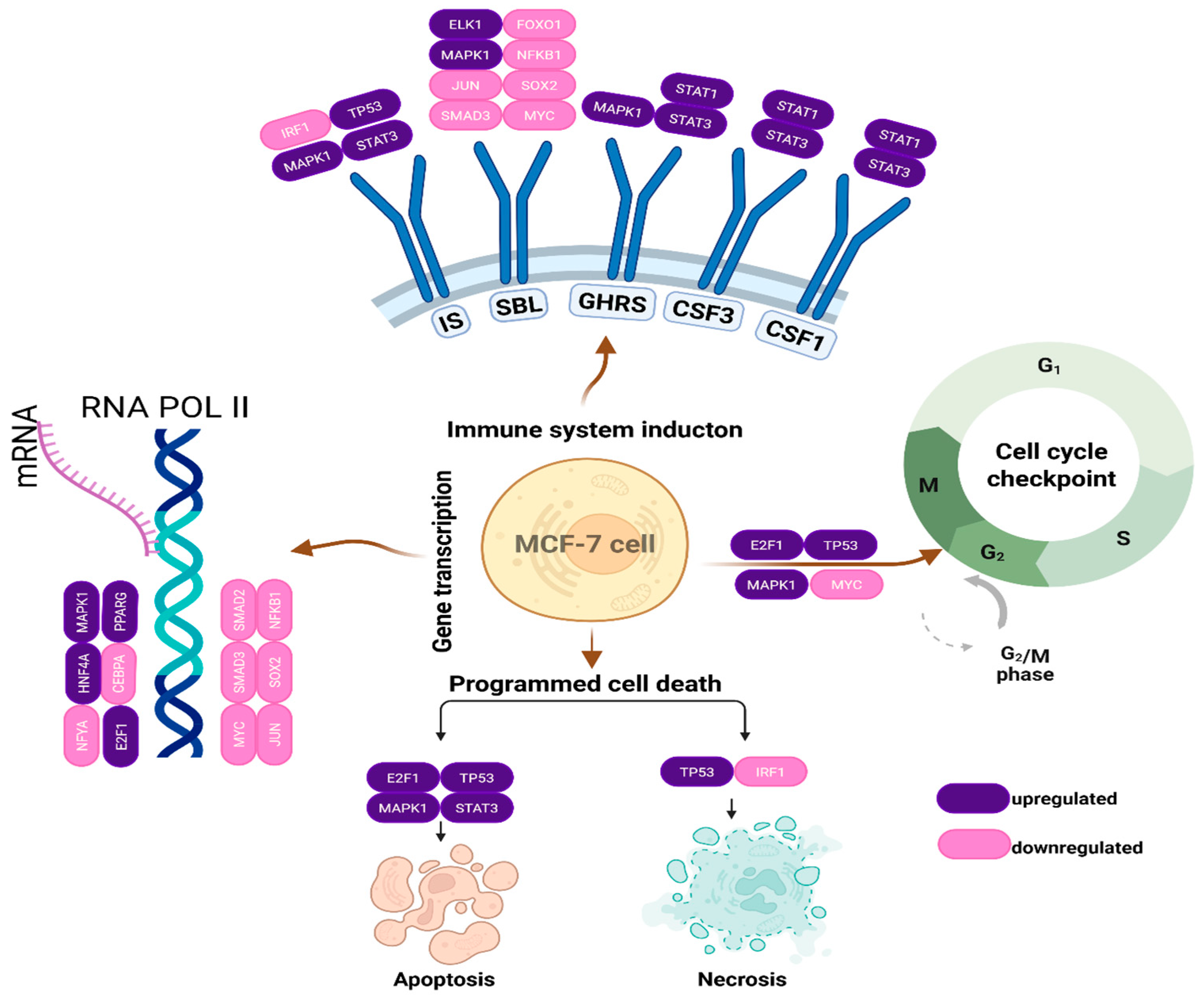
3.6. Metabolic Responses of Cancer Cells/Signaling Pathways
3.7. Flow Cytometry Analyses
3.7.1. Cell Cycle
3.7.2. Apoptosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RC | Rumex confertus |
| FRAP | Ferric reducing antioxidant power |
| CUPRAC | Cupric reducing antioxidant capacity |
| ABTS | 2,2′-azino-bis(3ethylbenzothiazoline-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| Ant | Total anthocyanin content |
| Carb | Total soluble carbohydrates |
| Prot | Total soluble proteins |
| MTT | [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] |
| PCR | Polymerase chain reaction |
| DMEM | Dulbecco’s modified eagle medium |
| EDTA | Ethylenediaminetetraacetic acid |
| DMSO | Dimethyl sulfoxide |
References
- Umarov, D.; Tseomashko, N.; Abidova, A.; Abrekova, N.; Beknazarova, N.; Turaboev, S.; Sagdullaev, B. Wound Healing and Antimicrobial Properties of Extract of the Rumex confertus Willd. Res. J. Pharm. Technol. 2024, 17, 2256–2264. [Google Scholar] [CrossRef]
- Eshbakova, K.; Ashirmatova, N.; Mamarasulov, B.; Khasanova, K.; Komilov, B.; Davranov, K. Total Phenol and Flavonoid Content, Antibacterial and Antioxidant Activity of Extract and Fractions of Medicinal Plants of the Rumex (Polygonaceae) Family in the Flora of Uzbekistan. Ann. Phytomed. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, M.M.; Bouajila, J.; Alouani, K. Phenolic Compounds of Rumex roseus L. Extracts and Their Effect as Antioxidant and Cytotoxic Activities. Biomed. Res. Int. 2021, 2021, 2029507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, N.; Zhu, H.; Wang, D.; Zhang, Y. The Genus Rumex (Polygonaceae): An Ethnobotanical, Phytochemical and Pharmacological Review. Nat. Prod. Bioprospect. 2022, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.V.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Kozhahmetova, M.; Lebedeva, L. Overview of the Biological Activity of Anthraquinones and Flavonoids of the Plant Rumex Species. Molecules 2022, 27, 1204. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.M.; Elsharkawy, E.R.; He, Y.; Li, X. Importance of Advanced Detection Methodologies from Plant Cells to Human Microsystems Targeting Anticancer Applications. Int. J. Mol. Sci. 2025, 26, 4691. [Google Scholar] [CrossRef] [PubMed]
- Emam, J.A.; Yaya, E.E.; Choudhary, M.I.; Yousuf, S.; Gebremedihn, T.M. In Vitro Antifungal, Anti-inflammatory and Cytotoxic Activities of Rumex abyssinicus Rhizome Extract and Bioassay-Guided Isolation of Cytotoxic Compounds from Rumex abyssinicus. Bull. Chem. Soc. Ethiop. 2022, 36, 879–892. [Google Scholar] [CrossRef]
- Tesfay, T.; Tekluu, B.; Chaithanya, K.K.; Varma, D.L.; Palleti, J.D.; Rai, S.; Kamalakararao, K. In Vitro Anti-cancer Activity of Rumex abyssinicus Root Extracts on Breast Cancer MCF-7 Cell Lines. Res. J. Pharm. Technol. 2023, 16, 5410–5415. [Google Scholar] [CrossRef]
- Mohammadhosseinpour, S.; Bhandari, M.; Lee, D.; Clack, B. Anti-Proliferative and Apoptotic Activities of Rumex crispus. Life 2023, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Lockwood, H.N.; Clack, B. Induction of Apoptosis in DLD-1 Colorectal Cancer Cells Using Water Soluble Compounds From Rumex crispus. FASEB J. 2016, 30, 1090.10. [Google Scholar] [CrossRef]
