Abstract
Herpes simplex virus type 1 (HSV-1) is a widespread pathogen responsible for recurrent infections, primarily affecting the skin and mucous membranes. With the aim of targeting both the viral infection and the associated inflammatory response, biotechnologically produced Lavandula angustifolia Miller (L. angustifolia) extract, rich in rosmarinic acid, was incorporated into liposomal formulations intended for topical application. Lavender is known for its strong anti-inflammatory, antioxidant, wound-healing, and antiviral properties. However, its low stability under certain conditions limits its therapeutic potential. Four different formulations were developed: conventional liposomes, glycerosomes, hyalurosomes, and glycerohyalurosomes. The vesicles were characterized for size, stability, and entrapment efficiency. Glycerosomes were the smallest (~58 nm), while the other formulations ranged around 77 nm, all maintaining a highly negative surface charge, ensuring stability and reduced aggregation. Glycerol-containing formulations demonstrated superior stability over 12 months, while liposomes and hyalurosomes increased their size after only two months. Entrapment efficiency reached up to 100% for most vesicles, except for glycerohyalurosomes (~54%). In vitro studies on Normal Human Dermal Fibroblasts (NHDFs) demonstrated that all formulations were biocompatible and enhanced cell viability under oxidative stress. Glycerosomes, hyalurosomes, and glycerohyalurosomes exhibited significant anti-inflammatory activity by reducing MMP-1 and IL-6 levels in LPS-stimulated fibroblasts. Furthermore, these preliminary results highlighted promising antiviral activity against HSV-1 of the obtained formulations, particularly when applied during or post-infection. Overall, these phospholipid vesicles offer a dual therapeutic approach, combining antioxidant, anti-inflammatory, and antiviral effects, positioning them as promising candidates for the treatment of HSV-induced skin lesions and related inflammatory conditions.
1. Introduction
The Herpes simplex virus (HSV), an enveloped double-stranded DNA virus and part of the Herpesviridae family, has afflicted humanity for thousands of years, leaving an indelible mark on human health, infecting its hosts with long-lasting life effects [1]. The two types, HSV-1 and HSV-2, are mainly responsible for human infections, each of them with distinct epidemiological and clinical characteristics [2]. The prevalence of HSV infections is alarming, with around 67% of the population under the age of 50 (3.7 billion) infected by HSV-1, which is mostly associated with oral lesions [3]. On the other hand, it is estimated that almost 520 million people aged 15–49 (around 13%) are infected by HSV-2, the main cause of genital herpes, contributing to a substantial burden of sexually transmitted infections [2,4]. Following primary infection at the entry site, the viral lytic replication results in a cytopathic effect that leads the host cell to death, jeopardizing the tissue integrity. The main clinical manifestation of HSV infections is the formation of painful blisters and ulcers, especially in mucous membranes [5]. The virus can spread through direct contact with wounds or infected body fluids [6]. These infections are not limited to a specific anatomical site, because both viruses can infect several tissues and organs, leading to oral, ocular, neurological, and systemic diseases [7].
Additionally, HSV remaining latent within the host cells causes persistent infections for the entire life of the host, and it can evade the immune system [8]. Stress, illness, UV light exposure, and other factors may cause the viral reactivation from latency, often resulting in recurrent episodes of disease [9]. HSV has also been associated with a range of complications, like neonatal herpes, encephalitis, and increased susceptibility to other sexually transmitted infections [8,10,11].
Despite being among the most prevalent viral infections worldwide, HSV infections still represent a significant challenge to medical science and public health. To date, there is no approved cure for HSV infection, and the available treatments are limited to the symptoms [12]. Approved antiviral medications (e.g., acyclovir, ganciclovir) primarily reduce viral replication and symptom duration but do not eliminate the virus or prevent latency reactivation [12]. Moreover, drug resistance and systemic toxicity further limit their long-term efficacy [13,14]. Consequently, there is a pressing need for novel topical agents that can address both viral activity and tissue damage.
In recent years, the research has provided unprecedented insights into the biology of HSV, sparking renewed efforts to uncover novel strategies for prevention and treatment [15].
Thanks to their low side effects and consolidated uses over the years in traditional medicine, natural products and extracts have garnered increasing attention for their therapeutic applications [16]. Lavandula angustifolia (L. angustifolia), commonly known as lavender, has been extensively studied due to its rich phytochemical profile and associated biological activities. Lavender is rich in terpenes, flavonoids, and phenolic acids [17]. Several studies have demonstrated the strong antioxidant activity of lavender extracts or essential oils, largely attributed to high concentrations of phenolic compounds, such as rosmarinic acid, isoferulic acid, luteolin, and caffeic acid derivatives [17,18,19]. Moreover, growing evidence suggests that lavender extracts also exhibit antiviral activity, particularly against enveloped viruses like HSV-1 [20,21,22]. Among these bioactive compounds, rosmarinic acid stands out due to its well-documented antioxidant and anti-inflammatory effects [23,24].
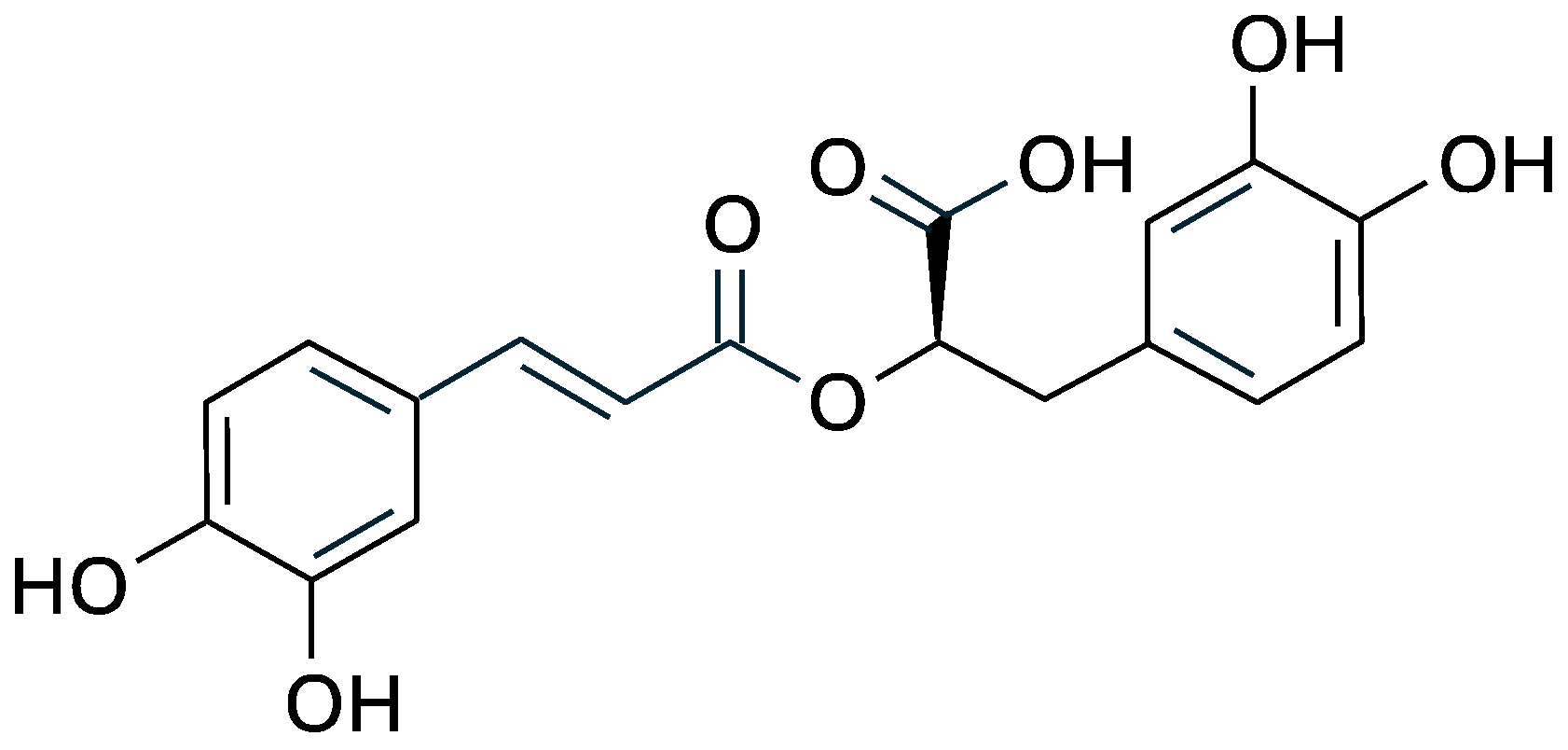
Chemically, rosmarinic acid is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, and its chemical structure includes two catechol groups (Figure 1) that confer high antioxidant capacity by donating hydrogen atoms to free radicals and chelating metal ions involved in oxidative reactions [25,26].
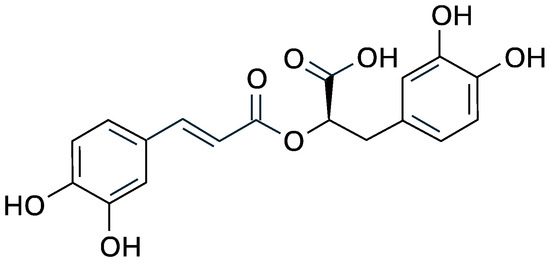
Figure 1.
Structural formula of rosmarinic acid.
Because of these properties, rosmarinic acid has been widely studied for its therapeutic potential against several diseases, such as psoriasis, arthritis, atherosclerosis, and herpes infections [27,28,29].
Recent advancements in biotechnology have enabled the production of high yield extracts from L. angustifolia cell suspensions, offering an alternative to the traditional extraction methods [30]. These innovative approaches allow for the selective enrichment of specific bioactive molecules, particularly rosmarinic acid. For instance, Koycheva et al. reported a rosmarinic content of approximately 100.16 mg/g in an extract obtained from cell cultures, as confirmed by HPLC and NMR analyses [29]. This is notably higher than the concentrations typically found in flower extracts of L. angustifolia, where rosmarinic acid concentrations are reported in the range of 829.68–1229.33 µg/g. This highlights the potential of biotechnological approaches to obtain enriched extracts with significantly higher concentrations of target phenolic compounds. Taken together, these bioactivities, along with the extract tolerance profile, make L. angustifolia a promising candidate for topical delivery in the treatment of viral and inflammatory skin conditions.
However, despite its significant therapeutic potential, L. angustifolia extracts, rich in rosmarinic acid and many other phenolic compounds, face challenges commonly associated with natural products, such as high chemical instability, poor permeability, and, consequently, low bioavailability, which limit its efficacy in both cosmetic and pharmaceutical applications [31,32,33,34].
Over the years, several methods based on nanotechnology have been used to overcome these limitations. Among them, liposomes and liposome-like vesicles have demonstrated the ability to protect sensitive compounds from degradation, enhance their solubility, and facilitate targeted delivery to specific tissues [35,36,37]. Notably, liposome-like vesicles modified with glycerol or sodium hyaluronate (called glycerosomes and hyalurosomes, respectively) are able to enhance skin penetration and dermal delivery of various bioactive compounds [38,39,40,41].
Considering these previous findings, with the aim of targeting both the viral infection and the associated inflammatory response, a biotechnologically produced Lavandula angustifolia Miller extract, rich in rosmarinic acid, was incorporated into four types of lipid-based vesicles. Conventional liposomes were used as a control, while glycerosomes, hyalurosomes, and glycerohyalurosomes were designed to evaluate the effects of glycerol and/or sodium hyaluronate on vesicle properties and efficacy.
The obtained vesicles were characterized in terms of size, surface charge, entrapment efficiency, and stability over time. Their biocompatibility and ability to protect cells from oxidative damage and inflammatory responses were assessed using Normal Human Dermal Fibroblasts (NHDFs). Finally, the antiviral and virucidal effects of L. angustifolia extract alone or loaded in the aforementioned vesicles were evaluated against the cytopathic effect related to the HSV-1 infection.
2. Materials and Methods
2.1. Materials
The Lipoid S 75 (S75) was purchased from Lipoid AG (Cologne, Germany) with the support of its Italian agent AVG srl (Milan, Italy). Methanol, ethanol, and all other products with analytical grade were purchased from Sigma-Aldrich (Milan, Italy). Medium, fetal bovine serum, penicillin, streptomycin, and the other reagents and plastics, unless otherwise specified, used for in vitro cell culture studies were supplied by Thermo Fisher Scientific Inc. (Waltham, MA, USA). Water was purified through a Milli-Q system from Millipore (conductivity: 18.2 MΩ cm at 25 ± 1 °C; Milford, MA, USA).
2.2. Preparation and Chemical Characterization of Lavandula angustifolia Miller Extract
Lavandula angustifolia Miller extract was biotechnologically produced as previously reported [30]. Briefly, L. angustifolia cell suspension was incubated at 26 ± 1 °C, using Linsmayer and Skoog (LS) as the growth medium, supplemented with sucrose (30 g/L) and 2,4-dichlorophenoxyacetic acid (0.2 mg/L), in the dark and under constant agitation (100 rpm). The biomass was then collected, dried, and treated with a mixture of water and ethanol (50:50 v/v) in an ultrasonic bath for 20 min at room temperature (25 ± 1 °C) to ensure complete extraction of the targeted compounds. The resulting extractive solution was filtered, concentrated under vacuum at 40 ± 1 °C, lyophilized, and stored at −20 ± 1 °C prior to use. The dry extract powder was analyzed by means of high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) imaging as previously described [29].
