Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Design
2.2. Sample Gatherings
2.3. Intestinal Morphology
2.4. Analysis of Oxidative Stress Markers
2.5. RNA Extraction and qPCR Analysis
2.6. Western Blotting Analysis
2.7. SCFA Analysis
2.8. The 16S rRNA Sequencing
2.9. Statistical Assessment
3. Results
3.1. Maternal GML Supplementation Enhanced Fetal Intrauterine Development
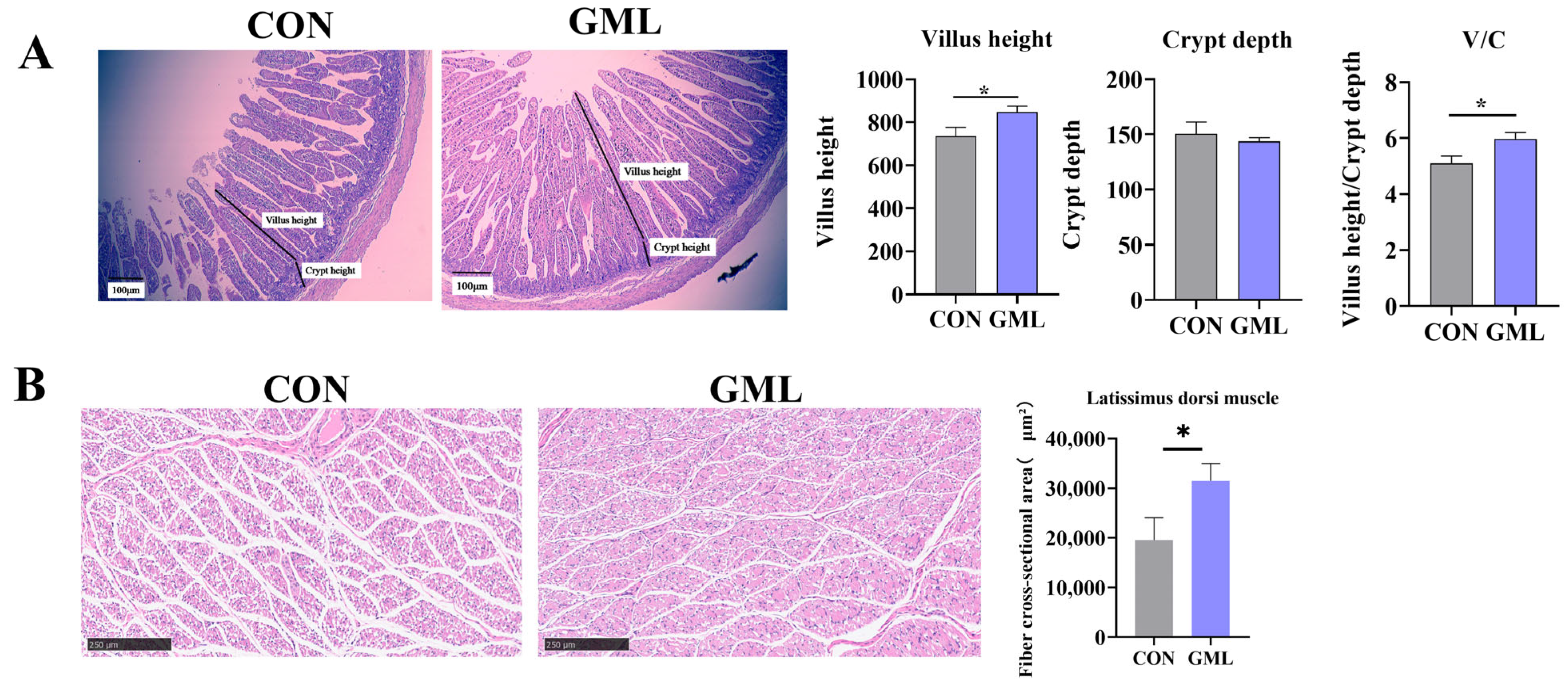
3.2. Maternal GML Supplementation Influenced Jejunum and Muscle Development in Newborn Piglets
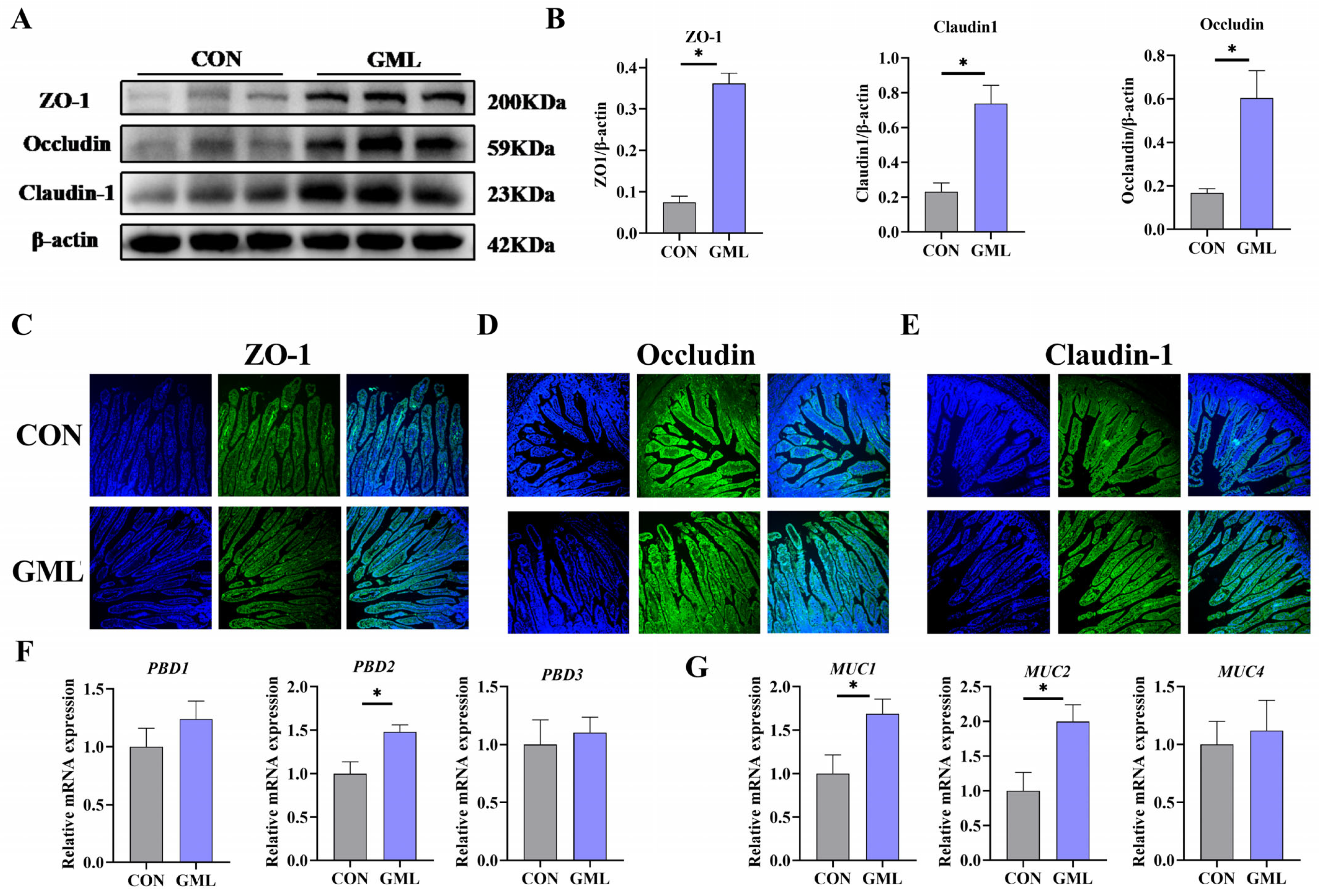
3.3. Maternal GML Supplementation Enhanced Intestinal Barrier Function in Neonatal Piglets
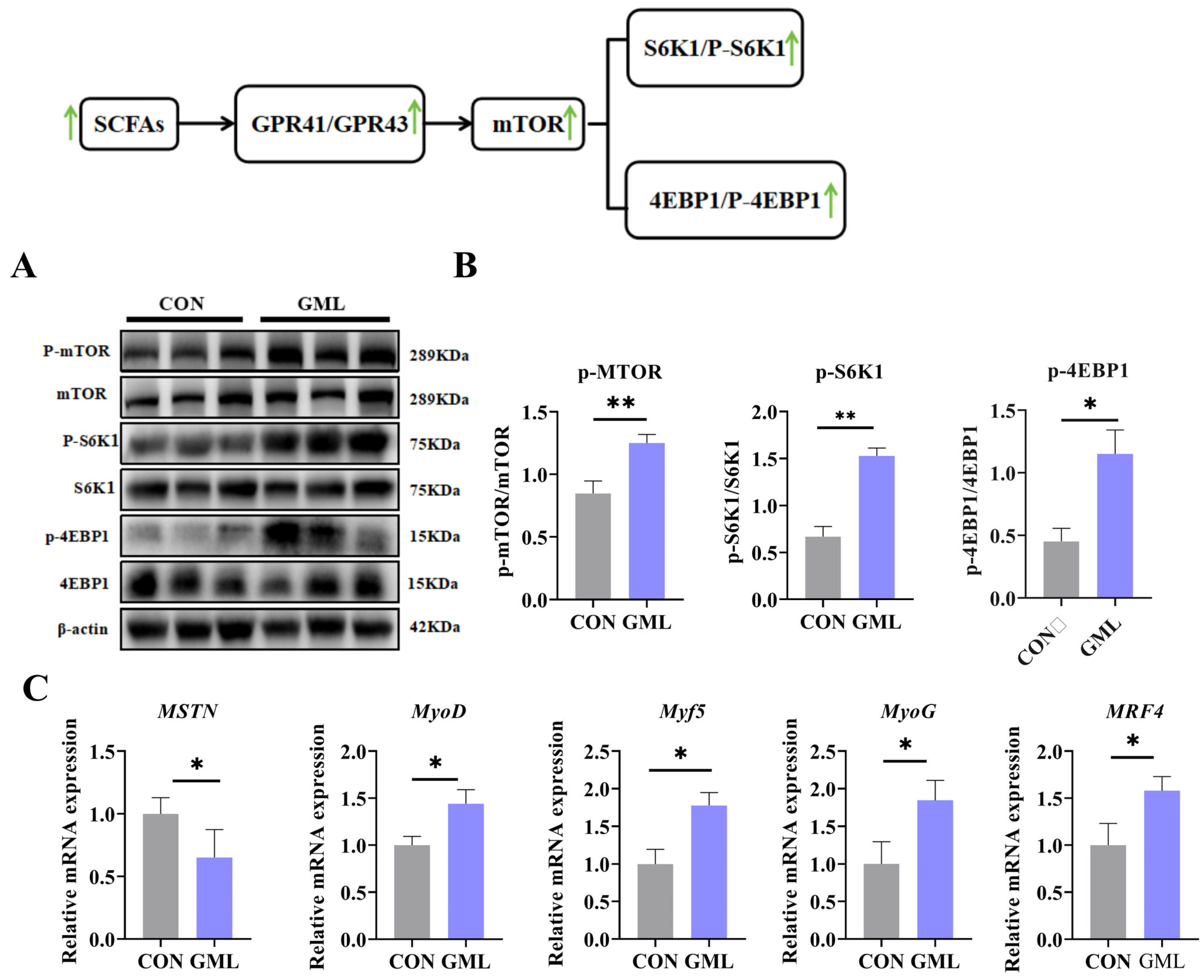
3.4. Maternal GML Supplementation Promoted Longissimus Dorsi Muscle Development in Neonatal Piglets
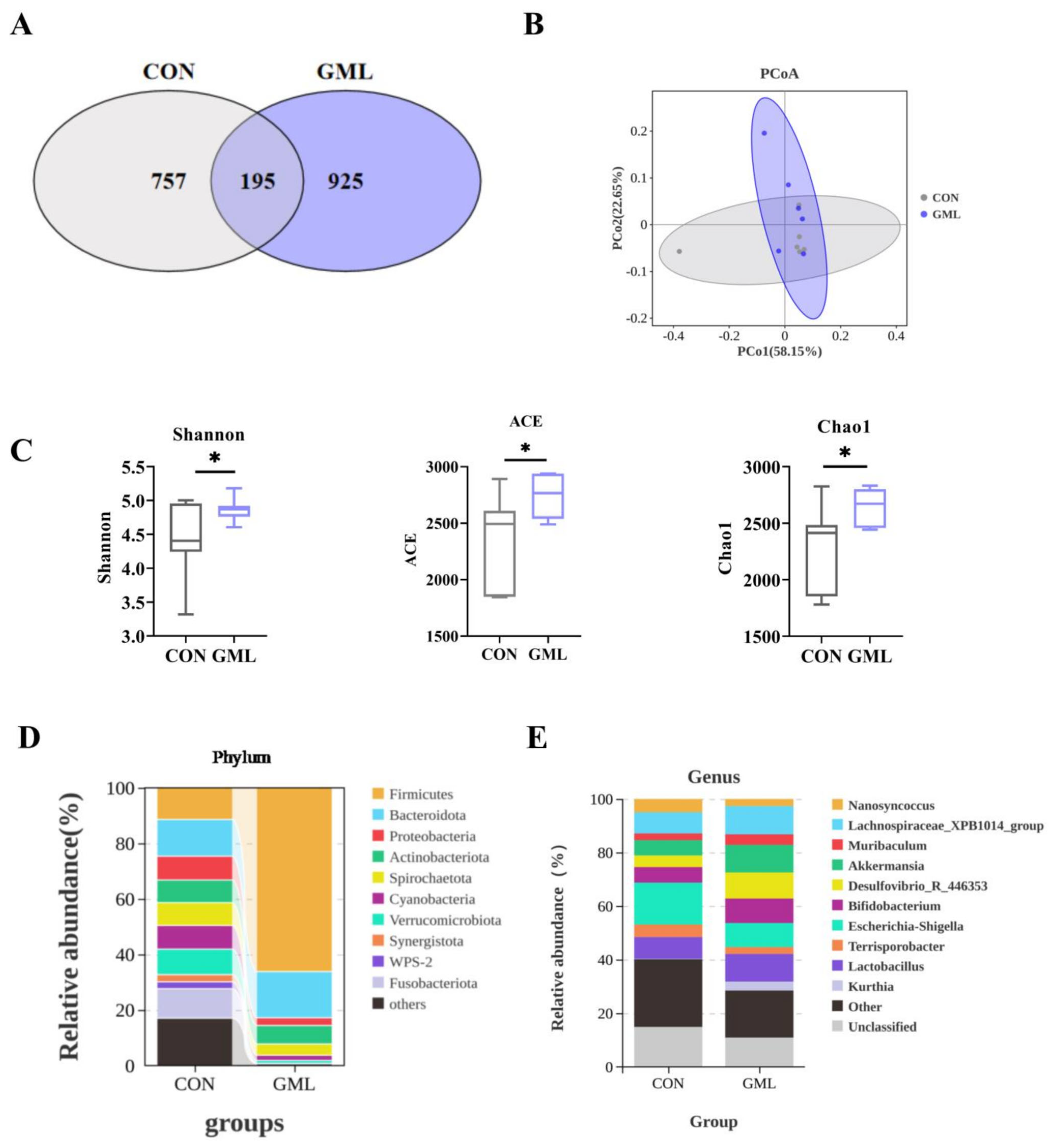
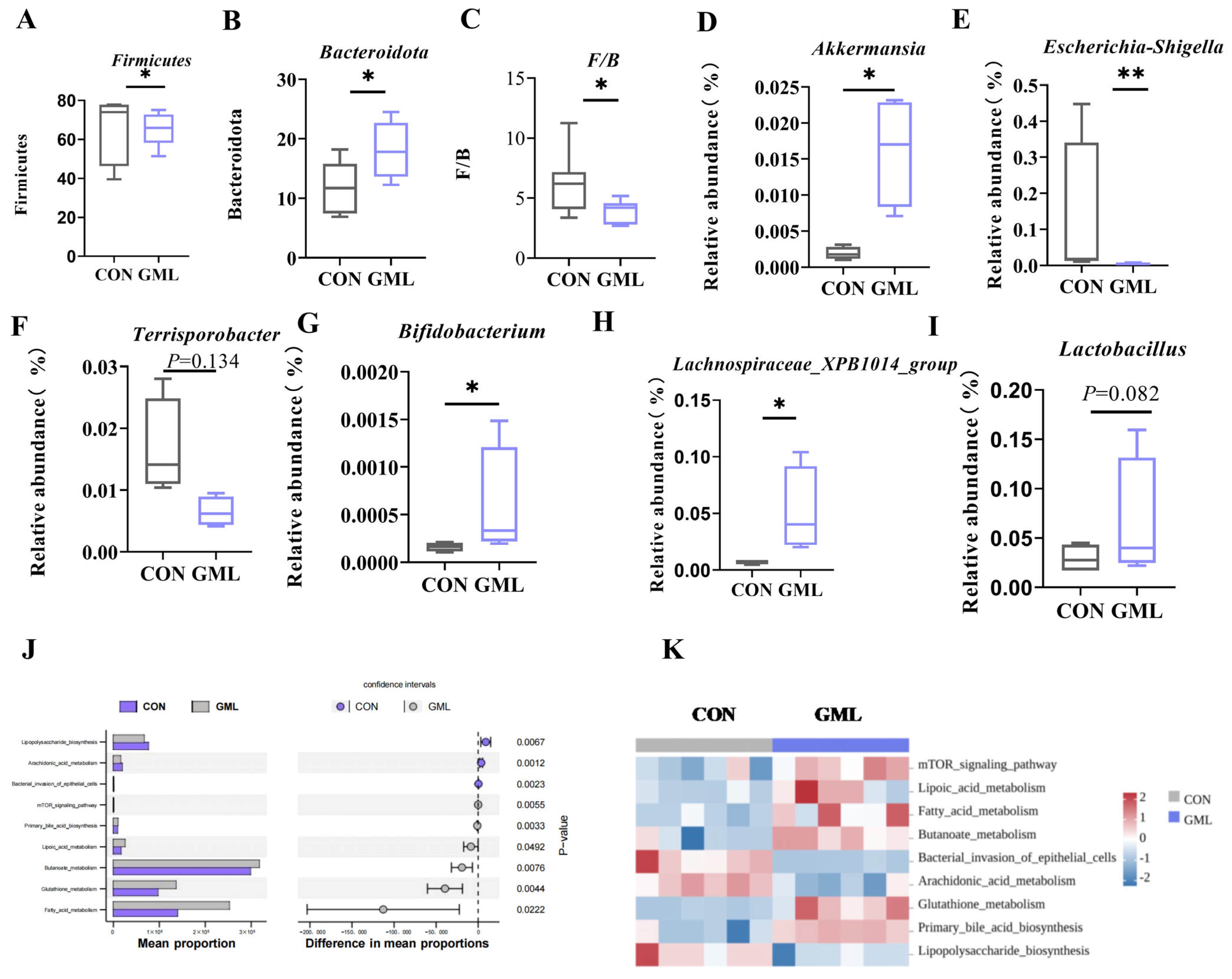
3.5. Maternal GML Supplementation Altered the Intestinal Microbiota of Sows
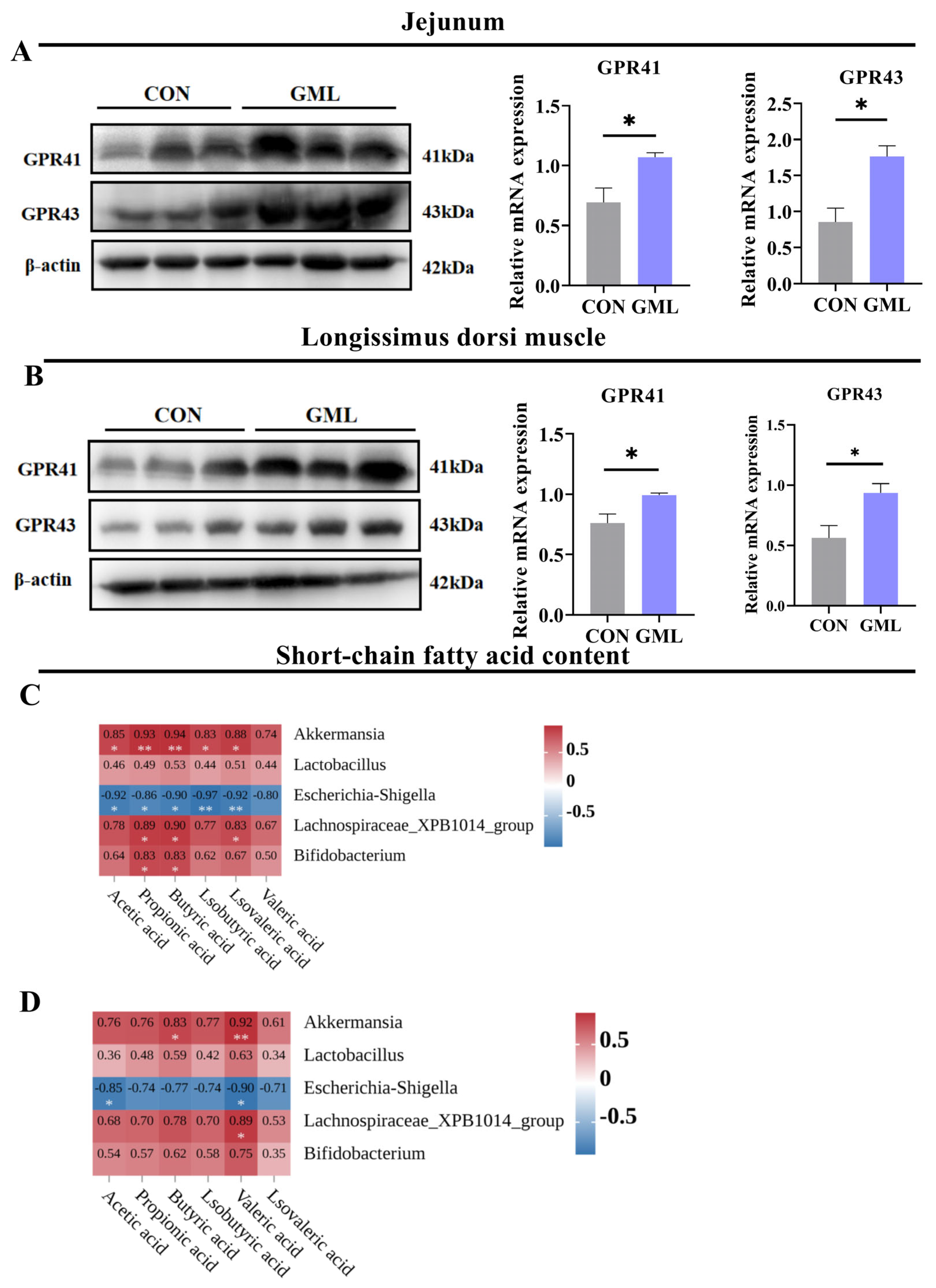
3.6. Maternal GML Supplementation Increased SCFA Levels and Fatty Acid Receptor Expression
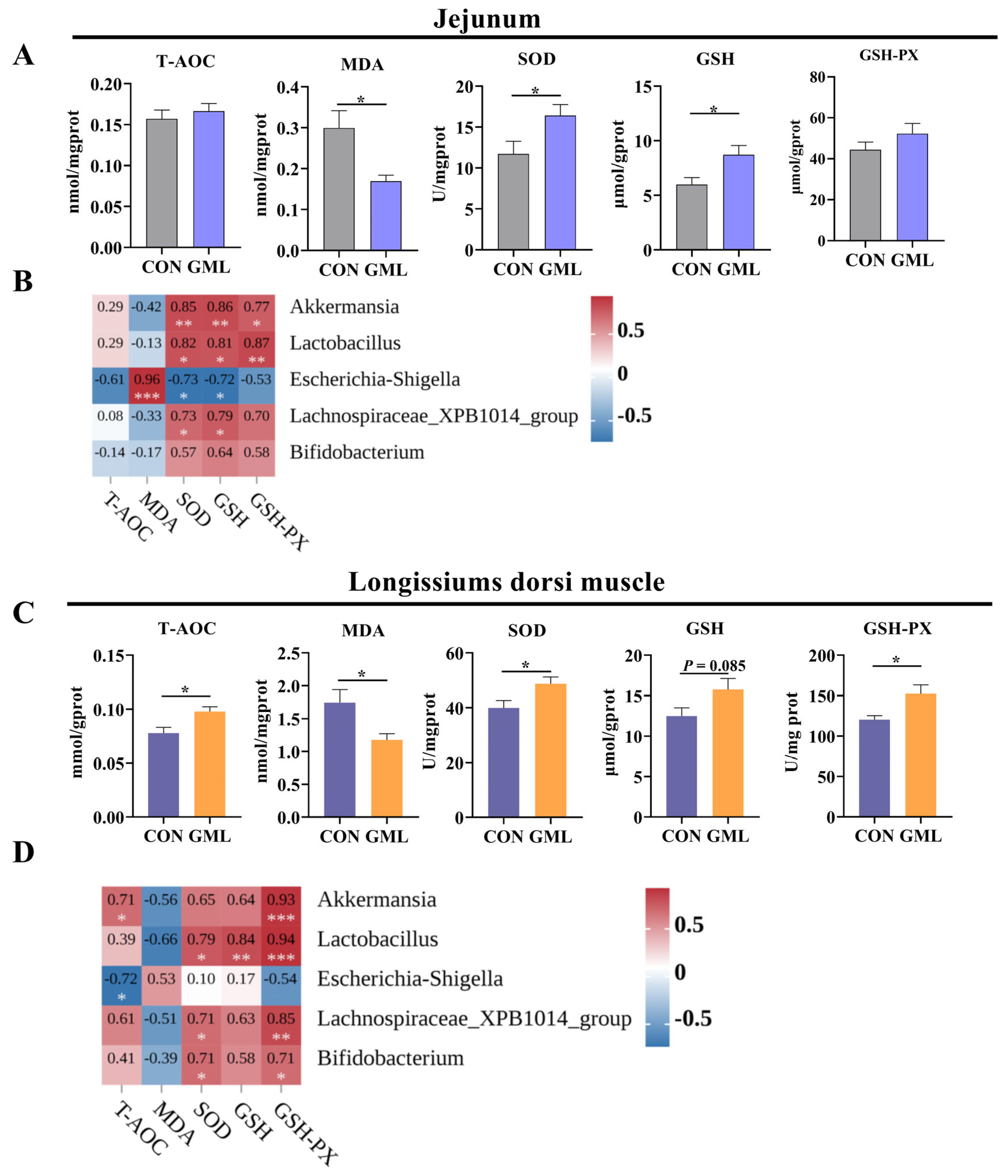
3.7. Maternal GML Supplementation Reduced Oxidative Stress in Newborn Piglets
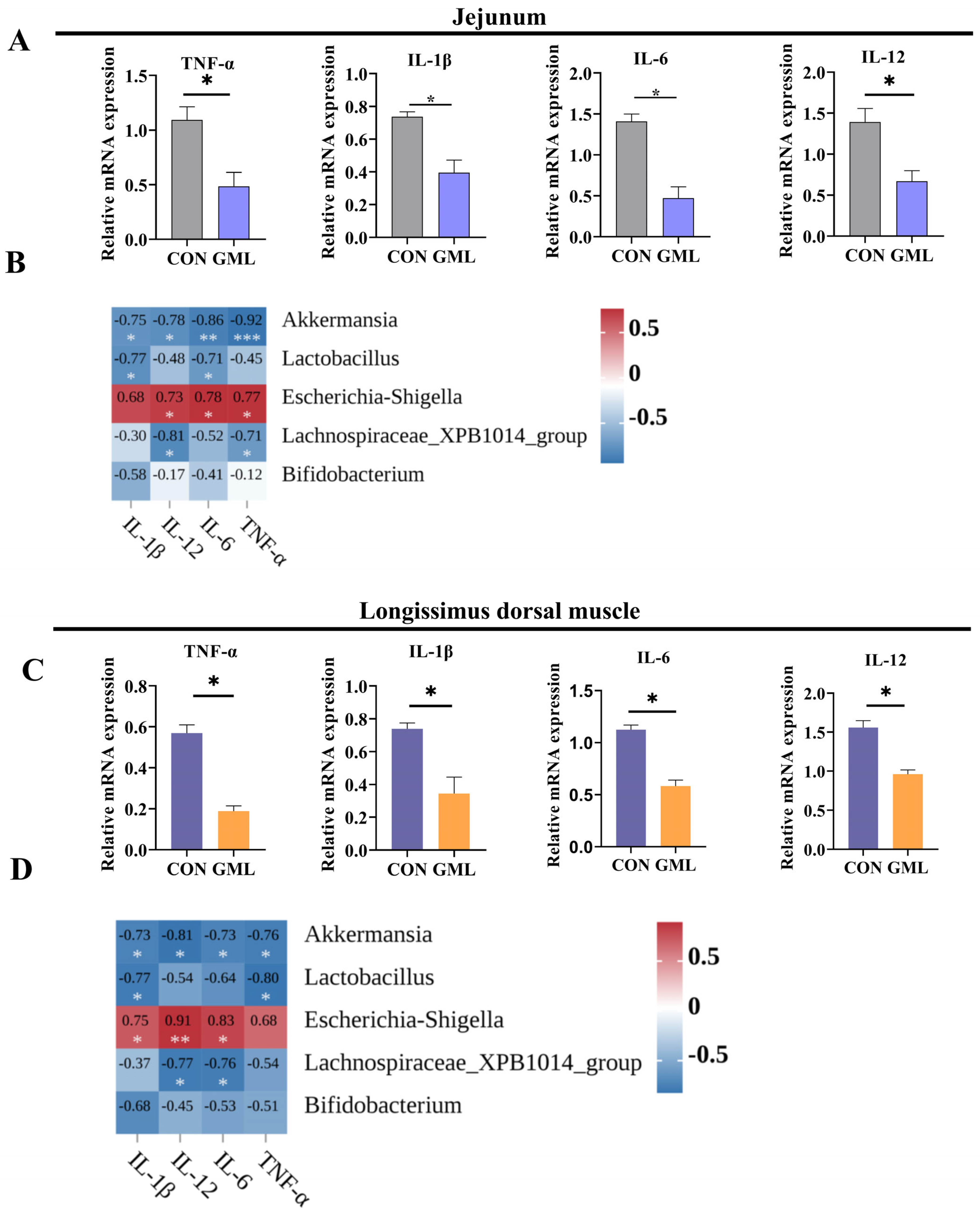
3.8. Maternal GML Supplementation Attenuated Inflammation in Neonatal Piglets
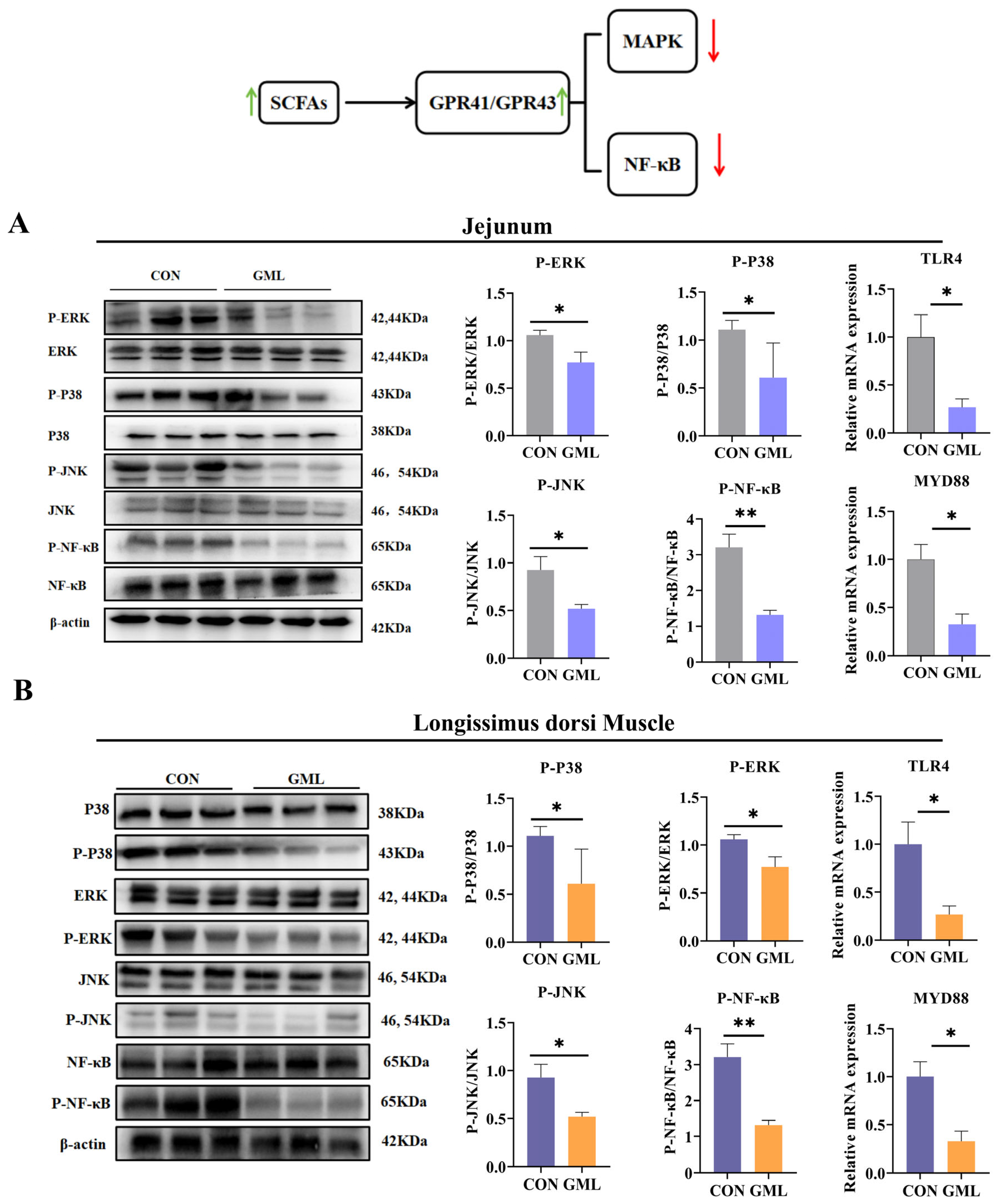
3.9. Maternal GML Supplementation Suppressed MAPK/NF-κB Pathways
4. Discussion
4.1. Maternal GML Supplementation Enhances Fetal Development and Growth
4.2. Impact of Maternal GML on Intestinal Barrier and Muscle Development
4.3. Gut Microbiota Modulation and Its Role in Maternal–Fetal Health