- Tikhomirov, A.S.; Shtil, A.A.; Shchekotikhin, A.E. Advances in the Discovery of Anthraquinone-Based Anticancer Agents. Recent Pat. Anticancer Drug Discov. 2018, 13, 159–183. [Google Scholar] [CrossRef] [PubMed]
- Hafaz, M.F.; Soliman, H.M.; Abbas, M.A.; Gebreil, A.S.; El-Amier, Y.A. Potential Assessment of Rumex spp. as a Source of Bioactive Compounds and Biological Activity. Biointerface Res. Appl. Chem. 2022, 12, 1824–1834. [Google Scholar]
- Shermatova, G.D.; Bobakulov, H.M.; Shamuratov, B.A.; Mavlyanov, S.M.; Zhang, Y.J.; Eshbakova, K.A.; Azimova, S.S.; Sasmakov, S.A. Phenolic Compounds of Rumex L: Aerial Part Fractions and Essential Oil Results of In Vitro Screening for Antimicrobial Activity. Chem. Sci. Int. J. 2022, 31, 15–20. [Google Scholar] [CrossRef]
- Sharma, G.; Poudel, P.; Thapa, R.; Lamichhane, S.; Poudel, H.R.; Devkota, H.P.; Paniagua-Zambrana, N.Y. Rumex nepalensis Spreng., Rumex hastatus D. Don, Rumex longifolius DC. Polygonaceae. In Ethnobotany of the Himalayas; Springer: Cham, Germany, 2021; pp. 1–19. [Google Scholar]
- Wegiera, M.; Grabarczyk, P.; Baraniak, B.; Smolarz, H.D. Antiradical Properties of Extracts From Roots, Leaves and Fruits of Six Rumex L. Species. Acta Biol. Crac. Ser. Bot. 2011, 53, 29–37. [Google Scholar] [CrossRef]
- Mishra, A.P.; Sharifi-Rad, M.; Shariati, M.A.; Mabkhot, Y.N.; Al–Showiman, S.S.; Rauf, A.; Salehi, B.; Župunski, M.; Sharifi-Rad, M.; Gusain, P.; et al. Bioactive Compounds and Health Benefits of Edible Rumex Species—A Review. Cell. Mol. Biol. 2018, 64, 5–14. [Google Scholar] [CrossRef]
- Özenver, N.; Erkmen, Y.; Boyalı, F.; Demirezer, Ö. Cytotoxicity Screening and Antioxidant Capacity Assessment of the Inner Perianth Segments of 14 Rumex Species Grown in Türkiye. J. Fac. Pharm. Ankara Univ. 2024, 48, 456–469. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Current Protocols in Food Analytical Chemistry. AL Waterhouse 2002, 6, I1-1. [Google Scholar]
- Guler, E. Differentiations in Total Contents of Major Components in Grape Leaves Influenced by Cultivar. Ști. Educ. Cultură 2025, 1, 518–522. [Google Scholar]
- Atanacković, M.; Cvejić, J.; Gojković-Bukarica, L.; Veljović, M.; Despotović, S.; Pecić, S.; Leskošek-Čukalović, I. Quantitative Determination of Total Anthocyanins and Flavonoids in Natural Products Obtained from Grapes and Malt. In Proceedings of the CE-Food 2012-Proceedings of 6th Central European Congress on Food, Novi Sad, Serbia, 23–26 May 2012; pp. 183–188. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Geater, C.W.; Fehr, W.R. Association of Total Sugar Content with Other Seed Traits of Diverse Soybean cultivars. Crop Sci. 2000, 40, 1552–1555. [Google Scholar] [CrossRef]