2.3. Antioxidant Activity of Lavandula angustifolia Miller Extract
The antioxidant activity of the L. angustifolia extract was measured as a function of its ability to scavenge l-2,2-diphenyl-1-picrylhydrazyl (DPPH), a stable free radical characterized by an intense purple-red color, which turns yellow when scavenged by an antioxidant molecule. The methanolic solution of the extract (10 µL) was diluted (1:100) with a methanolic solution of DPPH (40 µg/mL), the diluted samples were kept at room temperature and in the dark for 30 min, then the absorbance of the solution was measured at 517 nm, using a microplate reader (Synergy 4, BioTek Instruments, AHSI S.p.A, Bernareggio, Italy). The antioxidant power of the samples was calculated according to the formula below:
2.4. Preparation of Vesicles
The composition of the vesicular formulations was selected based on previous studies reporting their efficiency in delivering phytochemicals and ensuring nanosystem stability [42,43]. Four different formulations were prepared (Table 1), each of them containing 20 mg/mL of L. angustifolia extract and 120 mg/mL of S75, differing in the dispersing medium used. Liposomes were obtained using water, glycerosomes using a mixture of glycerol and water (50:50 v/v), hyalurosomes using a sodium hyaluronate solution at 0.2% w/v, and glycerohyalurosomes using a mixture of glycerol and sodium hyaluronate solution at 0.4% w/v (50:50 v/v). The obtained dispersions were immediately sonicated (13 µm, 20 cycles, 5 s on and 2 s off) using a Soniprep 150 ultrasonic disintegrator (MSE Crowley, London, UK) to obtain small and homogeneous vesicles. Each formulation was prepared in triplicate and independently repeated in at least three separate batches.

Table 1.
Composition of the Lavandula angustifolia Miller extract loaded vesicles.
2.5. Characterization of Vesicles
Freshly prepared samples were diluted 1:100 in water and analyzed using a Zetasizer Ultra (Malvern Instrument, Worcestershire, UK) at 25 ± 1 °C to determine the mean diameter and polydispersity index (PI) through the dynamic light-scattering method. Zeta potential was measured using the M3-PALS (Mixed Mode Measurement-Phase Analysis Light Scattering) technique, which measures the electrophoretic mobility of particles.
Unentrapped phytochemicals were removed by means of dialysis. Briefly, 1 mL of each sample was placed into a Spectra/Por® dialysis tube (12–14 kDa MW cut-off, 3 nm pore size; Spectrum Laboratories Inc., Breda, The Netherlands) and purified for 2 h at room temperature under constant stirring in 2 L of water, which was refreshed after 1 h. The entrapment efficiency (EE%), i.e., the percentage of bioactives that are successfully entrapped into vesicles, was calculated as the percentage of the concentration of phytochemicals after dialysis versus the concentration before dialysis. The concentration of phytochemicals was measured as a function of the antioxidant activity of the dispersions with the DPPH assay, as described in Section 2.2.
Samples were stored at 4 ± 1 °C for 12 months. To evaluate their stability over time, each month mean diameter, polydispersity index, and zeta potential were measured.
2.6. Cell Viability and Protection Against Oxidative Stress
Normal Human Dermal Fibroblasts (NHDFs) were isolated from skin fragments. The skin specimens were obtained from healthy tissue taken from plastic surgery patients from Faculty Hospital Olomouc after informed consent. The study was performed according to the Code of Ethics of the World Medical Association (ref. number 41/09). These cells were chosen to respect the future potential of selected substances for wound healing. NHDFs were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and antibiotics (penicillin (100 mg/mL) and streptomycin (100 U/mL)), at 37 °C with 100% humidity and 5% CO2. The medium was changed every 72 h. The NHDFs between 2–3 passages were used for the experiments [44].
NHDFs were seeded in 96 well plates at the final density of 1 × 104 cells/mL. The following day, the tested samples (L. angustifolia extract dispersion, liposomes, glycerosomes, hyalurosomes, and glycerohyalurosomes), in a concentration range from 0.04 µg/mL to 40 µg/mL in serum-free DMEM, were applied to the cells for 24 h. After incubation period, cell viability was assessed using the MTT assay, which measured the mitochondrial reduction of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan at 540 nm.
The in vitro protective effect of the formulations against cell damages caused by oxidative stress was evaluated as well. The NHDFs were seeded into 96-well plates at a density of 1 × 104 cells/mL. After 24 h of incubation, the cells were stressed with hydrogen peroxide (250 µM) and simultaneously treated for 1 h with sub-toxic concentration (40 µg/mL) of L. angustifolia extract, in dispersion or loaded into vesicles, diluted in serum-free DMEM medium. Untreated NHDFs were used as control (100% viability). NHDFs untreated and stressed with hydrogen peroxide (250 µM; for 1 h) were used as the positive control. After incubation, the NHDFs were washed with phosphate buffered saline (PBS, pH 7.4), and their viability was determined by the MTT assay. The results are expressed as the percentage of control (100% viability). NHDFs were carefully washed with PBS after both the treatment phase and the incubation with MTT to ensure the complete removal of residual formulation components.
2.7. Scratch Assay Infected by Lipopolysaccharide
For scratch assay, the NHDFs were seeded in 6-well plates at a cell density of 0.5 × 105 cells/mL and cultivated until reaching confluence. When the NHDFs reached the confluence, the lipopolysaccharide (LPS, Pseudomonas aeruginosa, final concentration 10 µg/mL) was added to half of the plates for 6 h to stimulate the pathological inflammatory response [45]. Next, all plates with NHDFs (included plates with LPS) were scratched by using a 5 mL pipette to produce a wounded area. The culture medium was removed and tested samples (L. angustifolia extract water solution or formulations) were applied (in a concentration range from 4 µg/mL to 40 µg/mL) in serum-free DMEM for 24 h. At the end of the incubation period, the cell culture medium was collected and stored at −80 ± 1 °C. The following three controls were used: (1) NHDFs untreated and not scratched as negative control (C); (2) NHDFs untreated and scratched as positive control (CS); (3) NHDFs untreated and scratched that were stressed with LPS (CL). Levels of interleukin-6 (IL-6), matrix metalloproteinase-1 (MMP-1) and matrix metalloproteinase-2 (MMP-2) in the cell supernatant after the treatment were examined using ELISA Human IL-6 Kit (900-K16), Human Total MMP-1 DuoSet ELISA (DY901B-05) and Human MMP-2 Duo-Set ELISA (DY902) (R&D Systems, Minneapolis, MI, USA) according to the manufacturer’s protocol.
2.8. Antiviral Activity Tests
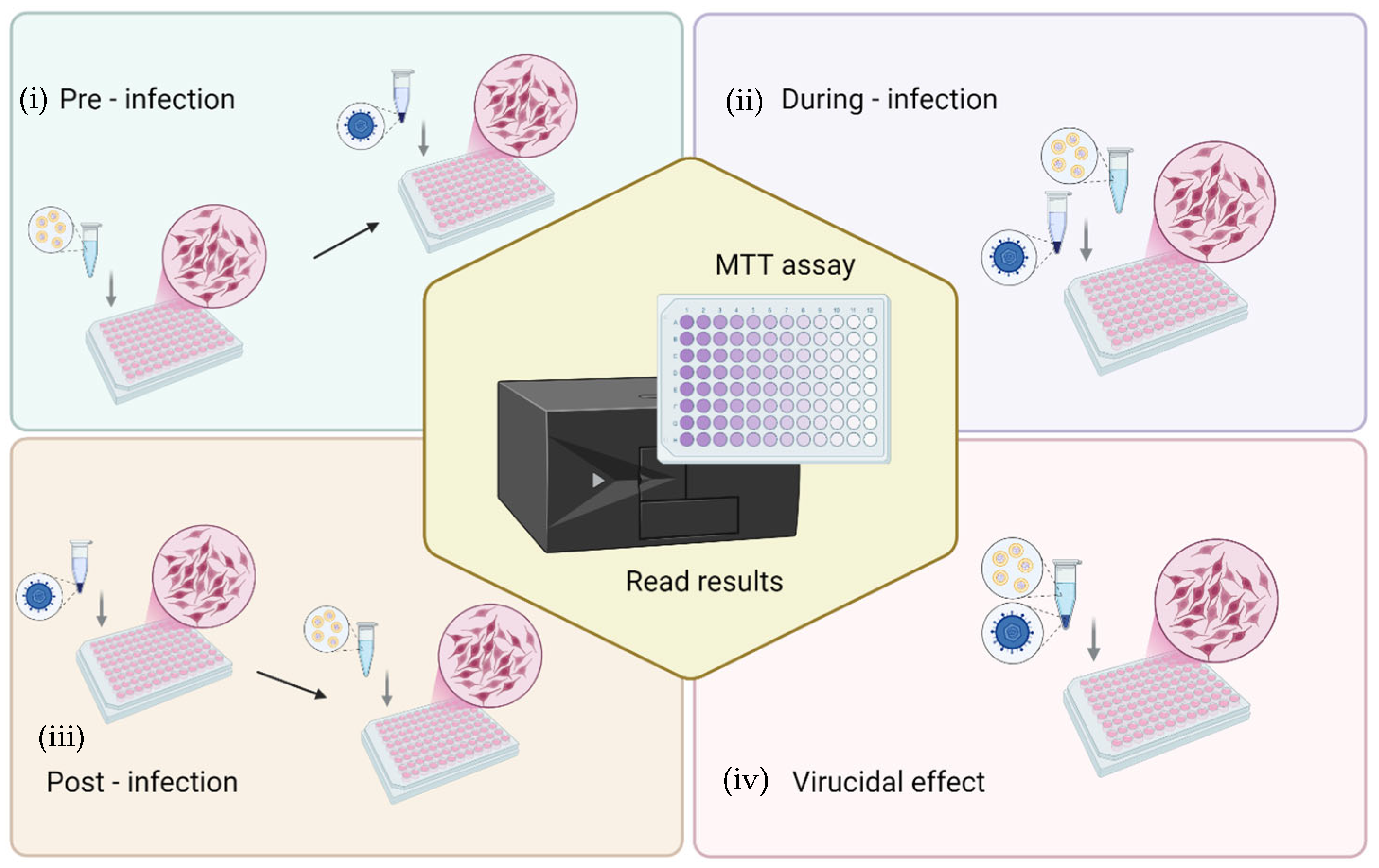
The antiviral activity of formulations was evaluated by the cytopathic effect assay, which is based on infecting cell cultures and measuring the ability to protect cells from virus-induced cytopathic effects by cell viability. In brief, African green monkey kidney (Vero) cells (ATCC collection, Manassas, VA, USA) were grown in DMEM (Corning Inc., Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and incubated at 37 °C with 100% humidity and 5% CO2. Cells were seeded in 96-well plates at a density of 3.0 × 104 cells/well and, after 24 h of incubation, were processed for antiviral activity testing against HSV-1 strain KOS (ATCC VR-1493). Four different challenging experiments were performed to investigate the step of the viral cycle in which the formulations exerted action, as follows: (i) pre-infection: the cell monolayer was first treated with formulations for 3 h, then washed and viral suspension added for the viral absorption time; (ii) during-infection: formulations and virus at multiplicity of infection (MOI) of 0.01, were added simultaneously to the cell monolayer; (iii) post-infection: the cell monolayer was first infected with the virus for 1 h and then the cells were washed and treated with formulations; (iv) finally, to verify the virucidal effect, the viral suspension was incubated with the formulations for 1 h at room temperature; thereafter, this suspension was added to the cells for 1 h. The experimental setups used to assess the protective effect of formulations against HSV-induced cytopathic effects are shown in Figure 2. In each experiment, after the viral adsorption, the cells were washed with PBS, and fresh culture medium was added. After 72 h incubation, the cells were washed with PBS, and 450 μM MTT was added to each well. After 2–3 h, cells were washed with PBS and the formazan crystals were dissolved in DMSO, and the optical density was measured at 570 nm with a microplate reader (Tecan Infinite 200, Männedorf, Switzerland) [46,47]. All the experiments were repeated at least three times and in duplicate. The results are presented as the percentage of protection from viral cytotoxic effect as calculated by comparing the viability observed in treated samples vs. the infected control cells and/or mock infected untreated cells. Moreover, to discriminate the formulation concentration-associated cytotoxicity from the infection-related cytopathic effect, the cytotoxicity of the formulations on Vero cells and the percent of cell viability of uninfected treated cells vs. untreated cells were also evaluated. Finally, the half-maximal effective concentration (EC50) of the formulations was inferred by curve-fitting interpolation and compared to the reference compound (acyclovir).
