Upcycling of By-Products from Autochthonous Red Grapes and Commercial Apples as Ingredients in Baked Goods: A Comprehensive Study from Processing to Consumer Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Flours from Grape Pomace and Apple Skin
2.3. Chemical Characterization of By-Products and Baked Goods
2.3.1. Sample Preparation for Analysis
2.3.2. Extraction of Samples
2.3.3. Spectrophotometric Assays of Fresh and Dried By-Products
Total Polyphenol Content: TPC
Total Anthocyanins: TA
Ferric-Reducing Antioxidant Potential: FRAP
2.3.4. Mass Spectrometric Analyses of Fresh and Dried By-Products
Single Polyphenol Compounds: Grape Pomace
Single Polyphenol Compounds: Apple Skins
2.3.5. Total Fat Content: Grape Pomace
2.3.6. Fatty Acid Methyl Esters (FAME): Grape Pomace
2.4. Baked Goods
2.4.1. Food System Breadsticks
2.4.2. Food System Focaccia
2.4.3. Food System Cookies
2.5. Technological Characterization of Baked Goods
2.5.1. Color Analysis
2.5.2. Texture Profile Analysis
2.6. Impact of Baking on Bioactive Compounds
2.7. Sensory Evaluation and Consumer Test
2.7.1. Sensory Test