- Gülüm, L.; Güler, E.; Zırhlı, Ç.B.; Çelik, A.B.; Tutar, Y. Phytochemical Profiling and Anticancer Potential of Pistachio Wastes Against MCF-7 Breast Cancer Cells: A Metabolic and Apoptotic Pathway Analysis. Res. Sq. 2025; preprint. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of Antioxidant Capacity Assays and The CUPRAC (Cupric Ion Reducing Antioxidant Capacity) Assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Bulut, M.; Akpolat, H.; Tunçtürk, Y.; Alwazeer, D.; Türkhan, A. Determination of Optimum Ethanolic Extraction Conditions and Phenolic Profiles of Thyme, Mint, Uckun, Grape Seeds and Green Tea Waste Fiber. Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2020, 6, 605–614. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Tapia, O.; Espinoza, J.A.; Leal, P.; Buchegger, K.; Sandoval, A.; Bizama, C.; Araya, J.C.; Peek, R.M.; Roa, J.C. The Gene Expression Status of the PI3K/AKT/mTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathol. Oncol. Res. 2016, 22, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Çapan, İ.; Hawash, M.; Qaoud, M.T.; Gülüm, L.; Tunoglu, E.N.Y.; Çifci, K.U.; Çevrimli, B.S.; Sert, Y.; Servi, S.; Koca, İ.; et al. Synthesis of Novel Carbazole Hydrazine-Carbothioamide Scaffold as Potent Antioxidant, Anticancer and Antimicrobial Agents. BMC Chem. 2024, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Aghajanyan, A.; Nikoyan, A.; Trchounian, A. Biochemical Activity and Hypoglycemic Effects of Rumex obtusifolius L. Seeds Used in Armenian Traditional Medicine. BioMed Res. Int. 2018, 2018, 4526352. [Google Scholar] [CrossRef] [PubMed]
- Çoruh, İ.; Görmez, A.; Erçışlı, S.; Şengül, M. Total Phenolic Content, Antioxidant, and Antibacterial Activity of Rumex Crispus Grown Wild in Turkey. Pharm. Biol. 2008, 46, 634–638. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ferreira Azevedo, P.I.; Ferrareze Veeck, J.P. Rumex obtusifolius Is a Wild Food Plant with Great Nutritional Value, High Content of Bioactive Compounds and Antioxidant Activity. Emir. J. Food Agric. 2019, 31, 315–320. [Google Scholar] [CrossRef]
- Ereifej, K.; Feng, H.; Rababah, T.; Almajwal, A.; Alu’datt, M.H.; Gammoh, S.; Oweis, L.I. Chemical Composition, Phenolics, Anthocyanins Concentration and Antioxidant Activity of Ten Wild Edible Plants. Food Nutr. Sci. 2015, 6, 581–590. [Google Scholar] [CrossRef]
- Gıdık, B. Antioxidant, Antimicrobial Activities and Fatty Acid Compositions of Wild Berberis Spp. by Different Techniques Combined with Chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Oris, J.T. Anthracene Photoinduced Toxicity to PLHC-1 Cell Line (Poeciliopsis lucida) and the Role of Lipid Peroxidation in Toxicity. Environ. Toxicol. Chem. 2000, 19, 2699–2706. [Google Scholar] [CrossRef]
- Lehmler, H.; Telu, S.; Vyas, S.; Shaikh, N.S.; Rankin, S.E.; Knutson, B.L.; Parkin, S. Synthesis and Solid State Structure of Fluorous Probe Molecules for Fluorous Separation Applications. Tetrahedron 2010, 66, 2561–2569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Evidence in Support of Potential Applications of Lipid Peroxidation Products in Cancer Treatment. Oxidative Med. Cell. Longev. 2013, 2013, 931251. [Google Scholar] [CrossRef] [PubMed]
- Tekin, U.; Kısa, Ü.; Güven, O.; Kurku, H. Malondialdehyde Levels in Dental Follicles of Asymptomatic Impacted Third Molars. J. Oral Maxillofac. Surg. 2011, 69, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Jeyashanthi, N.; Ravikumar, P.; Baalakumar, N.N. Effect of Glycated Hemoglobin Induced Lipid Peroxidation on Membrane Bound Acetyl Cholinesterase. SciExplore Int. J. Res. Sci. 2017, 4, 18. [Google Scholar] [CrossRef]