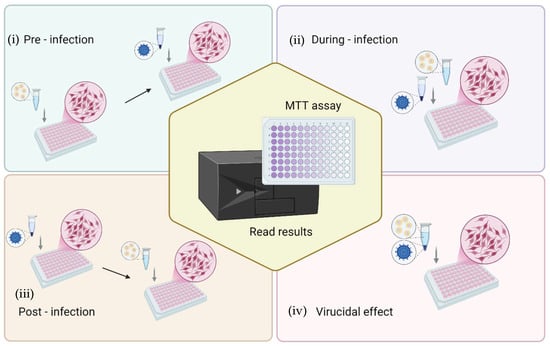
Figure 2.
Schematic representation of the experimental setups for evaluating the protective effect of L. angustifolia formulations against the cytopathic effect of HSV-1 in Vero cells. Four experimental conditions were tested: (i) pre-infection (cells treated with formulations prior to viral exposure), (ii) during-infection (formulations and virus added simultaneously), (iii) post-infection (cells treated with formulations after viral infection), and (iv) virucidal effect (virus pre-incubated with formulations before cell infection). After treatment and infection, cell viability was determined by the MTT assay to quantify protection against virus-induced cytopathic effects.
2.9. Statistical Analysis
Results are expressed as the means ± standard deviations. Multiple comparisons of means (ANOVA) were used to substantiate statistical differences between groups, while Student’s t-test was used to compare two samples. Significance was tested at the 0.05 level of probability (p). Data analysis was carried out with the software GraphPad Prism v10 (GraphPad Software, San Diego, CA, USA) for macOS.
3. Results
3.1. Extract Characterization
The biotechnological extract was obtained from L. angustifolia cell suspension culture capable of producing rosmarinic acid [30]. The high amount of rosmarinic acid ((R)-a-[[3-(3,4-dihydroxyphenyl)-1-oxo-2E-propenyl]oxy]-3,4-dihydroxy-benzenepropanoic acid) in the dry extract powder was previously confirmed by means of HPLC and NMR, with HPLC analysis revealing a content of ~100.16 mg/g extract in the cell suspension [29].
The antioxidant activity of L. angustifolia extract at a final concentration of 200 µg/mL, (~20 µg/mL of rosmarinic acid), quantified with the DPPH assay, was ~80%.
3.2. Vesicle Preparation and Characterization and Stability
The physico-chemical properties (mean diameter, polydispersity index, and zeta potential) of vesicular systems enriched with glycerol, sodium hyaluronate, or with the combination of both additives, were studied and compared with those of conventional liposomes used as reference (Table 2).

Table 2.
Mean diameter (MD), polydispersity index (PI) and zeta potential (ZP) of Lavandula angustifolia Miller extract loaded vesicles. Each value represents the mean ± standard deviation of at least three replicates and independently repeated in at least three separate batches. The same letter (a, b, and c) indicates values not statistically different from each other (p > 0.05) and different from other values (p < 0.05).
The particle size of liposomes, hyalurosomes, and glycerohyalurosomes was ~77 nm in diameter. The use of glycerol alone as a co-solvent has led to smaller vesicles (~58 nm). The addition of additives has influenced the system in terms of homogeneity, as liposomes had the lowest polydispersity index (0.16), which increased up to 0.27 when both glycerol and sodium hyaluronate were used. Surface charge was highly negative in all vesicles, especially in the case of glycerosomes, hyalurosomes, and glycerohyalurosomes. In all cases, the vesicles were suitable for topical administration.
The entrapment efficiency (EE), calculated as the percentage of antioxidant activity of vesicle dispersions measured before and after removal of unentrapped phytochemicals, is remarkably high for liposomes, glycerosomes, and hyalurosomes, consistently equal to or greater than 94%. In the case of glycerohyalurosomes, the combination of glycerol and sodium hyaluronate ensures the formation of a viscous and structured system capable of retaining lower but substantial quantities of bioactive compounds contained in the extract, such as rosmarinic acid (EE = ~54%).
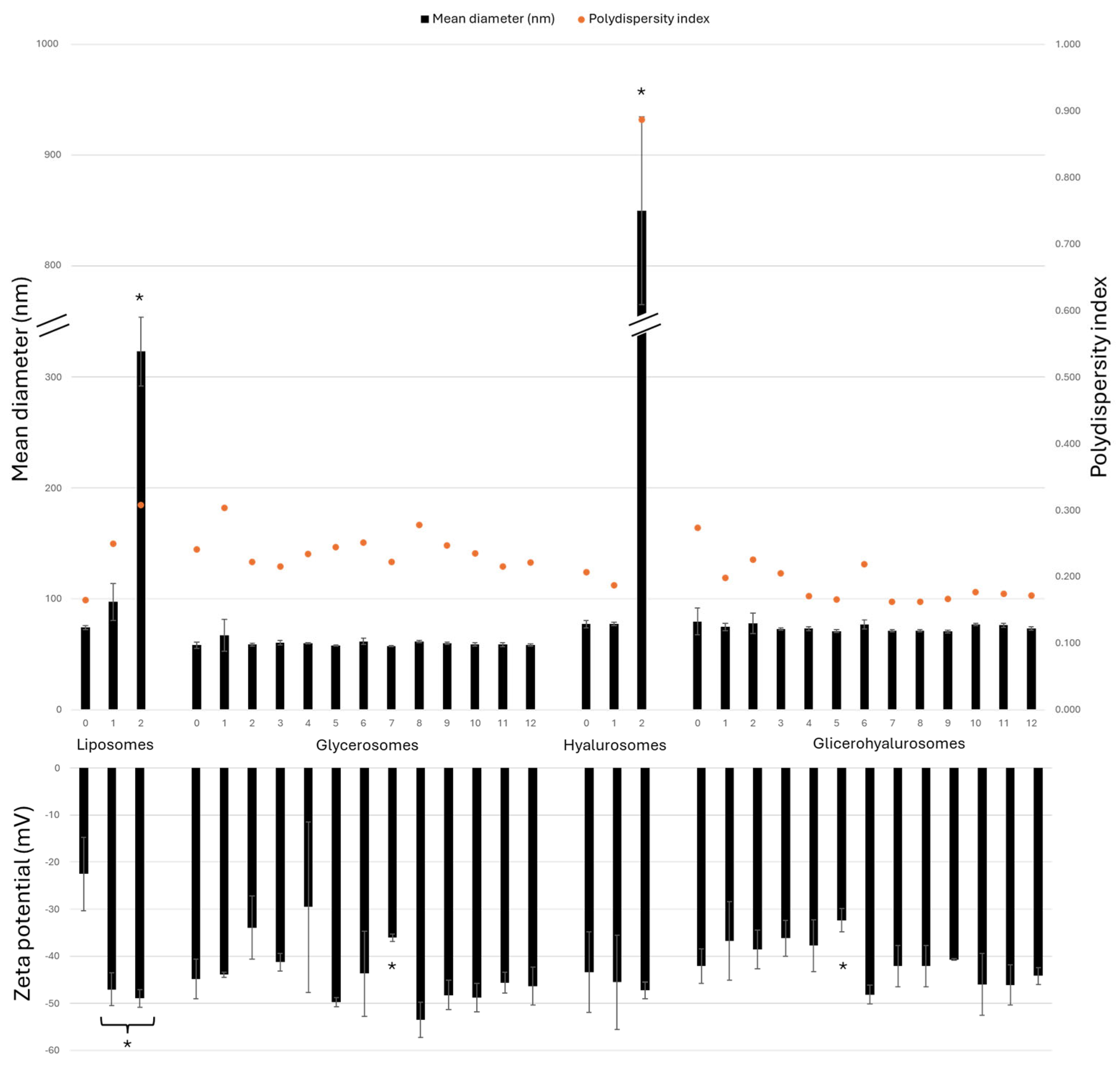
To confirm the stability of the vesicular formulations prepared during the study, they were stored at 4 ± 1 °C for a period of 12 months and evaluated for their mean diameter, polydispersity index, and zeta potential each month (Figure 3). Conventional liposomes, as well as hyalurosomes, experience a significant increase in size after 2 months, as the sizes are 4 times and 11 times larger, respectively, compared to the sizes of the corresponding freshly prepared formulations. As shown in Figure 2, glycerosomes and glycerohyalurosomes displayed remarkable stability, as their sizes did not statistically change throughout the storage period (~60 and ~74 nm, respectively).
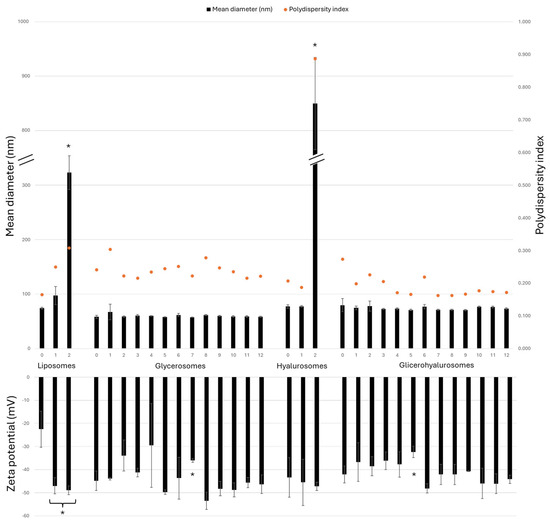
Figure 3.
Mean diameter (nm), polydispersity index, and zeta potential (mV) values of Lavandula angustifolia Miller extract loaded vesicles stored for 12 months at 4 ± 1 °C. Data are reported as mean values ± standard deviations (error bars) (n = 3). * p < 0.05 were considered significant compared to corresponding freshly prepared formulations (0 months).
On the other hand, the zeta potential remains highly negative for all tested formulations, with no big variations observed during the 12-month storage.
3.3. Cell Viability and Protection Against Oxidative Stress
The cytotoxicity of formulations on primary NHDFs was evaluated using the MTT test (Figure 3). The MTT test is a well-established, reliable, and widely accepted method for evaluating cell viability in response to liposomal formulations. Roggia et al. evaluated the possible interference between liposomes and MTT. They found that the spectra for liposomes were similar to the controls, indicating that there is no interference, thus showing reliability of the results obtained through this assay [48].
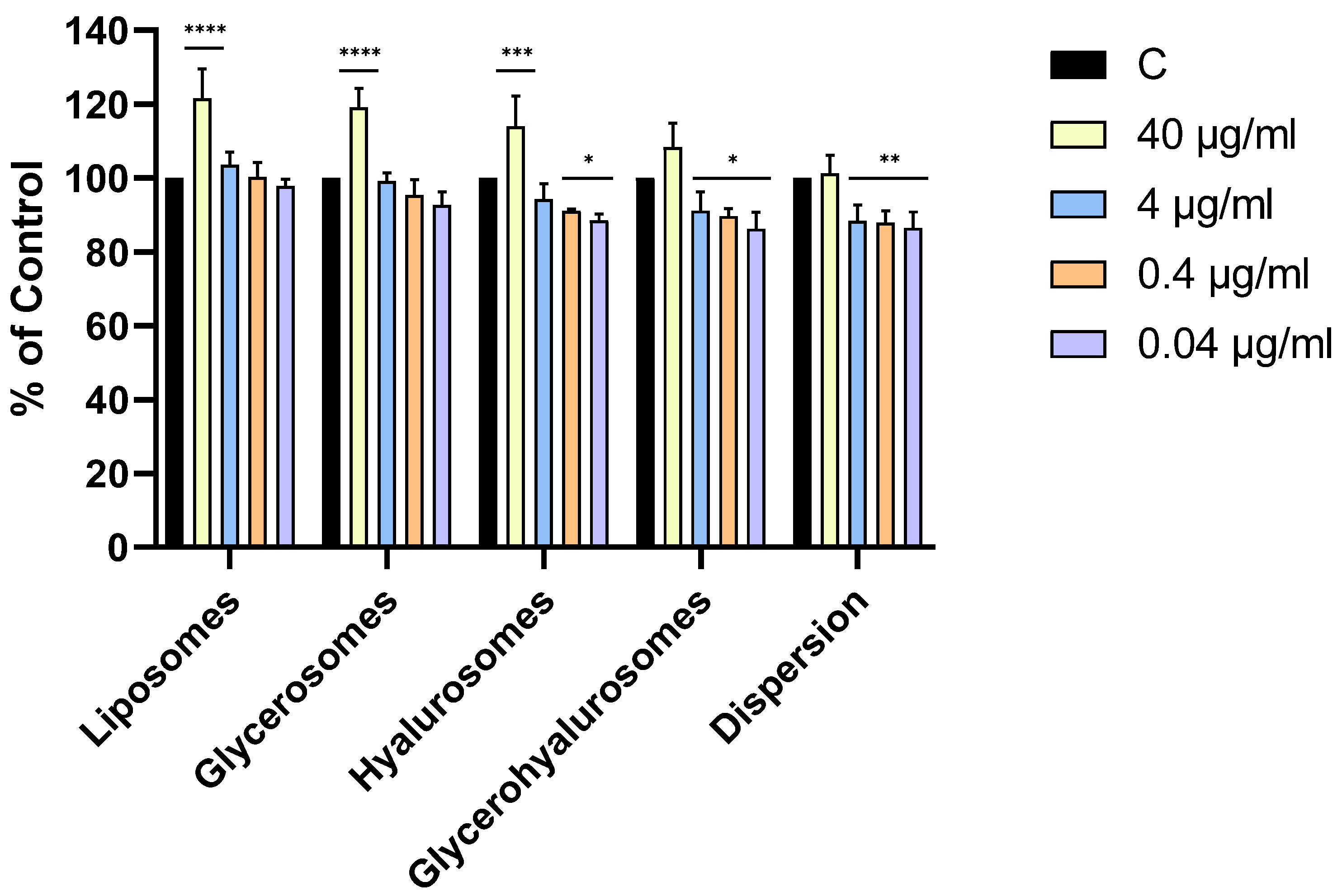
All the formulations were highly biocompatible, irrespective of the concentration. Cell viability was always higher than 70%, therefore the compounds are not cytotoxic to NHDFs according to ISO 10993-5:2009. Moreover, the treatment with liposomes, glycerosomes, and hyalurosomes at the highest concentration of L. angustifolia Miller extract (40 µg/mL) exerted a proliferative effect as the viability of primary NHDFs was ~120%.