2.7.2. Consumer Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Stability of Bioactive Compounds During Drying and Milling
3.2. Polyphenols and Anthocyanin Composition in Flours
3.3. Chemical and Technological Characterization of Baked Goods
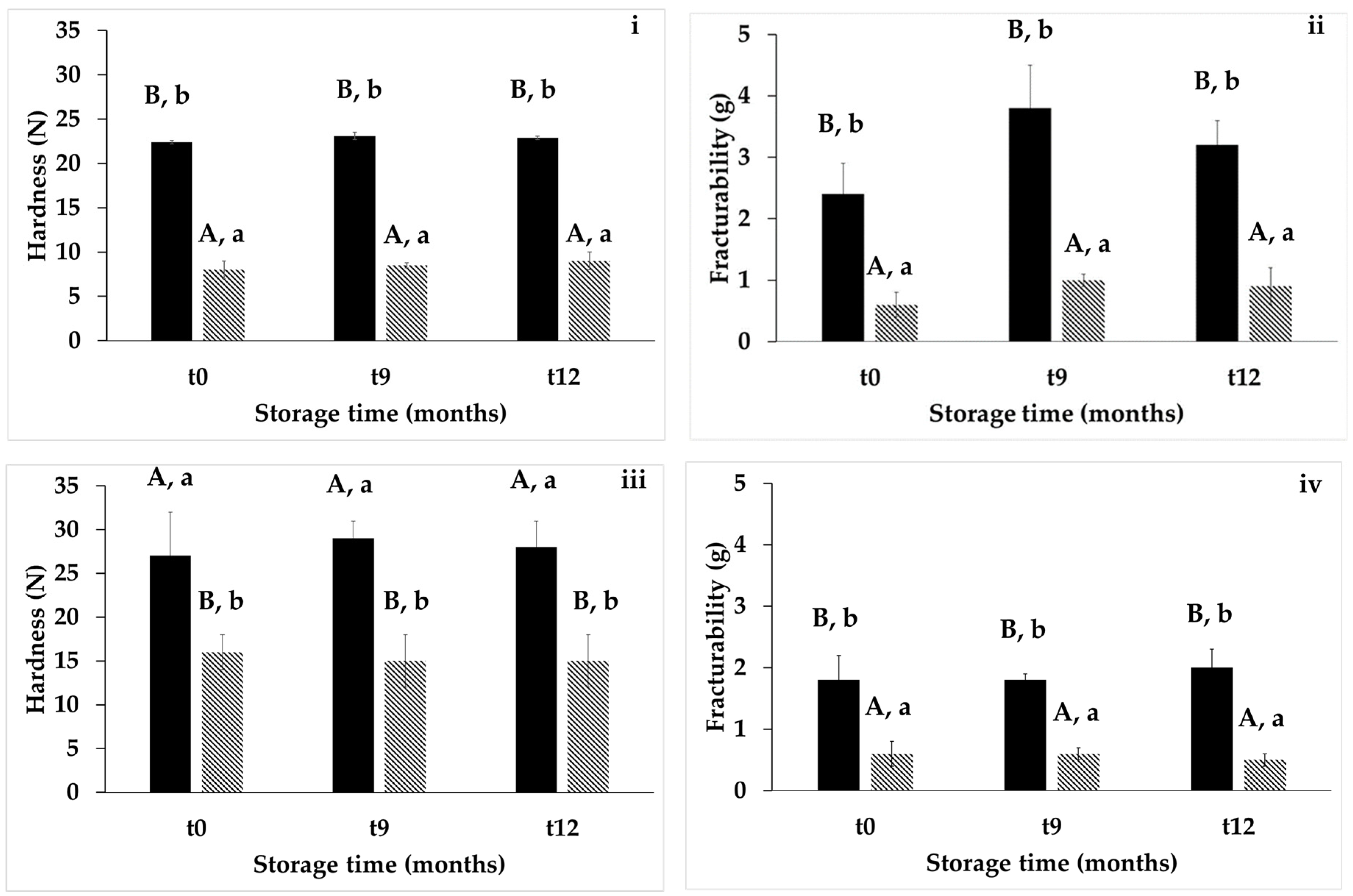
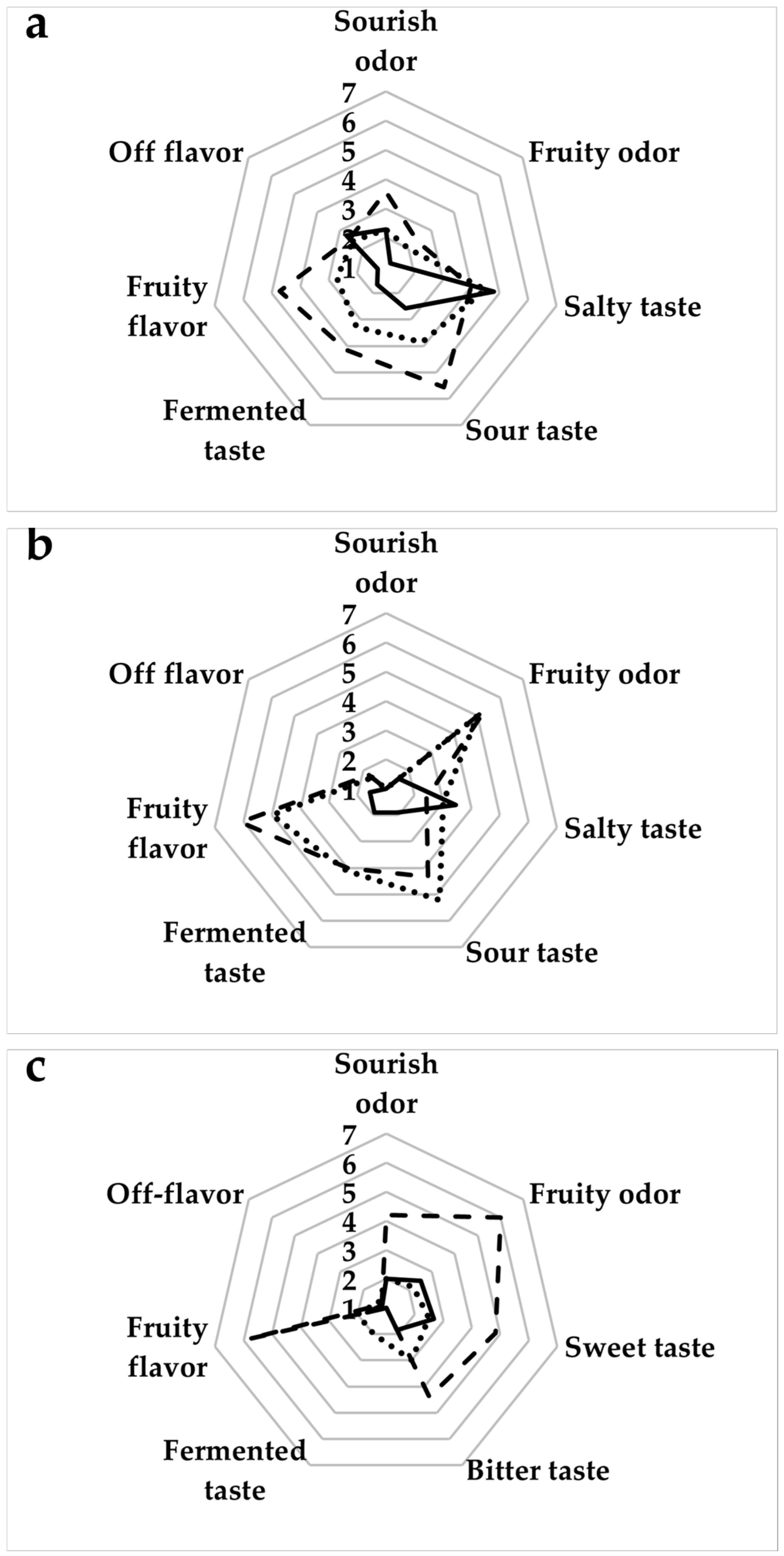
3.4. Texture, Color, and Sensory Attributes of Baked Goods
3.5. Consumer Acceptance and Perception of Sustainability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC-MS | High performance liquid chromatography–mass spectrometry |
| UHPLC | Ultra-high performance liquid chromatography |
| H-ESI | Heated–electrospray ionization |
| LD | Linear dichroism |
| SAFA | Saturated fatty acid |
| MUFA | Mono-unsaturated fatty acid |
| PUFA | Poly-unsaturated fatty acid |
| FRAP | Ferric-reducing antioxidant potential |
| TPC | Total polyphenol content |
| AC | Anthocyanin content |
| FAME | Fatty acid methyl esters |
| GC-FID | Gas chromatography–flame ionization detector |
| DW | Dry weight |
| ARB | Arbitrary unit |
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 22 May 2025).
