Mangosteen Pericarp Extract Mitigates Diquat-Induced Hepatic Oxidative Stress by NRF2/HO-1 Activation, Intestinal Barrier Integrity Restoration, and Gut Microbiota Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design and Diets
2.3. Sample Collection
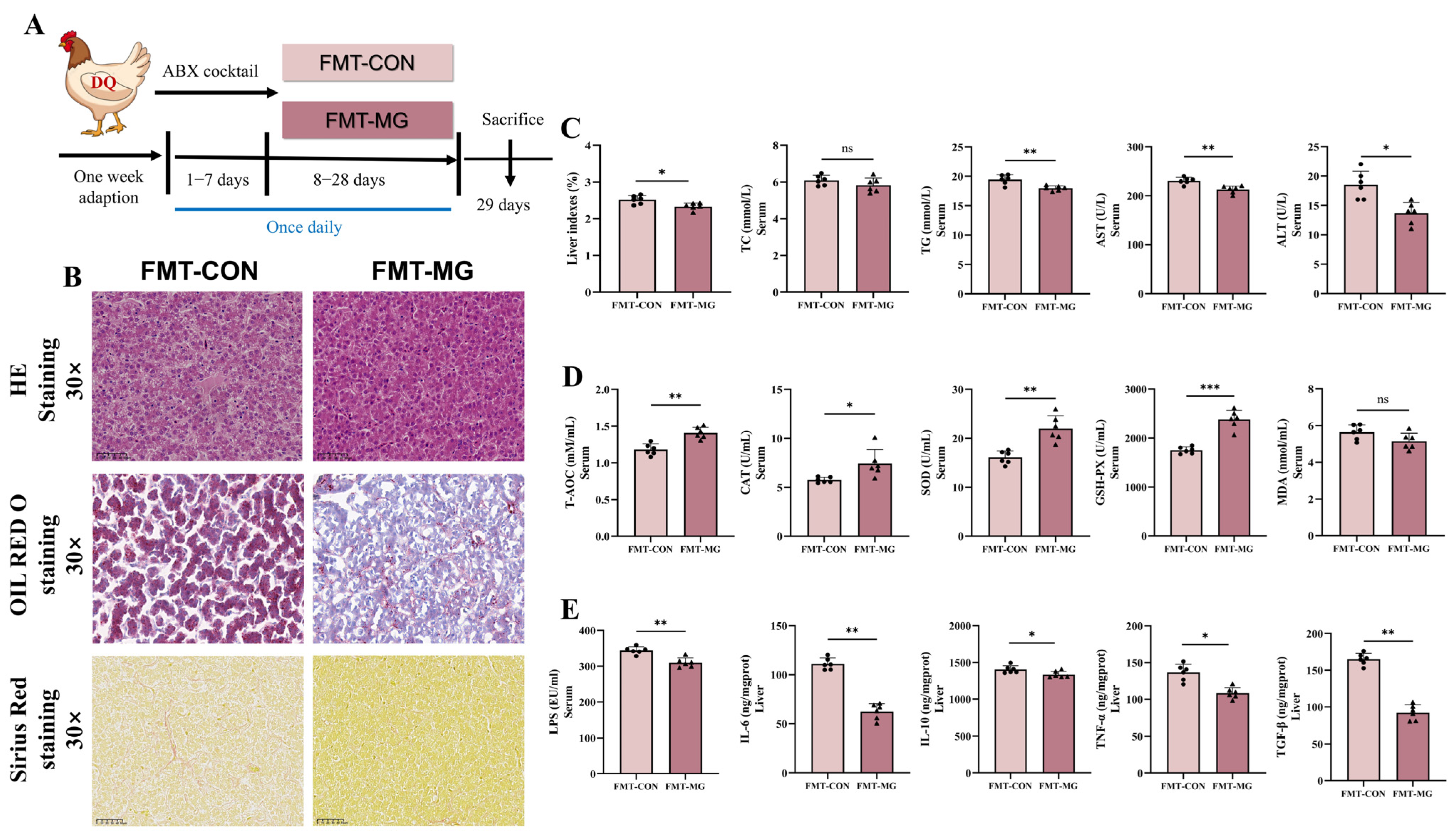
2.4. Fecal Microbiota Transplantation
2.5. Organ Index
2.6. Serum Biochemical Profiling
2.7. Histological Analysis
2.8. Oil Red O Staining and Sirius Red Staining
2.9. Transmission Electron Microscopy (TEM)
2.10. Intestinal Permeability Parameters
2.11. Oxidative Stress Parameter Assays
2.12. Immunofluorescence Analysis
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. RNA Extraction and Quantitative Real-Time PCR Analysis
2.15. Western Blotting
2.16. 16S rDNA Sequencing Analysis
2.17. Statistical Analysis
3. Results
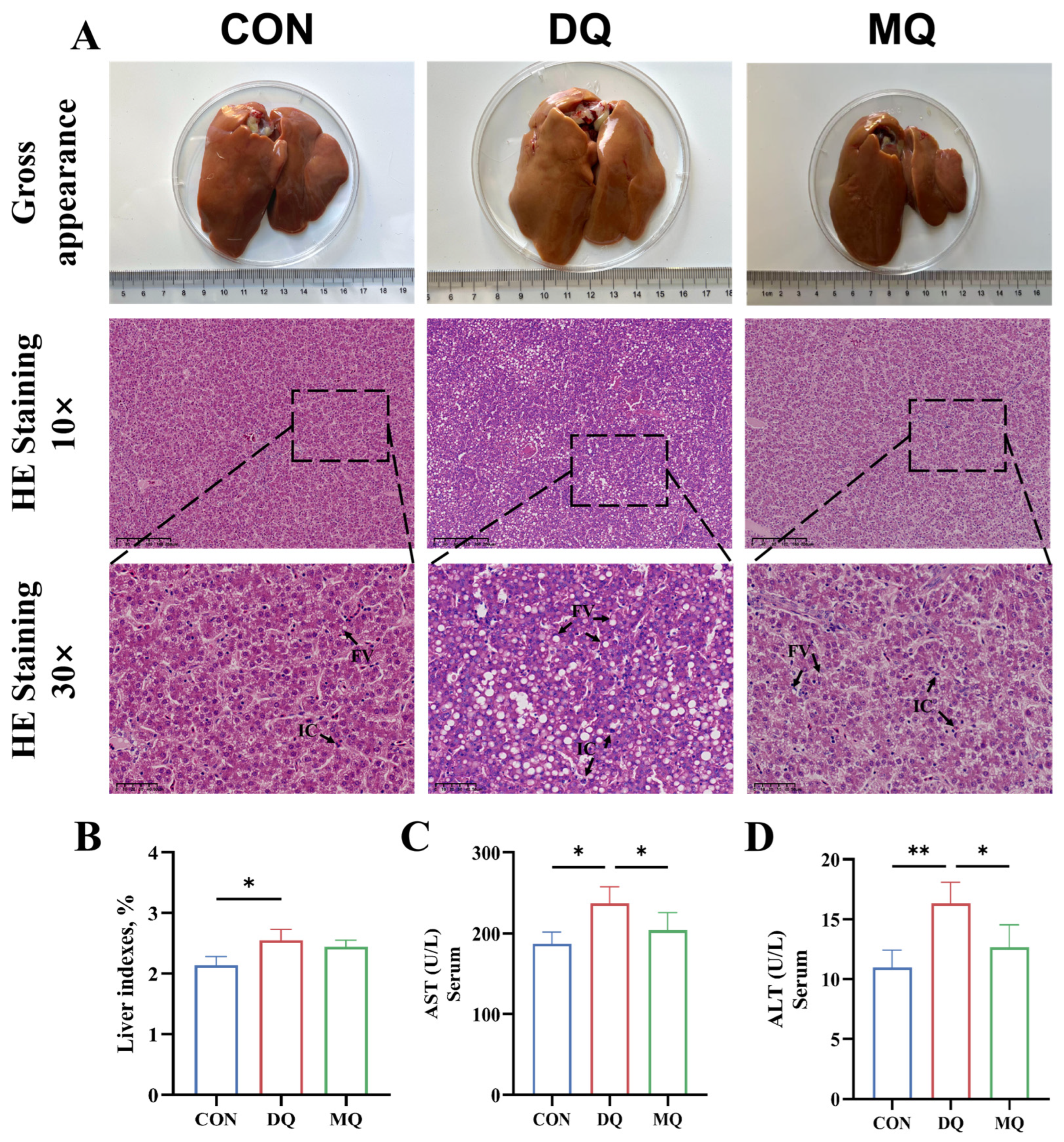
3.1. MPE Ameliorated DQ-Induced Hepatic Oxidative Injury
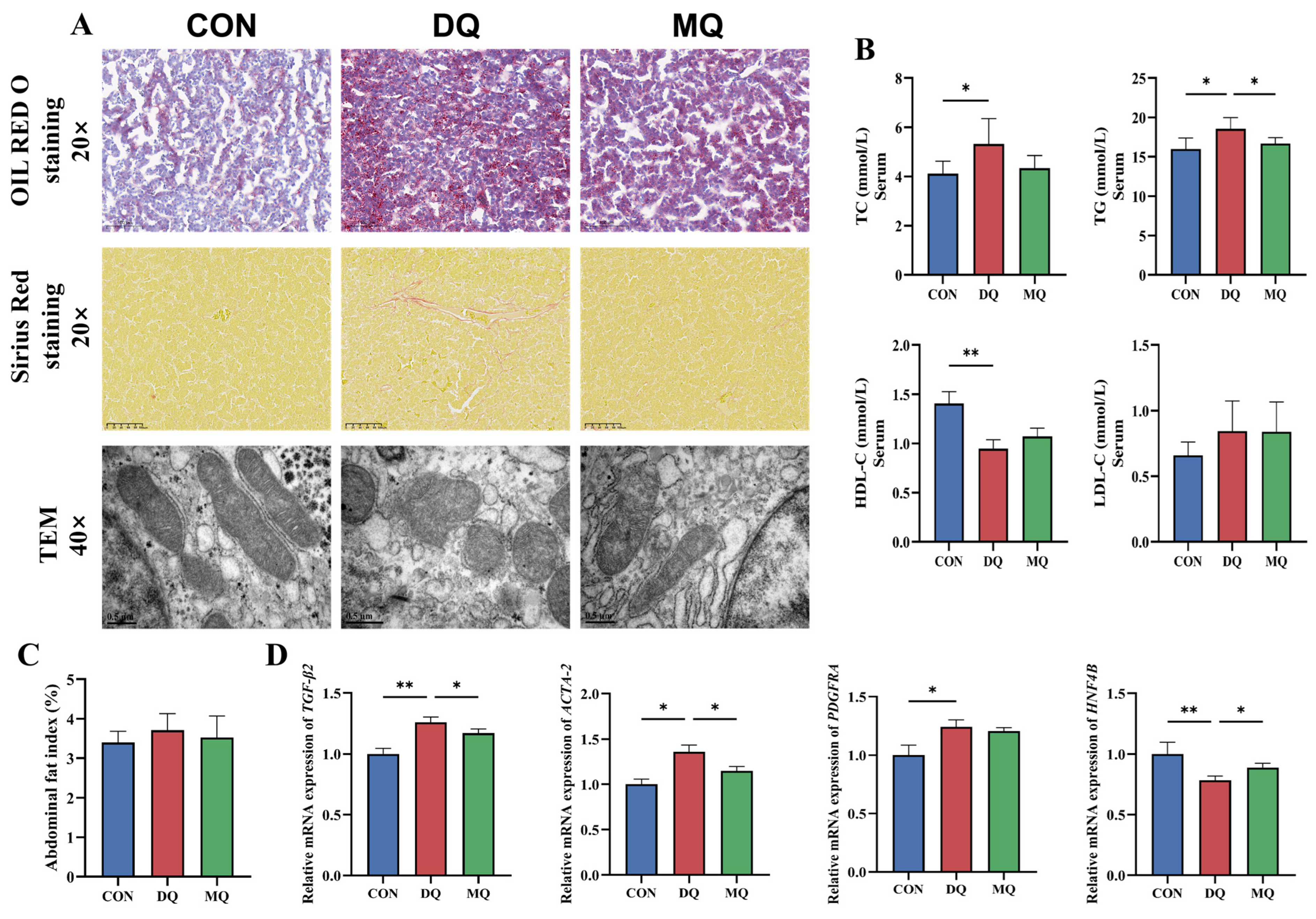
3.2. MPE Alleviated DQ-Induced Hepatic Fibrosis and Lipid Deposition
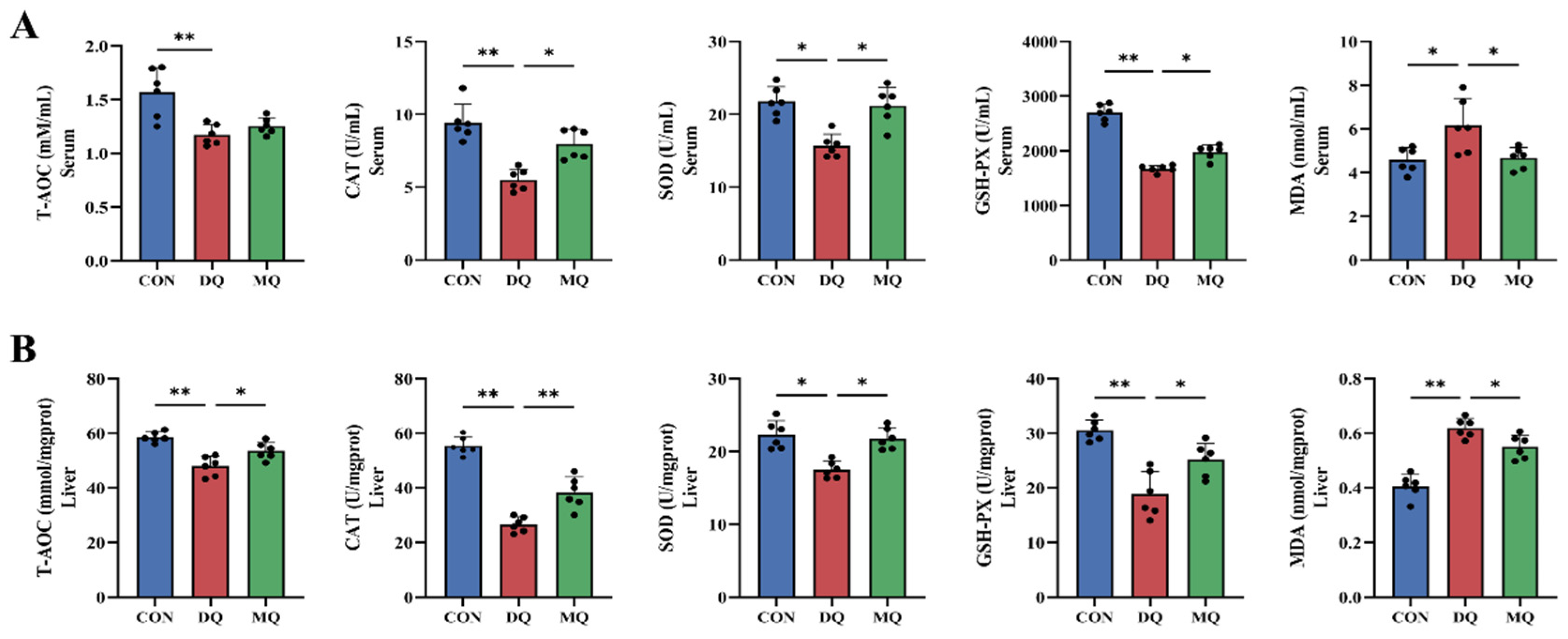
3.3. MPE Enhanced Antioxidant Capacity in DQ-Induced Oxidative Stress
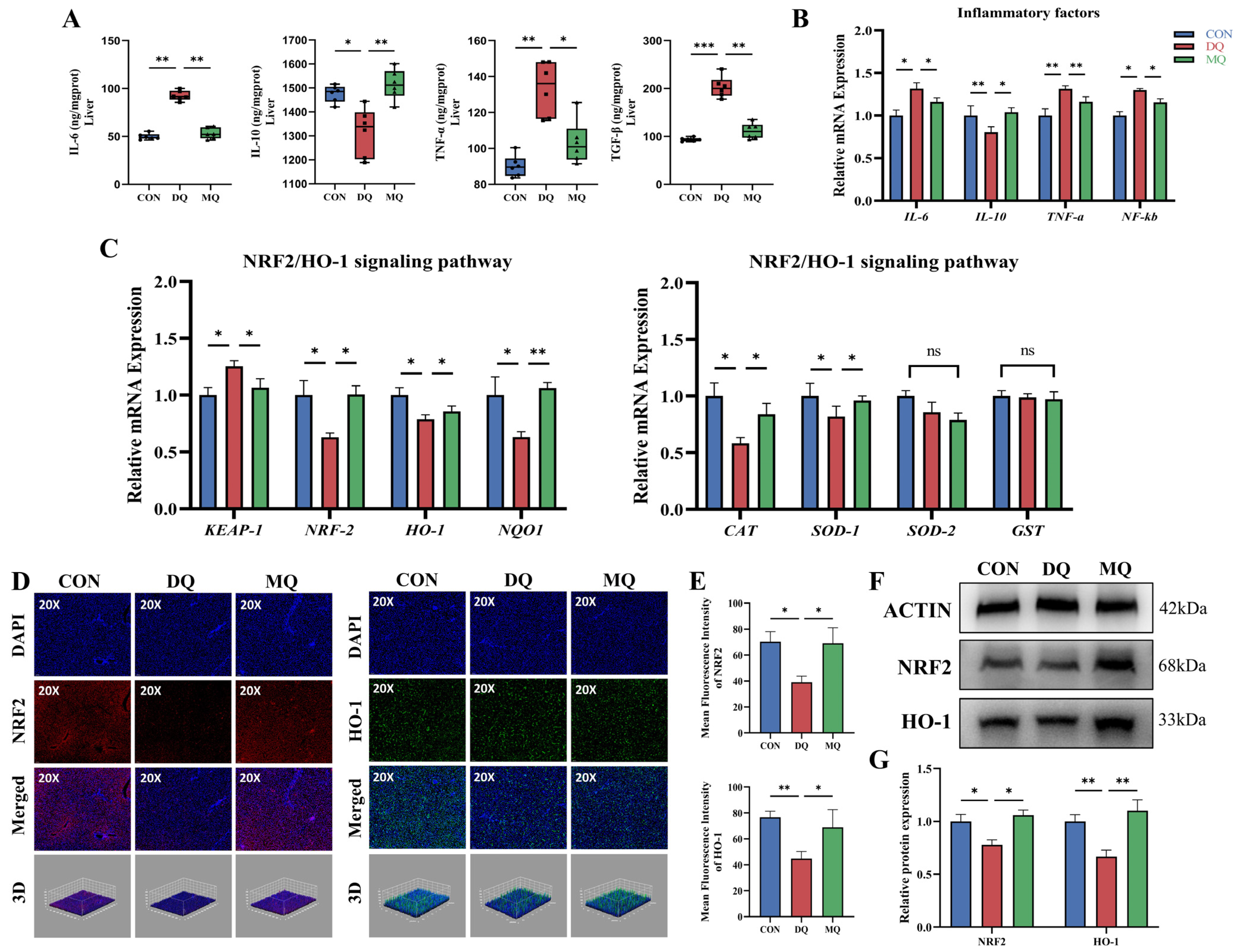
3.4. MPE Attenuated DQ-Induced Hepatic Oxidative Stress by Activating the NRF2/HO-1 Pathway
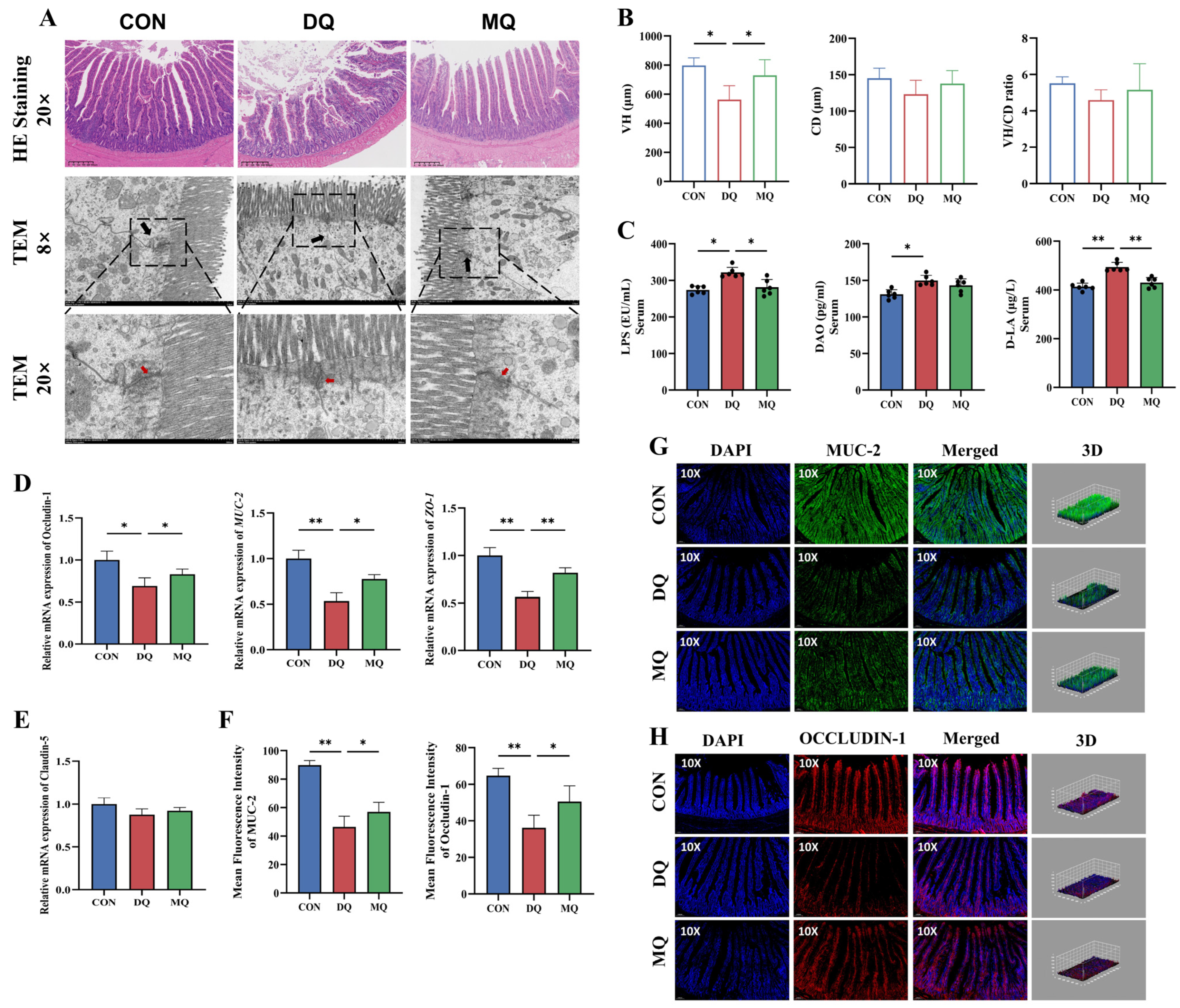
3.5. MPE Ameliorated DQ-Induced Intestinal Barrier Dysfunction and Mucosal Oxidative Injury
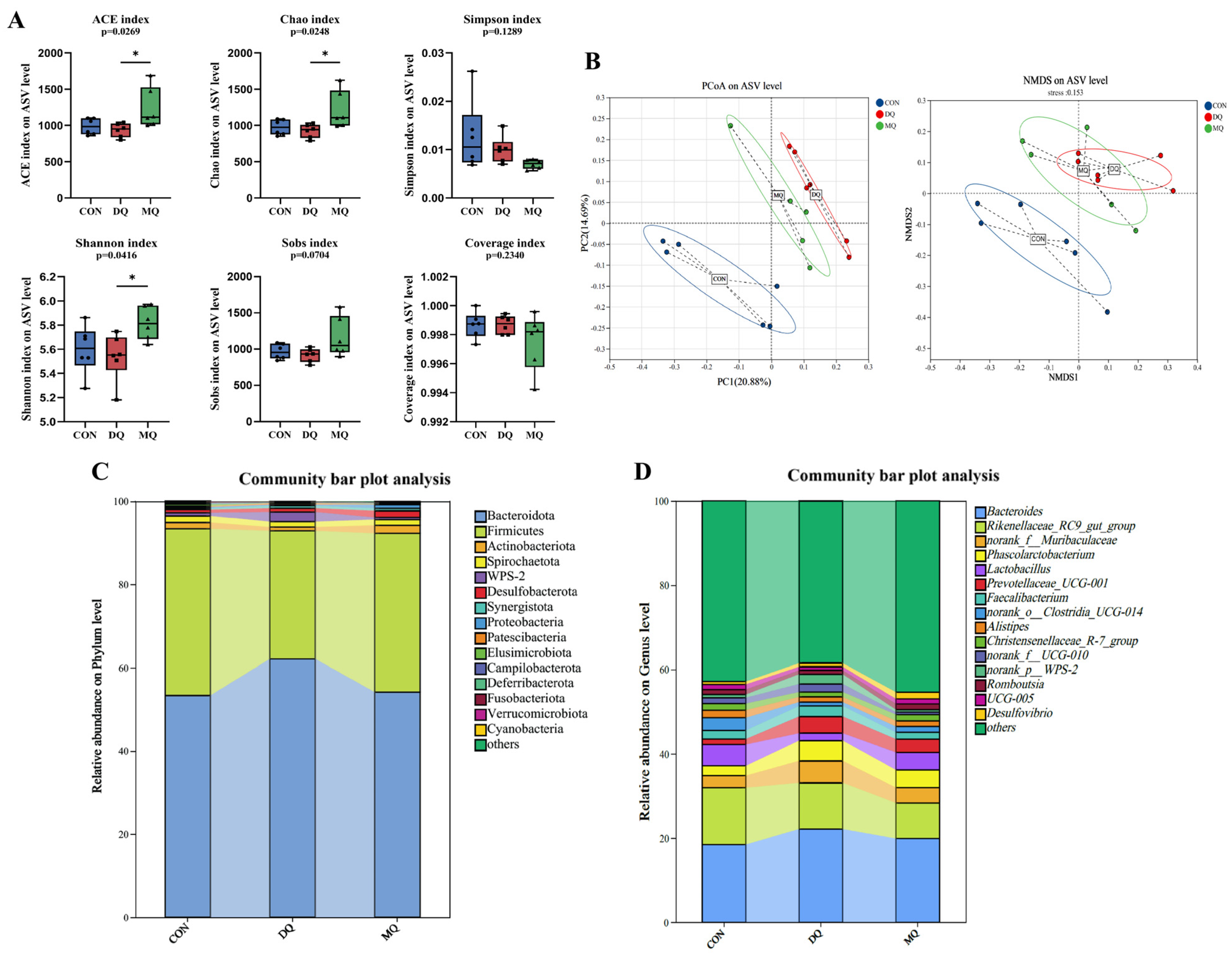
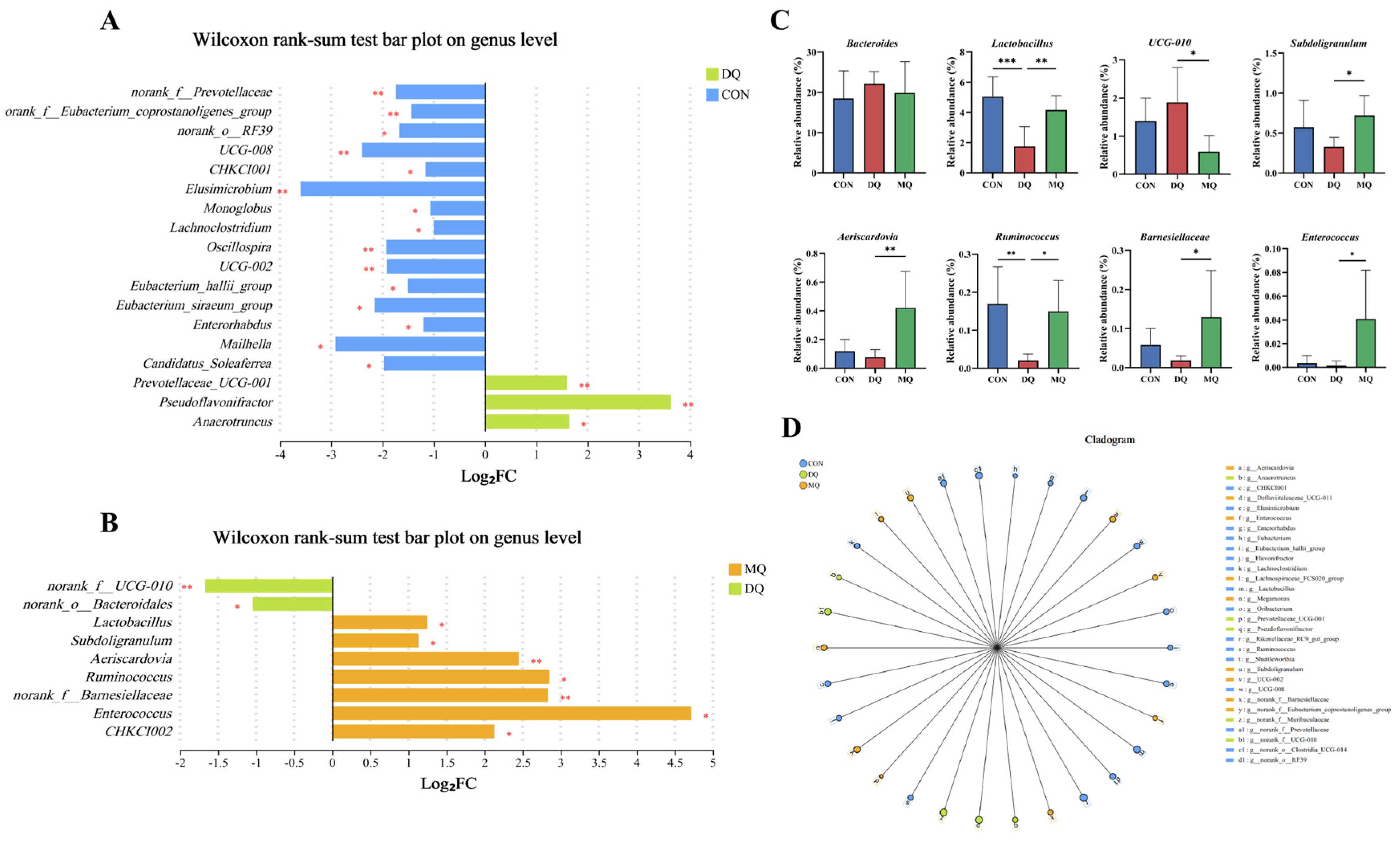
3.6. MPE Attenuated DQ-Induced Gut Microbiota Disorder
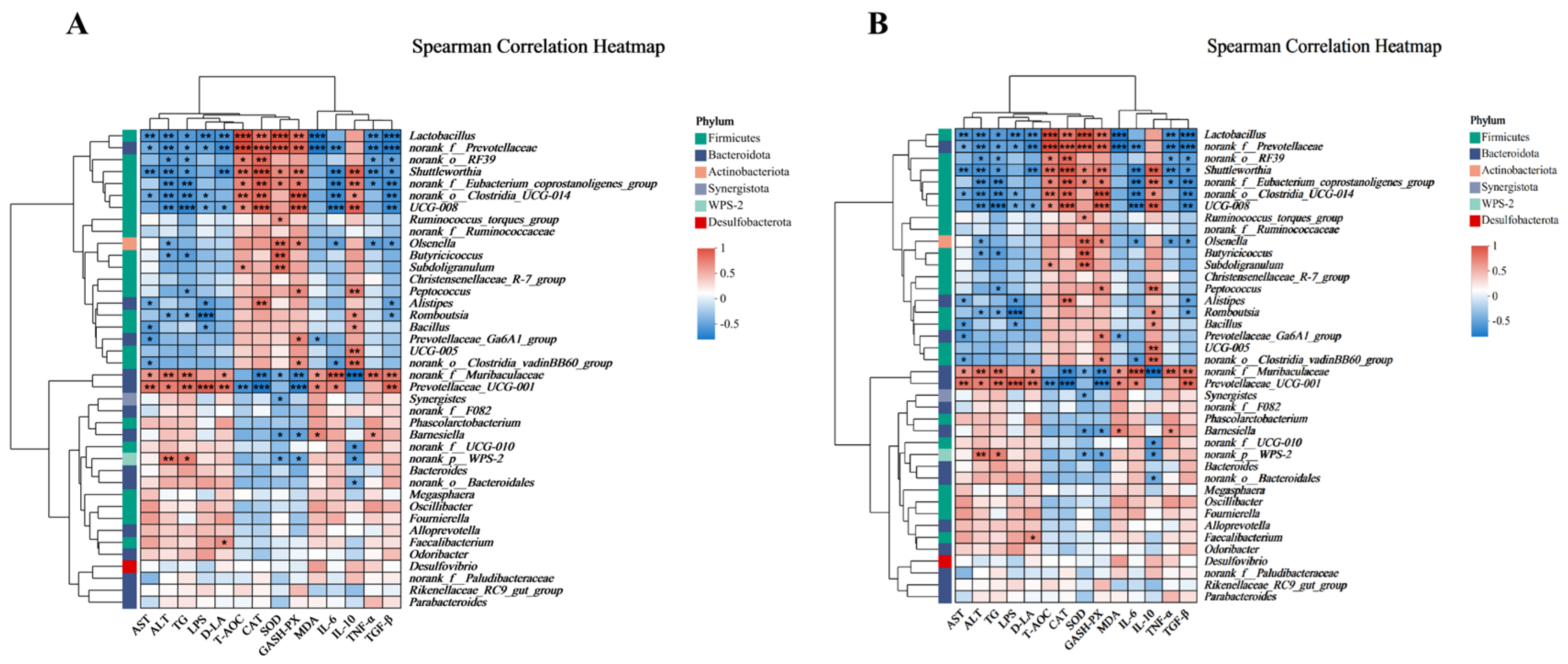
3.7. Correlation Analysis
3.8. FMT Further Verified MPE Attenuated Oxidative Stress Mediated by Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estevez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef]
- Guo, H.; Wan, H.; Lou, W.; Khan, R.U.; You, J.; Huang, B.; Hao, S.; Li, G.; Dai, S. Deoxynivalenol and T-2 toxin cause liver damage and egg quality degradation through endoplasmic reticulum stress in summer laying hens. Int. J. Biometeorol. 2024, 68, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I. Glutathione peroxidases in poultry biology: Part 2. Modulation of enzymatic activities. Worlds Poult. Sci. J. 2019, 74, 239–250. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, L.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Integrated analysis of microbiome and transcriptome reveals the mechanisms underlying the chlorogenic acid-mediated attenuation of oxidative stress and systemic inflammatory responses via gut-liver axis in post-peaking laying hens. J. Anim. Sci. Biotechnol. 