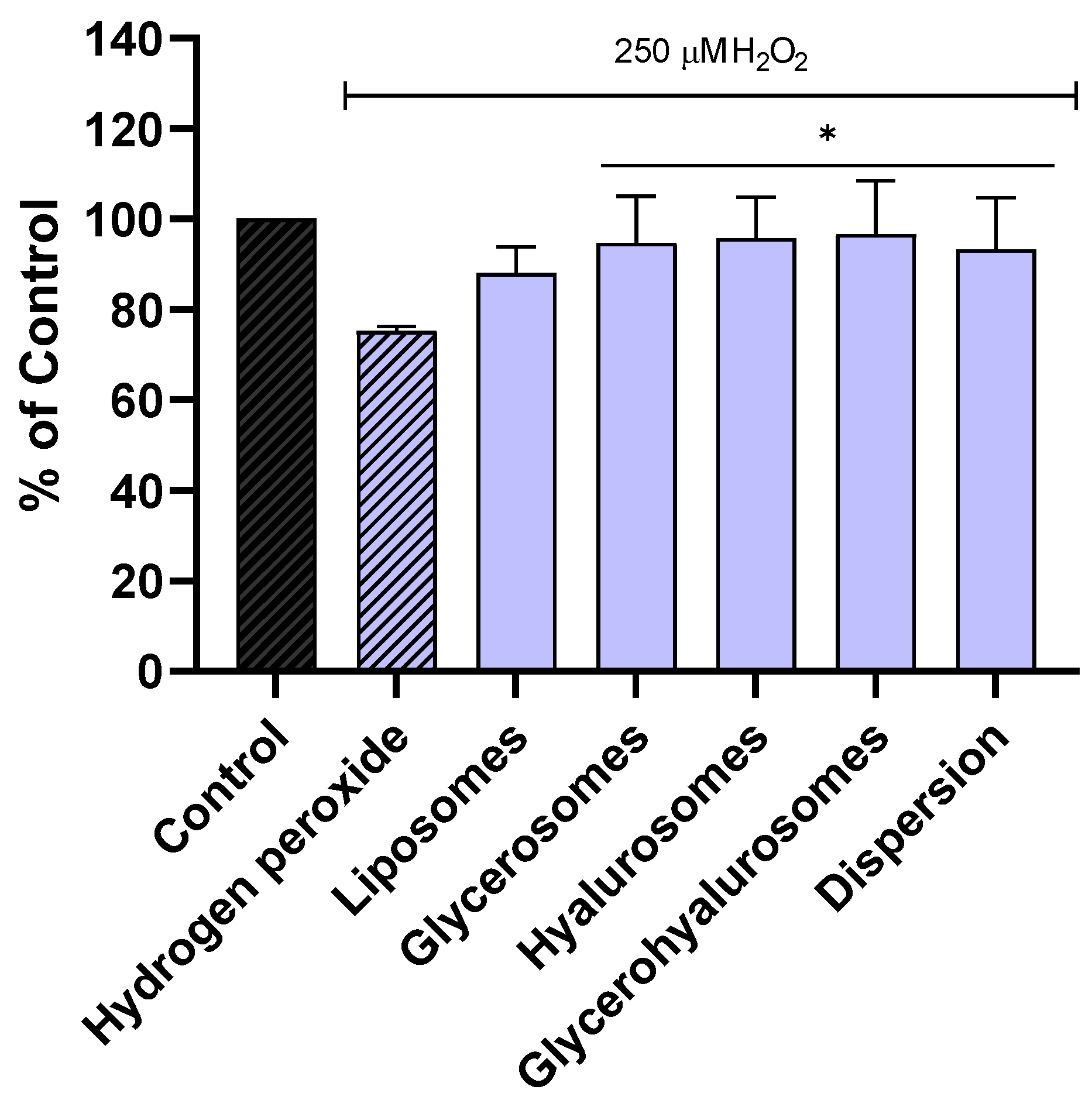
The ability of the formulations to protect NHDF from oxidative stress caused by hydrogen peroxide was evaluated as well (Figure 4). The viability of NHDF not treated, stressed with hydrogen peroxide was ~70%. On the contrary, when the cells were exposed to hydrogen peroxide and treated with formulations loading Lavandula angustifolia Miller extract, the viability was significantly increased, reaching almost 100%. These results confirmed the ability of the obtained vesicles to counteract the formation of peroxide radicals, thus avoiding cell damage and death.
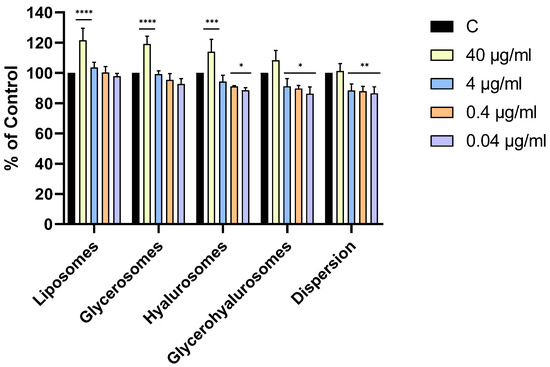
Figure 4.
Viability of NHDF treated with L. angustifolia extract in dispersion or loaded in liposomes, glycerosomes, hyalurosomes, and glycerohyalurosomes. Untreated NHDFs were used as a control (C). Results are expressed as a percentage of control viability and SD. Number of measurements: n = 3. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 were considered significant compared to control (C).
3.4. Scratch Assay: Determination of MMP-1, MMP-2 and IL-6
Matrix metalloproteinases (MMPs) and cytokines (ILs) are involved in the regulation of the healing process and play an important role in immune response and tissue repair.
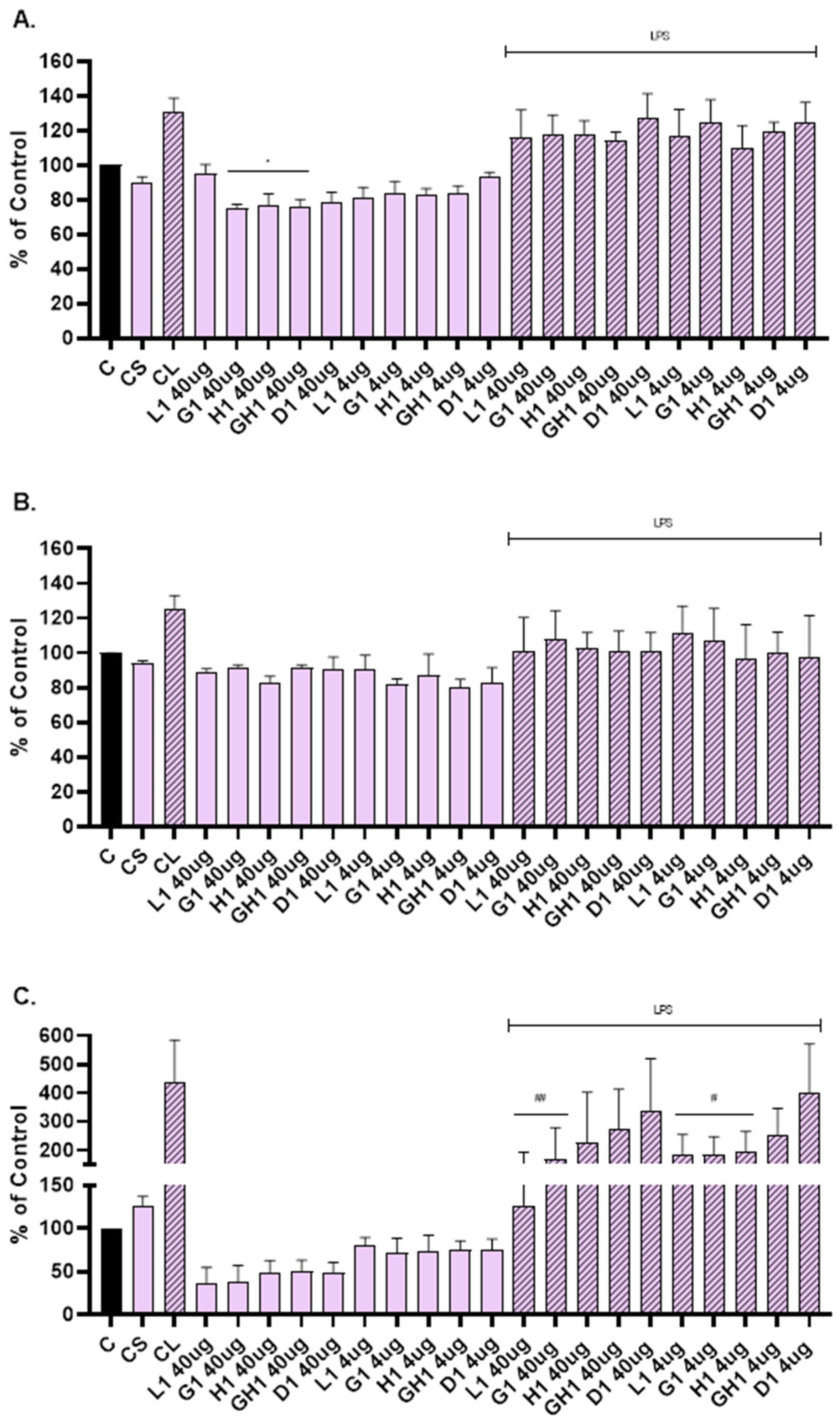
ELISA was used to determine levels of the MMP-1, MMP-2, and IL-6 released into the cultivation medium after scratch assay and treatment with extract loaded vesicles. Based on the MTT test results, two concentrations were selected: 4 µg/mL and 40 µg/mL (Figure 5).
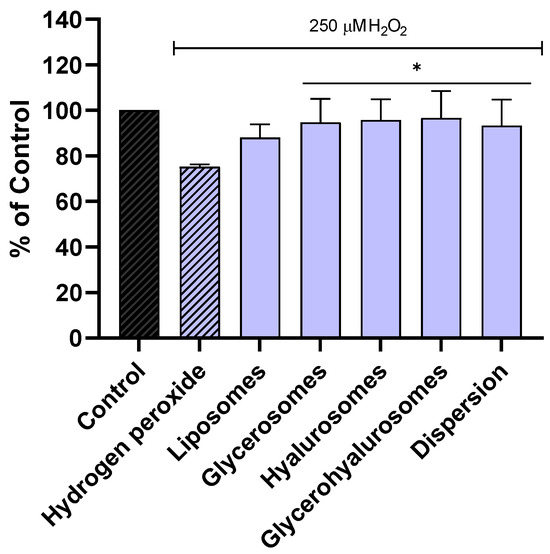
Figure 5.
Viability of NHDFs stressed with hydrogen peroxide and treated with L. angustifolia extract in dispersion or loaded in liposomes, glycerosomes, hyalurosomes, and glycerohyalurosomes. Untreated NHDFs were used as a control (C). Results are expressed as a percentage of control viability and SD. Number of measurements: n = 4. * p < 0.05 were considered significant compared to hydrogen peroxide.
Vesicles were able to suppress the level of MMP-1 (Figure 6A), MMP-2 (Figure 6B), and IL-6 (Figure 6C) compared to the cells that were not scratched and treated (C). The level of MMP-1 was significantly reduced when NHDFs were treated with glycerosomes, hyalurosomes, and glycerohyalurosomes at a concentration of 40 µg/mL (Figure 6A). Liposomes, glycerosomes, and hyalurosomes also significantly reduced the level of pro-inflammatory IL-6 compared to LPS-stimulated control (CL), (Figure 6C).