- Ahmed, O.H. The Cytotoxic Effect of Iraqi Rumex acetosella Against Breast and Esophagus Cancer Cells. Indian J. Forensic Med. Toxicol. 2021, 6, 929–940. [Google Scholar] [CrossRef]
- Batool, R.; Aziz, E.; Tan, B.K.H.; Mahmood, T. Rumex Dentatus Inhibits Cell Proliferation, Arrests Cell Cycle, and Induces Apoptosis in MDA-MB-231 Cells through Suppression of the NF-κB Pathway. Front. Pharmacol. 2017, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Ćebović, T.; Jakovljević, D.; Maksimović, Z.; Djordjevic, S.; Jakovljević, S.; Četojević-Simin, D. Antioxidant and Cytotoxic Activities of Curly Dock (Rumex crispus L., Polygonaceae) Fruit Extract. Vojn. Pregl. 2020, 77, 308–316. [Google Scholar] [CrossRef]
- Nasr, F.A.; Abutaha, N.; Al-Zahrani, M.; Farooq, M.; Wadaan, M.A. Anticancer Potential of Plant Extracts from Riyadh (Saudi Arabia) on MDA-MB-231 Breast Cancer Cells. Afr. J. Tradit. Complement. Altern. Med. 2018, 15, 46–53. [Google Scholar] [CrossRef][Green Version]
- Abou Elfotoh, M.A.; Shams, K.A.; Anthony, K.P.; Shahat, A.A.; Ibrahim, M.T.; Abdelhady, N.M.; Abdel Azim, N.S.; Hammouda, F.M.; El-Missiry, M.M.; Saleh, M.A. Lipophilic Constituents of Rumex vesicarius L. and Rumex dentatus L. Antioxidants 2013, 2, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Astuti, I.; Sofyannoor, G.M.; Oktriani, R.; Wardana, T.; Anwar, S.L.; Aryandono, T.; Haryana, S.M. MicroRNA-21 and PTEN Expression Levels Are Negatively Correlated in Doxorubicin Resistant McF-7 Breast Cancer Cell Line. Preprints 2019, 42, 281–290. [Google Scholar] [CrossRef]
- Aljabr, B.A.; Zihlif, M.; Abu-Dahab, R.; Zalloum, H. Effect of Quercetin on Doxorubicin Cytotoxicity in Sensitive and Resistant Human McF7 Breast Cancer Cell Lines. Biomed. Rep. 2024, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Desrini, S.; Mustofa, M.; Sholikhah, E.N. The Effect of Quercetin and Doxorubicin Combination in Inhibiting Resistance in McF-7 Cell. Bangladesh J. Med. Sci. 2017, 6, 91–97. [Google Scholar] [CrossRef][Green Version]
- Jang, H.; Baek, J.; Nam, K.; Kim, S.Y. Determination of the Optimal Time for Tamoxifen Treatment in Combination with Radiotherapy. Int. J. Oncol. 2016, 49, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, H.; Cheng, L.; Liu, R. The Oncolytic Herpes Simplex Virus Vector, G47Δ, Effectively Targets Tamoxifen-Resistant Breast Cancer Cells. Oncol. Rep. 2015, 5, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhang, C.; Chu, J.; Wu, Y.; Li, Y.; Liu, J.; Li, Q.; Li, S.; Shi, Q.; et al. Tamoxifen-Resistant Breast Cancer Cells Are Resistant to DNA-Damaging Chemotherapy Because of Upregulated BARD1 and BRCA1. Nat. Commun. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Mazarei, Z.; Sepehri, H.; Delphi, L.; McClements, D.J.; Rafati, H. Surface Modified Carvacrol-Rich Satureja Khuzestanica Essential Oil Nanoemulsion: A Novel Paclitaxel Formulation Induced Apoptosis on Paclitaxel-Resistant Breast Cancer Cells. Pharm. Sci. 2023, 30, 85–97. [Google Scholar] [CrossRef]