2025, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, L.; Zhao, Z.; Wu, Y.; Cao, J.; Cai, H.; Yang, P.; Wen, Z. Rubber (Hevea brasiliensis) seed oil supplementation attenuates immunological stress and inflammatory response in lipopolysaccharide-challenged laying hens. Poult. Sci. 2022, 101, 102040. [Google Scholar] [CrossRef]
- Abbas, A.O.; Alaqil, A.A.; El-Beltagi, H.S.; Abd El-Atty, H.K.; Kamel, N.N. Modulating Laying Hens Productivity and Immune Performance in Response to Oxidative Stress Induced by E. coli Challenge Using Dietary Propolis Supplementation. Antioxidants 2020, 9, 893. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Duan, Y.; Fu, W.; Wang, S.; Ni, Y.; Zhao, R. Cholesterol deregulation induced by chronic corticosterone (CORT) stress in pectoralis major of broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 176, 59–64. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Sun, X.; Li, Y.; Yang, X.; Liu, Y. Dietary betaine supplementation improved egg quality and gut microbes of laying hens under dexamethasone-induced oxidative stress. Poult. Sci. 2024, 103, 104178. [Google Scholar] [CrossRef]
- Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef]
- Arbi, D.; Wahyu, W.; Sutiman Bambang, S.; Bambang, P.; Marisca Evalina, G. Microstructural Characterization of the Garcinia mangostana Fruit at Different Maturity Level. J. Nat. Remedies 2018, 18, 63–70. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cardenas-Rodriguez, N.; Orozco-Ibarra, M.; Perez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Rohman, A.; Rafi, M.; Alam, G.; Muchtaridi, M.; Windarsih, A. Chemical composition and antioxidant studies of underutilized part of mangosteen (Garcinia mangostana L.) fruit. J. Appl. Pharm. Sci. 2019, 9, 47–52. [Google Scholar] [CrossRef]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Aizat, W.M.; Ahmad-Hashim, F.H.; Syed Jaafar, S.N. Valorization of mangosteen, “The Queen of Fruits,” and new advances in postharvest and in food and engineering applications: A review. J. Adv. Res. 2019, 20, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Manasathien, J.; Khanema, P. Antioxidant and cytotoxic activities of mangosteen garcinia mangostana pericarp extracts. Asia-Pac. J. Sci. Technol. 2015, 20, 381–392. [Google Scholar]
- Ovalle-Magallanes, B.; Eugenio-Perez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef]