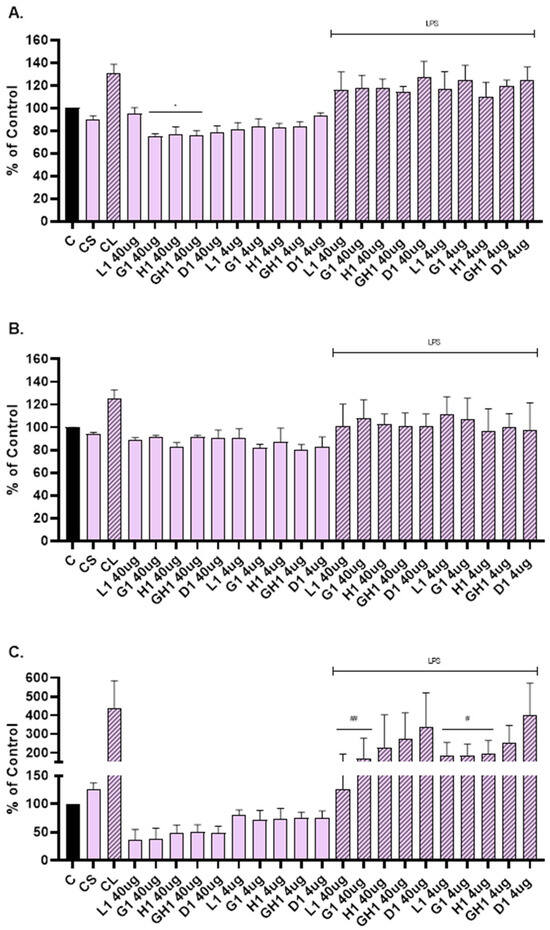
Figure 6.
Effect of L. angustifolia extract loaded liposomes (L1), glycerosomes (G1), hyalurosomes (H1), and glycerohyalurosomes (GH1) on MMP-1 (A), MMP-2 (B), and IL-6 (C) production by primary scratch NHDF. The control (C) was cells that were not scratched and treated. The other controls were untreated scratched cells (CS) and untreated scratched cells with LPS (CL). Number of measurements: n = 3. The symbol * indicates statistical significance with p < 0.05 vs. cells that were not scratched and treated (C). The symbol # indicates statistical significance with p < 0.05 vs. untreated scratched cells with LPS (CL). The symbol ## indicates statistical significance with p < 0.01 vs. untreated scratched cells with LPS (CL).
3.5. Cytotoxicity on Vero Cells
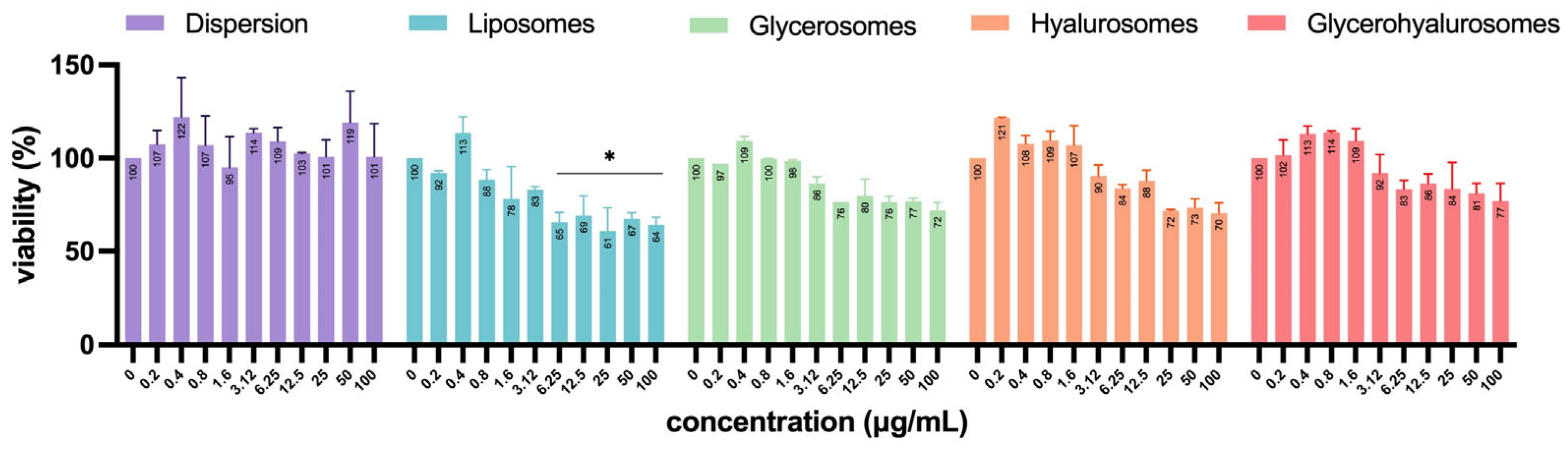
Vero cells are a well-established model for studying viral infections and are commonly used in antiviral and virucidal assays due to their high susceptibility to various viruses, including HSV-1 [49,50,51]. To exclude any formulation-associated toxic effects that might confound interpretation of infection-related cytopathic outcomes, the cytotoxicity of formulations at different concentrations (0–100 µg/mL) was also evaluated on Vero cells (Figure 7). Almost all formulations did not have significant cytotoxicity, as the percentage of cell viability was not significantly different from untreated control (100%). Despite a progressive decrease in viability with increasing concentration being observed for all the tested formulations, only the treatment with high concentrations of L. angustifolia extract loaded liposomes (from 6.25 up to 100 µg/mL) determined a significant cytotoxic effect, as Vero cells’ viability decreased up to ~35%.
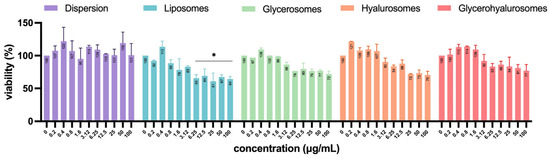
Figure 7.
Viability of Vero cells treated with L. angustifolia extract in dispersion or loaded in liposomes, glycerosomes, hyalurosomes, and glycerohyalurosomes. The symbol * indicates statistical significance with p < 0.05 vs. untreated infected control (0 µg/mL) as calculated by two-way ANOVA and Bonferroni’s post-hoc test.
3.6. Antiviral Activity Against HSV-1
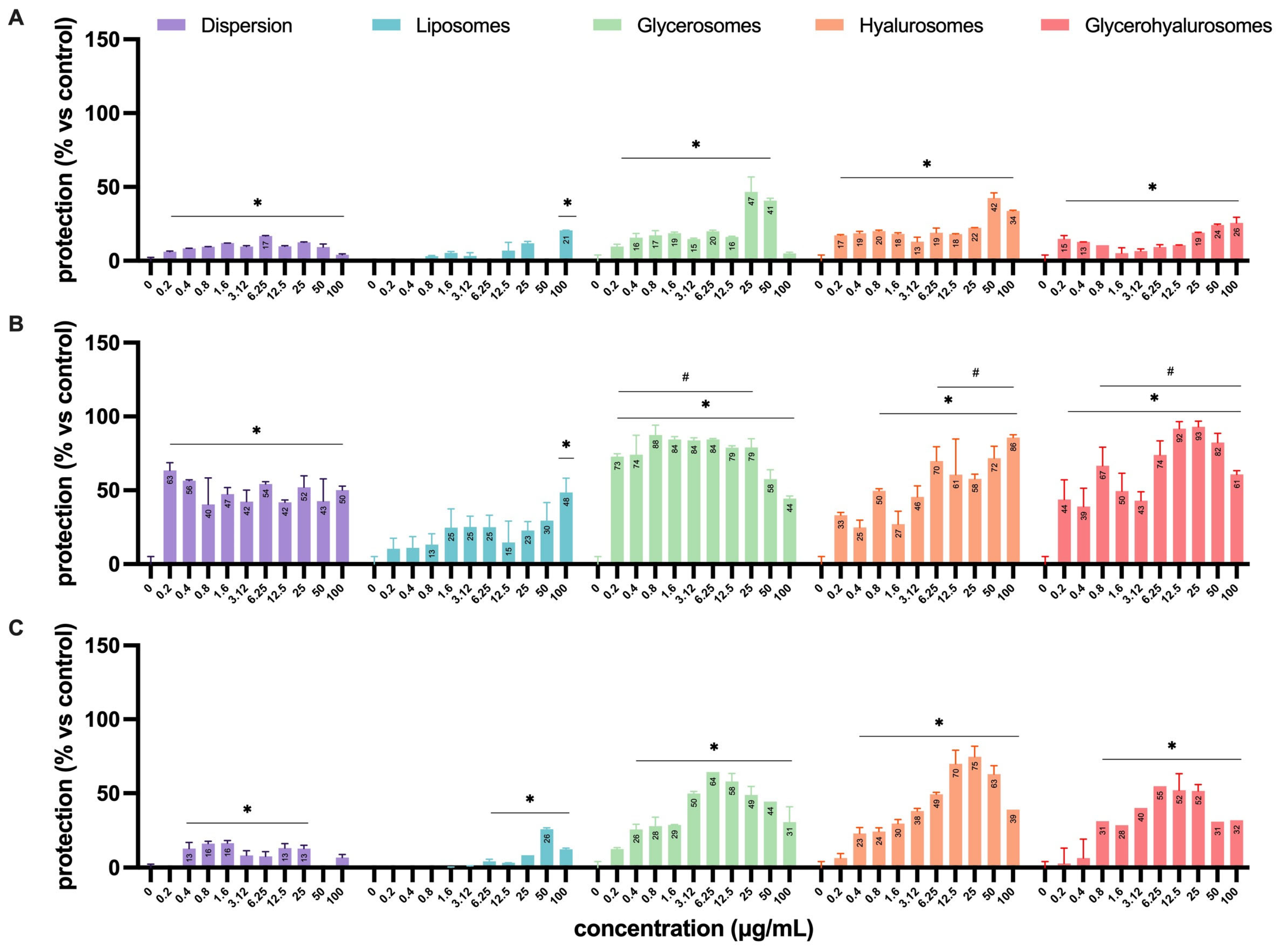
The antiviral activity was assessed by means of four different experimental configurations (Figure 2) that allowed us to define in which step of the virus life cycle the formulations exert their antiviral effect (Figure 8). The experiments performed by treating Vero cells with the formulations before the infection (Figure 8A) demonstrated a lower capacity to protect the cell monolayer from the cytopathic action of HSV-1 when compared to the simultaneous treatment during the infection (Figure 8B) or the treatment after the infection (Figure 8C). In particular, when cells were treated before the infection, the dispersion had a significant protective effect on cells from a concentration of 0.4 up to 50 µg/mL, with a percentage of protection of ~17%.
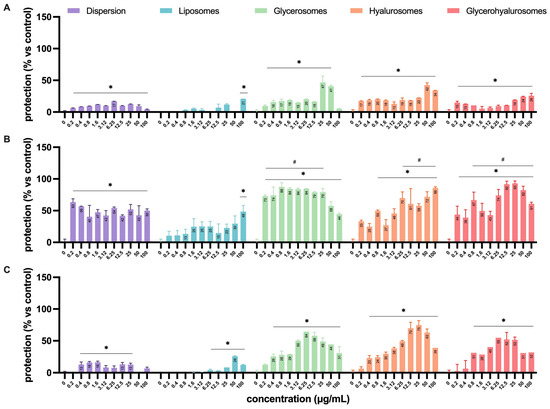
Figure 8.
Antiviral activity against HSV-1 of L. angustifolia extract in dispersion or loaded into formulations at different concentrations and in the different experimental setups: (A) pre-infection, (B) during-infection, and (C) post-infection formulation treatments. The symbol * indicates statistical significance with p < 0.05 vs. untreated and infected control (0 µg/mL). The symbol # indicates statistical non-significance vs. untreated and uninfected control (100%), as calculated by two-way ANOVA and Bonferroni’s post-hoc test.
When L. angustifolia extract was loaded into liposomes, it was able to exert a significant protective effect only at the highest concentration (100 µg/mL), with a percentage of protection that reached ~20%. On the contrary, the treatment with glycerosomes, hyalurosomes, or glycerohyalurosomes determined a protective effect irrespective of the concentration, with a percentage of protection ranging from ~17% to ~50% (Figure 8A).
When Vero cells were treated with formulations simultaneously with the infection (during infection), the L. angustifolia extract in dispersion, or loaded into glycerosomes and glycerohyalurosomes, exerted a significant protection against the cytopathic effect induced by HSV-1, irrespective of the concentration, with a percentage of protection close to 100%. Similarly, hyalurosomes were able to protect the Vero cells from HSV-1 at a concentration range of 0.8 up to 100 µg/mL, also reaching a percentage of protection of ~100%. In contrast, extract-loaded liposomes were able to exert a protective effect only at the highest concentration, 100 µg/mL (Figure 8B).
Finally, when cells were treated with the formulations after the infection, the L. angustifolia extract in dispersion exerted a significant protective effect at concentrations ranging from 0.4 to 25 µg/mL. The treatment with extract-loaded liposomes determined a significant protection in the range of concentrations from 6.25 to 100 µg/mL, with a percentage of protection of ~30% at 50 µg/mL. On the contrary, the post-infection treatment with L. angustifolia extract loaded glycerosomes or hyalurosomes exerted a significant protective effect at all tested concentrations, with the only exception being 0.2 µg/mL. If compared to the two previous formulations, glycerohyalurosomes exerted a slightly lower protective activity, at concentrations ranging from 0.8 to 100 µg/mL. All three formulations reached a percentage of protection of ~80%. For these last three formulations, it is interesting to note how some concentrations, even the lowest for glycerosomes (0.2 µg/mL), were so effective as to reach a protection comparable to 100% (Figure 6).