- Şekeroğlu, Z.A.; Şekeroğlu, V.; Aydın, B.; Yedier, S.K. Cerium Oxide Nanoparticles Exert Antitumor Effects and Enhance Paclitaxel Toxicity and Activity Against Breast Cancer Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 111, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Bal, Y.; Sürmeli, Y.; Şanlı-Mohamed, G. Antiproliferative and Apoptotic Effects of Olive Leaf Extract Microcapsules on MCF-7 and A549 Cancer Cells. ACS Omega 2023, 8, 28984–28993. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.T.X.; Saddi, V.A.; e Silva, K.S.F.; Sugita, D.M.; Guillo, L.A. Expression of the Cancer Stem Cell Marker OCT4 Is Associated with Worse Prognosis and Survival in Cutaneous Melanoma. Melanoma Res. 2021, 31, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.M.; Liu, S.; Lu, H.; Zhang, H.; Zhang, P.J.; Gimotty, P.A.; Xu, X. Acquired Cancer Stem Cell Phenotypes Through Oct4-Mediated Dedifferentiation. Oncogene 2012, 31, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Hai, Y.; Xi, Y.; Zhang, Y.; Liu, Z.; Chen, W.; Hu, X.; Zou, X.; Hao, J. Integrative Multi-Omics Analysis Unveils Stemness-Associated Molecular Subtypes in Prostate Cancer and Pan-Cancer: Prognostic and Therapeutic Significance. J. Transl. Med. 2023, 21, 789. [Google Scholar] [CrossRef] [PubMed]
- Bubin, R.; Uljanovs, R.; Štrumfa, I. Cancer Stem Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7030. [Google Scholar] [CrossRef] [PubMed]
- Alcantara Llaguno, S.R.; Parada, L.F. Cancer Stem Cells in Gliomas: Evolving Concepts and Therapeutic Implications. Curr. Opin. Neurol. 2021, 34, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.L.M.; Zhang, F.; Rao, R.; Adam, M.; Pollard, K.; Szabo, S.; Liu, X.; Belcher, K.A.; Luo, Z.; Ogurek, S.; et al. Single-Cell Multiomics Identifies Clinically Relevant Mesenchymal Stem-Like Cells and Key Regulators for MPNST Malignancy. Sci. Adv. 2022, 8, eabo5442. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-Associated MicroRNAs and Their Roles in Cancer Stemness and Drug Resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Chesnelong, C.; Hao, X.; Cseh, O.; Wang, A.Y.; Luchman, H.A.; Weiss, S. SLUG Directs the Precursor State of Human Brain Tumor Stem Cells. Cancers 2019, 11, 1635. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Tsai, J.T.; Chao, T.Y.; Ma, H.I.; Liu, W. The STAT3/Slug Axis Enhances Radiation-Induced Tumor Invasion and Cancer Stem-Like Properties in Radioresistant Glioblastoma. Cancers 2018, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Woosley, A.N.; Dalton, A.C.; Hussey, G.S.; Howley, B.V.; Mohanty, B.K.; Grelet, S.; Dincman, T.; Bloos, S.M.; Olsen, S.K.; Howe, P.H. TGFβ Promotes Breast Cancer Stem Cell Self-Renewal Through an ILEI/LIFR Signaling Axis. Oncogene 2019, 38, 3794–3811. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Zhang, M.; Sun, B.; Xu, C.; Yang, Y.; Zhang, Y.W.; Li, S.; Chen, G.; Chen, C.; Li, Y.; et al. LINC00115 Promotes Chemoresistant Breast Cancer Stem-Like Cell Stemness and Metastasis Through SETDB1/PLK3/HIF1α Signaling. Mol. Cancer 2024, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, J.; Tong, T.R.; Wu, X.; Nelson, R.A.; Yuan, Y.; Reno, T.; Liu, Z.; Yun, X.; Kim, J.Y.; et al. Loss of H2B Monoubiquitination Is Associated with Poor Differentiation and Enhanced Malignancy of Lung Adenocarcinoma. Int. J. Cancer 2017, 141, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Enhanced In Vitro Anti-Cancer Activity of Curcumin Encapsulated in Hydrophobically Modified Starch. Food Chem. 2010, 119, 669–674. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.H.; Kim, R.K.; Jung, U.; Kahm, Y.J.; Cho, E.; Kim, I. HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. Int. J. Mol. Sci. 2020, 21, 6957. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Long, Y.; Chong, T.; Cai, W.; Tsang, C.M.; Zhou, X.; Lin, Y.; Ding, T.; Zhou, W.; Zhao, H.; et al. EBV-miR-BART7-3p Imposes Stemness in Nasopharyngeal Carcinoma Cells by Suppressing SMAD7. Front. Genet. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Ooki, T.; Hayashi, T.; Yamamoto, Y.; Hatakeyama, M. YAP1-2α/TAZ Heterodimerization Drives Phase Separation, Activating TEAD-Positive Super-Enhancers That Enhance Cancer Stemness and Multidrug Resistance. BioRxiv 2025. [Google Scholar] [CrossRef]
- Shirakawa, Y.; Ohta, K.; Miyake, S.; Kanemaru, A.; Kuwano, A.; Yonemaru, K.; Uchino, S.; Yamaoka, M.; Ito, Y.; Ito, N.; et al. Glioma Cells Acquire Stem-Like Characters by Extrinsic Ribosome Stimuli. Cells 2021, 10, 2970. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Ali, N.M.; Akhtar, M.N.; Razak, N.A.; Chong, Z.X.; Ho, W.Y.; Boo, L.; Zareen, S.; Kurniawan, T.A.; Avtar, R.; et al. Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-Bis-(4-Hydroxy-3-Methoxybenzylidene)-Cyclohexanone (BHMC) Treatment on McF-7 Breast Cancer Cells. Molecules 2021, 26, 1277. [Google Scholar] [CrossRef] [PubMed]
- Razali, S.; Khan, A.F.; Khatib, A.; Ahmed, Q.U.; Hassan, H.; Murugesu, S.; Jalal, T.; Ichwan, M.; Selamat, J.; Khalifa, S.A.; et al. Evaluation of Anticancer Activity of Neolamarckia Cadamba Leaves and Its Metabolite Profile. Front. Pharmacol. 2021, 12, 741683. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, V.; Azad, N.; Yakisich, J.S.; Iyer, A.K. Antitumor Effects of Naturally Occurring Cardiac Glycosides Convallatoxin and Peruvoside on Human ER+ and Triple-Negative Breast Cancers. Cell Death Discov. 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Shi, A.; Fan, Z.; Du, Y. Salidroside Inhibits the Growth of Human Breast Cancer in Vitro and in Vivo. Oncol. Rep. 2015, 33, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Sarighieh, M.A.; Montazeri, V.; Shadboorestan, A.; Ghahremani, M.H.; Ostad, S.N. The Inhibitory Effect of Curcumin on Hypoxia Inducer Factors (HIFs) as a Regulatory Factor in the Growth of Tumor Cells in Breast Cancer Stem-Like Cells. Drug Res. 2020, 70, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Stan, S.D.; Zeng, Y.; Singh, S.V. Ayurvedic Medicine Constituent Withaferin A Causes G2 and M Phase Cell Cycle Arrest in Human Breast Cancer Cells. Nutr. Cancer 2008, 60, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Zaidi, D.; Shyam, H.; Sharma, R.; Balapure, A.K. Polyphenols Sensitization Potentiates Susceptibility of McF-7 and MDA MB-231 Cells to Centchroman. PLoS ONE 2012, 7, e37736. [Google Scholar] [CrossRef] [PubMed]