- Chavan, T.; Muth, A. The diverse bioactivity of alpha-mangostin and its therapeutic implications. Future Med. Chem. 2021, 13, 1679–1694. [Google Scholar] [CrossRef]
- Wang, F.; Ma, H.; Liu, Z.; Huang, W.; Xu, X.; Zhang, X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed. Pharmacother. 2017, 92, 672–680. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Zhan, S.; Wu, L.; Lv, Y.; Huang, W.; Ge, C.; Hu, Z.; Shen, X.; Lin, G.; Yu, D.; Liu, B. Lactobacillus reuteri alleviates diquat induced hepatic impairment and mitochondrial dysfunction via activation of the Nrf2 antioxidant system and suppression of NF-kappaB inflammatory response. Poult. Sci. 2025, 104, 104997. [Google Scholar] [CrossRef]
- Chen, L.; Zu, M.; Cao, Y.; Wang, Y.; Jiang, A.; Bao, S.; Yang, Q.; Liu, G.; Ge, L.; Xiao, B.; et al. Oral Plant-Derived Nanomedicines Mitigate Acetaminophen-Induced Liver Injury by Modulating the Gut-Liver Axis and Intestinal Microbiota Metabolism. Small 2025, 21, e2502001. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, X.; Chen, D.; Yu, B.; He, J. Dietary ferulic acid supplementation enhances antioxidant capacity and alleviates hepatocyte pyroptosis in diquat challenged piglets. J. Anim. Sci. Biotechnol. 2024, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Zha, P.; Wei, L.; Liu, W.; Chen, Y.; Zhou, Y. Effects of dietary supplementation with chlorogenic acid on growth performance, antioxidant capacity, and hepatic inflammation in broiler chickens subjected to diquat-induced oxidative stress. Poult. Sci. 2023, 102, 102479. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Xu, A.; Zhao, B.; Xia, Y.; He, Y.; Xue, H.; Li, S. Dietary selenomethionine reduced oxidative stress by resisting METTL3-mediated m6A methylation level of Nrf2 to ameliorate LPS-induced liver necroptosis in laying hens. J. Nutr. Biochem. 2024, 125, 109563. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.; Wu, Q.; Ma, W. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult. Sci. 2021, 100, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ge, C.; Wu, L.; Hu, Z.; Luo, X.; Huang, W.; Zhan, S.; Shen, X.; Yu, D.; Liu, B. Hepatoprotective effects of magnolol in fatty liver hemorrhagic syndrome hens through shaping gut microbiota and tryptophan metabolic profile. J. Anim. Sci. Biotechnol. 2024, 15, 120. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Z.; Lv, Y.; Ge, C.; Luo, X.; Zhan, S.; Huang, W.; Shen, X.; Yu, D.; Liu, B. Hericium erinaceus polysaccharides ameliorate nonalcoholic fatty liver disease via gut microbiota and tryptophan metabolism regulation in an aged laying hen model. Int. J. Biol. Macromol. 2024, 273, 132735. [Google Scholar] [CrossRef]