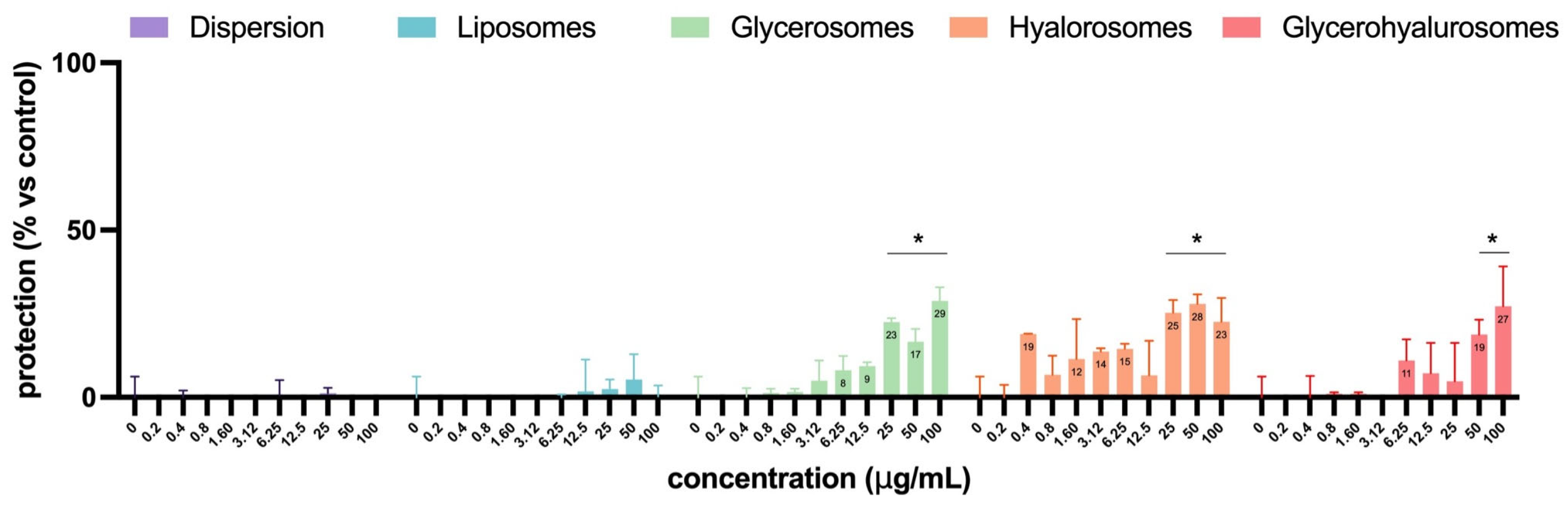
In Figure 9, the virucidal effect of L. angustifolia extract in dispersion or loaded into formulations is reported. The extract in dispersion or loaded into liposomes did not have any virucidal activity. Instead, glycerosomes, hyalurosomes, and glycerohyalurosomes were able to exert virucidal activity at higher concentrations, from 25 up to 100 µg/mL for glycerosomes and hyalurosomes and 50 and 100 µg/mL for glycerohyalurosomes (Figure 9). These results suggest that the antiviral activity observed is mainly attributable to substances activated following cellular metabolism rather than to their direct action on HSV-1.
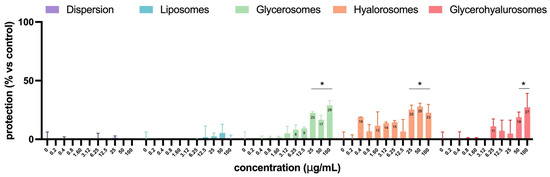
Figure 9.
Virucidal effect of L. angustifolia extract in dispersion or loaded into formulations at different concentrations. The symbol * indicates statistical significance with p < 0.05 vs. untreated and infected control (0 µg/mL), as calculated by two-way ANOVA and Bonferroni’s post-hoc test.
The antiviral efficacy of L. angustifolia extract, in dispersion or loaded into formulations, against HSV-1 infection was compared to acyclovir by measuring EC50 values in pre-infection, during infection, and post-infection conditions (Table 3). During infection, both the dispersion and glycerosomes exhibited the highest antiviral activity with EC50 values below 0.20 µg/mL, significantly outperforming acyclovir (9.80 µg/mL). Glycerohyalurosomes and hyalurosomes also demonstrated strong antiviral effects during infection, with EC50 values of 0.59 and 1.79 µg/mL, respectively. Pre-infection treatment showed moderate prophylactic efficacy for glycerosomes (23.82 µg/mL) and hyalurosomes (53.76 µg/mL), whereas acyclovir was ineffective. Post-infection, glycerosomes, hyalurosomes, and glycerohyalurosomes maintained antiviral activity with higher EC50, ranging from 3.53 to 5.76 µg/mL, if compared to acyclovir (1.91 µg/mL). Liposomes were the least effective formulation across all conditions.

Table 3.
Antiviral efficacy of L. angustifolia extract in dispersion or loaded into formulations against HSV-1 infection in comparison to acyclovir. Data are reported as the half-maximal effective concentration (EC50, µg/mL), and the symbol - indicates data not applicable.
4. Discussion
In this study, a biotechnologically produced L. angustifolia extract, rich in rosmarinic acid, has been loaded into phospholipid vesicles, specifically tailored for topical administration. The extract exhibited ~80% antioxidant activity, measured with DPPH assay at a final concentration of 200 µg/mL, corresponding to approximately 20 µg/mL of rosmarinic acid based on its quantified content (100.16 mg/g).
Similar results were observed by Aldoghachi et al. for pure rosmarinic acid. The authors observed that the antioxidant activity of pure rosmarinic acid, at a concentration of 25 µg/mL, was ~89% [52]. Moreover, the overall antioxidant capacity of the extract (88 µmol TE/g) falls in the lower range compared to values reported in the literature for similar extracts (104.58–206.77 µmol TE/g) [53]. These differences may be attributed to variations in extraction method, plant source, and the relative abundance of other phenolics or synergistic compounds. Despite this, the extract retained a consistent and measurable antioxidant effect, supporting its potential as a reliable component in functional formulations.
Four different formulations were obtained: conventional liposomes that were used as control; glycerosomes, characterized by a high glycerol content (10 to 50%) and a more flexible phospholipid membrane that improves penetration and transport of bioactives into the deeper layers of the skin [54,55]; hyalurosomes, innovative phospholipid vesicles modified with sodium hyaluronate, which improves skin permeation by promoting the therapeutic activity of the incorporated active ingredients [43,56]; and finally, glycerohyalurosomes, characterized by the combination of both glycerol and sodium hyaluronate, and capable of further enhancing the effect of the active ingredients incorporated at the skin level [57,58].
The first phase of this study was focused on the characterization of the vesicular systems by evaluating the possible influence of composition on the properties of the vesicles and their suitability for topical administration in terms of size, stability over time, and ability to incorporate high amounts of L. angustifolia extract.
Glycerosomes were the smallest vesicles (~58 nm) with a homogeneously monodisperse system (0.24), indicating that glycerol ensures good packing of phospholipid molecules, thus increasing curvature, resulting in smaller vesicles. This observation is consistent with previous findings by Manca et al., who reported that glycerosomes loaded with diclofenac had smaller diameters compared to conventional liposomes, with improved deformability and skin penetration properties [54]. Liposomes, hyalurosomes, and glycerohyalurosomes were slightly larger in size (~77 nm), with polydispersity index values consistently below 0.3 (0.16, 0.21, and 0.27, respectively), indicating narrow size distribution and good homogeneity, an essential characteristic for reproducible dermal application. Furthermore, with the exception of liposomes (~−23 mV), all the vesicles were characterized by a strongly negative surface charge of ~−43 mV. A surface charge of ±30 mV is generally associated with stable nanodispersions due to sufficient electrostatic repulsion, thus reducing the phenomena of vesicle aggregation and fusion [59,60]. These parameters confirm the physico-chemical stability of the prepared systems and their suitability for topical delivery.
It appears that glycerol has played a significant role in maintaining the stability of vesicles, as the formulations containing it (glycerosomes and glycerohyalurosomes) have proven to be the most stable over time, with their sizes remaining unchanged over a period of 12 months. This might be due to the ability of glycerol to increase the viscosity of the solution, thus exerting a positive effect on vesicles’ stability over time [54]. In contrast, for liposomes and hyalurosomes, it was only possible to measure their sizes up to 2 months. Similar results were reported by other authors [54,58,61]. For example, Manca et al. observed that glycerosomes were highly stable during the considered storage period, irrespective of the concentration of glycerol (10, 20, and 30%), while conventional liposomes underwent a significant increase of the mean diameter, which almost doubled, from ~81 nm at the beginning to ~180 nm after 1 month of storage [54]. Regarding hyalurosomes, Casula et al. reported the same stability issues, as after three months of storage the authors reported that the main physico-chemical characteristics of the obtained Zingiber officinalis extract loaded hyalurosomes become undetectable [61].
Regarding the entrapment efficiency, when both glycerol and sodium hyaluronate were used, the system was able to load lower amount of payload (EE = ~54%) when compared to liposomes, glycerosomes, and glycerohyalurosomes, which were able to load up to 100% of payload.
The in vitro evaluation of toxicity and efficacy represent one of the major factors to be considered when characterizing new molecules or delivery systems [62,63]. Dermal fibroblasts, one of the main cell types of the dermis, have proven to be one of the major players involved in the wound healing process [64]. For this reason, in this work, the biocompatibility of the obtained vesicular systems and their ability to counteract hydrogen peroxide-induced oxidative stress were evaluated in vitro, using Normal Human Dermal Fibroblasts (NHDFs). All formulations of L. angustifolia extract in the concentration range of 0.04–40 µg/mL were sub-toxic for primary NHDFs. Moreover, the concentration of 40 µg/mL of extract loaded into liposomes, glycerosomes, and hyalurosomes significantly increased the viability of primary NHDFs, up to ~120% (p < 0.05 versus control). Results agree with those previously reported by other authors for the biocompatibility of natural compounds loaded vesicles modified with glycerol and/or hyaluronic acid [57,58]. Furthermore, glycerosomes, hyalurosomes, and glycerohyalurosomes exhibited remarkable protective effects when NHDFs were stressed with hydrogen peroxide. These results demonstrated that the obtained formulations did not alter the ability of rosmarinic acid to inhibit both hydrogen peroxide oxidative damages and hydrogen peroxide-induced inflammatory response in NHDFs [65].
Both lavender extracts, particularly essential oils, and rosmarinic acid have proven to be able to exert strong anti-inflammatory and wound-healing effect both in vitro and in vitro [66,67,68,69,70]. Considering this, the ability of the L. angustifolia extract, in dispersion or loaded into the vesicles, to inhibit the production of inflammation mediators was evaluated in vitro on fibroblasts stressed with lipopolysaccharide (LPS), a toxic substance capable of triggering the cascade reactions characteristic of the inflammatory process [71]. Glycerosomes, hyalurosomes, and glycerohyalurosomes at a concentration of 40 µg/mL were the most effective in reducing the MMP-1 level. The level of IL-6 was most reduced by liposomes and glycerosomes (40 µg/mL), which significantly reduced IL-6 levels in LPS-stimulated NHDFs.
All formulations were tested at subtoxic concentrations (0.04–40 µg/mL), within which no pro-inflammatory or pro-oxidant effects were observed. These results are in line with previous findings reporting the anti-inflammatory activity of L. angustifolia extracts in different cell models, including macrophages and monocytes [72,73]. However, bell-shaped dose–response relationships have been described for various plant-derived polyphenols, including rosmarinic acid, where low concentrations exert protective effects, while higher doses may lead to paradoxical pro-oxidant or cytotoxic responses [74,75,76]. Moreover, studies have shown that the anti-inflammatory potency of L. angustifolia may vary depending on the flowering stage and phytochemical composition [72]. These considerations highlight the importance of dose optimization and extract standardization in the development of safe and effective topical formulations.
The involvement of matrix metalloproteinases (MMPs) in wound healing is well documented, as MMPs play a crucial role in extracellular matrix regulation, both for the inflammatory phase and tissue remodeling [77]. In particular, excessive MMP activity has been associated with delayed wound healing and increased tissue damage in various pathological conditions, including chronic wounds [78].
Lesions caused by HSV infection, similarly to others, are characterized by tissue damage and an intense inflammatory response [79]. Yang et al. demonstrated that following induction of keratitis in Balb/c mice by inoculating the cornea with HSV-1 (KOS strain), MMP-2 and MMP-9 expression significantly increased in the epithelium compared to uninfected controls, with localization in the superficial stromal tissue and inflammatory cells beneath the epithelium [80].
To further explore the potential of our formulations, we assessed their antiviral activity against HSV-1 through different experimental setups, allowing us to determine at which stage of the viral life cycle they exert their effect. Acyclovir, a well-established antiviral therapy, was used as reference to provide a benchmark for evaluating the efficacy of the tested formulations. Acyclovir remains the gold standard for HSV therapy, acting as a potent and selective inhibitor of viral DNA polymerase, and being highly effective in reducing HSV replication and lesion duration [81,82]. However, its use is limited by a lack of anti-inflammatory or wound-healing effects, the emergence of resistant viral strains (especially in immunocompromised patients), and, in systemic applications, potential renal and neurological toxicity [83].