- Alazzam, S.; Sharqi, M.M.; Almehemdi, A.F. Phytochemical Analysis, Antioxidant and Cytotoxic Potential of Rumex vesicarius Extracts. Med. Leg. Update 2020, 20, 449–454. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Y.; Wang, W.; Ma, M.; Li, M.; Chen, S. Effect of the Serum Inhibited Gene (Si1) on Autophagy and Apoptosis in McF-7 Breast Cancer Cells. Cell. Physiol. Biochem. 2017, 41, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Gharibvand, N.; Setayeshmehr, M.; Kazemi, M.; Safaee, A.; Khorsandi, L.S.; Nejad, D.B.; Hasheminia, S.J.; Hashemibeni, B. Pomegranate Seed Extract Enhances the Inhibitory Effect of Adipose-Derived Mesenchymal Stem Cells on Breast Cancer Cell Line in Co-Culture Conditions. Res. Pharm. Sci. 2022, 17, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.A.; Mahdavi, M.; Afsartala, Z.; Amani, L.; Semsarha, F. The Influence of Faradarmani Consciousness Field on the Survival and Death of McF-7 Breast Cancer Cells: An Optimization Perspective. J. Cosmointel. 2022, 1, 8–21. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, X.J.; Zhang, H.; Lu, Y.Y.; Su, S. Curcumin Enhanced Antiproliferative Effect of Mitomycin C in Human Breast Cancer McF-7 Cells in Vitro and in Vivo. Acta Pharmacol. Sin. 2011, 32, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Laka, K.; Makgoo, L.; Mbita, Z. Survivin Splice Variants in Arsenic Trioxide (As2O3)-Induced Deactivation of PI3K and MAPK Cell Signaling Pathways in MCF-7 Cells. Genes 2019, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Lin, W.C.; Ho, I.L.; Chang, H.C.; Lee, P.Y.; Chung, Y.T.; Hsieh, J.T.; Pu, Y.S.; Shi, C.S.; Huang, K.H. 2-Methoxyestradiol Induces Mitotic Arrest, Apoptosis, and Synergistic Cytotoxicity with Arsenic Trioxide in Human Urothelial Carcinoma Cells. PLoS ONE 2013, 8, e68703. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Sharma, D.; Sharma, R.; Tuli, H.S.; Haque, S.; Ramniwas, S.; Yadav, V. The Role of Phytonutrient Kaempferol in the Prevention of Gastrointestinal Cancers: Recent Trends and Future Perspectives. Cancers 2024, 16, 1711. [Google Scholar] [CrossRef] [PubMed]
- ul Islam, B.; Suhail, M.; Khan, M.S.; Ahmad, A.; Zughaibi, T.A.; Husain, F.M.; Tabrez, S. Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr. Med. Chem. 2021, 28, 8083–8097. [Google Scholar] [CrossRef] [PubMed]
- Peña-Jorquera, H.; Cid-Jofré, V.; Landaeta-Díaz, L.; Petermann-Rocha, F.; Martorell, M.; Zbinden-Foncea, H.; Cristi-Montero, C. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome—A Comprehensive Review. Nutrients 2023, 15, 3244. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Das, C.; Kityania, S.; Nath, D.; Das, S.; Choudhury, M.D.; Talukdar, A.D. Natural Flavonoids in the Prevention and Treatment of Lung Cancer: A Pharmacological Aspect. Comb. Chem. High Throughput Screen. 2023, 26, 863–879. [Google Scholar] [CrossRef]