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein Grape Pomace as a Source of Phenolic Compounds: Analysis of the Contents of Anthocyanins, Flavanols and Antioxidant Activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Area di Coltivazione Delle Mele Alto Adige. Available online: https://www.melaaltoadige.com/it/l-alto-adige-e-la-melicoltura/area-di-coltivazione-mele.html (accessed on 25 March 2025).
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Genisheva, Z.; Soares, M.; Oliveira, J.M.; Carvalho, J. Wine Production Wastes, Valorization, and Perspectives. In Advances and Challenges in Hazardous Waste Management; Saleh, H.M., Hassan, A.I., Sayed, R.F., Eds.; IntechOpen: London, UK, 2023; pp. 1–18. ISBN 978-0-85466-030-8. [Google Scholar]
- Sawicka, M.; Latocha, P.; Łata, B. Peel to Flesh Bioactive Compounds Ratio Affect Apple Antioxidant Potential and Cultivar Functional Properties. Agriculture 2023, 13, 478. [Google Scholar] [CrossRef]
- Mendoza-Wilson, A.M.; Castro-Arredondo, S.I.; Espinosa-Plascencia, A.; Robles-Burgueño, M.d.R.; Balandrán-Quintana, R.R.; Bermúdez-Almada, M.d.C. Chemical Composition and Antioxidant-Prooxidant Potential of a Polyphenolic Extract and a Proanthocyanidin-Rich Fraction of Apple Skin. Heliyon 2016, 2, e00073. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S. De Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Gianotti, A.; Marin, V.; Cardone, G.; Bordoni, A.; Mancini, E.; Magni, M.; Pichler, A.; Ciani, S.; Polenghi, O.; Cerne, V.L.; et al. Personalized and Precise Functional Assessment of Innovative Flatbreads toward the Colon Microbiota of People with Metabolic Syndrome: Results from an in Vitro Simulation. Food Res. Int. 2025, 209, 116197. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Pellegrini, N.; Agostoni, C. Nutritional Aspects of Gluten-Free Products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef]
- Giosuè, A.; Siano, F.; Di Stasio, L.; Picariello, G.; Medoro, C.; Cianciabella, M.; Giacco, R.; Predieri, S.; Vasca, E.; Vaccaro, O.; et al. Turning Wastes into Resources: Red Grape Pomace-Enriched Biscuits with Potential Health-Promoting Properties. Foods 2024, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Aghajanzadeh, S. Effect of Dried Fruits and Vegetables Powder on Cakes Quality: A Review. Trends Food Sci. Technol. 2020, 95, 162–172. [Google Scholar] [CrossRef]
- Yalcin, E.; Ozdal, T.; Gok, I. Investigation of Textural, Functional, and Sensory Properties of Muffins Prepared by Adding Grape Seeds to Various Flours. J. Food Process. Preserv. 2022, 46, e15316. [Google Scholar] [CrossRef]
- Fontana, M.; Murowaniecki Otero, D.; Pereira, A.M.; Santos, R.B.; Gularte, M.A. Grape Pomace Flour for Incorporation into Cookies: Evaluation of Nutritional, Sensory and Technological Characteristics. J. Culin. Sci. Technol. 2024, 22, 850–869. [Google Scholar] [CrossRef]
- Shewry, P.R.; Popineau, Y.; Lafiandra, D.; Belton, P. Wheat Glutenin Subunits and Dough Elasticity: Findings of the EUROWHEAT Project. Trends Food Sci. Technol. 2000, 11, 433–441. [Google Scholar] [CrossRef]
- Antoniolli, A.; Becerra, L.; Piccoli, P.; Fontana, A. Phenolic, Nutritional and Sensory Characteristics of Bakery Foods Formulated with Grape Pomace. Plants 2024, 13, 590. [Google Scholar] [CrossRef]
- Difonzo, G.; de Gennaro, G.; Pasqualone, A.; Caponio, F. Potential Use of Plant-Based By-Products and Waste to Improve the Quality of Gluten-Free Foods. J. Sci. Food Agric. 2022, 102, 2199–2211. [Google Scholar] [CrossRef]
- Baldán, Y.; Riveros, M.; Fabani, M.P.; Rodriguez, R. Grape Pomace Powder Valorization: A Novel Ingredient to Improve the Nutritional Quality of Gluten-Free Muffins. Biomass Convers. Biorefinery 2023, 13, 9997–10009. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis; Food Science Text Series; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-45774-1. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Commission Directive 98/64/EC of 3 September 1998 Establishing Community Methods of Analysis for the Determination of Amino-Acids, Crude Oils and Fats, and Olaquindox in Feeding stuffs and Amending Directive 71/393/EEC. Off. J. Eur. Communities 1998, 257, 14. [Google Scholar]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils Gas Chromatography of Fatty Acid Methyl Esters Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/72142.html (accessed on 22 May 2025).
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: https://www.iso.org/standard/63503.html (accessed on 22 May 2025).