- Ge, C.; Luo, X.; Lv, Y.; Wu, L.; Hu, Z.; Huang, W.; Zhan, S.; Shen, X.; Hui, C.; Yu, D.; et al. Essential oils ameliorate the intestinal damages induced by nonylphenol exposure by modulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota regulation. Chemosphere 2024, 362, 142571. [Google Scholar] [CrossRef]
- Liu, B.; Xiong, Y.L.; Jiang, J.; Yu, D.; Lin, G. Cellular antioxidant mechanism of selenium-enriched yeast diets in the protection of meat quality of heat-stressed hens. Food Biosci. 2021, 39, 100798. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, J.; Zhou, Q.; Yu, D. Tolerance and safety evaluation of sodium sulfate: A subchronic study in laying hens. Anim. Nutr. 2021, 7, 576–586. [Google Scholar] [CrossRef]
- Acar, A. In vivo toxicological assessment of diquat dibromide: Cytotoxic, genotoxic, and biochemical approach. Environ. Sci. Pollut. Res. Int. 2021, 28, 47550–47561. [Google Scholar] [CrossRef]
- Cao, S.; Wu, H.; Wang, C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cui, S.; Wang, W.; Jian, T.; Kan, B.; Jian, X. Kidney and lung injury in rats following acute diquat exposure. Exp. Ther. Med. 2022, 23, 275. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Lv, M.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Wang, Q.; Chen, D. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 2014, 5, 49. [Google Scholar] [CrossRef]
- Gou, F.; Lin, Q.; Tu, X.; Zhu, J.; Li, X.; Chen, S.; Hu, C. Hesperidin Alleviated Intestinal Barrier Injury, Mitochondrial Dysfunction, and Disorder of Endoplasmic Reticulum Mitochondria Contact Sites under Oxidative Stress. J. Agric. Food Chem. 2024, 72, 16276–16286. [Google Scholar] [CrossRef]
- Khosravi, S.; Alavian, S.M.; Daryani, N.E.; Zare, A.; Fereshtehnejad, S.M.; Taba, S.; Vakili, T.; Daryani, N.E.; Abdollahzade, S. 1325 Non-Alcoholic Fatty Liver Disease and Correlation of Serum Alanin Aminotransferase (Alt) Level with Histopathologic Findings. J. Hepatol. 2012, 56, S521. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Rana, J.S.; Stroes, E.S.; Despres, J.P.; Shah, P.K.; Kastelein, J.J.; Wareham, N.J.; Boekholdt, S.M.; Khaw, K.T. Beyond low-density lipoprotein cholesterol: Respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J. Am. Coll. Cardiol. 2009, 55, 35–41. [Google Scholar] [CrossRef]
