Abstract
As two pivotal regulatory factors in cancer biology, oxidative stress and inflammation interact dynamically through complex network mechanisms to influence tumor initiation, progression, and treatment resistance. Oxidative stress induces genomic instability, oncogenic signaling activation, and tumor microenvironment (TME) remodeling via the abnormal accumulation of reactive oxygen species (ROS) or reactive nitrogen species (RNS). Conversely, inflammation sustains malignant phenotypes by releasing pro-inflammatory cytokines and chemokines and promoting immune cell infiltration. These processes create a vicious cycle via positive feedback loops whereby oxidative stress initiates inflammatory signaling, while the inflammatory milieu further amplifies ROS/RNS production, collectively promoting proliferation, migration, angiogenesis, drug resistance, and immune evasion in tumor cells. Moreover, their crosstalk modulates DNA damage repair, metabolic reprogramming, and drug efflux pump activity, significantly impacting the sensitivity of cancer cells to chemotherapy, radiotherapy, and targeted therapies. This review systematically discusses these advances and the molecular mechanisms underlying the interplay between oxidative stress and inflammation in cancer biology. It also explores their potential as diagnostic biomarkers and prognostic indicators and highlights novel therapeutic strategies targeting the oxidative stress–inflammation axis. The goal is to provide a theoretical framework and translational roadmap for developing synergistic anti-tumor therapies.
1. Introduction
Cancer remains a formidable challenge to global health, with its intricate etiology and heterogeneous nature posing significant obstacles to effective treatment [1]. Among various factors driving tumor occurrence and progression, oxidative stress and inflammation have emerged as two pivotal regulatory factors that interact dynamically through intricate network mechanisms, which significantly influence tumor initiation, progression, and therapeutic resistance [2,3]. To achieve efficient cancer treatment, it is essential to understand their interplay.
Oxidative stress, an imbalance between reactive ROS/RNS and antioxidant defenses, plays a dual role in cancer biology. At low levels, ROS act as signaling molecules, modulating cell proliferation and survival via pathways like NF-κB and mitogen-activated protein kinase (MAPK) [4,5]. However, excessive ROS accumulation, often triggered by genetic mutations (e.g., in TP53 or KEAP1) or environmental insults, can induce DNA damage, genomic instability, and epigenetic alterations, directly facilitating oncogenesis [4]. Significantly, ROS also drive TME remodeling through the activation of hypoxia-inducible factors (HIFs) and the promotion of angiogenesis, thereby creating a niche that supports tumor growth and immune evasion [6,7].
Inflammation, a physiological response to tissue injury or infection, becomes pathogenic when unresolved [8]. Chronic inflammation in the TME promotes tumorigenesis through the secretion of pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and chemokines, which can recruit immune suppressive cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) [3,9]. These mediators not only suppress anti-tumor immunity but also directly drive malignant phenotypes by inducing epithelial–mesenchymal transition (EMT), metastasis, and metabolic reprogramming [10]. For instance, TNF-α and interleukin-1β (IL-1β) can enhance ROS production through NF-κB signaling, establishing a positive feedback loop that sustains both oxidative stress and inflammatory signaling [11].
The synergistic interplay between oxidative stress and inflammation further augments cancer’s aggressiveness [12]. On the one hand, DNA damage derived from ROS triggers the activation of inflammatory sensors, such as AIM2-like receptors (ALRs); on the other hand, inflammatory cytokines can amplify ROS production via nicotinamide adenine dinucleotide phosphate (NADPH) oxidases’ activation, thereby establishing a self-reinforcing cycle [11]. This intricate axis facilitates tumor cell survival, confers resistance to chemotherapeutic agents, and enables immune escape. For example, ROS-induced NF-κB activation enhances CXCL12 secretion, promoting metastasis to lymph nodes in a pancreatic tumor model [13]. In another report, in a colitis-associated colorectal cancer (CRC) model, chronic inflammation resulting in the upregulation of Cyclooxygenase-2 (COX-2) greatly elevates ROS levels and contributes to genomic instability [14].
Despite the increasing acknowledgment of these biological mechanisms, current therapies targeting either oxidative stress or inflammation alone often fail to achieve sustained therapeutic responses [3,15]. Antioxidants like N-acetylcysteine (NAC) may paradoxically promote tumor growth by scavenging ROS-dependent apoptotic signals [16]. Similarly, anti-inflammatory agents, such as aspirin or COX-2 inhibitors, struggle to address the TME’s complexity, often suppressing beneficial immune responses [17,18]. A paradigm shift is needed toward context-dependent therapeutic strategies that exploit the oxidative–inflammatory axis. Recent advances highlight the potential of dual-targeting approaches. For instance, combining Nrf2 activators (e.g., sulforaphane) with immune checkpoint inhibitors enhances T cell infiltration in hepatocellular carcinoma [19,20]. Pharmacological inhibition of NOX enzymes or KEAP1-NRF2 pathway regulators disrupts the oxidative stress–inflammatory feedback loop, sensitizing tumors to chemotherapy [21,22].
This review emphasizes current knowledge regarding the oxidative stress–inflammation axis, emphasizing its dual regulatory roles throughout tumor initiation, progression, and therapy resistance. We discuss mechanistic insights into their crosstalk, highlight diagnostic and prognostic biomarkers, and critically assess therapeutic strategies aimed at breaking the pro-tumorigenic cycle. Future studies should focus on designing precise medicines based on patient-specific biomarkers given the heterogeneity of tumors. Ultimately, targeting this dual axis offers a transformative opportunity to overcome therapeutic limitations and improve patient outcomes.
2. The Complex and Dual Relationships Between Oxidative Stress and Cancer
The complex relationship between oxidative stress and inflammation in cancer is shown in Figure 1. Oxidative stress mainly refers to the imbalance between the production of ROS/RNS, including superoxide anion (O2•−), hydroxyl radical (OH•), nitrogen dioxide (NO2•), alkoxyl/alkyl peroxyl (RO•/ROO•), hydrogen peroxide (H2O2), nitric oxide (NO), hypochlorous acid (HOCl), etc. [4,23,24], and the endogenous antioxidant defense system in the body, leading to a pathological state of oxidative damage [25]. The disruption of redox homeostasis has been associated with a wide range of human diseases, such as cancer, in which elevated levels of ROS are thought to play a pivotal role in the development and progression of tumors [7,25,26,27]. Researchers speculate that tumor tissues generate relatively higher levels of ROS due to metabolic changes, inflammation, hypoxic conditions, and the upregulation of ROS-generating enzymes driven by oncogenes [26,28]. Elevated ROS levels can lead to DNA mutations and facilitate oncogenic signaling pathways, thereby propelling tumor initiation and progression. However, excessively high ROS levels can also induce tumor cell death [29,30], suggesting that cancer prevention could potentially be achieved by reducing these relatively high ROS levels. In this section, we comprehensively discuss the dual effects of oxidative stress on promoting and inhibiting tumorigenesis and development.
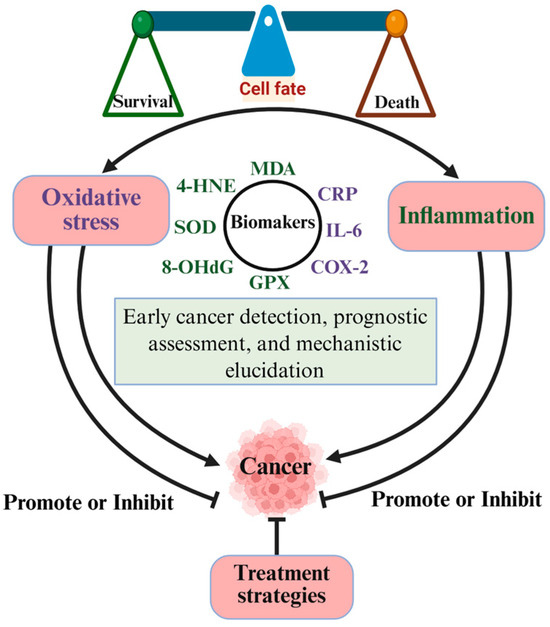
Figure 1.
The complex relationship between oxidative stress and inflammation in cancer. Oxidative stress and inflammation mutually promote each other. They promote or inhibit the occurrence and development of cancer by regulating the levels of different biomarkers. Created with BioRender.com.
2.1. The Generation of ROS
ROS are highly reactive free radicals, ions, or molecules characterized by a single unpaired electron, including species like 1O2, O2•−, H2O2, and OH• [31]. As pivotal signaling molecules, the regulation of ROS levels and redox homeostasis is critical for determining cellular functions and cell fate [32]. In cancer cells, elevated ROS levels can arise from diverse signaling pathways (Figure 2), including the activation of oncogenes or tumor suppressor genes, self-sustaining growth factor signaling, chronic inflammatory microenvironments, exposure to radiotherapy and chemotherapy, increased metabolic demands, and mitochondrial dysfunction [7,33,34].
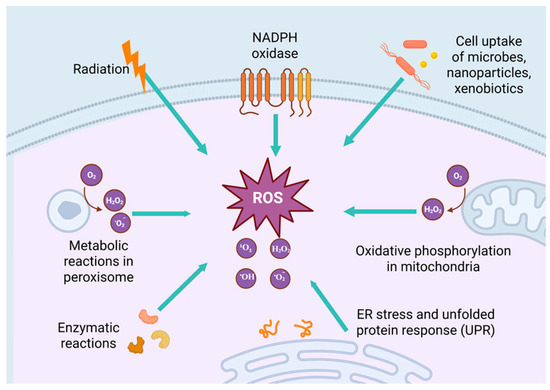
Figure 2.
Elevated ROS levels are associated with many factors. In the process of mitochondrial oxidative phosphorylation, when the electron transport chain (such as complex I and III) is dysfunctional, electrons easily combine with oxygen to form O2•−, which in turn converts to H2O2 and other ROS. NADPH oxidase is a transmembrane enzyme complex. Its core function is to catalyze the transfer of electrons from NADPH to oxygen to generate O2•−, which is further converted to H2O2, OH•, and other ROS. Cellular uptake of microorganisms, nanoparticles, and xenobiotics is an important physiological process for organisms to cope with external stimuli. However, the intracellular responses triggered by the uptake of microorganisms, nanoparticles, and xenobiotics are often accompanied by ROS generation and oxidative stress. Radiation can directly damage DNA, lipids, and proteins and induce cells to produce ROS. Peroxisomes play a key role in maintaining cellular REDOX homeostasis by catalyzing the generation and scavenging of ROS, the balance of oxidative metabolic pathways, and the interaction with other organelles. Enzymatic reactions generate ROS (such as 1O2, O2•−, H2O2 and OH•) through catalytic processes, disrupting cellular redox balance and leading to oxidative stress. ER stress and unfolded protein response interfering with steady-state, metabolic balance, and intracellular calcium signaling pathways ultimately lead to ROS excess generation. Created with BioRender.com.
Mitochondrial-derived ROS in cancer cells. Cancer cells utilize glucose through aerobic glycolysis (the Warburg effect) while maintaining mitochondrial activity [35]. Mutations in mitochondrial DNA and genes associated with the electron transport chain are commonly observed in tumors, leading to improper assembly of respiratory chain complexes and subsequent electron leakage [36,37]. It is widely acknowledged that mitochondrial ROS are primarily generated by Complexes I and III of the electron transport chain as superoxide within the mitochondrial matrix. Superoxide dismutases (SODs) present in the cytoplasm and mitochondrial matrix can convert O2•− into O2 and H2O2, which regulate diverse cellular signaling pathways.