4.4. Anti-Inflammatory and Antioxidant Effects of Maternal GML Supplementation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediators Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, H.; Cao, L.; Mou, D.; Ding, D.; Qin, B.; Che, L.; Fang, Z.; Xu, S.; Zhuo, Y.; et al. Maternal organic selenium supplementation during gestation enhances muscle fiber area and muscle fiber maturation of offspring in porcine model. J. Anim. Sci. Biotechnol. 2022, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Buey, B.; Grasa, L.; Mesonero, J.E.; Latorre, E. Protective role of short-chain fatty acids on intestinal oxidative stress induced by TNF-alpha. Cell Stress. Chaperones 2024, 29, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiong, Y.; Guo, D.; Deng, G.; Wu, H. The gut-reproductive axis: Bridging microbiota balances to reproductive health and fetal development. Int. Immunopharmacol. 2024, 144, 113627. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut-Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Zhang, H.; Zha, X.; Zhang, B.; Zheng, Y.; Elsabagh, M.; Wang, H.; Wang, M. Gut microbiota contributes to bisphenol A-induced maternal intestinal and placental apoptosis, oxidative stress, and fetal growth restriction in pregnant ewe model by regulating gut-placental axis. Microbiome 2024, 12, 28. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3987–4002. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Wang, J.; et al. Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow-Piglet Model. Vet. Sci. 2025, 12, 24. [Google Scholar] [CrossRef]