- Li, S.; Hou, L.; Zhu, S.; Yi, Q.; Liu, W.; Zhao, Y.; Wu, F.; Li, X.; Pan, A.; Song, P. Lipid Variability and Risk of Cardiovascular Diseases and All-Cause Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2022, 14, 2450. [Google Scholar] [CrossRef]
- Blas-Garcia, A.; Apostolova, N. Novel Therapeutic Approaches to Liver Fibrosis Based on Targeting Oxidative Stress. Antioxidants 2023, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Sabaj, M.; Tolosa, G.; Herrera Vielma, F.; Zuniga, M.J.; Gonzalez, D.R.; Zuniga-Hernandez, J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-kappaB, Reducing Oxidative Stress and Inflammation. Cells 2021, 10, 3406. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of liver fibrosis and its role in liver cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Chida, T.; Ito, M.; Nakashima, K.; Kanegae, Y.; Aoshima, T.; Takabayashi, S.; Kawata, K.; Nakagawa, Y.; Yamamoto, M.; Shimano, H.; et al. Critical role of CREBH-mediated induction of transforming growth factor beta2 by hepatitis C virus infection in fibrogenic responses in hepatic stellate cells. Hepatology 2017, 66, 1430–1443. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, X.L.; Xu, D.H.; Li, S.; Yang, Q.; Feng, X.; Wei, Y.G.; Li, H.; Yang, L.; Zhang, Y.J.; et al. NPM promotes hepatotoxin-induced fibrosis by inhibiting ROS-induced apoptosis of hepatic stellate cells and upregulating lncMIAT-induced TGF-beta2. Cell Death Dis. 2023, 14, 575. [Google Scholar] [CrossRef]
- Ding, Z.; Cheng, R.; Liu, J.; Zhao, Y.; Ge, W.; Yang, Y.; Xu, X.; Wang, S.; Zhang, J. The suppression of pancreatic lipase-related protein 2 ameliorates experimental hepatic fibrosis in mice. Biochim. Biophys. Acta 2022, 1867, 159102. [Google Scholar] [CrossRef]
- Wei, Q.; Kong, N.; Liu, X.; Tian, R.; Jiao, M.; Li, Y.; Guan, H.; Wang, K.; Yang, P. Pirfenidone attenuates synovial fibrosis and postpones the progression of osteoarthritis by anti-fibrotic and anti-inflammatory properties in vivo and in vitro. J. Transl. Med. 2021, 19, 157. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer cells in the pathogenesis of liver disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hsieh, P.L.; Chao, S.C.; Liao, Y.W.; Liu, C.M.; Yu, C.C. alpha-Mangostin Inhibits the Activation of Myofibroblasts via Downregulation of Linc-ROR-Mediated TGFB1/Smad Signaling. Nutrients 2023, 15, 1321. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, J.; Gao, L.; Lin, H.; Yang, Z.; Liu, X.; Niu, Y. alpha-Mangostin reduces hypertension in spontaneously hypertensive rats and inhibits EMT and fibrosis in Ang II-induced HK-2 cells. Int. J. Med. Sci. 2024, 21, 1681–1688. [Google Scholar] [CrossRef]