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough Fermentation of Whole and Sprouted Lentil Flours: In Situ Formation of Dextran and Effects on the Nutritional, Texture and Sensory Characteristics of White Bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef] [PubMed]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadstick Fortification with Red Grape Pomace: Effect on Nutritional, Technological and Sensory Properties. J. Sci. Food Agric. 2022, 102, 2545–2552. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 11136:2014; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. International Organization for Standardization: Geneva, Switzerland, 2014.
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of Bioactive Compounds from Grape Pomace (Vitis Vinifera and Vitis Labrusca) by Spectrophotometric, Chromatographic and Spectral Analyses. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1007, 72–80. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Stafussa, A.P.; Makara, C.N.; Branco, I.G.; Maciel, G.M.; Haminiuk, C.W.I. Exploratory Analysis of Bioactive Compounds and Antioxidant Potential of Grape (Vitis vinifera) Pomace. Acta Sci. Technol. 2022, 44, e56934. [Google Scholar] [CrossRef]
- Yu, J. Impacts of Different Drying Methods on Mold Viability and Ochratoxin A Content of Grape Pomace. Int. J. Appl. Agric. Sci. 2018, 4, 35. [Google Scholar] [CrossRef]
- Lim, H.S.; Park, S.H.; Ghafoor, K.; Hwang, S.Y.; Park, J. Quality and Antioxidant Properties of Bread Containing Turmeric (Curcuma longa L.) Cultivated in South Korea. Food Chem. 2011, 124, 1577–1582. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Cheng, K.-W.; Jiang, Y.; Chen, F.; Wang, M. The Effects of Grape Seed Extract Fortification on the Antioxidant Activity and Quality Attributes of Bread. Food Chem. 2010, 119, 49–53. [Google Scholar] [CrossRef]
- Pramsohler, M.; Spitaler, R.; Thaler, K.; Dordevic, N.; Robatscher, P.; Oberhuber, M. Trocknungsfaktoren Bei Kräutern Und Einfluss Der Probennahme Und Probenvorbereitung Auf Den Gehalt an Pflanzenschutzmittel-Rückständen. Laimbg. J. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Sokač, T.; Gunjević, V.; Pušek, A.; Tušek, A.J.; Dujmić, F.; Brnčić, M.; Ganić, K.K.; Jakovljević, T.; Uher, D.; Mitrić, G.; et al. Comparison of Drying Methods and Their Effect on the Stability of Graševina Grape Pomace Biologically Active Compounds. Foods 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-García, R.; González-SanJosé, M.L.; Rivero-Pérez, M.D.; García-Lomillo, J.; Muñiz, P. The Effects of Heat Treatment on the Phenolic Composition and Antioxidant Capacity of Red Wine Pomace Seasonings. Food Chem. 2017, 221, 1723–1732. [Google Scholar] [CrossRef]
- Henríquez, C.; Almonacid, S.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Cabezas, L.; Simpson, R.; Speisky, H. Determinación de La Capacidad Antioxidante, Contenido de Fenoles Totales y Composición Mineral de Diferentes Tejidos de Frutos de Cinco Variedades de Manzana Cultivadas En Chile. Chil. J. Agric. Res. 2010, 70, 523–536. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mura, M.; Chasseur, M.; Freguglia, M.; Valentini, S.; Palombo, D.; Perego, P. Polyphenols from Grape and Apple Skin: A Study on Non-Conventional Extractions and Biological Activity on Endothelial Cell Cultures. Chem. Eng. Trans. 2015, 44, 205–210. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors Affecting the Stability of Anthocyanins and Strategies for Improving Their Stability: A Review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef]