Growth factor and inflammatory cytokine signaling derived ROS in tumor cells. Self-sufficiency in growth signal transduction and chronic inflammation are two key hallmarks of tumor progression [38]. Signaling from growth factors and inflammatory cytokines increases the activity or expression of NOXs, membrane-bound enzyme complexes that generate O2•− using electrons from NADPH [39]. Growth factors and cytokines, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor β (TGFβ), and TNFα, promote ROS production by activating NOXs. In the TME, elevated ROS levels originate not only from tumor cells but also from tumor-infiltrating immune cells (e.g., neutrophils and macrophages) [40]. For example, macrophage-derived NOX1 expression enhances inflammation and hepatocarcinogenesis in mouse models [41]. Thus, targeting NOXs to mitigate ROS-dependent pro-tumorigenic signaling has been proposed as a promising anticancer strategy.
ROS associated with oncogenic transformation. Oncogenic transformation promotes oxidative stress by remodeling cellular metabolism and enhancing mitochondrial ROS production [41]. The oncogenic activation of K-Ras in colon cancer cells redirects cellular metabolism towards aerobic glycolysis in the cytoplasm and enhances the activity of tricarboxylic acid (TCA) cycle activity within mitochondria [41,42]. Sustained energy generation via the TCA cycle generates NADH/FADH2, which amplifies electron flow through the electron transport chain and ROS generation, thereby facilitating tumorigenesis. Nevertheless, in normal human fibroblasts, the enhanced expression of c-Myc and H-RasV12 induces oxidative stress while simultaneously reducing mitochondrial ETC activity, resulting in non-mitochondrial superoxide [43], indicating that the origin of oncogene-induced oxidative stress may depend on the cellular context. Oncogenic signaling can also differentially regulate the expression of ETC subunits, giving rise to imbalanced ETC complex assembly and unstable electron transfer [43]. Significantly, mutations in mitochondrial-encoded ETC genes caused by ROS-induced mitochondrial DNA damage further disrupt mitochondrial redox homeostasis and promote cancer progression [44].
2.2. The Mechanism of ROS Based on Oxidative Stress Promoting Tumor Development
Oxidative stress drives tumorigenesis. Genomic instability, coupled with mutational accumulation, represents a fundamental characteristic of carcinogenesis [45]. This variability is caused by internal factors, such as the spontaneous accumulation of mutations and external factors (e.g., the environment and radiation) [46]. ROS, one of the common cellular mediators for both intrinsic and extrinsic carcinogenic factors, can directly impair nuclear DNA, resulting in genomic instability, such as single/double-strand breaks, point mutations, or chromosomal breaks, which subsequently activate the expression of proto-oncogenes (such as RAS and MYC) or inactivate tumor suppressor genes (such as TP53 and PTEN), along with DNA damage-repair-related genes, thereby driving tumorigenesis [47,48]. Moreover, ROS induces mutagenic lesions by damaging DNA bases (e.g., the oxidation of guanine to 8-oxo-dG) or inhibiting repair enzymes (e.g., ATM, BRCA1), thus triggering the repair of mutagenic lesions and SOS response, a stress-induced DNA damage response. This halts replication forks and promotes a transition of DNA polymerases from high-fidelity to error-prone forms, leading to additional oncogenic mutations [49,50]. Research has demonstrated that ROS can oxidize cysteine residues to activate the three most prevalent oncogenic switch genes (HRAS, NRAS, and KRAS) in human cancers, ultimately influencing tumorigenesis [51]. In a Kras-mediated lung cancer mouse model, mitochondrial ROS promotes tumor development via the ERK-MAPK signaling pathway [42]. In Kras-driven pancreatic cancer mouse models, the development and progression of premalignant lesions were significantly suppressed upon inhibition of ROS using NAC and MitQ [52]. Epigenetic regulation is another critical mechanism associated with the expression of tumor-associated genes [53]. ROS has been identified as a catalyst for DNA methylation modifications, upregulating DNA methyltransferase (DNMT) expression or forming DNMT-containing complexes. These processes extensively participate in the regulation of aberrant hypermethylation of tumor suppressor gene (TSG) promoters (e.g., CDX1, which governs intestinal epithelial proliferation and differentiation) and global hypomethylation, thereby downregulating TSG expression and promoting tumorigenesis [54,55,56]. In summary, oxidative-stress-driven DNA damage, mutations, or epigenetic modifications (including methylation and histone modifications) alter gene expression, acting as key determinants of tumor initiation, angiogenesis, metastasis, and treatment resistance (Figure 2).
Oxidative stress promotes tumor cell proliferation. Oxidative stress, mediated by ROS, directly influences the characteristic features of malignant tumors, including uncontrolled proliferation and growth [57,58]. For example, ROS induced by 5-lipoxygenase (5-LO) and NADPH oxidase 4 (NOX4) significantly augment pancreatic cancer cell survival [59]. The mechanisms of oxidative-stress-promoting tumor growth are multifarious. For instance, ROS generated under oxidative stress can stimulate tumor proliferation by activating pro-survival signaling pathways. Researchers have observed that administering the antioxidant NAC to mice bearing tumor xenografts decelerated tumor growth, which was attributed to reduced HIF-1α expression, demonstrating the critical role of ROS in driving cancer progression [60]. Thus, oxidative signaling-activated HIF-1 enhances tumor survival and progression by upregulating genes that govern glycolysis, angiogenesis, and cellular metabolism. Simultaneously, ROS can activate the PI3K/Akt/mTOR and NF-κB pathways by modifying kinases like MAPK and PI3K/AKT, facilitating tumor cell proliferation and suppressing apoptosis [61]. Moreover, ROS function as a second messenger molecule to directly mediate the activation of growth factors, such as PDGF [62], EGF [63], and MAPK [62], or induce the inactivation of phosphatase and tensin homolog (PTEN), thereby promoting tumor cell proliferation [64]. For example, the copper chaperone for superoxide dismutase (CCS) promotes breast cancer cell proliferation via ROS-mediated MAPK/ERK signaling [65]. H2O2-induced PTEN inactivation hyperactivates the PI3K/AKT/mTOR pathway, further facilitating breast cancer cell proliferation [66].
Oxidative stress promotes and maintains tumor metastasis. Emerging evidence suggests that elevated ROS levels are essential for promoting and sustaining invasive behaviors in cancer cells [67]. In the early stages, tumor cells often utilize the EMT process to invade adjacent stromal tissue [68,69]. During this transition, ROS promotes tumor metastasis through multiple mechanisms [64]. First, ROS induces Rho family GTPase-dependent cytoskeletal reorganization. Rho family proteins govern the dynamic changes in the cytoskeleton, and ROS can enhance their activity to alter cell morphology and motility, facilitating tumor cell migration [64]. Second, ROS promote the degradation of the extracellular matrix (ECM) through protease-dependent mechanisms (e.g., via matrix metalloproteinases and serine proteases), thereby driving tumor cell metastasis [70,71]. As the ECM serves as a critical microenvironmental scaffold for cell survival, its degradation would disrupt the structural support, enabling tumor cells to breach basement membranes and infiltrate surrounding tissues, thereby enhancing invasive and metastatic phenotypes [64]. Emerging evidence highlights the critical role of ROS in tumor metastasis. For instance, in clear cell renal cell carcinoma (ccRCC), elevated ROS production activates the MAPK signaling pathway, leading to the overexpression of matrix metalloproteinase-2 (MMP2), a protease responsible for degrading ECM components. This overexpression enhances the invasiveness of ccRCC cells, driving the progression of renal cell carcinoma [72]. Conversely, aberrant expression of serine-threonine kinase A (AURKA) is observed in oral squamous cell carcinoma (OSCC). Knockdown of AURKA increases intracellular ROS levels, which, in turn, inhibits the EMT process, demonstrating that the regulatory mechanisms of ROS-interacting proteins on EMT vary among different tumor types, reflecting the complexity of oxidative stress in cancer progression. Furthermore, ROS accelerate HIF-1α-dependent angiogenesis, as tumor metastasis requires adequate blood supply, and ROS-activated HIF-1α promotes new blood vessel formation, thus providing pathways for distant tumor cell metastasis [64]. Additionally, elevated ROS levels in tumor cells activate HSF1, matrix metalloproteinases, and NF-κB, all of which collectively promote metastasis [72,73,74]. For example, ROS-induced activation of NF-κB can downregulate epithelial cadherin (E-cadherin) to disrupt intercellular adhesion while upregulating N-cadherin and vimentin, thereby triggering EMT and driving tumor cell metastasis. Moreover, ROS, a signaling molecule, can activate the ERK signaling pathway by downregulating DUSP6 expression, further enhancing tumor invasiveness and metastasis [75].
ROS facilitate tumor multidrug resistance. In cancer therapy, chemotherapy drugs and radiotherapy rely on inducing excessive ROS production within cancer cells as a fundamental mechanism to eliminate them. However, many cancer cells gradually develop resistance to chemotherapeutic agents after treatment, significantly limiting therapeutic efficacy [76,77,78]. Research suggests that ROS-dependent drug resistance may arise from the activation of ROS-negative feedback regulatory systems, including HIF-1α, glutathione (GSH), xCT, thioredoxin reductase (TrxR), Nrf2, NADPH/NADP+, MnSOD, and catalase [78]. For example, Nrf2 is a key transcription factor for antioxidant defense. Under normal conditions, Nrf2 remains inactive and bound to Kelch-like ECH-associated protein 1 (Keap1). Upon elevation of intracellular ROS levels, ROS can oxidize cysteine residues on Keap1, causing Nrf2 dissociation. Free Nrf2 then translocates to the nucleus, binds to antioxidant response elements (AREs), and activates the transcription of antioxidant genes, such as glutathione S-transferase (GST) and heme oxygenase-1 (HO-1). These antioxidant proteins enhance cellular antioxidant defenses, alleviating chemotherapy-induced oxidative damage and, consequently, endowing cancer cells with drug resistance. Recent studies have revealed that chemotherapy-induced excessive ROS are sensed by the transcription factor aryl hydrocarbon receptor (AhR). The free sulfhydryl group of cysteine at position 300 (C300) in AhR undergoes sulfenylation (SH → S-OH), causing the modified AhR to dissociate from the HSP90 complex and be recruited to glycogen-targeting protein (PTG). By competing for the PP1 phosphatase binding site, the modified AhR inhibits the dephosphorylation of glycogen phosphorylase liver form (PYGL), thereby promoting glycogen breakdown. This process redirects glucose-6-phosphate (G6P) derived from glycogenolysis into the pentose phosphate pathway (PPP), generating NADPH to eliminate ROS produced by the CYP450-mediated metabolism of chemotherapeutic agents. This mechanism allows tumor cells to evade oxidative-stress-induced lethality and drive drug resistance in cancer patients [77]. Furthermore, ROS can activate signaling pathways like PI3K/AKT and AMPK, which enhance tumor cell survival under chemotherapeutic stress [79]. For instance, ROS-induced PI3K/Akt signaling and abnormal nuclear activation of β-catenin can promote HIF-1α overexpression, conferring resistance to 5-fluorouracil (5-FU) in colorectal cancer cells [79]. These findings highlight the complex interplay between ROS signaling and cellular adaptive mechanisms in chemotherapy resistance. ROS may also stimulate cancer stem cell differentiation [80], promote EMT [81], and induce metabolic reprogramming [82], all of which contribute to the increased chemoresistance of cancer cells. The overexpression of ATP-binding cassette (ABC) transporter family members is a key mechanism underlying tumor drug resistance. ROS may upregulate ABC transporter expression, enabling these proteins to utilize the energy derived from ATP hydrolysis to efflux chemotherapeutic agents from cells, thereby reducing intracellular drug concentrations and conferring resistance [78]. Additionally, ROS promote the development of multidrug resistance (MDR) by modulating intracellular metabolic pathways. For instance, ROS-induced metabolic reprogramming shifts cancer cells towards aerobic glycolysis, providing rapid energy and biosynthetic precursors for tumor growth while enhancing tolerance to chemotherapeutic agents. Simultaneously, ROS influence the autophagy of cancer cells [83]. Autophagy, a cellular self-degradation mechanism, helps cancer cells in eliminating damaged organelles and proteins to maintain homeostasis. ROS activates autophagy-related signaling pathways, promoting autophagy, which enables cancer cells to survive chemotherapeutic stress, thereby establishing drug resistance [84,85].