- Liu, F.; Peng, Y.; Qiao, Y.; Huang, Y.; Song, F.; Zhang, M. Consumption of Flavonoids and Risk of Hormone-Related Cancers: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. J. 2022, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, F.; Zhang, Y.; Chang, W.; Zhou, M. The Effects and Mechanisms of Flavonoids on Cancer Prevention and Therapy: Focus on Gut Microbiota. Int. J. Biol. Sci. 2022, 18, 1451. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Huang, L. Structural Insights and Biological Activities of Flavonoids: Implications for Novel Applications. Food Front. 2025, 6, 218–247. [Google Scholar] [CrossRef]
- Islam, R.; Mamat, Y.; Ismayil, I.; Yan, M.; Kadir, M.; Abdugheny, A.; Rapkat, H.; Niyaz, M.; Ali, Y.; Abay, S. Toxicity of Anthraquinones: Differential Effects of Rumex Seed Extracts on Rat Organ Weights and Biochemical and Haematological Parameters. Phytother. Res. 2015, 29, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, M.M.; Bouajila, J.; Rahmani, R.; Alouani, K. Phytochemical Composition, Antioxidant, Antiacetylcholinesterase, and Cytotoxic Activities of Rumex crispus L. Int. J. Anal. Chem. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Čižmáriková, M.; Takáč, P.; Spengler, G.; Kincses, A.; Nové, M.; Vilková, M.; Mojžíš, J. New Chalcone Derivative Inhibits ABCB1 in Multidrug Resistant T-Cell Lymphoma and Colon Adenocarcinoma Cells. Anticancer Res. 2019, 39, 6499–6505. [Google Scholar] [CrossRef] [PubMed]




| Variation Source | DF | Sum of Squares | F Ratio | p-Value |
|---|---|---|---|---|
| Solvent | 2 | 471.71 | 21.95 | 7.55 × 10−9 |
| Time | 1 | 67,143.11 | 6247.31 | 2.4 × 10−105 |
| Concentration | 9 | 2481.76 | 115.46 | 1.48 × 10−88 |
| Solvent × Time | 2 | 44,472.31 | 459.77 | 1.09 × 10−28 |
| Solvent × Concentration | 18 | 5444.79 | 28.14 | 1.19 × 10−34 |
| Time × Concentration | 9 | 965.34 | 9.98 | 2.5 × 10−11 |
| Solvent × Time × Concentration | 18 | 9495.71 | 49.08 | 1.24 × 10−46 |
| Source | 48 h | 96 h |
|---|---|---|
| Hexane | 46.22 ± 6.85 a | 3.93 ± 0.03 c |
| Ethyl acetate | 29.74 ± 5.58 b | 10.43 ± 2.74 b |
| Methanol | 42.19 ± 8.4 a | 20.61 ± 5.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gülüm, L.; Güler, E.; Aktaş, F.L.; Çelik, A.B.; Yılmaz, H.; Tutar, Y. In Vitro Effects of Rumex confertus Extracts on Cell Viability and Molecular Pathways in MCF-7 Breast Cancer Cells. Antioxidants 2025, 14, 879. https://doi.org/10.3390/antiox14070879
Gülüm L, Güler E, Aktaş FL, Çelik AB, Yılmaz H, Tutar Y. In Vitro Effects of Rumex confertus Extracts on Cell Viability and Molecular Pathways in MCF-7 Breast Cancer Cells. Antioxidants. 2025; 14(7):879. https://doi.org/10.3390/antiox14070879
Chicago/Turabian StyleGülüm, Levent, Emrah Güler, Fatma Lale Aktaş, Ayşe Büşranur Çelik, Hilal Yılmaz, and Yusuf Tutar. 2025. "In Vitro Effects of Rumex confertus Extracts on Cell Viability and Molecular Pathways in MCF-7 Breast Cancer Cells" Antioxidants 14, no. 7: 879. https://doi.org/10.3390/antiox14070879
APA StyleGülüm, L., Güler, E., Aktaş, F. L., Çelik, A. B., Yılmaz, H., & Tutar, Y. (2025). In Vitro Effects of Rumex confertus Extracts on Cell Viability and Molecular Pathways in MCF-7 Breast Cancer Cells. Antioxidants, 14(7), 879. https://doi.org/10.3390/antiox14070879