- Tobolka, A.; Škorpilová, T.; Beňo, F.; Podskalská, T.; Rajchl, A. Effect of Various Carbohydrates in Aqueous Solutions on Color Stability and Degradation Kinetics of Selected Anthocyanins During Storage. Foods 2024, 13, 3628. [Google Scholar] [CrossRef]
- Ma, Q.; Bi, J.; Yi, J.; Wu, X.; Li, X.; Zhao, Y. Stability of Phenolic Compounds and Drying Characteristics of Apple Peel as Affected by Three Drying Treatments. Food Sci. Hum. Wellness 2021, 10, 174–182. [Google Scholar] [CrossRef]
- Heras-Ramírez, M.E.; Quintero-Ramos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muñoz, J.V.; Salas-Muñoz, E. Effect of Blanching and Drying Temperature on Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Bai, R.; Cui, Y.; Luo, L.; Yuan, D.; Wei, Z.; Yu, W.; Sun, B. A Semisynthetic Approach for the Simultaneous Reaction of Grape Seed Polymeric Procyanidins with Catechin and Epicatechin to Obtain Oligomeric Procyanidins in Large Scale. Food Chem. 2019, 278, 609–616. [Google Scholar] [CrossRef]
- Guaita, M.; Panero, L.; Motta, S.; Mangione, B.; Bosso, A. Effects of High-Temperature Drying on the Polyphenolic Composition of Skins and Seeds from Red Grape Pomace. LWT 2021, 145, 111323. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An Apple a Day Keeps the Doctor Away”: The Potentials of Apple Bioactive Constituents for Chronic Disease Prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.-L. Proanthocyanidin Composition and Antioxidant Potential of the Stem Winemaking Byproducts from 10 Different Grape Varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Firestone, D. Physical and Chemical Characteristics of Oils, Fats, and Waxes, 3rd ed.; Amer Oil Chemists Society: Urbana, IL, USA, 2013; ISBN 0983079196. [Google Scholar]
- Poiana, M.A.; Alexa, E.; Radulov, I.; Raba, D.N.; Cocan, I.; Negrea, M.; Misca, C.D.; Dragomir, C.; Dossa, S.; Suster, G. Strategies to Formulate Value-Added Pastry Products from Composite Flours Based on Spelt Flour and Grape Pomace Powder. Foods 2023, 12, 3239. [Google Scholar] [CrossRef]
- Bressa, F.; Tesson, N.; Dalla Rosa, M.; Sensidoni, A.; Tubaro, F. Antioxidant Effect of Maillard Reaction Products: Application to a Butter Cookie of a Competition Kinetics Analysis. J. Agric. Food Chem. 1996, 44, 692–695. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of Grape Pomace Powder Addition on Chemical, Nutritional and Technological Properties of Cakes. LWT 2020, 134, 109950. [Google Scholar] [CrossRef]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat Flour Replacement by Wine Grape Pomace Powder Positively Affects Physical, Functional and Sensory Properties of Cookies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of Grape Pomace: Extraction of Bioactive Phenolics with Antioxidant Properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of Vegetable and Fruit By-Products as Functional Ingredient and Food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for Recycling and Valorization of Grape Marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Walker, R.; Tseng, A.; Cavender, G.; Ross, A.; Zhao, Y. Physicochemical, Nutritional, and Sensory Qualities of Wine Grape Pomace Fortified Baked Goods. J. Food Sci. 2014, 79, S1811–S1822. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. Effects of Baking Conditions and Dough Formulations on Phenolic Compound Stability, Antioxidant Capacity and Color of Cookies Made from Anthocyanin-Rich Corn Flour. LWT 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Santetti, G.S.; Dacoreggio, M.V.; Silva, A.C.M.; Biduski, B.; Bressiani, J.; Oro, T.; de Francisco, A.; Gutkoski, L.C.; Amboni, R.D.D.M.C. Effect of Yerba Mate (Ilex paraguariensis) Leaves on Dough Properties, Antioxidant Activity, and Bread Quality Using Whole Wheat Flour. J. Food Sci. 2021, 86, 4354–4364. [Google Scholar] [CrossRef]
- Chi, C.-H.; Cho, S.-J. Improvement of Bioactivity of Soybean Meal by Solid-State Fermentation with Bacillus Amyloliquefaciens versus Lactobacillus Spp. and Saccharomyces Cerevisiae. LWT 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Zhou, S. Effect of Flaxseed Marc Flour on High-Yield Wheat Bread Production: Comparison in Baking, Staling, Antioxidant and Digestion Properties. LWT 2022, 169, 113979. [Google Scholar] [CrossRef]
- Gumul, D.; Korus, J.; Ziobro, R.; Kruczek, M. Enrichment of Wheat Bread with Apple Pomace as a Way to Increase Pro-Health Constituents. Qual. Assur. Saf. Crop. Foods 2019, 11, 231–240. [Google Scholar] [CrossRef]
- Hayta, M.; Özuǧur, G.; Etgü, H.; Şeker, I.T. Effect of Grape (Vitis vinifera L.) Pomace on the Quality, Total Phenolic Content and Anti-Radical Activity of Bread. J. Food Process. Preserv. 2014, 38, 980–986. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat Bread Fortification by Grape Pomace Powder: Nutritional, Technological, Antioxidant, and Sensory Properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality Characteristics of Wheat Flour Dough and Bread Containing Grape Pomace Flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Jannati, N.; Hojjatoleslamy, M.; Hosseini, E.; Mozafari, H.R.; Siavoshi, M. Effect of Apple Pomace Powder on Rheological Properties of Dough and Sangak Bread Texture. Carpathian J. Food Sci. Technol. 2018, 10, 77–84. [Google Scholar]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Gallina Toschi, T.; Monteleone, E. Functional and Sensory Properties of Phenolic Compounds from Unripe Grapes in Vegetable Food Prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef]
- Rosales Soto, M.U.; Brown, K.; Ross, C.F. Antioxidant Activity and Consumer Acceptance of Grape Seed Flour-containing Food Products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- Boff, J.M.; Strasburg, V.J.; Ferrari, G.T.; de Oliveira Schmidt, H.; Manfroi, V.; de Oliveira, V.R. Chemical, Technological, and Sensory Quality of Pasta and Bakery Products Made with the Addition of Grape Pomace Flour. Foods 2022, 11, 3812. [Google Scholar] [CrossRef]
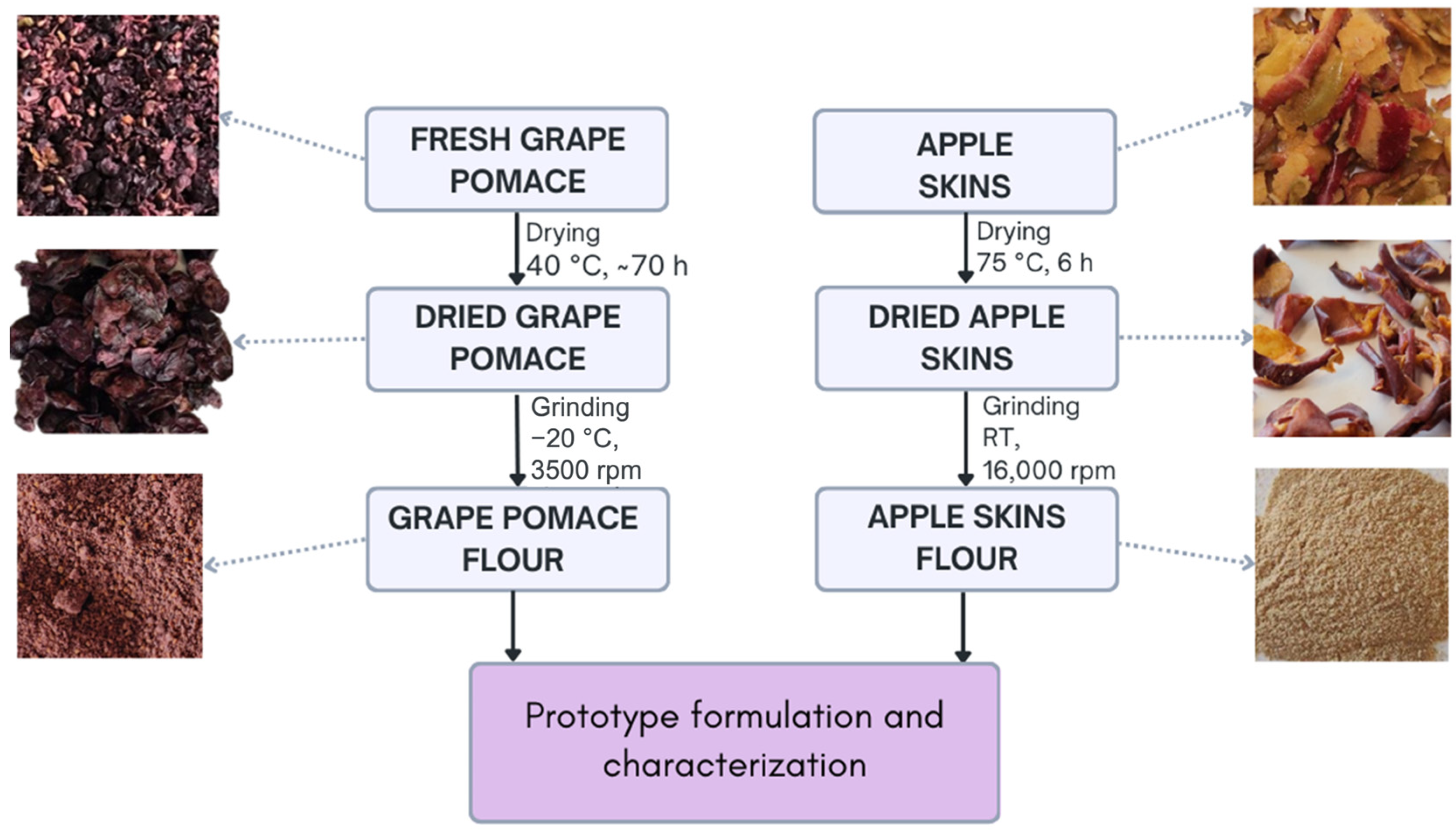
| Food Systems | Grape Pomace Flour | Apple Skin Flour | Other Ingredients |
|---|---|---|---|
| Unenriched breadsticks | - | - | See Section 2.4.1 |
| Enriched breadsticks | 5% | 3% | |
| Unenriched focaccia | - | - | See Section 2.4.2 |
| Enriched focaccia | 5% | 3% | |
| Unenriched cookies | - | - | See Section 2.4.3 |
| Enriched cookies | 5% | 10% |
| Fresh | Dried | Flour | ||
|---|---|---|---|---|
| Grape pomace | Total polyphenols | 4045.3 ± 105.5 b | 3661.1 ± 127.3 a | 4369.9 ± 173.3 b |