2.3. Mechanisms of ROS Based on Oxidative Stress Impeding Tumor Development
Under normal physiological conditions, high ROS levels during oxidative stress can inhibit tumor cell proliferation or promote tumor cell death through multiple mechanisms [30]. Excessively high ROS levels can induce diverse forms of tumor cell death (Figure 3). In the mitochondrial apoptotic pathway triggered by excessive ROS, oxidative stress disrupts the permeability of the mitochondrial inner membrane and mitochondrial membrane potential, activating signaling pathways like Bax/Bcl-2-cyt c, p38 MAPK, and phosphorylated HSP27. This leads to the release of cytochrome c and activation of the caspase family, ultimately driving the apoptotic death of cancer cells [86,87,88,89]. Moreover, studies have demonstrated that ROS can disrupt calcium homeostasis in the endoplasmic reticulum (ER) by impairing the gating functions of ryanodine receptors (RyR) and inositol 1,4,5-trisphosphate receptors (IP3R) while simultaneously inhibiting Ca2+-ATPase activity, thereby inducing cancer cell apoptosis [90,91]. Recent research has further highlighted that the lethal accumulation of lipid ROS in multiple tumor types triggers ferroptosis, an iron-dependent form of cell death [92,93]. Elevated iron concentrations and ROS levels stimulate the expression of tumor suppressor p14ARF (CDKN2A), activate p53, and inhibit NRF2 activity, which collectively promote ferroptosis [94]. Under oxidative stress conditions, high levels of ROS can also suppress tumor cell proliferation by inhibiting proliferation-associated signaling pathways. For instance, excessive ROS inhibits AKT-dependent signaling pathways, thereby restraining proliferation in colorectal cancer cells [95]. Additionally, the persistent activation of cell cycle inhibitors by ROS can inhibit tumor growth [96,97]. The most commonly reported inhibitors are p21 (CDKN1A) and p16 (CDKN2A). Their accumulation leads to hypophosphorylation of the retinoblastoma protein (RB), which can inhibit the transactivation of E2F genes involved in nucleotide metabolism and DNA synthesis [98]. This results in cell cycle arrest and ultimately suppresses cell proliferation [99]. A notable phenomenon under oxidative stress is the translocation of p53 to the mitochondria, where it regulates mitochondrial membrane potential and triggers apoptosis [100,101]. Furthermore, studies have demonstrated that several proteins interacting with p53 are localized to the mitochondria, including MDM2, FOXO3a, and Parkin [102,103,104].
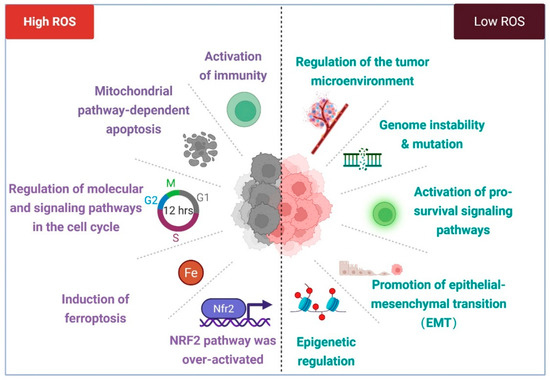
Figure 3.
ROS in cancer biology. Excessive levels of ROS can inhibit tumor growth by triggering apoptosis, ferroptosis, or cell cycle arrest and even activate immune cells to recognize abnormal cells. In addition, it can inhibit the occurrence and development of tumors by activating Nrf2. However, relatively low levels of ROS can lead to genomic instability or mutation, thereby inducing the activation of proto-oncogenes or the inactivation of tumor suppressor genes and promoting the transformation of normal cells into precancerous lesions. At the same time, low levels of ROS can also promote the occurrence and development of cancer by activating pro-survival signaling pathways, promoting epithelial–mesenchymal transition and regulating epigenetics. Created with BioRender.com.
In addition to directly killing tumor cells, ROS can indirectly inhibit tumor progression through mechanisms like enhancing therapeutic sensitivity, modulating immune responses [105], and suppressing tumor stem-like properties. For instance, radiotherapy generates massive ROS, which collaborate with chemotherapeutic agents like cisplatin, doxorubicin, and oxaliplatin to induce DNA crosslinking damage, ultimately leading to cancer cell death [106,107,108,109]. Additionally, acute oxidative-stress-induced ROS promote the release of tumor antigens [110], activate dendritic cells (DCs) and CD8+ T cells [105,111], and enhance the efficacy of immune checkpoint inhibitors [112]. Furthermore, oxidative stress can suppress tumor stem-like characteristics, thereby affecting tumor cell survival [113,114,115].
2.4. Spatiotemporal Regulation of ROS Under Oxidative Stress
ROS play a complex and dynamic dual role in tumorigenesis and tumor progression, exhibiting both pro-tumorigenic and anti-tumorigenic activities, a long-standing paradox in the field. Discrepancies and even contradictory findings reported by different researchers have ignited controversy regarding the role of antioxidants in cancer therapy. These discrepancies likely stem from differences in experimental models and methodologies [75]. A recent study demonstrated that ROS exhibit distinct functions at different stages of pancreatic cancer development; they suppress pre-neoplastic lesions yet promote metastasis in pancreatic cancer. This dynamic regulatory mechanism may be attributed to the pivotal regulatory factor TIGAR [75]. The spatiotemporal regulation of ROS has become a critical focus in tumor biology research, underscoring the necessity to elucidate the context-dependent mechanisms that govern their dual roles in cancer.
From a temporal perspective, oxidative stress levels exhibit dynamic changes throughout tumor progression. During the initiation phase of tumorigenesis, localized elevation of ROS facilitates the transformation of normal cells into malignant phenotypes by inducing DNA oxidative damage and genomic instability, thereby activating oncogenes and inactivating tumor suppressor genes [25,29,75,116,117]. Simultaneously, ROS activate redox-sensitive survival pathways, such as NF-κB, HIF-1α, and Nrf2, through spatiotemporal dynamic fluctuations to remodel the TME to enhance the release of inflammatory cytokines, thus supporting the survival and proliferation of tumor cells [25,29,75,116,117]. As tumors advance to advanced stages, the continuous accumulation of ROS may surpass cellular antioxidant defenses, leading to genomic instability and cell death. Paradoxically, ROS may also promote the acquisition of invasive and metastatic phenotypes [7,26,33,118,119,120,121]. Notably, therapeutic interventions significantly alter redox kinetics; radiotherapy, many chemotherapeutic agents, and photodynamic therapy exert their anti-tumor effects by further increasing ROS levels [78,122,123,124,125,126,127]. Nevertheless, cancer cells may develop resistance by upregulating antioxidant defense systems, such as the glutathione system and the thioredoxin system, to counteract ROS-induced cytotoxicity [27,118,128,129,130,131,132,133].
From a spatial perspective, oxidative stress levels differ significantly across distinct tumor regions [83,134,135,136]. The tumor core region typically exhibits chronic hypoxia due to the abnormal vasculature and insufficient blood perfusion, leading to mitochondrial electron transport chain dysfunction. This results in increased ROS production, accompanied by reduced ROS scavenging capacity, thereby facilitating the emergence of invasive phenotypes [26,83]. For example, after cisplatin treatment, the core region shows a marked elevation in Z-RNA levels, triggering necroptosis, while the margin region may demonstrate a weaker response due to non-uniform drug distribution or microenvironmental barriers [137]. Conversely, the tumor margin region features relatively abundant vasculature and elevated oxygen tension but undergoes intermittent hypoxia–reoxygenation cycles, further amplifying oxidative stress fluctuations. In glioblastoma (GBM), tumor-associated microglia undergo immunosuppressive transformation under oxidative stress, resulting in reduced antigen-presenting capacity. This phenomenon is more pronounced in the tumor’s core, whereas the margin region may exhibit lower oxidative stress because of differences in vascularization and immune cell infiltration [135]. Moreover, variations in extracellular matrix density and GSH levels within the tumor influence the distribution of oxidative stress. Nanoparticles, for instance, can deplete GSH in the core region and catalyze H2O2 to generate hydroxyl radicals, thereby intensifying oxidative stress in the core. However, the margin region may exhibit weaker responses due to differences in drug permeability [134]. At the subcellular level, mitochondria, the ER, peroxisomes, and plasma membranes are primary sites of ROS generation. ROS produced by different organelles exhibit distinct chemical properties and biological effects. For example, mitochondria predominantly generate O2•−, while the ER generates H2O2 during protein-folding processes.
Clinical assays demonstrate that in patients with high-grade serous ovarian cancer (HGSOC), downregulation of VPS35 in hypoxic regions leads to mitochondrial translation inhibition, contributing to resistance against multiple ROS-dependent anticancer drugs [83]. Additionally, during pre-invasive stages, tumor cells enhance local ROS clearance by upregulating antioxidant enzymes like SOD2 and GPX4, which aid in evading immune surveillance and enhance metastatic potential [26,117]. These findings suggest that the spatiotemporal regulation of ROS and its dynamic effects throughout tumor progression stages may partially elucidate the controversial roles of antioxidants in cancer therapy. Understanding the spatiotemporal dynamics of ROS balance in tumors could guide the formulation of stage-specific therapeutic strategies. For instance, antioxidants might be employed in early preventive interventions, while pro-oxidant agents engineered to circumvent tumor antioxidant defenses could be applied in late-stage metastatic phases. Such approaches could exploit the context-dependent roles of ROS to optimize therapeutic efficacy.
3. Inflammation in Cancer
3.1. The Link Between Inflammation and Cancer
Since its discovery, inflammation has emerged as a research focus due to its significant role in regulating various diseases, particularly in cancer biology [138,139]. Over the past decades, numerous studies have extensively explored the influence of inflammation on tumor initiation, invasion, metastasis, and drug resistance. Among the crucial factors contributing to tumorigenesis, the role of inflammation, especially chronic inflammation, has been the subject of extensive investigation. Although inflammation and cancer manifest as separate pathological conditions, they are, in fact, closely intertwined. Inflammation, an inherent defense mechanism, is triggered by tissue injury or pathogen invasion, aiming to facilitate repair and eliminate harmful entities. However, when inflammation becomes persistent and progresses to a chronic state, it gives rise to a permissive microenvironment that promotes carcinogenesis [138].
Chronic inflammation and tumor progression are intricately linked via the TME, which is shaped by inflammatory mediators, including inflammatory cells and cytokines. Inflammatory cells, such as macrophages and lymphocytes, accumulate and continuously secrete pro-inflammatory cytokines like TNF-α and IL-6 (Figure 4). On the one hand, these cytokines can stimulate genetic mutations in normal cells and disrupt normal intracellular signaling pathways, such as activating the NF-κB pathway, which dysregulates the balance of meticulously controlled cell proliferation and apoptosis processes, ultimately leading to abnormal cell proliferation. On the other hand, these cytokines also promote tumor angiogenesis, continuously supplying nutrients to cancer cells and facilitating their growth and metastasis. A well-documented example is the elevated risk of CRC associated with ulcerative colitis and Crohn’s disease [140,141]. Moreover, infections with Helicobacter pylori, hepatitis B, and hepatitis C viruses are also associated with increased cancer risk [142,143]. The recurrent tissue damage and repair caused by chronic inflammation drive continuous cellular proliferation. During this process, the probability of DNA replication errors increases, and mutations in pivotal tumor suppressor genes or oncogenes can ultimately propel normal cells towards malignant transformation.