Results revealed that pre-infection treatment of cells with the formulations provided limited protection against the virus-induced cytopathic effect. However, when the formulations were applied simultaneously with infection or post-infection, a significantly stronger protective effect was observed. Glycerosomes and hyalurosomes exhibited the highest antiviral activity, with protection reaching nearly 100% during infection and up to 80% post-infection, even at low concentrations. Interestingly, despite a slightly lower entrapment efficiency, glycerohyalurosomes exhibited high antiviral activity as well. This might be due to a synergistic effect of glycerol and sodium hyaluronate, which contribute to enhanced skin interaction, bioadhesion, and cellular uptake. In fact, glycerol has been shown to increase the deformability and fluidity of vesicle bilayers, facilitating their passage through the stratum corneum and improving dermal delivery of bioactive compounds [54,84]. Moreover, its humectant and anti-irritant properties support skin hydration and barrier function, creating a more favorable microenvironment for antiviral efficacy. Sodium hyaluronate, on the other hand, confers strong bioadhesive properties that prolong vesicle residence time on the skin and enable sustained release. It also interacts with CD44 receptors expressed on keratinocytes and fibroblasts, promoting endocytosis and intracellular delivery of the encapsulated extract [85,86]. In contrast, liposomes and L. angustifolia extract dispersion displayed a more limited effect, requiring higher concentrations to achieve moderate levels of protection. Finally, when compared with acyclovir, the obtained formulations exhibited notable antiviral activity against HSV-1, with EC50 values markedly lower than that of acyclovir. In particular, with the only exception of liposomes, they demonstrated superior efficacy during infection, with EC50 values below 1.79 µg/mL, compared to 9.80 µg/mL for acyclovir.
The antiviral activity observed in the obtained vesicular formulations loaded with L. angustifolia extract are in agreement with previous research describing the efficacy of lavender-derived compounds against HSV-1. Several studies have demonstrated the antiviral properties of Lavandula species, including L. angustifolia and L. austroapennina, which showed significant inhibition of HSV-1 replication in vitro [20,22]. In particular, lavender stem extract, rich in oleanolic acid, was able to completely inhibit viral replication at low concentrations, especially when administered prior to viral adsorption [22]. Additionally, an essential oil, rich in linalyl acetate and linalool, obtained from L. angustifolia exhibited promising antiviral effects against H5N1 influenza virus replication [87]. Considering both the results obtained in this work and previous findings, the antiviral effect is likely the result of multiple constituents acting through different pathways. Rosmarinic acid, reported as a major component, may play a key role, but synergistic interactions with other molecules cannot be excluded.
5. Conclusions
In light of these preliminary findings, the obtained vesicular formulations, particularly those containing glycerol and/or sodium hyaluronate, demonstrated promising dual benefits for treating herpetic sores. On the one hand, they support wound healing and reduce local oxidative damages and inflammation by modulating MMPs and pro-inflammatory cytokines, which are crucial for tissue repair and regeneration. On the other hand, they were able to exert a direct antiviral effect against HSV-1, providing an additional layer of therapeutic efficacy. Overall results highlight the potential of glycerosomes, hyalurosomes, and glycerohyalurosomes as promising delivery systems that enhance the antiviral potential of L. angustifolia extract against HSV-1 and may serve as effective alternatives or adjuncts to conventional antiviral therapies. Further studies are needed to evaluate the pharmacological efficacy, skin penetration, and safety of the formulations in more physiologically relevant models, such as ex vivo human skin and in vivo dermal infection models. Moreover, in-depth mechanistic studies, including viral replication kinetics and RT-PCR analysis, will be performed to better elucidate how they interfere with HSV-1 infection pathways.
Author Contributions
Conceptualization, F.F.; methodology, F.A., M.P., I.D., S.P., R.N., M.I.G., J.F., M.L., A.M., M.M. and M.L.M.; validation, F.A., M.P., I.D., S.P., R.N., M.I.G., J.F., M.L., A.M., M.M. and M.L.M.; formal analysis, F.F.; investigation, F.F., F.A., M.P., I.D., S.P. and R.N.; data curation, F.F.; writing—original draft preparation, F.F.; writing—review and editing, F.A., M.P., I.D., S.P., R.N., M.I.G., J.F., M.L., A.M., M.M. and M.L.M.; supervision, F.A., M.M. and M.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The present work has been supported by EU funding within the Next Generation EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT) (F.A. and A.M.). M.I.G. acknowledges financial support by the European Regional Development Fund through Programme Research Innovation and Digitalisation for Smart Transformation, Grant agreement No. BG16RFPR002-1.014-0003-C01. R.N. and J.F. acknowledge financial support by the institutional support of Palacký University in Olomouc RVO:61989592.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors acknowledge Ivanka Koycheva (Laboratory of Metabolomics, Institute of Microbiology, Bulgarian Academy of Sciences) for the preparation of L. angustifolia extract. Authors acknowledge BioRender (Toronto, ON, Canada) by which Figure 1 has been created [Angius, F. (2025) https://BioRender.com/8yw439l accessed on 18 April 2025].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bradshaw, M.J.; Venkatesan, A. Herpes Simplex Virus-1 Encephalitis in Adults: Pathophysiology, Diagnosis, and Management. Neurotherapeutics 2016, 13, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Koe, K.H.; Maharajan, M.K.; Panda, S. A Comprehensive Overview of Epidemiology, Pathogenesis and the Management of Herpes Labialis. Viruses 2023, 15, 225. [Google Scholar] [CrossRef]
- Herpes Simplex Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed on 13 January 2025).
- Whitley, R.; Kimberlin, D.W.; Prober, C.G. Pathogenesis and Disease. In Rhizoctonia Solani, Biology and Pathology: Based on an American Phytopathological Society Symposium on Rhizoctonia solani held at the Miami meeting of the Society, October, 1965; American Phytopathological Society (APS): St. Paul, MN, USA, 2007; pp. 161–171. [Google Scholar] [CrossRef]
- Miller, C.S.; Berger, J.R.; Mootoor, Y.; Avdiushko, S.A.; Zhu, H.; Kryscio, R.J. High Prevalence of Multiple Human Herpesviruses in Saliva from Human Immunodeficiency Virus-Infected Persons in the Era of Highly Active Antiretroviral Therapy. J. Clin. Microbiol. 2006, 44, 2409–2415. [Google Scholar] [CrossRef]
- Chentoufi, A.A.; Benmohamed, L. Mucosal Herpes Immunity and Immunopathology to Ocular and Genital Herpes Simplex Virus Infections. J. Immunol. Res. 2012, 2012, 149135. [Google Scholar] [CrossRef]
- Price, R.W.; Antoinette Walz, M.; Wohlenberg, C.; Notkins, A.L. Latent Infection of Sensory Ganglia with Herpes Simplex Virus: Efficacy of Immunization. Science 1975, 188, 938–940. [Google Scholar] [CrossRef]
- Stoeger, T.; Adler, H. “Novel” Triggers of Herpesvirus Reactivation and Their Potential Health Relevance. Front. Microbiol. 2019, 10, 429807. [Google Scholar] [CrossRef] [PubMed]
- Pieknik, J.R.; Bertke, A.S.; Krause, P.R. Herpes Simplex Virus 2 in Autonomic Ganglia: Evidence for Spontaneous Reactivation. J. Virol. 2019, 93, e00227-19. [Google Scholar] [CrossRef]
- Kaul, R.; Nagelkerke, N.J.; Kimani, J.; Ngugi, E.; Bwayo, J.J.; MacDonald, K.S.; Rebbaprgada, A.; Fonck, K.; Temmerman, M.; Ronald, A.R.; et al. Prevalent Herpes Simplex Virus Type 2 Infection Is Associated with Altered Vaginal Flora and an Increased Susceptibility to Multiple Sexually Transmitted Infections. J. Infect. Dis. 2007, 196, 1692–1697. [Google Scholar] [CrossRef]
- Chang, J.Y.; Balch, C.; Puccio, J.; Oh, H.S. A Narrative Review of Alternative Symptomatic Treatments for Herpes Simplex Virus. Viruses 2023, 15, 1314. [Google Scholar] [CrossRef]
- Reusser, P. Herpesvirus Resistance to Antiviral Drugs: A Review of the Mechanisms, Clinical Importance and Therapeutic Options. J. Hosp. Infect. 1996, 33, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir Resistance in Herpes Simplex Viruses: Prevalence and Therapeutic Alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral Activity of Some Plant Oils against Herpes Simplex Virus Type 1 in Vero Cell Culture. J. Acute Med. 2015, 5, 62–68. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Barar, A.; Bensebia, O. Surfactant Assisted Extraction for the Recovery of Bioactive Compounds from Solid Wastes of Lavender (Lavandula Angustifolia Mill): Optimization by Box–Behnken Design. Process Biochem. 2025, 153, 170–181. [Google Scholar] [CrossRef]
- Kovatcheva, E.G.; Koleva, I.I.; Ilieva, M.; Pavlov, A.; Mincheva, M.; Konushlieva, M. Antioxidant Activity of Extracts from Lavandula Vera MM Cell Cultures. Food Chem. 2001, 72, 295–300. [Google Scholar] [CrossRef]
- Yucharoen, R.; Chansakaow, S.; Tragoolpua, Y. Inhibitory Effect of Aromatic Herbs, Lavender, Sage and Chamomile against Herpes Simplex Virus Infection. Afr. J. Biotechnol. 2011, 10, 15394–15401. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative Study on the Antiviral Activity of Selected Monoterpenes Derived from Essential Oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Chianese, A.; Gravina, C.; Morone, M.V.; Ambrosino, A.; Formato, M.; Palma, F.; Foglia, F.; Nastri, B.M.; Zannella, C.; Esposito, A.; et al. Lavandula Austroapennina: Assessment of the Antiviral Activity of Lipophilic Extracts from Its Organs. Viruses 2023, 15, 1648. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Madroñal, M.; Caro-León, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vázquez-Lasa, B. Chitosan–Rosmarinic Acid Conjugates with Antioxidant, Anti-Inflammatory and Photoprotective Properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Yassin, A.; Nehmeh, B.; Labaki, L.; Mitri, S.; Naser Aldine, F.; Hirko, A.; Caballero, S.; Monck, E.; Garcia-Maruniak, A.; et al. Deciphering the Therapeutical Potentials of Rosmarinic Acid. Sci. Rep. 2022, 12, 15489. [Google Scholar] [CrossRef]
- Samy, C.R.A.; Karunanithi, K.; Sheshadhri, J.; Rengarajan, M.; Srinivasan, P.; Cherian, P. (R)-(+)-Rosmarinic Acid as an Inhibitor of Herpes and Dengue Virus Replication: An In Silico Assessment. Rev. Bras. Farmacogn. 2023, 33, 543. [Google Scholar] [CrossRef]
- Koycheva, I.K.; Vasileva, L.V.; Amirova, K.M.; Marchev, A.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Biotechnologically Produced Lavandula Angustifolia Mill. Extract Rich in Rosmarinic Acid Resolves Psoriasis-Related Inflammation Through Janus Kinase/Signal Transducer and Activator of Transcription Signaling. Front. Pharmacol. 2021, 12, 680168. [Google Scholar] [CrossRef]
- Georgiev, M.; Pavlov, A.; Ilieva, M. Selection of High Rosmarinic Acid Producing Lavandula Vera MM Cell Lines. Process Biochem. 2006, 41, 2068–2071. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken Design Optimization of Mucoadhesive Chitosan-Coated Nanoemulsions for Rosmarinic Acid Nasal Delivery—In Vitro Studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Casanova, F.; Estevinho, B.N.; Santos, L. Preliminary Studies of Rosmarinic Acid Microencapsulation with Chitosan and Modified Chitosan for Topical Delivery. Powder Technol. 2016, 297, 44–49. [Google Scholar] [CrossRef]
- Sardarabadi, H.; Darvishi, M.H.; Zohrab, F.; Javadi, H. Nanophytomedicine: A Promising Practical Approach in Phytotherapy. Phytother. Res. 2024, 38, 3607–3644. [Google Scholar] [CrossRef] [PubMed]