| Total anthocyanins | 389.6 ± 8.0 c | 262.4 ± 9.5 a | 335.2 ± 15.8 b | |
| Antioxidant capacity | 80.4 ± 4.1 b | 71.2 ± 4.0 a | 76.9 ± 3.3 ab | |
| Apple skin | Total polyphenols | 683.6 ± 52.8 a | 587.9 ± 24.0 a | 556.3 ± 4.2 a |
| Total anthocyanins | 32.1 ± 2.3 b | 21.0 ± 1.2 a | 20.5 ± 1.0 a | |
| Antioxidant capacity | 10.6 ± 0.1 c | 9.2 ± 0.0 b | 7.9 ± 0.0 a |
| Compound Class | Compound | Fresh Grape Pomace | Grape Pomace Flour | Fresh Apple Skin | Apple Skin Flour |
|---|---|---|---|---|---|
| Flavan-3-ols | Catechin | 64.9 ± 0.2 | 51.1 ± 4.1 ns | 2.1 ± 0.1 | 1.4 ± 0.0 ** |
| Epicatechin | 46.8 ± 1.2 | 23.8 ± 9.1 ns | 26.8 ± 1.0 | 21.3 ± 0.0 * | |
| Procyanidin B1 | 19.3 ± 2.1 | 47.2 ± 4.3 * | 3.4 ± 0.2 | 2.6 ± 0.0 * | |
| Procyanidin B2 | 17.3 ± 0.1 | 19.0 ± 3.9 ns | 29.7 ± 1.1 | 30.4± 0.1 * | |
| Procyanidin C1 | 13.4 ± 0.7 | 14.3 ± 1.0 ns | 26.5 ± 1.0 | 19.5 ± 0.2 ** | |
| Anthocyanins | Cyanidin-3-glucoside | 1.4 ± 0.1 | 2.9 ± 0.3 * | n.d. | n.d. |
| Cyanidin-3-galactoside | n.d. | n.d. | 19.6 ± 0.8 | 10.2 ± 0.3 ** | |
| Cyanidin-3-arabinoside | n.d. | n.d. | 1.0 ± 0.0 | 0.4 ± 0.1 * | |
| Petunidin-3-glucoside | n.d. | 11.3 | - | - | |
| Delphinidin-3-glucoside | 7.2 ± 0.0 | 6.7 ± 0.7 ns | - | - | |
| Flavonols | Quercetin | 12.5 ± 4.8 | 9.7 ± 2.7 ns | 8.3 ± 0.0 | 9.3 ± 0.2 * |
| Quercetin-3-arabinoside | n.d. | n.d. | 13.2 ± 0.2 | 7.9 ± 0.3 ** | |
| Quercetin-3-galactoside | 1.1 ± 0.0 | 6.9 ± 0.8 ** | 51.4 ± 0.2 | 27.4 ± 2.1 ** | |
| Quercetin-3-rhamnoside | 0.7 ± 0.0 | 1.5 ± 0.2 * | 11.2 ± 0.2 | 7.8 ± 0.2 ** | |
| Quercetin-3,4-diglucoside | 1.3 ± 0.0 | 1.2 ± 0.1 ns | - | - | |
| Quercetin-3-glucoside | 3.5 ± 0.1 | 2.4 ± 0.4 * | 7.8 ± 0.3 | 5.0 ± 0.5 * | |
| Quercetin-3-glucuronide | 8.4 ± 0.0 | 5.2 ± 0.2 *** | - | - | |
| Quercetin-3-xyloside | n.d. | n.d. | 26.7 ± 0.5 | 15.0 ± 0.5 ** | |
| Rutin | n.d. | n.d. | 3.3 ± 0.1 | 2.1 ± 0.3 * | |
| Isorhamnetin-3-glucoside | 0.6 ± 0.0 | 2.0 ± 0.3 * | - | - | |
| Kaempferol | n.d. | 1.4 ± 0.2 | - | - | |
| Kaempferol-3-glucoside | n.d. | 2.0 ± 0.3 | - | - | |
| Kaempferol-3-glucuronide | 0.2 ± 0.0 | 2.7 ± 0.4 * | - | - | |
| Kaempferol-3-rutenoside | 0.6 ± 0.0 | n.d. | - | - | |
| Myricetin | 5.2 ± 0.2 | 8.1 ± 1.5 ns | - | - | |
| Myricetin-3-glucoside | 2.9 ± 0.0 | 2.5 ± 0.5 ns | - | - | |
| Hydroxycinnamic acids | Caftaric acid | 1.7 ± 0.3 | 2.9 ± 0.2 * | - | - |
| Sum of Chlorogenic acids | - | - | 2.7 ± 0.1 | 2.6 ± 0.0 ns | |
| Sum of Dihydrochalcones | - | - | 27.4 ± 0.3 | 25.0 ± 0.7 * | |
| Total Polyphenols | Total Anthocyanin | Antioxidant Capacity | ||
|---|---|---|---|---|
| Breadsticks | unenriched | 26.1 ± 0.6 | 1.6 ± 0.1 | 0.3 ± 0.0 |
| enriched | 215.3 ± 8.5 *** | 15.7 ± 0.3 *** | 4.1 ± 0.0 *** | |
| Focaccia | unenriched | 59.2 ± 3.9 | 1.1 ± 0.1 | 0.6 ± 0.3 |
| enriched | 310.2 ± 3.0 *** | 20.0 ± 0.7 *** | 5.4 ± 0.1 *** | |
| Cookies | unenriched | 87.3 ± 22.3 | 4.1 ± 0.0 | 1.3 ± 0.0 |
| enriched | 349.4 ± 7.2 *** | 29.5 ± 1.0 *** | 4.8 ± 0.2 *** | |
| Luminosity | Redness | Yellowness | ||
|---|---|---|---|---|
| Breadsticks | unenriched | 83.0 ± 0.9 | −3.0 ± 0.3 | 19.9 ± 0.6 |
| enriched | 30.3 ± 2.3 *** | 6.5 ± 0.2 *** | 8.1 ± 0.3 *** | |
| Focaccia | unenriched | 70.2 ± 1.3 | 3.0 ± 0.2 | 27.6 ±1.7 |
| enriched | 31.9 ± 1.7 *** | 7.4 ± 0.3 *** | 6.3 ± 0.4 *** | |
| Cookies | unenriched | 69.9 ± 0.7 | 3.2 ± 0.2 | 8.6 ± 0.3 |