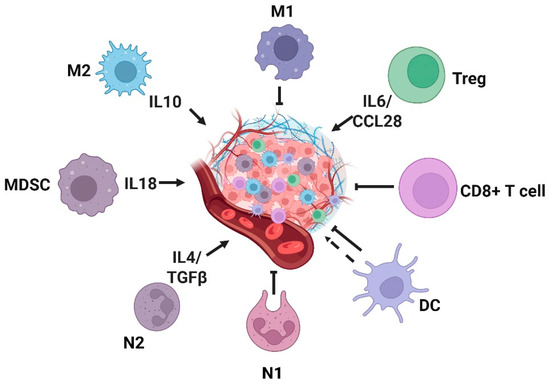
Figure 4.
Tumor microenvironment and inflammatory cells. Inflammatory cells and inflammatory factors are involved in the development of tumors. M2, MDSC, N2, and Treg cells promote the occurrence and development of tumors in the tumor microenvironment, while M1, CD8+T, and N1 cells inhibit the occurrence and development of tumors. M1: M1-type macrophages; M2: M2-type macrophages; MDSC: myeloid-derived suppressor cells; N1: N1-type neutrophils; N2: N2-type neutrophils; DC: dendritic cell. Created with BioRender.com.
From a mechanistic perspective, chronic inflammation drives carcinogenesis through diverse pathways. For example, in chronic inflammation induced by microbial infections, immune cells, such as macrophages at inflammatory sites, can produce ROS, leading to sustained DNA damage and subsequent mutations [144]. Moreover, cytokines secreted by immune cells, including TNF-α and macrophage migration inhibitory factor (MIF), can inhibit the activation of p53 and the Rb-E2F pathway, thereby facilitating tumorigenesis [145]. Components of the inflammatory process form self-reinforcing positive feedback loops that support cancer progression. Inflammatory and growth factors further stimulate transcription factors, such as NF-κB, collectively promoting the formation of an inflammatory TME [146,147]. The inflammatory TME is highly dynamic and complex, consisting of cellular components, such as tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), DCs, MDSCs, and T lymphocytes [147]. These tumor-infiltrating cells collectively maintain an inflammatory milieu that enables tumor growth and induces immune suppression during tumor progression.
3.2. Role of Key Inflammatory Cells in Tumorigenesis and Progression
Inflammatory cells serve as a critical mediator of the body’s immune response, playing pivotal roles in recognizing and phagocytosing pathogens, as well as clearing damaged tissues to combat infections and promote tissue repair. Within the TME, distinct populations of inflammatory cells exert diverse effects on tumorigenesis and progression. Neutrophils, the principal effector cells during inflammatory responses and the most abundant white blood cells, are categorized into N1 and N2 subtypes. N1 neutrophils exhibit anti-tumor activity, whereas N2 neutrophils promote tumor growth [148]. Notably, TANs in the TME predominantly display an N2 phenotype and drive tumor metastasis through multiple mechanisms. Studies have demonstrated that TANs may promote tumor angiogenesis by inducing sustained release of vascular endothelial growth factor (VEGF) from peripheral endothelial cells or by secreting pro-inflammatory and immunosuppressive factors, such as IL-1β, IL-17, TNF-α, CCL4, MMP-9, CXCL8, and ANG1 [149]. Tumor-derived cytokines IFN-γ and GM-CSF drive TANs’ polarization by upregulating the expression of specific neutrophil activation markers, thereby enhancing anti-tumor activity [150]. Additionally, tumor-secreted TGF-β promotes the paracrine production of CCL2 and CCL17, recruiting N2-polarized neutrophils and establishing an immunosuppressive microenvironment [151,152]. Moreover, tumor cells induce tumor-associated neutrophils to release neutrophil extracellular traps (NETs) through autocrine signaling via IL-8/CXCL8, CXCR1/CXCR2 agonists, G-CSF, and TGF-β pathways. NETs can promote metastasis by inducing EMT [153,154,155,156,157,158,159]. However, the functional roles of NETs released by tumor-associated neutrophils are not fixed, and they are contingent on the TME context. For instance, in vitro-generated NETs have been shown to inhibit tumor growth in CRC and head and neck squamous cell carcinoma (HNSCC) by inducing apoptosis and suppressing proliferation [160,161,162]. Furthermore, co-culture experiments with melanoma cells revealed that NETs induced necrosis in melanoma cells [162]. These findings highlight the biphasic regulation of tumor-associated neutrophils in tumorigenesis and progression, with their effects determined by the dynamic state of the TME.
Similarly to tumor-associated neutrophils, TAMs can be classified into distinct subtypes, including M1-type macrophages and M2-type macrophages [163], with their polarization determined by molecular cues within the TME. Tumor cells use the plasticity of macrophages to secure their survival. In the early stages of tumor development, macrophages polarize towards the M1 phenotype, showing anti-tumor responses; however, in advanced stages, they shift towards the M2 phenotype to promote tumor progression [164]. This shift in polarization probably reflects variations in the molecular composition of TME across different phases of tumor development. Studies demonstrate that cytokines, such as IL-4, M-CSF/CSF1, IL-10, IL-33, IL-21, and TGF-β, drive TAMs towards the M2 phenotype, whereas M1-type TAMs are activated by TNF-α or GM-CSF [138]. M1 macrophages exert anti-tumor effects through multiple mechanisms, including inducing direct cytotoxicity via the secretion of ROS and NO [165] and mediating antibody-dependent cell-mediated cytotoxicity (ADCC) [166]. Conversely, M2 macrophages predominantly facilitate tumor progression by activating endothelial cell responses to growth factor signaling via CXCL12, IL-1β, IL-8, and Sema4d, thereby leading to the upregulation of angiogenesis-related genes and enhanced vascularization [167,168,169]. Additionally, M2 TAMs assist in tumor invasion and metastasis by indirectly degrading the tumor extracellular matrix [170].
DCs, originating from the bone marrow, are indispensable inflammatory cells that exert significant functions within inflammatory TMEs. In the context of the TME, tumor-infiltrating DCs exhibit compromised capacity to present tumor antigens, leading to T cell tolerance and the loss of anti-tumor immune function [171]. Moreover, cytokines secreted by tumor cells can modulate the maturation state of DCs, promoting pro-tumorigenic inflammatory responses. For instance, tumor-secreted IL-6 and M-CSF can transform mature DCs into macrophages and impede the priming of tumor-specific T cells [172]. Additionally, the expression of PD-L1 and PD-L2 on the surface of DC may inhibit the production of cytokines required for T cell activation [173].
MDSCs have been extensively characterized in the context of inflammatory tumors. Research has shown that MDSCs suppress anti-tumor immunity by either directly interacting with T cells or remodeling the TME through immunosuppressive cellular and molecular networks [138]. Upon being recruited to inflammatory tumor tissues in response to chemokines like CCL2, CCL5, CXCL8, and CXCL12, MDSCs rapidly produce immunosuppressive mediators, including ARG1, NO, TGF-β, and IL-10, which further impair anti-tumor immunity [174,175]. Additionally, tumor-derived factors, such as VEGF, IL-6, and IL-10, can recruit MDSCs to the inflammatory TME, where they activate the STAT3 signaling pathway to produce more VEGF, establishing a positive feedback loop that promotes tumor angiogenesis [176,177].
Additionally, regulatory T cells (Tregs) significantly drive tumor initiation, progression, and metastasis by establishing an immunosuppressive tumor microenvironment, remodeling inflammatory networks, and intervening in metabolic homeostasis [178]. Tregs directly inhibit the activation and proliferation of CD8+ cytotoxic T cells and Th1 cells by highly expressing immune checkpoint molecules (such as CTLA-4, PD-1) and secreting suppressive cytokines (IL-10, TGF-β) [179]. They also induce dendritic cells (DCs) to differentiate into an immunologically incompetent phenotype and recruit myeloid-derived suppressor cells (MDSCs) and M2-type macrophages to the tumor microenvironment (TME), forming an “immune desert”-type microenvironment that allows tumor cells to escape immune surveillance [180,181,182]. In chronic-inflammation-associated tumors, Tregs weaken the ability of inflammation to eliminate abnormal cells by suppressing Th1/Th17 cell-mediated pro-inflammatory responses while secreting chemokines, such as CCL22, to establish a “pro-cancer inflammation-immunosuppression” positive feedback loop. For example, they cooperate with macrophages via the CXCL12–CXCR4 axis to promote angiogenesis [183]. At the metabolic level, Tregs degrade ATP into immunosuppressive adenosine by highly expressing CD39, preferentially uptake glucose and tryptophan, deprive effector T cells of energy sources, and deplete their function [184]. In addition, VEGF and TGF-β secreted by Tregs can directly promote tumor angiogenesis and EMT and enhance the invasion ability of tumor cells. In the therapeutic scenario, Tregs mediate chemotherapy, radiotherapy, and immunotherapy resistance by maintaining PD-L1 expression or recruiting MDSCs [185]. Clinical studies have shown that the infiltration level of FOXP3+ Tregs in tumor tissues is negatively correlated with the prognosis of patients. Immune intervention strategies targeting Tregs, such as the depletion of Tregs by anti-CD25 monoclonal antibody and the blockade of their function by CTLA-4 inhibitors, have become the key direction of tumor immunotherapy [186,187,188].
Vascular endothelial cells are now widely recognized as pivotal inflammatory cells involved in the regulation of inflammatory tumors. Beyond their conventional role as “gatekeepers,” these cells can be subverted by inflammatory or cancer signals to facilitate immune cell infiltration and tumor dissemination. As the crucial barrier separating blood from tissues, endothelial cells actively participate in inflammatory responses. Upon stimulation by inflammatory signals such as TNF-α and IL-1, vascular endothelial cells undergo activation, during which they release chemotactic signals (e.g., CXCL8, CXCL12, complement C5a, and platelet-activating factor) and upregulate surface adhesion molecules, thus facilitating the transmigration of leukocytes into tissues [189,190]. Nevertheless, chronic inflammation can weaken vascular wall integrity, paradoxically enabling easier leukocyte transmigration and exacerbating inflammation. This creates a conducive microenvironment for cancer metastasis. Tumor cells metastasize by breaching vascular barriers, a process similar to leukocyte migration. However, due to their larger size, cancer cells often become trapped within blood vessels [191]. To overcome this obstacle, they utilize adhesion proteins, such as E-selectin, to “roll” along the vascular endothelium, much like a rolling mechanism, to identify suitable exit sites [192,193,194,195,196,197].
3.3. Role of Key Inflammatory Cytokines in Tumorigenesis and Progression
Cytokines, a class of low-molecular-weight peptides or glycoproteins, play critical roles in TME signaling by mediating pro-inflammatory or anti-inflammatory responses. Research has evidenced that numerous inflammatory cytokines are closely associated with tumor initiation and progression [198]. Under the distinctive pathophysiological conditions of the TME, cancer-associated inflammatory cytokines, including TNF-α, TGF-β, IFN-I, IL-1, IL-6, and IL-10, often exhibit upregulated expression, thereby modulating the biological behaviors of tumor cells [199].