- Li, X.; Qu, S.; Song, X.; Wu, C.; Shen, J.; Zhu, K. In Situ Neutralization and Detoxification of LPS to Attenuate Hyperinflammation. Adv. Sci. 2023, 10, e2302950. [Google Scholar] [CrossRef]
- Kisioglu, B.; Onal, E.; Karabulut, D.; Onbasilar, I.; Akyol, A. Neuroprotective Roles of Lauric Acid and Resveratrol: Shared Benefits in Neuroinflammation and Anxiety, Distinct Effects on Memory Enhancement. Food Sci. Nutr. 2024, 12, 9735–9748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-Monolaurate-Mediated Attenuation of Metabolic Syndrome is Associated with the Modulation of Gut Microbiota in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801417. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial Emulsifier-Glycerol Monolaurate Induces Metabolic Syndrome, Gut Microbiota Dysbiosis, and Systemic Low-Grade Inflammation in Low-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yi, Y.; Wu, J.; Yang, Q.; Tan, B.; Chi, S. Effects of Plant-Derived Glycerol Monolaurate (GML) Additive on the Antioxidant Capacity, Anti-Inflammatory Ability, Muscle Nutritional Value, and Intestinal Flora of Hybrid Grouper (Epinephelus fuscoguttatusfemale symbol x Epinephelus lanceolatusmale symbol). Metabolites 2022, 12, 1089. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Dong, S.; Ma, Y. Supplementation with alpha-glycerol monolaurate during late gestation and lactation enhances sow performance, ameliorates milk composition, and improves growth of suckling piglets. J. Anim. Sci. Biotechnol. 2023, 14, 47. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Weng, M.; Shen, Y.; Lai, W.; Hao, T.; Yao, C.; Bu, X.; Du, J.; Li, Y.; et al. Glycerol monolaurate improved intestinal barrier, antioxidant capacity, inflammatory response and microbiota dysbiosis in large yellow croaker (Larimichthys crocea) fed with high soybean oil diets. Fish. Shellfish. Immunol. 