- Zhou, Q.; Ali, S.; Shi, X.; Cao, G.; Feng, J.; Yang, C.; Zhang, R. Protective impacts of bamboo leaf flavonoids in stressed broilers induced by diquat: Insight of antioxidant, immune response and intestinal barrier function. Anim. Nutr. 2025, 20, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Sozio, C.; La Rosa, G.; Guida, B.; Faraonio, R.; Santillo, M.; Mondola, P. Metabolism Regulation and Redox State: Insight into the Role of Superoxide Dismutase 1. Int. J. Mol. Sci. 2020, 21, 6606. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update. Antioxidants 2019, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.; Mancini, A. The Double-Edged Sword of Total Antioxidant Capacity: Clinical Significance and Personal Experience. Antioxidants 2024, 13, 933. [Google Scholar] [CrossRef]
- Mooli, R.G.R.; Mukhi, D.; Ramakrishnan, S.K. Oxidative Stress and Redox Signaling in the Pathophysiology of Liver Diseases. Compr. Physiol. 2022, 12, 3167–3192. [Google Scholar] [CrossRef]
- Simos, Y.V.; Zerikiotis, S.; Lekkas, P.; Zachariou, C.; Halabalaki, M.; Ververidis, F.; Trantas, E.A.; Tsamis, K.; Peschos, D.; Angelidis, C.; et al. Hydroxytyrosol produced by engineered Escherichia coli strains activates Nrf2/HO-1 pathway: An in vitro and in vivo study. Exp. Biol. Med. 2023, 248, 1598–1612. [Google Scholar] [CrossRef]
- Xu, L.; Sang, R.; Yu, Y.; Li, J.; Ge, B.; Zhang, X. The polysaccharide from Inonotus obliquus protects mice from Toxoplasma gondii-induced liver injury. Int. J. Biol. Macromol. 2019, 125, 1–8. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond repression of Nrf2: An update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Öllinger, R.; Wang, H.; Yamashita, K.; Wegiel, B.; Thomas, M.; Margreiter, R.; Bach, F.H. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid. Redox Signal. 2007, 9, 2175–2186. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y.; et al. Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Gao, X.; Li, T.; Qiao, B.; Tang, L.; Lan, J.; Su, Q.; Ruan, Z.; Tang, Z.; et al. Curcumin alleviates AFB1-induced nephrotoxicity in ducks: Regulating mitochondrial oxidative stress, ferritinophagy, and ferroptosis. Mycotoxin Res. 2023, 39, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.N.; Lu, M.H.; Guo, Y.N.; Liang, S.S.; Mou, R.W.; He, Y.M.; Tang, L.P. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 249, 114411. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Gul, H.; Khan, S.; Nassar, N.; Khalid, A.; Swelum, A.A.; Wang, Z. Green tea polyphenol epigallocatechin-3-gallate mediates an antioxidant response via Nrf2 pathway in heat-stressed poultry: A review. Poult. Sci. 2025, 104, 105071. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shen, Z.; Wang, C.; Zhang, Q.; Hong, Q.; He, Y.; Hu, C. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets(1). Food Funct. 2019, 10, 344–354. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary Tributyrin Attenuates Intestinal Inflammation, Enhances Mitochondrial Function, and Induces Mitophagy in Piglets Challenged with Diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef]
- Pan, H.; Ding, B.; Jiang, Z.; Wang, J.; Li, D.; Yu, F.; Wang, L.; Hu, S.; Zhao, Y.; Xu, H. Hydrogel Derived from Decellularized Porcine Small Intestinal Submucosa as a Physical Shielding Repaired the Gut Epithelial Barrier of Murine Ulcerative Colitis. Adv. Funct. Mater. 2024, 34, 2405601. [Google Scholar] [CrossRef]
- Yang, R.; Harada, T.; Li, J.; Uchiyama, T.; Han, Y.; Englert, J.A.; Fink, M.P. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005, 31, 709–717. [Google Scholar] [CrossRef]
- Nong, K.; Qin, X.; Liu, Z.; Wang, Z.; Wu, Y.; Zhang, B.; Chen, W.; Fang, X.; Liu, Y.; Wang, X.; et al. Potential effects and mechanism of flavonoids extract of Callicarpa nudiflora Hook on DSS-induced colitis in mice. Phytomedicine 2024, 128, 155523. [Google Scholar] [CrossRef]
- Ran, X.; Hu, G.; He, F.; Li, K.; Li, F.; Xu, D.; Liu, J.; Fu, S. Phytic Acid Improves Hepatic Steatosis, Inflammation, and Oxidative Stress in High-Fat Diet (HFD)-Fed Mice by Modulating the Gut-Liver Axis. J. Agric. Food Chem. 2022, 70, 11401–11411. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhan, Y.; Gao, S.; Li, T.; Xuan, H. Hepatotoxicity of imidacloprid in zebrafish and the alleviating role of 10-hydroxy-2-decenoi acid: Insights into oxidative stress, inflammation, and gut microbiota. J. Hazard. Mater. 2025, 494, 138695. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zou, X.; Mao, M.; Zhang, M.; Tu, W.; Jin, M. Low Ca diet leads to increased Ca retention by changing the gut flora and ileal pH value in laying hens. Anim. Nutr. 2023, 13, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, X.; Cen, S.; Duan, H.; Feng, S.; Xue, Y.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; et al. Beneficial effect of GABA-rich fermented milk on insomnia involving regulation of gut microbiota. Microbiol. Res. 2020, 233, 126409. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y.; Dai, G.C.; Zhou, B.B.; Yan, X.N.; Tan, R.X. Abietic acid ameliorates psoriasis-like inflammation and modulates gut microbiota in mice. J. Ethnopharmacol. 2021, 272, 113934. [Google Scholar] [CrossRef]