Interleukin-1 (IL-1), a key pro-inflammatory cytokine, is aberrantly upregulated in diverse cancer types and correlates with adverse clinical outcomes in patients [199]. This cytokine exhibits a dual role in tumor biology, mediating both pro-tumorigenic and context-dependent anti-tumor effects. Mechanistically, IL-1, secreted by tumor cells or immunosuppressive myeloid populations, such as MDSCs and Tregs, promotes oncogenesis through multiple pathways; it stimulates the production of pro-tumorigenic factors (e.g., IL-6, TNF-α) to drive autocrine/paracrine tumor growth [200,201], facilitates immune evasion by recruiting MDSCs to suppress effector T cell activity [202], and induces VEGF to promote tumor angiogenesis [203,204]. Conversely, under specific microenvironmental contexts, IL-1 can also activate anti-tumor immune pathways, such as inducing Th1-type immune responses and enhancing dendritic cell maturation [205]. Despite these pro-immunity functions, IL-1 exerts a predominantly pro-tumorigenic role in most cancer settings. It orchestrates an immunosuppressive TME by fostering the accumulation of regulatory immune cells (e.g., Tregs, MDSCs), inhibiting effector T cell cytotoxicity, and promoting the expression of immune checkpoint molecules. These effects collectively enable tumors to evade immune surveillance and develop resistance to immunotherapies like PD-1/PD-L1 blockade. Given this mechanistic duality, targeting IL-1β, the biologically active isoform primarily associated with pathological inflammation, has emerged as a promising adjunct to combination immunotherapy. Preclinical studies demonstrate that IL-1β inhibition synergizes with immune checkpoint inhibitors to enhance anti-tumor efficacy in murine models, likely by reversing TME-mediated immune suppression [206]. However, clinical translation must account for the context-dependent effects of IL-1, as its dual pro- and anti-tumor functions vary across cancer types and stages. Therapeutic strategies should therefore be meticulously tailored to specific malignancies, integrating molecular profiling of the TME to optimize benefits while mitigating off-target inflammatory risks.
IL-6, a pro-inflammatory cytokine produced by diverse cell types, including tumor cells and inflammatory cells, drives tumorigenesis and progression through multiple molecular pathways. Functioning as a “conspirator” in tumor development, IL-6 not only promotes the uncontrolled growth and drug resistance of tumors but also facilitates metastasis and colonization, ultimately making cancer more challenging to treat. Research has demonstrated that IL-6 expedites tumor cell proliferation by upregulating cyclins, downregulating the expression of the cyclin-dependent kinase inhibitor p21, and activating STAT3 protein. Meanwhile, IL-6 activates the Ras-ERK and PI3K-Akt signaling pathways to further accelerate tumor growth [207,208]. Furthermore, IL-6 promotes the expression of the anti-apoptotic protein Bcl-2 through STAT3 signaling, enabling tumor cells to evade chemotherapy-induced cytotoxicity [209,210]. Other pro-tumorigenic mechanisms of IL-6 also involve the suppression of tumor senescence [211,212], collaboration with other growth factors to amplify malignant behaviors [213], the induction of EMT, and the promotion of angiogenesis [214,215,216].
As a pivotal mediator of anti-inflammatory responses, IL-10 cytokine is primarily produced by tumor cells and leukocytes. IL-10 is a cytokine with a “Janus-faced” nature. As an anti-inflammatory molecule, it suppresses excessive inflammatory reactions, such as by reducing the release of pro-inflammatory cytokines of certain immune cells, thereby facilitating the maintenance of tissue homeostasis [217]. Nevertheless, IL-10 derived from either tumor or immune cells suppresses immune function by creating an “immunosuppressive shield” that allows tumors to evade immune attack [218]. Under certain contexts, IL-10 can activate immune cells and directly stimulate anti-tumor responses by increasing IFN-γ secretion [219]. This dual functionality likely arises from cancer-type-specific and immune-cell-state-dependent mechanisms. Consequently, its role must be evaluated in the context of the specific TME and patient conditions when formulating therapeutic strategies.
TNF-α, a pro-inflammatory cytokine produced by immune cells and tumor cells [220], exerts a double-edged sword role in cancer. At low concentrations, TNF-α acts as a tumor “conspirator”, promoting cancer cell proliferation, metastasis and angiogenesis, and chemoresistance [221], along with reducing the infiltration of CD8+ T cells into tumor tissues [222] and enhancing the function of immunosuppressive Treg cells through the TNF receptor 2 (TNFR2) [223]. However, at high concentrations, TNF-α may directly induce tumor cell death [224]. Nevertheless, the TME predominantly sustains low TNF-α concentrations, where its pro-tumorigenic effects dominate. Hence, targeting TNF-α or its receptors TNFR2 has emerged as a viable anticancer strategy. Some studies have demonstrated that blocking TNFR2 can inhibit ovarian cancer cell growth [225] and that the combination of TNF-α inhibition with immune checkpoint inhibitors could enhance therapeutic outcomes. Nevertheless, meticulous consideration of its dual nature is imperative to prevent interference with beneficial immune responses. In the majority of instances, TNF-α acts as an oncogenic ally, facilitating tumorigenesis, metastasis, and immune evasion.
The Janus-faced role of TGF-β in cancer evolves dynamically according to the disease stage [226,227,228,229,230]. Under physiological conditions and during early tumorigenesis, TGF-β acts as a tumor suppressor, operating much like a “brake” to inhibit tumor growth. As tumors advance, TGF-β undergoes a paradoxical role switch, altering its function to promote tumor proliferation, metastasis, angiogenesis, and immune evasion. Specifically, TGF-β induces EMT, enabling cancer cells to detach from primary lesions [231,232,233], and facilitates immune escape by suppressing anti-tumor immune responses [234]. These mechanisms collectively render cancer cells more resistant to therapeutic eradication [226,228,229,230].
Type I interferons (IFN-I), which function as versatile mediators, also exhibit complex and paradoxical roles in the context of cancer. As pro-inflammatory signals, IFN-I can promote tumor growth and metastasis via multiple mechanisms, including modulation of the TME [235], the regulation of cancer cell stemness [236], and the induction of EMT in endothelial cells [237]. In chronic inflammation, IFN-I establishes a tumor-supportive microenvironment conducive to growth and progression [235,238] and facilitates tumor cell immune evasion [236,239,240]. Additionally, evidence has shown that IFN-I can enhance cancer cell stemness via the regulation of the epigenetic regulator KDM1B [236]. However, the anti-tumor activities of IFN-I have also been well-documented, as studies have demonstrated that IFN-I exert their anticancer effects by negatively regulating the formation of premetastatic niches within the TME [241].
Consequently, the regulatory roles of various inflammatory cytokines in tumorigenesis are not one-dimensional, as the majority of inflammatory cytokines possess Janus-faced characteristics; they can either serve as allies in the fight against cancer or act as traitors in promoting tumor development. Their ultimate effects are contingent upon factors like cancer types, disease stage, and other elements within the TME. In the development of therapeutic strategies, it is critical to precisely evaluate their mechanistic roles to avoid “one-size-fits-all” approaches. The cornerstone of effective therapy lies in capitalizing on their context-dependent properties to devise targeted interventions that are customized to the specific circumstances of individual tumors.
4. Crosstalk Between Oxidative Stress and Inflammation in Cancer
As two critical biological processes, oxidative stress and inflammation not only exert individual influences on physiological states but also act synergistically under pathological conditions, co-regulating the initiation and progression of cancer [242]. Understanding their intricate interplay is fundamental to elucidating the mechanisms underlying tumorigenesis [243]. Oxidative stress refers to an imbalance between oxidative and antioxidant defense systems, resulting in elevated levels of ROS or RNS [242]. Inflammation, a protective response of the body to harmful stimuli, entails the activation of immune cells, the release of inflammatory mediators, and tissue repair processes [242]. However, dysregulated inflammation can precipitate the pathogenesis of numerous diseases. In cancers, oxidative stress and inflammation establish a vicious cycle, both directly and indirectly promoting tumor cell proliferation, migration, invasion, and metastasis. Moreover, they jointly shape the TME, creating a milieu conducive to tumor progression [243].
ROS have been demonstrated to influence both cancer and inflammation [244,245,246]. Therefore, targeting ROS in tumors may represent a potential cancer treatment strategy [181]. Oxidative stress prompts the overproduction of ROS or RNS within the body, which can be eliminated by the body’s endogenous antioxidant system under normal physiological circumstances [242]. However, when the body’s antioxidant system fails to maintain the balance between the production and clearance of ROS or RNS, the excessive accumulation of ROS or RNS can trigger inflammatory responses [242,247]. Prolonged exposure to oxidative stress is associated with many inflammation-related diseases, such as chronic inflammatory disorders, inflammatory bowel disease (IBD), and cancer [242]. Oxidative stress activates or enhances inflammatory responses through multiple pathways, thereby facilitating the occurrence and development of tumors [11,242,248]. Excessive ROS or RNS can cause damage upon cellular structures by destroying the membrane lipids [243]. Subsequently, the damaged cells release a cascade of damage-associated molecular patterns (DAMPs), which initiate innate immune responses and trigger inflammation [243]. Moreover, ROS can activate key signaling molecules, such as the NF-κB, PI3K/Akt and MAPK inflammatory signaling pathways, leading to the release of inflammatory cytokines and further exacerbating local inflammatory responses [249,250,251]. Notably, the activation of the transcription factor NF-κB plays a critical role in regulating inflammation, angiogenesis, adaptive metabolism, and treatment resistance [252,253], and is involved in multiple stages of cancer development. This transcription factor serves as both a trigger and a target for ROS, leading to the deterioration of the redox status in tumor tissues [254]. Additionally, NF-κB can induce the release of pro-inflammatory cytokines, promote the formation of an inflammatory tumor microenvironment, contribute to cancer progression, and facilitate tumorigenesis. This indicates that NF-κB acts as a crossroads between oxidative stress and inflammation in cancer. Therefore, targeting ROS production and the formation of the tumor inflammatory microenvironment mediated by NF-κB represents a promising cancer treatment strategy [255,256]. Based on the current research landscape, several bioactive natural products and small molecules have been tested for their anti-tumor effects due to their regulatory roles in the ROS/NF-κB signaling pathway [257,258,259,260]. Furthermore, growing evidence demonstrates that mitochondrial ROS play a key role in the tumor inflammatory microenvironment. Numerous studies have shown that oxidative stress impairs mitochondrial function [261,262], and the release of mitochondrial DNA can activate inflammasomes, thus promoting the secretion of inflammatory factors [263,264,265]. In the tumor microenvironment (TME), active oncogenic signaling prompts cancer cells to secrete inflammatory factors to surrounding cells, thereby facilitating tumorigenesis. Elevated reactive oxygen species (ROS) in the TME can enhance NADPH production by secreting inflammatory cytokines, stabilizing hypoxia-inducible factor (HIF) and activating the AMPK signaling pathway, thus promoting tumor metastasis and angiogenesis [266]. ROS are also capable of directly inducing oxidative damage to DNA, exemplified by the generation of 8-hydroxy-2′-deoxyguanosine (8-OHdG), and causing gene mutations, such as those in the Ras gene, which may give rise to abnormal cell function and trigger the activation of inflammatory signaling pathways. For example, mutated Ras proteins can lead to the expression of pro-inflammatory cytokines, such as IL-1 and IL-6, and recruit inflammatory cells, to promote angiogenesis [243,267]. This process underscores the pivotal role of ROS not only in causing direct cellular damage but also in fostering an environment conducive to inflammation and tumor progression. Significantly, persistent oxidative stress results in a decline in the activity of antioxidant enzymes (e.g., SOD and GPx) and a reduction in GSH levels, thereby impeding the effective scavenging of ROS. This, in turn, activates pathways, such as NF-κB, and promotes the development of chronic-inflammation-related diseases, such as cancer and cardiovascular diseases [243,251,267]. Notably, oxidative-stress-mediated chronic inflammation not only promotes genetic instability during the early stages of tumorigenesis but also provides a favorable environment for tumor cell survival, facilitating tumor progression by inducing angiogenesis and evading immune surveillance. In addition, there is a feedback loop between ROS and chronic inflammation, which is mainly initiated by immune cells caused by chronic inflammation. These immune cells not only promote chronic inflammation by releasing cytokines but also further aggravate inflammation by producing ROS [11]. In a chronic inflammatory environment, myeloid cells can secrete ROS and inflammatory mediators to aggravate chronic inflammation, suggesting that myeloid cells may play a key role in the inflammatory tumor microenvironment, especially in tumor metastasis [268]. It can be concluded that elevated ROS levels induce oxidative stress and inflammation, which in turn promote the occurrence and development of tumors. Targeting ROS may be an effective strategy for cancer therapy.