| enriched | 32.4 ± 2.0 *** | 9.6 ± 0.8 *** | 2.38 ± 2.0 *** | |
| Sample | Unenriched | Grape Pomace Flour | Grape Pomace + Apple Skin Flours | |
|---|---|---|---|---|
| Breadsticks | 71.7 ± 2.1 a | 72.3 ± 1.4 a | 74.1 ± 1.6 a | |
| Focaccia | 63.0 ± 1.8 b | 55.7 ± 1.6 a | 58.9 ± 1.9 ab | |
| Breadsticks | Color | 93.8 ± 1.5 c | 70.4 ± 1.7 a | 79.4 ± 1.3 b |
| Flavor | 91.2 ± 1.4 b | 84.7 ± 1.5 a | 85.2 ± 1.1 a | |
| Mouthfeel | 87.5 ± 1.6 b | 84.1 ± 1.4 a | 91.6 ± 1.6 c | |
| Focaccia | Color | 99.0 ± 1.0 b | 56.2 ± 1.6 a | 56.4 ± 1.7 a |
| Flavor | 87.4 ± 1.4 b | 68.4 ± 1.1 ab | 63.3 ± 1.4 a | |
| Mouthfeel | 87.3 ± 1.7 c | 65.9 ± 1.5 a | 83.1 ± 1.2 b | |
| Item Pool | Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree |
|---|---|---|---|---|---|
| 1. Food plays an important role in my personal health | - | - | - | 15 | 85 |
| 2. I think I would buy a product enriched with food industry by-products, such as grapes and apples, which are rich in antioxidants and/or fiber, in order to improve their nutritional characteristics | - | 5 | 16 | 45 | 33 |
| 3. Functional foods are likely to have a beneficial impact on my personal health | - | 3 | 19 | 45 | 33 |
| 4. Functional foods are a convenient way of meeting recommended daily intakes, which I would never meet with my conventional diet | 7 | 31 | 27 | 24 | 12 |
| 5. I do believe that enriched foods are considered healthier than traditional ones | 12 | 33 | 40 | 12 | 3 |
| 6. Functional foods are acceptable for me, even if they taste worse than the conventional alternative foods | 4 | 31 | 39 | 21 | 5 |
| 7. According to my personal opinion, I would purchase a product enriched with food industry by-products to enhance and promote the concept of reusing such resources in the production of new foods | 3 | 7 | 15 | 40 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, G.; Magni, M.; Marin, V.; Pichler, A.; Zatelli, D.; Robatscher, P.; Polenghi, O.; Cerne, V.L.; Oberhuber, M.; Ciani, S. Upcycling of By-Products from Autochthonous Red Grapes and Commercial Apples as Ingredients in Baked Goods: A Comprehensive Study from Processing to Consumer Consumption. Antioxidants 2025, 14, 798. https://doi.org/10.3390/antiox14070798
Cardone G, Magni M, Marin V, Pichler A, Zatelli D, Robatscher P, Polenghi O, Cerne VL, Oberhuber M, Ciani S. Upcycling of By-Products from Autochthonous Red Grapes and Commercial Apples as Ingredients in Baked Goods: A Comprehensive Study from Processing to Consumer Consumption. Antioxidants. 2025; 14(7):798. https://doi.org/10.3390/antiox14070798
Chicago/Turabian StyleCardone, Gaetano, Martina Magni, Veronica Marin, Andrea Pichler, Daniele Zatelli, Peter Robatscher, Ombretta Polenghi, Virna Lucia Cerne, Michael Oberhuber, and Silvano Ciani. 2025. "Upcycling of By-Products from Autochthonous Red Grapes and Commercial Apples as Ingredients in Baked Goods: A Comprehensive Study from Processing to Consumer Consumption" Antioxidants 14, no. 7: 798. https://doi.org/10.3390/antiox14070798
APA StyleCardone, G., Magni, M., Marin, V., Pichler, A., Zatelli, D., Robatscher, P., Polenghi, O., Cerne, V. L., Oberhuber, M., & Ciani, S. (2025). Upcycling of By-Products from Autochthonous Red Grapes and Commercial Apples as Ingredients in Baked Goods: A Comprehensive Study from Processing to Consumer Consumption. Antioxidants, 14(7), 798. https://doi.org/10.3390/antiox14070798