2023, 141, 109031. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, H.; Mi, Y.; Xue, Y.; Li, J.; Ma, Y. Effects of essential oil coated with glycerol monolaurate on growth performance, intestinal morphology, and serum profiles in weaned piglets. Anim. Biosci. 2023, 36, 753–760. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol Monolaurate Ameliorated Intestinal Barrier and Immunity in Broilers by Regulating Intestinal Inflammation, Antioxidant Balance, and Intestinal Microbiota. Front. Immunol. 2021, 12, 713485. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, M.; Xiong, L.; Lin, T.; Zhang, S.; Yue, X.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal supplementation with glycerol monolaurate improves the intestinal health of suckling piglets by inhibiting the NF-kappaB/MAPK pathways and improving oxidative stability. Food Funct. 2023, 14, 3290–3303. [Google Scholar] [CrossRef]
- Liu, H.; Xu, K.; Wang, H.; Lin, H.; Yang, X.; Wang, X.; Zhao, J.; Ma, B.; Shu, Q.; Lu, Y.; et al. Effects of different forms of amino acid supplementation on the performance and intestinal barrier function of laying hens fed a low-protein diet. Poult. Sci. 2024, 103, 104375. [Google Scholar] [CrossRef]
- Bryan, E.E.; Chen, X.; Smith, B.N.; Dilger, R.N.; Dilger, A.C. Maternal immune activation and dietary soy isoflavone supplementation influence pig immune function but not muscle fiber formation. J. Anim. Sci. 2022, 100, skac134. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liang, Y.; Geng, J.; Yan, Y.; Ding, R.; He, M. Gestational Interrelationships among Gut-Metabolism-Transcriptome in Regulating Early Embryo Implantation and Placental Development in Mice. Microorganisms 2024, 12, 1902. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.J.; Feng, J.T.; Xiang, T.; Liao, C.L.; Zhou, Y.P.; Xuan, R.R. Expression and clinical significance of short-chain fatty acids in patients with intrahepatic cholestasis of pregnancy. World J. Hepatol. 2024, 16, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.Y. Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am. J. Clin. Nutr. 1999, 70, 557–565. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Ma, Z.; Fu, Z.; Zhao, Y.; Zeng, X.; Lin, G.; Zhang, S.; Guan, W.; Chen, F. Enhanced Antioxidative Capacity Transfer between Sow and Fetus via the Gut-Placenta Axis with Dietary Selenium Yeast and Glycerol Monolaurate Supplementation during Pregnancy. Antioxidants 2024, 13, 141. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, T.; Li, S.; Song, Z.; Zhang, H.; Zhang, L.; Wang, T. Effects of Early Resveratrol Intervention on Skeletal Muscle Mitochondrial Function and Redox Status in Neonatal Piglets with or without Intrauterine Growth Retardation. Oxid. Med. Cell Longev. 2020, 2020, 4858975. [Google Scholar] [CrossRef]
- He, K.J.; Dong, J.H.; Ouyang, X.M.; Huo, Y.N.; Cheng, X.S.; Lin, Y.; Li, Y.; Gong, G.; Liu, J.; Ren, J.L.; et al. Glycerol monolaurate ameliorates DSS-induced acute colitis by inhibiting infiltration of Th17, neutrophils, macrophages and altering the gut microbiota. Front. Nutr. 2022, 9, 911315. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Zou, D.; Yang, Y.; Ji, F.; Lv, R.; Xu, T.; Hu, C. DUOX2-Induced Oxidative Stress Inhibits Intestinal Angiogenesis through MMP3 in a Low-Birth-Weight Piglet Model. Antioxidants 2023, 12, 1800. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Cortes-Araya, Y.; Stenhouse, C.; Salavati, M.; Dan-Jumbo, S.O.; Ho, W.; Ashworth, C.J.; Clark, E.; Esteves, C.L.; Donadeu, F.X. KLB dysregulation mediates disrupted muscle development in intrauterine growth restriction. J. Physiol. 2022, 600, 1771–1790. [Google Scholar] [CrossRef] [PubMed]
- Spreeuwenberg, M.A.; Verdonk, J.M.; Gaskins, H.R.; Verstegen, M.W. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 2001, 131, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- McCracken, B.A.; Spurlock, M.E.; Roos, M.A.; Zuckermann, F.A.; Gaskins, H.R. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J. Nutr. 1999, 129, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterology Res. 2018, 11, 261–263. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahceci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Alim, A.; Ren, D.; Zhao, Y.; Yang, X. Regulatory Effects of Stachyose on Colonic and Hepatic Inflammation, Gut Microbiota Dysbiosis, and Peripheral CD4(+) T Cell Distribution Abnormality in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2019, 67, 11665–11674. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Jayakumar, T.; Lin, K.C.; Chang, C.C.; Hsia, C.W.; Manubolu, M.; Huang, W.C.; Sheu, J.R.; Hsia, C.H. Targeting MAPK/NF-kappaB Pathways in Anti-Inflammatory Potential of Rutaecarpine: Impact on Src/FAK-Mediated Macrophage Migration. Int. J. Mol. Sci. 2021, 2, 92. [Google Scholar] [CrossRef]