Current research suggests that there is a vicious cycle between chronic inflammation and ROS. Chronic inflammation can promote ROS production, and, in turn, ROS can facilitate the formation of chronic inflammation. Inflammation constitutes a pivotal innate defense mechanism against detrimental stimuli, and the inflammatory response triggers massive ROS production to combat pathogens. The defense process is mainly mediated by the activation of immune cells caused by a variety of factors. Activated immune cells produce large amounts of ROS, causing DNA damage, which is a cancer signal [269]. Therefore, ROS, as well as activation of ROS-mediated signaling pathways, may cause oncogenic effects. For example, during the inflammatory cascade, phagocytes, such as neutrophils and macrophages, harness the respiratory burst mechanism to produce ROS as an integral component of their pathogen-elimination strategy [3]. Nevertheless, in chronic inflammation, the overproduction of ROS not only targets foreign invaders but also damages surrounding healthy tissues and exacerbates oxidative stress levels [9]. As an illustration, in IBD, ROS actively participate in the inflammatory response and intensify oxidative stress [8,10]. Significantly, inflammatory responses and oxidative stress exhibit a bidirectional relationship, mutually reinforcing each other and thereby facilitating tumor progression [3,10]. During acute inflammation, immune cells, such as neutrophils and macrophages, deploy the respiratory burst mechanism to generate copious amounts of ROS for pathogen clearance. For example, activated phagocytes like neutrophils and macrophages produce ROS as a key weapon in their arsenal to combat pathogens during the inflammatory response [3,10,139,270]. However, in chronic inflammation, the excessive production of ROS transcends its role in pathogen elimination, causing extensive damage to surrounding normal tissues and escalating oxidative stress. TAMs, which are prominent in the TME, often adopt an M2 phenotype [3,10,138,139,267]. While secreting anti-inflammatory factors, they also release ROS and other oxidants, contributing to the maintenance of oxidative pressure within the TME [271]. In addition, inflammatory mediators produced during the inflammatory response can directly or indirectly promote oxidative stress. For example, pro-inflammatory cytokines, such as TNF-α and IL-1β, can activate NADPH oxidase, a major source of ROS in the body. Moreover, prostaglandins generated during inflammation can disrupt mitochondrial function, diminishing their antioxidant capacity and rendering cells more vulnerable to oxidative damage. Persistent chronic inflammation can lead to repeated cycles of tissue injury and repair during tissue regeneration, leading to the accumulation of genetic mutations and an elevated risk of cancer development. More importantly, the inflammatory microenvironment appears to favor the survival and self-renewal of cancer stem cells, potentially due to the selective pressure exerted by sustained oxidative stress, which promotes the emergence of tumor cell subpopulations with enhanced resistance and adaptability. It is worth noting that during inflammation, white blood cells and mast cells are recruited to the site of injury, triggering a “respiratory burst” that leads to the production and accumulation of large amounts of ROS [272,273]. Zhou et al. showed that all known activators of inflammasomes containing LRR-, NOD-, and NLRP3-based vesicles can induce ROS production. Importantly, studies have demonstrated that the mutagenic effects of ROS generated by inflammatory cells can promote tumor development [274]. Therefore, within the TME, oxidative stress and inflammation synergistically reinforce each other’s effects, creating a vicious cycle that is central to tumor initiation and progression. Both oxidative stress and inflammation are implicated at every stage of tumorigenesis, from genetic mutations and epigenetic alterations to immune escape. A deeper understanding of this intricate relationship not only helps to uncover the molecular mechanisms underlying tumor formation but also provides a theoretical foundation for the development of novel therapeutic strategies. Future research should prioritize the exploration of strategies to effectively disrupt this vicious cycle, with the ultimate goal of preventing and controlling cancer.
5. Diagnostic and Therapeutic Implications
5.1. Biomarkers
Oxidative-stress- and inflammation-associated tumor biomarkers exhibit multifaceted scientific and clinical relevance in early cancer detection, prognostic assessment, and mechanistic elucidation of tumorigenesis (Table 1). For early screening, noninvasive biomarkers, including 8-OHdG, C-reactive protein (CRP), and gut-microbiota-derived metabolites (e.g., the butyrate-to-deoxycholic acid ratio), serve as molecular indicators of DNA oxidative damage, chronic inflammatory states, and dysbiosis in host–microbiota interactions [275,276,277]. By quantifying these pathophysiological alterations, these markers significantly enhance the sensitivity and specificity of screening protocols for high-risk populations [275]. In prognostic assessment, longitudinal monitoring of the glutathione redox status (GSH/GSSG ratio), lactate levels, and IL-6 concentrations allows for quantitative characterization of spatiotemporally dynamic tumor redox stress and inflammatory microenvironments. This analytical framework enables precise prediction of treatment resistance and recurrence risks by capturing heterogeneous tumor biology [278,279]. Mechanistically, these biomarkers act as molecular sentinels, unveiling core regulatory modules within the oxidative stress–inflammation axis that govern tumor progression—including the ROS–NF-κB positive feedback circuitry and NLRP3 inflammasome activation thresholds [254,274]. Furthermore, these biomarkers illuminate cross-kingdom interaction mechanisms within microbiota metabolic reprogramming–epigenetic modification axes, exemplified by Fusobacterium nucleatum-mediated ROS production via the FadA/β-catenin signaling axis [280]. While persistent technical challenges exist in characterizing spatiotemporal heterogeneity and establishing detection standardization, emerging methodologies, including single-cell multi-omics profiling, artificial-intelligence-driven biomarker network modeling, and microbiome-targeted engineering strategies, are propelling the field toward high-resolution, dynamically integrated systems and medicine paradigms. These advancements foster a closed-loop evidence framework that bridges mechanistic discoveries with clinical translation, enabling precision cancer prevention and therapy through iterative refinement of diagnostic and therapeutic strategies.
8-OHdG, a well-characterized biomarker of DNA oxidative damage, arises from ROS-mediated guanine oxidation, directly contributing to genomic instability. This lesion induces mutagenic G → T transversions in key cancer-related genes, including tumor-suppressor loci (e.g., TP53) and oncogenes (e.g., KRAS) [276]. Mechanistically, 8-OHdG disrupts base excision repair (BER) pathways by inhibiting OGG1 glycosylase activity, thereby escalating the mutational burden in proliferating cells [281]. Accumulated 8-OHdG levels exhibit a strong positive correlation with tumor genomic instability, underscoring its role as a critical driver of mutagenesis. Notably, in chronically inflamed microenvironments, proinflammatory cytokines, such as TNF-α, synergize with ROS to upregulate APOBEC3B deaminase expression, amplifying 8-OHdG-associated mutational signatures by, for example, enriching TCW motif mutations in breast cancer genomes [282]. Furthermore, recent investigations have uncovered that oxidative stress induces extracellular vesicle release via the OGG1–SYT7 signaling axis, thereby facilitating tumor metastasis. As a pivotal mediator of oxidative signaling, 8-OHdG enhances NF-κB pathway activation through epigenetic reprogramming, establishing a self-reinforcing cycle of “oxidative damage–inflammatory activation–metastasis initiation”. In pleural effusion-derived cells from lung cancer patients, 8-OHdG coexists with KRAS and TP53 mutations at frequencies of 64.2% and 69.8%, respectively, findings that provide empirical evidence for its synergistic contribution to tumor progression.
In early cancer detection, 8-OHdG has emerged as a promising noninvasive biomarker with substantial clinical utility. Elevated serum and urinary 8-OHdG concentrations can be detected in prelesional stages, preceding the appearance of imaging-identifiable lesions. For instance, in lung cancer high-risk cohorts, combined measurement of serum 8-OHdG and IL-6 has been shown to significantly enhance early diagnostic sensitivity compared to conventional modalities [276]. Moreover, 8-OHdG levels exhibit a dose-dependent correlation with the malignant potential of pulmonary nodules, supporting their use in risk stratification [283]. In CRC screening, fecal 8-OHdG measurement combined with SDC2/TFPI2 gene methylation detection has been demonstrated to improve the identification rate of advanced adenomas, outperforming traditional fecal occult blood tests in detecting preinvasive lesions [284]. Notably, recent data from China’s National Cancer Center demonstrate a dose-dependent association between occupational exposures (e.g., steelworkers) and both elevated 8-OHdG levels and TP53 mutation frequencies, underscoring their utility in identifying environment-associated tumor risks. In prognostic stratification, dynamic 8-OHdG expression profiles in tumor tissues offer critical insights for predicting treatment resistance and recurrence risk. For CRC patients, low intratumoral 8-OHdG levels correlate with a higher risk of lymph node metastasis and poorer 5-year survival outcomes, whereas high expression is associated with improved sensitivity to chemotherapeutic regimens [285]. In hepatocellular carcinoma (HCC) patients who undergo radical resection, elevated postoperative serum 8-OHdG levels are significantly associated with an increased risk of early tumor recurrence [286]. This clinical correlation is hypothesized to arise from persistent oxidative stress-driven EMT activation within residual micrometastases, facilitating metastatic seeding [287]. Furthermore, 8-OHdG acts as a dynamic biomarker of therapeutic efficacy for guiding antioxidant intervention strategies. By reducing 8-OHdG levels, these therapies restore cellular redox homeostasis, a mechanism corroborated by their demonstrated ability to lower HbA1c levels in diabetic populations [276]. Such findings highlight the translational potential of 8-OHdG-targeted redox modulation as an adjuvant strategy in oncological care.

Table 1.
Biomarkers related to oxidative stress or inflammation in cancer biology.
Table 1.
Biomarkers related to oxidative stress or inflammation in cancer biology.