- Qin, X.; Liu, Z.; Nong, K.; Fang, X.; Chen, W.; Zhang, B.; Wu, Y.; Wang, Z.; Shi, H.; Wang, X.; et al. Porcine-derived antimicrobial peptide PR39 alleviates DSS-induced colitis via the NF-kappaB/MAPK pathway. Int. Immunopharmacol. 2024, 127, 111385. [Google Scholar] [CrossRef]
- Wang, N.; Pei, Z.; Wang, H.; Zhao, J.; Lu, W. Bifidobacterium longum Ameliorates Intestinal Inflammation and Metabolic Biomarkers in Mice Fed a High-Fat Diet with Gliadin by Indoleacrylic Acid. Probiotics Antimicrob. Proteins 2025, 1–16. [Google Scholar] [CrossRef]
- Qin, Q.; Li, Z.; Zhang, M.; Dai, Y.; Li, S.; Wu, H.; Zhang, Z.; Chen, P. Effects of melittin on production performance, antioxidant function, immune function, heat shock protein, intestinal morphology, and cecal microbiota in heat-stressed quails. Poult. Sci. 2023, 102, 102713. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Lv, Y.; Zou, C.; Ge, C.; Zhan, S.; Shen, X.; Wu, L.; Wang, X.; Yuan, H.; Lin, G.; et al. Mangosteen Pericarp Extract Mitigates Diquat-Induced Hepatic Oxidative Stress by NRF2/HO-1 Activation, Intestinal Barrier Integrity Restoration, and Gut Microbiota Modulation. Antioxidants 2025, 14, 1045. https://doi.org/10.3390/antiox14091045
Huang W, Lv Y, Zou C, Ge C, Zhan S, Shen X, Wu L, Wang X, Yuan H, Lin G, et al. Mangosteen Pericarp Extract Mitigates Diquat-Induced Hepatic Oxidative Stress by NRF2/HO-1 Activation, Intestinal Barrier Integrity Restoration, and Gut Microbiota Modulation. Antioxidants. 2025; 14(9):1045. https://doi.org/10.3390/antiox14091045
Chicago/Turabian StyleHuang, Weichen, Yujie Lv, Chenhao Zou, Chaoyue Ge, Shenao Zhan, Xinyu Shen, Lianchi Wu, Xiaoxu Wang, Hongmeng Yuan, Gang Lin, and et al. 2025. "Mangosteen Pericarp Extract Mitigates Diquat-Induced Hepatic Oxidative Stress by NRF2/HO-1 Activation, Intestinal Barrier Integrity Restoration, and Gut Microbiota Modulation" Antioxidants 14, no. 9: 1045. https://doi.org/10.3390/antiox14091045
APA StyleHuang, W., Lv, Y., Zou, C., Ge, C., Zhan, S., Shen, X., Wu, L., Wang, X., Yuan, H., Lin, G., Yu, D., & Liu, B. (2025). Mangosteen Pericarp Extract Mitigates Diquat-Induced Hepatic Oxidative Stress by NRF2/HO-1 Activation, Intestinal Barrier Integrity Restoration, and Gut Microbiota Modulation. Antioxidants, 14(9), 1045. https://doi.org/10.3390/antiox14091045