- Charest-Pekeski, A.J.; Sheta, A.; Taniguchi, L.; McVey, M.J.; Floh, A.; Sun, L.; Aujla, T.; Cho, S.K.S.; Ren, J.; Crawford-Lean, L.; et al. Achieving sustained extrauterine life: Challenges of an artificial placenta in fetal pigs as a model of the preterm human fetus. Physiol. Rep. 2021, 9, e14742. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Miles, J.R.; Freking, B.A. Development of the pig placenta. Soc. Reprod. Fertil. Suppl. 2009, 66, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Sheela, D.L.; Narayanankutty, A.; Nazeem, P.A.; Raghavamenon, A.C.; Muthangaparambil, S.R. Lauric acid induce cell death in colon cancer cells mediated by the epidermal growth factor receptor downregulation: An in silico and in vitro study. Hum. Exp. Toxicol. 2019, 38, 753–761. [Google Scholar] [CrossRef]
| Item | CON | GML | SEM | p-Value |
|---|---|---|---|---|
| Organ index (%) | ||||
| n | 6 | 6 | ||
| Heart | 0.008 | 0.008 | 0.00021 | 0.971 |
| Liver | 0.029 | 0.028 | 0.00069 | 0.660 |
| Spleen | 0.001 | 0.001 | 0.00011 | 0.531 |
| Kidney | 0.009 | 0.010 | 0.00032 | 0.122 |
| Stomach | 0.016 | 0.012 | 0.00187 | 0.244 |
| Intestine | 0.054 | 0.002 | 0.00262 | 0.001 |
| Lung | 0.017 | 0.030 | 0.00245 | 0.002 |
| Organ weight (g) | ||||
| n | 6 | 6 | ||
| Heart | 14.65 | 12.55 | 0.75 | 0.172 |
| Liver | 53.09 | 44.36 | 3.14 | 0.176 |
| Spleen | 2.53 | 2.51 | 0.25 | 0.968 |
| Kidney | 17.22 | 16.15 | 0.88 | 0.565 |
| Stomach | 29.80 | 18.15 | 3.29 | 0.074 |
| Intestine | 95.60 | 126.95 | 5.95 | 0.002 |
| Lung | 30.88 | 33.69 | 1.98 | 0.506 |
| Body weight | 1830.6 | 1548.95 | 0.21 | 0.089 |
| Item | CON | GML | SEM | p-Value |
|---|---|---|---|---|
| N | 6 | 6 | ||