| Biomarkers | Description of Correlation | Significance in the Occurrence and Development of Tumors | Significance in Clinical Diagnosis and Treatment | References |
|---|---|---|---|---|
| CRP | Inflammation | High levels of CRP are associated with an increased risk of cancers. | An indicator to evaluate a chronic inflammatory state, it is helpful for cancer risk assessment. | [288,289,290,291,292] |
| IL-6 | Inflammation | Promoting the growth and metastasis of tumor cells and inhibiting the surveillance of tumor cells by the immune system. | It can be used to monitor disease progression and treatment efficacy in patients with cancer. | [293,294,295,296] |
| COX-2 | Inflammation | It induces inflammation by promoting prostaglandin synthesis, which in turn supports tumor cell proliferation, invasion, and angiogenesis. | It can be used as an important reference for tumor diagnosis, prognosis, and treatment target selection. | [297,298,299,300] |
| MDA | Oxidative stress | Elevated concentrations indicate excessive oxidative stress in the body, which may impair normal cellular structure and function, thus creating the conditions for tumorigenesis. | The detection of MDA can help to understand the individual oxidative stress status and indirectly indicate the risk of cancer. | [301,302,303] |
| 8-OHdG | Oxidative stress | High levels of 8-OHdG mean the DNA has been subjected to more oxidative attack, increasing the probability of genetic mutations. | It is helpful to evaluate the degree of oxidative damage in individuals and has potential value for predicting cancer susceptibility. | [303,304,305] |
| 4-HNE | Oxidative stress | It combines with a variety of biological macromolecules, such as proteins and DNA, leading to cell dysfunction and participating in the occurrence and development of tumors. | The determination of 4-HNE can reflect the lipid peroxidation status in vivo, which is helpful for evaluating the stage of tumor development and its prognosis. | [306,307,308] |
| SOD | Antioxidant defense system | It can catalyze the disproportionation of superoxide anion free radicals into hydrogen peroxide and oxygen, reduce the damage caused by oxidative stress to cells, and inhibit the formation and development of tumors to a certain extent. | It can be used as an important index to evaluate the antioxidant capacity of the body, and it has guiding significance for tumor prevention and treatment. | [309,310] |
| GPX | Antioxidant defense system | By reducing peroxides to water or alcohol, cells are protected from oxidative damage and the likelihood of tumorigenesis is reduced. | The level of GPX activity is closely related to the risk and severity of cancer, so it can be used as one of the bases for early warning and intervention measures of cancer. | [310,311,312,313] |
Lipid peroxidation-derived metabolites, including malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), arise from oxidative peroxidation of polyunsaturated fatty acids (PUFAs), a hallmark of oxidative stress. These endogenous electrophilic molecules serve as critical biomarkers of redox dysregulation, exhibiting substantial utility in deciphering tumor progression and prognostic stratification. Mechanistic investigations have established that PUFA peroxidation represents a key mechanistic driver of ferroptosis, an oxidative form of regulated cell death. The resultant MDA and 4-HNE compromise cellular membrane integrity and trigger proinflammatory signaling cascades, thereby amplifying inflammatory responses within the TME [314]. Specifically, MDA covalently binds to guanine residues to form M1G adducts, directly inducing mutagenic G → T transversions in tumor-suppressor genes, such as TP53. Concurrently, MDA inhibits the catalytic activity of key BER enzymes, including OGG1 and APE1, thereby escalating genomic instability. This mechanistic link is supported by clinical evidence demonstrating a positive correlation between serum MDA levels and tumor genomic instability scores in CRC patients [315,316]. In parallel, 4-HNE undergoes covalent modification of mitochondrial cardiolipin (CL), disrupting electron transport chain function and eliciting burst-like ROS production. This bioactive aldehyde further activates proinflammatory signaling axes, including NF-κB and NLRP3 inflammasome pathways, driving the transcriptional upregulation and secretion of proinflammatory cytokines, such as IL-6 and TNF-α. This establishes an “oxidative damage–inflammatory activation” positive feedback circuitry, whereby amplified inflammatory responses drive tumor cell proliferation, invasive potential, and immune evasion capabilities [317,318,319]. Mechanistically, S100P orchestrates acetyl-CoA carboxylase 1 (ACC1)-dependent lipid metabolic reprogramming to promote the accumulation of polyunsaturated fatty acid (PUFA)-derived phospholipids in HCC cells, thereby enhancing the generation of lipid peroxidation byproducts. This metabolic rewiring suppresses ferroptosis and facilitates tumor progression [320]. Additionally, 4-HNE covalently modifies β-catenin, enhancing its nuclear translocation efficiency and inducing EMT in HCC cells [321]. Additionally, 4-HNE is also central to the survival and treatment resistance of cancer stem cells. These cells upregulate ROS detoxification through enhanced GSH biosynthesis, thereby reducing intracellular 4-HNE accumulation and augmenting their tolerance to radiation and chemotherapy [322]. Clinically, elevated serum levels of MDA and 4-HNE correlate significantly with adverse prognoses in multiple solid tumors, including hepatocellular carcinoma, lung cancer, and CRC [323]. Their increased concentrations are directly associated with resistance to targeted agents, such as sorafenib, underscoring their potential as biomarkers for molecular subtyping and therapeutic stratification [320]. Notably, recent investigations have uncovered an inverse correlation between diminished 4-HNE levels and enhanced tumor aggressiveness in non-small cell lung cancer (NSCLC), with such reductions prognosticating poor clinical outcomes. This phenomenon is mechanistically attributed to FOXO4, a tumor suppressor gene whose expression and nuclear activity exhibit a negative correlation with tumor invasiveness and malignant potential. Specifically, reduced intracellular 4-HNE concentrations induce downregulation of FOXO4 expression and impair its transcriptional activity, thereby facilitating uncontrolled tumor cell proliferation and enhancing invasive capacity [324]. Furthermore, lipid-peroxidation-derived metabolites have emerged as critical mediators of synergistic interactions with immunotherapeutic modalities. For example, 4-HNE potentiates the therapeutic efficacy of immune checkpoint inhibitors (ICIs) by augmenting CD8+ T cell effector function and proliferation [279,318]. Notably, pharmacological inhibition of lipid peroxidation through targeting GPX4 or ACSL4 has been shown to overcome tumor resistance to immunotherapy and improve clinical outcomes, thereby presenting a promising strategy for developing combinatorial treatment regimens [318,322]. These cumulative findings collectively establish a robust theoretical framework for integrating lipid peroxidation biomarkers and modulators into precision oncology strategies.
Superoxide dismutase (SOD) and glutathione peroxidase (GPx) represent pivotal antioxidant enzymes with context-dependent bifunctional roles in tumor initiation, progression, and therapeutic response. SOD catalyzes the dismutation of O2•− into H2O2, whereas GPx family members utilize GSH to reduce H2O2 and organic hydroperoxides, collectively maintaining intracellular redox homeostasis [325,326]. Paradoxically, tumor cells frequently exhibit selective upregulation of SOD and GPx isoforms to evade oxidative-stress-induced apoptosis, thereby facilitating uncontrolled proliferation, enhanced invasive potential, and metastatic dissemination [327,328]. Preclinical evidence demonstrates that genetic or pharmacological downregulation of overexpressed SOD in colorectal and nasopharyngeal cancer models promotes tumor cell apoptosis, suppresses migratory capacity, and inhibits metastatic seeding [329]. GPx4, a pivotal regulator of ferroptosis, attenuates lipid peroxidation upon overexpression, thereby conferring resistance to ferroptosis-inducing agents, such as RSL3 [330]. In cancer stem cells (CSCs), GPx4 maintains lipid peroxidation homeostasis, sustaining stem cell pluripotency and chemotherapeutic resistance; preclinical studies demonstrate that pharmacological or genetic targeting of GPx4 potently suppresses CSC’s self-renewal capacity [331,332]. Clinically, aberrant serum or tissue enzymatic activity of SOD and GPx family members serves as a potential biomarker panel for early cancer detection and prognostic stratification [333]. For example, elevated GPx4 expression in CRC tissues correlates significantly with adverse clinical outcomes and resistance to 5-FU-based chemotherapy [334,335]. Elevated GPx1 levels in pancreatic-cancer-derived exosomes correlate significantly with advanced tumor stages and adverse prognoses [336,337]. Conversely, SOD activity in lung cancer tissues demonstrates a strong association with EGFR mutation status, thereby facilitating molecular subtyping of NSCLC [338]. Pharmacological targeting of SOD and GPx enzymatic activities has emerged as a promising therapeutic strategy to enhance anticancer efficacy. For instance, the SOD mimetic EUK-134, when combined with chemotherapeutic agents, induces synergistic apoptosis in pancreatic cancer cells, while GPx4-specific inhibitors, such as RSL3, trigger ferroptosis and exhibit potentiated anti-tumor effects when paired with sorafenib in hepatocellular carcinoma and other solid malignancies [339,340,341]. Notably, differential expression of SOD and GPx isoforms within the TME is strongly correlated with immunosuppressive signaling landscapes, potentially influencing responses to immune checkpoint inhibitors [342,343]. However, therapeutic modulation of these antioxidant systems necessitates careful mechanistic consideration due to their context-dependent roles. For example, mitochondrial SOD2 suppresses EMT by regulating ROS homeostasis in specific cancer subtypes; conversely, genetic or pharmacological depletion of SOD2 has been shown to exacerbate tumor progression through compensatory redox imbalances in these contexts [344,345]. These findings underscore the intricate tumor type and TME-specific functions of SOD and GPx family members, emphasizing the critical need for precision therapeutic strategies informed by multi-omic profiling and longitudinal biomarker monitoring.
C-reactive protein, a hepatocyte-derived acute-phase reactant, mediates tumor progression by facilitating the establishment of chronically inflamed microenvironments. Elevated systemic CRP levels activate pro-tumorigenic signaling cascades, including NF-κB and MAPK pathways, inducing tumor cells to secrete proinflammatory cytokines, such as IL-6 and TNF-α. These cascades promote angiogenesis, inhibit anti-tumor immune surveillance, and enhance tumor cell invasion and metastatic dissemination [346]. In cancer screening paradigms, CRP levels exhibit a dose-dependent association with malignant transformation risk. A large Japanese prospective cohort study demonstrated that individuals in the highest CRP quartile exhibited a 28% increased risk of overall cancer incidence compared to the lowest quartile, with significant associations observed for colorectal, lung, and breast cancer development [347]. Cancer risk increases in a CRP-concentration-dependent manner, with progressive elevation of systemic CRP levels associated with heightened malignant potential. In NSCLC, preoperative serum CRP levels correlate significantly with tumor invasive characteristics and histological malignancy grade, serving as an adjunctive biomarker to improve early diagnostic accuracy [348]. However, the inherent lack of specificity of CRP limits its utility as a standalone tumor marker, necessitating combinatorial approaches that integrate tumor-specific antigens (e.g., carcinoembryonic antigen, CEA) or advanced imaging techniques to enhance screening precision. In prognostic stratification, CRP functions as an independent prognostic factor; elevated preoperative levels in colorectal cancer patients reflect intensified tumor microenvironmental inflammation, which is strongly associated with increased postoperative recurrence risk and shortened overall survival [349]. In hematological and gastrointestinal malignancies, elevated C-reactive protein/albumin (CRP/ALB) ratios reflect progressive systemic inflammation and malnutrition, which are strongly associated with shortened overall survival [350,351]. Notably, clinical interpretation of CRP must account for tumor histotype and microenvironmental context. The CALLY index, a composite score incorporating CRP, serum albumin, and lymphocyte count, has been validated as an independent prognostic biomarker for digestive system neoplasms. A multicenter clinical investigation involving 7916 patients demonstrated that lower CALLY scores correlate with poorer overall, disease-free, and recurrence-free survival outcomes, with consistent findings across diverse tumor histotypes and demographic populations [351]. However, CRP elevation in pancreatic cancer may complicate differential diagnosis due to overlap with severe acute pancreatitis, necessitating integrated clinical and imaging evaluations. Collectively, these findings establish a theoretical framework for CRP as a broadly applicable tumor-associated inflammatory biomarker while emphasizing the need for prospective validation of its specificity and sensitivity in large-scale, multi-center trials.