- Yihan, W.; Jinjin, D.; Yingqi, W.; Guanai, M.; Xiwu, Z. Advances in Plant Essential Oils and Drug Delivery Systems for Skincare. Front. Pharmacol. 2025, 16, 1578280. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Fulgheri, F.; Perra, M.; Bacchetta, G.; Fancello, L.; Corrias, F.; Usach, I.; Peris, J.E.; Manca, M.L.; Manconi, M. A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products. Nanomaterials 2023, 13, 2485. [Google Scholar] [CrossRef] [PubMed]
- Perra, M.; Fancello, L.; Castangia, I.; Allaw, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Manca, M.L.; Koycheva, I.K.; Georgiev, M.I.; et al. Formulation and Testing of Antioxidant and Protective Effect of Hyalurosomes Loading Extract Rich in Rosmarinic Acid Biotechnologically Produced from Lavandula Angustifolia Miller. Molecules 2022, 27, 2423. [Google Scholar] [CrossRef]
- Taléns-Visconti, R.; Perra, M.; Ruiz-Saurí, A.; Nácher, A. New Vehiculation Systems of Mometasone Furoate for the Treatment of Inflammatory Skin Diseases. Pharmaceutics 2022, 14, 2558. [Google Scholar] [CrossRef]
- Elhalmoushy, P.M.; Elsheikh, M.A.; Matar, N.A.; El-Hadidy, W.F.; Kamel, M.A.; Omran, G.A.; Elnaggar, Y.S.R. Novel Berberine-Loaded Hyalurosomes as a Promising Nanodermatological Treatment for Vitiligo: Biochemical, Biological and Gene Expression Studies. Int. J. Pharm. 2022, 615, 121523. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Gaafar, P.M.E.; Khattab, M.A.; Mohamed, M.K.; Noureldin, M.H.; Abbas, H. Dual-Effects of Caffeinated Hyalurosomes as a Nano-Cosmeceutical Gel Counteracting UV-Induced Skin Ageing. Int. J. Pharm. X 2023, 5, 100170. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liang, R.; Liu, C.; Yang, C. Improved Stability and Skin Penetration through Glycethosomes Loaded with Glycyrrhetinic Acid. Int. J. Cosmet. Sci. 2022, 44, 249–261. [Google Scholar] [CrossRef]
- Anwer, M.K.; Alshdefat, R.; Akhtar, J.; Aleemuddin, M. Punica Granatum Loaded Glycerosomes for Antibacterial Effect in Skin Infections: Preparation, Optimization, In Vitro and In Vivo Characterization. Bionanoscience 2025, 15, 294. [Google Scholar] [CrossRef]
- Melis, V.; Manca, M.L.; Bullita, E.; Tamburini, E.; Castangia, I.; Cardia, M.C.; Valenti, D.; Fadda, A.M.; Peris, J.E.; Manconi, M. Inhalable Polymer-Glycerosomes as Safe and Effective Carriers for Rifampicin Delivery to the Lungs. Colloids Surf. B Biointerfaces 2016, 143, 301–308. [Google Scholar] [CrossRef]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of Curcumin Loaded Sodium Hyaluronate Immobilized Vesicles (Hyalurosomes) and Their Potential on Skin Inflammation and Wound Restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Juráňová, J.; Aury-Landas, J.; Boumediene, K.; Baugé, C.; Biedermann, D.; Ulrichová, J.; Franková, J. Modulation of Skin Inflammatory Response by Active Components of Silymarin. Molecules 2018, 24, 123. [Google Scholar] [CrossRef]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juráňová, J.; Ulrichová, J. Effects of Silver Nanoparticles on Primary Cell Cultures of Fibroblasts and Keratinocytes in a Wound-Healing Model. J. Appl. Biomater. Funct. Mater. 2016, 14, e137–e142. [Google Scholar] [CrossRef]
- Angius, F.; Floris, A. Liposomes and MTT Cell Viability Assay: An Incompatible Affair. Toxicol. Vitr. 2015, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-Chemical Investigation and Antimicrobial Efficacy of Ozonated Oils: The Case Study of Commercial Ozonated Olive and Sunflower Seed Refined Oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef] [PubMed]
- Roggia, I.; Gomes, P.; Dalcin, A.J.F.; Ourique, A.F.; Mânica da Cruz, I.B.; Ribeiro, E.E.; Mitjans, M.; Vinardell, M.P. Profiling and Evaluation of the Effect of Guarana-Loaded Liposomes on Different Skin Cell Lines: An In Vitro Study. Cosmetics 2023, 10, 79. [Google Scholar] [CrossRef]
- Zangooie, S.; Ghanbari, R.; Jalilian, F.A.; Mahmoudvand, S.; Teimoori, A. Antiviral Potential of Phenolic Compounds against HSV-1: In-Vitro Study. Antivir. Ther. 2024, 29, 13596535241271589. [Google Scholar] [CrossRef]
- Zhu, Y.; Binder, J.; Yurgelonis, I.; Rai, D.K.; Lazarro, S.; Costales, C.; Kobylarz, K.; McMonagle, P.; Steppan, C.M.; Aschenbrenner, L.; et al. Generation of a VeroE6 Pgp Gene Knock out Cell Line and Its Use in SARS-CoV-2 Antiviral Study. Antivir. Res. 2022, 208, 105429. [Google Scholar] [CrossRef]
- Toscani, A.; Denaro, R.; Pacheco, S.F.C.; Biolatti, M.; Anselmi, S.; Dell’oste, V.; Castagnolo, D. Synthesis and Biological Evaluation of Amidinourea Derivatives against Herpes Simplex Viruses. Molecules 2021, 26, 4927. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha Piperita. Arch. Razi Inst. 2021, 76, 1279. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula Angustifolia and Lavandula x Intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Manca, M.L.; Cencetti, C.; Matricardi, P.; Castangia, I.; Zaru, M.; Sales, O.D.; Nacher, A.; Valenti, D.; Maccioni, A.M.; Fadda, A.M.; et al. Glycerosomes: Use of Hydrogenated Soy Phosphatidylcholine Mixture and Its Effect on Vesicle Features and Diclofenac Skin Penetration. Int. J. Pharm. 2016, 511, 198–204. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Zaru, M.; Valenti, D.; Peris, J.E.; Matricardi, P.; Maccioni, A.M.; Fadda, A.M. Glycerosomes: Investigation of Role of 1,2-Dimyristoyl-Sn-Glycero-3-Phosphatidycholine (DMPC) on the Assembling and Skin Delivery Performances. Int. J. Pharm. 2017, 532, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Caddeo, C.; Manca, M.L.; Casu, L.; Latorre, A.C.; Díez-Sales, O.; Ruiz-Saurí, A.; Bacchetta, G.; Fadda, A.M.; Manconi, M. Delivery of Liquorice Extract by Liposomes and Hyalurosomes to Protect the Skin against Oxidative Stress Injuries. Carbohydr. Polym. 2015, 134, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Razavi, S.H.; Nácher, A.; Díez-Sales, O.; Peris, J.E.; Allaw, M.; Terencio, M.C.; Usach, I.; Manconi, M. Canthaxanthin Biofabrication, Loading in Green Phospholipid Vesicles and Evaluation of In Vitro Protection of Cells and Promotion of Their Monolayer Regeneration. Biomedicines 2022, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Firoznezhad, M.; Castangia, I.; Tuberoso, C.I.G.; Cottiglia, F.; Marongiu, F.; Porceddu, M.; Usach, I.; Escribano-Ferrer, E.; Manca, M.L.; Manconi, M. Formulation and In Vitro Efficacy Assessment of Teucrium Marum Extract Loading Hyalurosomes Enriched with Tween 80 and Glycerol. Nanomaterials 2022, 12, 1096. [Google Scholar] [CrossRef]
- Mehdipour, A.; Reza, M.A.S.; Rasouli, A.; Baravati, M.H.J.; Jafari, G.A.; Heidari, F. Green Synthesis of Zinc Nanoparticles by Hydroalcoholic Extract of Lavender (Lavandula stoechas L.), Characterization, and Cytotoxic Effects on Human Breast and Colon Cancer. Sci. Rep. 2024, 14, 29543. [Google Scholar] [CrossRef]
- Souza, I.D.L.; Saez, V.; Mansur, C.R.E. Lipid Nanoparticles Containing Coenzyme Q10 for Topical Applications: An Overview of Their Characterization. Colloids Surf. B Biointerfaces 2023, 230, 113491. [Google Scholar] [CrossRef]
- Casula, E.; Manca, M.L.; Perra, M.; Pedraz, J.L.; Lopez-Mendez, T.B.; Lozano, A.; Calvo, E.; Zaru, M.; Manconi, M. Nasal Spray Formulations Based on Combined Hyalurosomes and Glycerosomes Loading Zingiber Officinalis Extract as Green and Natural Strategy for the Treatment of Rhinitis and Rhinosinusitis. Antioxidants 2021, 10, 1109. [Google Scholar] [CrossRef]
- Oliveira, S.; Chaleix, V.; Baccile, N.; Helary, C. Cytotoxicity Evaluation of Microbial Sophorolipids and Glucolipids Using Normal Human Dermal Fibroblasts (NHDF) in Vitro. Toxicol. Rep. 2025, 14, 101862. [Google Scholar] [CrossRef]
- Zhao, C. Cell Culture: In Vitro Model System and a Promising Path to in Vivo Applications. J. Histotechnol. 2023, 46, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Stunova, A.; Vistejnova, L. Dermal Fibroblasts—A Heterogeneous Population with Regulatory Function in Wound Healing. Cytokine Growth Factor. Rev. 2018, 39, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.J.; Kim, K.B.; An, I.S.; Ahn, K.J.; Han, H.J. Protective Effects of Rosmarinic Acid against Hydrogen Peroxide-Induced Cellular Senescence and the Inflammatory Response in Normal Human Dermal Fibroblasts. Mol. Med. Rep. 2017, 16, 9763–9769. [Google Scholar] [CrossRef]
- Matwiejczuk, N.; Galicka, A.; Zaręba, I.; Brzóska, M.M. The Protective Effect of Rosmarinic Acid against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12, 1282. [Google Scholar] [CrossRef] [PubMed]
- Erdal, A.; Özdemir, D.; Özdemir, Ş.; Bakırtaş, M.; Ağrı, İ. The Effect of Rosmarinic Acid on Wound Healing of Nasal Mucosa in the Rats. Am. J. Rhinol. Allergy 2024, 38, 133–139. [Google Scholar] [CrossRef]
- Lu, Y.H.; Hong, Y.; Zhang, T.Y.; Chen, Y.X.; Wei, Z.J.; Gao, C.Y. Rosmarinic Acid Exerts Anti-Inflammatory Effect and Relieves Oxidative Stress via Nrf2 Activation in Carbon Tetrachloride-Induced Liver Damage. Food Nutr. Res. 2022, 66, 66. [Google Scholar] [CrossRef]
- Ben Djemaa, F.G.; Bellassoued, K.; Zouari, S.; El Feki, A.; Ammar, E. Antioxidant and Wound Healing Activity of Lavandula aspic L. Ointment. J. Tissue Viability 2016, 25, 193–200. [Google Scholar] [CrossRef]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound Healing Potential of Lavender Oil by Acceleration of Granulation and Wound Contraction through Induction of TGF-β in a Rat Model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef]
- Guo, X.; Wu, S.; Shang, J.; Dong, W.; Li, Y.; Peng, Q.; Xie, Z.; Chen, C. The Effects of Lipoic Acid on Respiratory Diseases. Int. Immunopharmacol. 2023, 116, 109713. [Google Scholar] [CrossRef]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-Inflammatory Effect of Lavender (Lavandula angustifolia Mill.) Essential Oil Prepared during Different Plant Phenophases on THP-1 Macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef]
- Huang, M.Y.; Liao, M.H.; Wang, Y.K.; Huang, Y.S.; Wen, H.C. Effect of Lavender Essential Oil on LPS-Stimulated Inflammation. Am. J. Chin. Med. 2012, 40, 845–859. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hirao, Y.; Kawanishi, S.; Kato, S.; Mori, Y.; Murata, M.; Oikawa, S. Rosmarinic Acid, a Natural Polyphenol, Has a Potential pro-Oxidant Risk via NADH-Mediated Oxidative DNA Damage. Genes Environ. 2024, 46, 13. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Keane, T.J.; Horejs, C.M.; Stevens, M.M. Scarring vs. Functional Repair: Matrix-Based Strategies to Regulate Tissue Healing. Adv. Drug Deliv. Rev. 2018, 129, 407. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225. [Google Scholar] [CrossRef]
- Koujah, L.; Suryawanshi, R.K.; Shukla, D. Pathological Processes Activated by Herpes Simplex Virus-1 (HSV-1) Infection in the Cornea. Cell Mol. Life Sci. 2018, 76, 405. [Google Scholar] [CrossRef]
- Yuan, X.; Mitchell, B.M.; Wilhelmus, K.R. Expression of Matrix Metalloproteinases during Experimental Candida Albicans Keratitis. Invest. Ophthalmol. Vis. Sci. 2009, 50, 737. [Google Scholar] [CrossRef]
- Elion, G.B. Mechanism of Action and Selectivity of Acyclovir. Am. J. Med. 1982, 73, 7–13. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Whitley, R.J. Antiviral Therapy of HSV-1 and -2. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Cambridge University Press: Cambridge, UK, 2007; pp. 1153–1174. [Google Scholar] [CrossRef]
- Brandariz-Nuñez, D.; Correas-Sanahuja, M.; Maya-Gallego, S.; Martín Herranz, I. Neurotoxicity Associated with Acyclovir and Valacyclovir: A Systematic Review of Cases. J. Clin. Pharm. Ther. 2021, 46, 918–926. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A.M. Glycerosomes: A New Tool for Effective Dermal and Transdermal Drug Delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Prestwich, G.D. Hyaluronic Acid-Based Clinical Biomaterials Derived for Cell and Molecule Delivery in Regenerative Medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of Hyaluronic Acid as Carriers in Drug Delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral Activity of Lavandula angustifolia L. and Salvia officinalis L. Essential Oils against Avian Influenza H5N1 Virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).