| Feces, μmol/g | ||||
| Acetic acid | 15.73 | 36.19 | 2.823 | 0.0069 |
| Propionic acid | 4.80 | 8.43 | 1.323 | 0.0443 |
| Butyric acid | 1.83 | 4.78 | 0.618 | 0.1144 |
| Isobutyric acid | 2.77 | 2.80 | 0.107 | 0.8945 |
| Valeric acid | 1.37 | 2.59 | 0.057 | 0.0043 |
| Isovaleric acid | 1.45 | 5.86 | 0.886 | 0.0144 |
| Plasma, μmol/g | ||||
| Acetic acid | 41.80 | 54.41 | 0.683 | 0.0002 |
| Propionic acid | 3.24 | 6.17 | 0.247 | 0.0013 |
| Butyric acid | 1.38 | 2.05 | 0.055 | 0.0017 |
| Isobutyric acid | 0.93 | 1.31 | 0.017 | 0.0001 |
| Valeric acid | 1.32 | 1.51 | 0.044 | 0.0428 |
| Isovaleric acid | 1.56 | 2.45 | 0.064 | 0.0007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Wang, J.; Zhao, Y.; Deng, T.; Ma, Z.; Guan, W.; Zeng, X.; Chen, F. Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation. Antioxidants 2025, 14, 783. https://doi.org/10.3390/antiox14070783
Fu Z, Wang J, Zhao Y, Deng T, Ma Z, Guan W, Zeng X, Chen F. Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation. Antioxidants. 2025; 14(7):783. https://doi.org/10.3390/antiox14070783
Chicago/Turabian StyleFu, Zhichao, Jun Wang, Yueqi Zhao, Tanyi Deng, Ziwei Ma, Wutai Guan, Xiangfang Zeng, and Fang Chen. 2025. "Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation" Antioxidants 14, no. 7: 783. https://doi.org/10.3390/antiox14070783
APA StyleFu, Z., Wang, J., Zhao, Y., Deng, T., Ma, Z., Guan, W., Zeng, X., & Chen, F. (2025). Dietary Glyceryl Monolaurate Supplementation During Pregnancy Enhances Fetal Intrauterine Development and Antioxidant Capacity in Sows via Microbiota Modulation. Antioxidants, 14(7), 783. https://doi.org/10.3390/antiox14070783