COX-2, a key enzyme in the arachidonic acid pathway, catalyzes the conversion of arachidonic acid into bioactive eicosanoids, including prostaglandins (PGs) and thromboxanes, which are central to regulating inflammatory responses and tissue homeostasis [352]. While exhibiting low basal expression in most normal tissues, COX-2 is aberrantly overexpressed in a wide spectrum of malignancies, including lung, gastrointestinal, breast, and hepatocellular carcinomas. This dysregulated expression drives carcinogenesis through multiple mechanisms: excessive production of pro-inflammatory prostaglandin E2 (PGE2) promotes tumor cell proliferation, inhibits apoptosis, and facilitates angiogenesis; concurrently, COX-2-mediated remodeling of the TME induces immunosuppressive signaling, reduces anti-tumor immune surveillance, and enhances metastatic potential [353]. Clinically, COX-2 overexpression is associated with increased recurrence risk, shortened overall survival, and resistance to chemotherapeutic and radiotherapeutic interventions, solidifying its role as a pivotal oncogenic driver across diverse cancer types. COX-2-catalyzed synthesis of PGE2 triggers a cascade of pro-tumorigenic events by engaging EP2/4 prostanoid receptors. This ligand-receptor interaction activates NF-κB and STAT3 signaling axes, concurrently inhibiting p53-mediated apoptotic pathways and upregulating secretion of proinflammatory cytokines such as IL-6 and TNF-α. Collectively, these processes establish a chronically inflamed TME that promotes uncontrolled tumor cell proliferation, invasive migration, and EMT [354]. Furthermore, COX-2-mediated immune evasion occurs through dual mechanisms: suppression of macrophage phagocytic activity dampens anti-tumor immune surveillance, while VEGF upregulation drives angiogenesis in CRC, facilitating metastatic niche formation [355]. In cancer risk assessment, elevated COX-2 expression has been causally linked to increased susceptibility to colorectal and lung carcinogenesis. A 2022 prospective cohort study by Japan’s National Cancer Center demonstrated that COX-2, as a mechanistic inflammatory biomarker, enhances risk stratification for tumor initiation when integrated with traditional markers like CRP, providing incremental value beyond standalone CRP assessment [347]. In a landmark study, Keisuke Kosumi, et al. employed Cox proportional hazards regression to analyze data from 1,708 stage I–IV CRC patients, revealing a statistically significant interaction between COX-2 expression and BRAF mutation status. Among BRAF-mutant tumors, high COX-2 expression was independently associated with significantly poorer overall survival compared to COX-2-negative counterparts, whereas this prognostic association was absent in BRAF-wild-type cases. These findings validate COX-2 as a robust prognostic biomarker specific to BRAF-mutant CRC, underscoring its utility in molecular subtyping for personalized risk stratification [356]. Clinically, selective COX-2 inhibitors such as celecoxib have demonstrated chemopreventive and therapeutic benefits: long-term use is associated with reduced incidence of colorectal, lung, esophageal, and hepatic malignancies, while adjuvant administration enhances response to cytotoxic chemotherapy by sensitizing tumor cells to apoptosis. Mechanistic studies attribute these effects to COX-2-mediated suppression of pro-inflammatory prostaglandin signaling and modulation of drug resistance pathways [357,358]. Combination strategies integrating COX-2 inhibitors with cytotoxic chemotherapeutics (e.g., alkylating agents, antimetabolites, anti-tumor antibiotics), monoclonal antibodies, and other antineoplastic agents are being actively investigated to optimize therapeutic efficacy while minimizing treatment-related toxicities [358]. Indeed, the FDA has approved celecoxib for the management of familial adenomatous polyposis (FAP), and other COX-2 inhibitors are also being explored for cancer treatment and prevention. Meanwhile, mechanistic investigations further reveal synergistic interactions with conventional cytotoxins. For example, aspirin enhances cisplatin sensitivity in colorectal cancer cells by disrupting NF-κB binding to the COX-2 promoter, thereby suppressing COX-2-dependent pro-survival signaling [359]. These findings underscore the translational promise of targeting the COX-2-inflammatory axis as part of multi-modal cancer treatment strategies, emphasizing the need for biomarker-driven patient selection to maximize clinical benefit.
Furthermore, a multitude of additional tumor biomarkers associated with oxidative stress and inflammatory pathways have been implicated in critical roles across tumor pathogenesis, progression, and prognostic evaluation. The persistent activation of NF-κB represents a core component of the oxidative stress–inflammation axis, serving to regulate the expression of pro-inflammatory mediators such as IL-6 and COX-2. In HCC and gastric cancer, dysregulated NF-κB signaling has been consistently linked to enhanced tumor cell proliferation and chemoresistance, whereas pharmacological inhibition of NF-κB has been shown to augment the therapeutic efficacy of 5-FU [360]. Nrf2, a pivotal transcription factor governing antioxidant responses, exerts biphasic roles in carcinogenesis: its activation during the initiation phase suppresses tumorigenesis by upregulating antioxidant genes, whereas sustained activation at advanced stages promotes chemoresistance through enhanced GSH biosynthesis and upregulation of multidrug efflux transporters [351]. Clinical evidence demonstrates that elevated Nrf2 expression is associated with cisplatin resistance in NSCLC and sorafenib resistance in HCC. Consequently, combinatorial strategies targeting both antioxidant depletion and Nrf2 inhibition may potentiate chemotherapeutic sensitivity by disrupting adaptive resistance mechanisms [361,362]. Additionally, elevated Nrf2 expression is associated with poor prognosis in non-small cell lung cancer (NSCLC), and the activation status of Nrf2 in KEAP1-mutant lung cancer patients serves as a predictor of tumor sensitivity to antioxidant therapies [361,362]. Fusobacterium nucleatum drives tumorigenesis through the FadA adhesin, which activates the β-catenin/NF-κB signaling axis to induce the production of ROS [363,364]. High F. nucleatum abundance in CRC tissues has been shown to correlate with resistance to 5-FU [365]. Detection of this bacterium in fecal samples is emerging as a potential biomarker for early-stage CRC screening. Moreover, metabolites derived from the gut microbiota, such as butyrate and secondary bile acids, are implicated in critical regulatory roles during tumor progression. Butyrate exerts anti-tumor effects by inhibiting histone deacetylases (HDACs), thereby enhancing the expression of the transcription factor ID2 in CD8+ T cells and potentiating anti-tumor immune responses [366,367,368]. In HCC, butyrate suppresses tumor cell proliferation and metastasis while augmenting the therapeutic efficacy of sorafenib [367]. However, its biological actions are contingent upon microbial origin and concentration. For instance, Bifidobacterium-derived butyrate inhibits non-alcoholic fatty liver disease-associated HCC development, whereas butyrate generated under a high-fructose diet activates pro-inflammatory signaling through the SCFA receptor GPR43, thereby promoting HCC progression [366,367,368]. Secondary bile acids, including deoxycholic acid (DCA), promote CRC cell proliferation and EMT by activating the farnesoid X receptor (FXR)-NF-κB signaling axis. Conversely, lithocholic acid (LCA), a secondary metabolite derived from cholic acid, inhibits the activation of the NLRP3 inflammasome, thereby reducing the secretion of pro-inflammatory cytokines IL-1β and TNF-α. This dual action alleviates intestinal inflammation and diminishes the risk of CRC development [366,367,368].
5.2. Therapeutic Strategies Targeting Oxidative Stress–Inflammation Axis
5.2.1. Oxidative-Stress-Based Cancer Intervention Strategies
In cancer therapeutics, redox homeostasis modulation strategies encompass two primary approaches: ROS-depleting therapies that employ antioxidant agents, and ROS-promoting strategies that elevate intracellular ROS levels through direct or indirect inhibition of cellular antioxidant systems [16,369]. In normal somatic cells, endogenous antioxidant mechanisms serve to mitigate toxic free radical-induced damage and safeguard against malignant transformation. Conversely, within established neoplastic tissues, the multifaceted role of oxidative stress in tumor initiation, progression, and microenvironmental adaptation highlights the limitations of oversimplified ROS-modulating therapeutic paradigms. In the context of cancer biology, while ROS are implicated in driving neoplastic progression, initial hypotheses proposing that antioxidant therapies could suppress tumors have not been unequivocally validated. Emerging evidence instead highlights that pharmacological ROS suppression may inadvertently facilitate cancer progression and enhance treatment resistance mechanisms [7,33]. Mechanistically, if ROS-mediated carcinogenesis proceeds via induction of mutagenesis and by acting as second messengers in cell proliferation signaling pathways, antioxidant intervention to mitigate oxidative stress could theoretically impede tumor initiation, a premise underpinning the development of certain antioxidant-based cancer therapies. However, clinical and preclinical data indicate that antioxidant drugs, by scavenging ROS, may attenuate the cytotoxic efficacy of established chemotherapeutics and radiotherapy [370,371]. Moreover, dietary strategies incorporating natural antioxidants have been explored for cancer prophylactic applications (Table 2). Nutrient components with antioxidant activities, including vitamins A/D, genistein, sulforaphane, curcumin, tannins, and flavonoids, have demonstrated potential to inhibit tumor initiation and progression in breast, liver, and pancreatic cancers, among others [29,372,373]. A landmark large-scale randomized double-blind trial examining the chemopreventive effects of selenium, vitamin E, and β-carotene supplementation reported that this combination significantly reduced all-cause mortality in esophageal and gastric cancer patients, with protective effects persisting for over a decade following intervention discontinuation [374,375]. Concurrently, epidemiological evidence has established an inverse association between long-term vitamin E intake and HCC risk, with dose-dependent reductions in incidence observed across prospective cohort studies [376]. Paradoxically, longitudinal investigations in smoking populations revealed that sustained supplementation with β-carotene alongside vitamins A/E was associated with elevated lung cancer incidence, overriding any putative chemopreventive effects in this high-risk subgroup [377,378]. While initial reports suggested selenium may mitigate prostate cancer progression, subsequent mechanistic studies demonstrated that this protective effect is exclusively confined to aggressive metastatic phenotypes and exhibits strong dependence on genetic polymorphisms in selenoprotein-coding genes [379]. A large-scale epidemiological study involving 35,533 males with a long-term follow-up period demonstrated that supplementation with selenium or vitamin E in healthy populations failed to show a significant reduction in prostate cancer risk. Conversely, prolonged intake of vitamin E was associated with an increased risk of prostate cancer among healthy males [380]. These conflicting findings underscore the intricate dynamics of redox balance in determining cell fate within normal versus neoplastic cellular microenvironments. Future research and translational strategies must incorporate molecular subtyping of tumors, individual metabolic profiles, and interindividual variability to develop precision-oriented antioxidant strategies that optimize outcomes in cancer prevention and therapeutic interventions.

Table 2.
Anticancer treatments according to their role in regulating ROS levels.
Elevated ROS levels disrupt cellular homeostasis, whereas oxidative stress can exert tumor-suppressive effects, driving cancer cells to evolve robust antioxidant defense mechanisms for adaptive survival. Despite upregulating intracellular antioxidant systems, cancer cells maintain higher basal ROS levels than normal cells. This metabolic disparity establishes a therapeutic window, as malignant cells may exhibit heightened sensitivity to agents that further induce ROS accumulation [29]. Indeed, numerous clinically approved chemotherapeutic compounds exert their cytotoxic effects by elevating ROS levels (Table 2), although such treatments inherently carry risks of promoting off-target tumorigenesis. Taxanes (paclitaxel, docetaxel), vinca alkaloids (vincristine, vinblastine), and antimetabolites (fluorouracil, fludarabine) act through dual mechanisms: inducing cytochrome c release from mitochondria to trigger apoptotic cell death and disrupting mitochondrial electron transport chains to generate free radicals [388,390,394]. Anthracyclines, including doxorubicin, epirubicin, and daunorubicin, exert anti-tumor effects by inducing excessive ROS and RNS, which trigger DNA damage, mitochondrial dysfunction, and impaired protein synthesis [391]. Arsenic trioxide (As2O3), 2-methoxyestradiol, and N-(4-hydroxyphenyl)-retinamide (4-HPR) mediate apoptosis across multiple cancer types, including leukemia, multiple myeloma, and breast cancer, via ROS overproduction [383,384,385]. Additionally, platinum-based anticancer agents (cisplatin, carboplatin, oxaliplatin) induce oxidative stress through their high-affinity binding of platinum (Pt) moieties to sulfur and selenium atoms in proteins/peptides, thereby disrupting key cellular antioxidant systems such as GSH and thioredoxin reductase (TrxR) [395]. Cisplatin resistance is primarily mediated by its covalent interaction with GSH, as GSH readily forms conjugates with the drug’s platinum center, thereby facilitating drug detoxification [396]. Paradoxically, this detoxification process depletes intracellular GSH stores, disrupting redox homeostasis and inducing ROS overproduction. Notably, elevated GSH levels in neoplastic cells correlate with resistance not only to cisplatin but also to anthracyclines and alkylating agents. Additionally, tumors adapting to chronic oxidative stress through upregulation of SOD2, peroxidase 1, and B-cell lymphoma-2 (BCL-2) protein exhibit enhanced chemoresistance to agents like 5-FU [393]. Targeting tumor redox homeostasis, via inhibition of GSH/SOD2 biosynthesis or induction of their metabolic depletion, represents a promising therapeutic strategy to potentiate anticancer drug efficacy [16]. NOV-002, a glutathione disulfide (GSSG) mimetic compound, modulates the intracellular GSSG/GSH redox balance to induce oxidative stress, thereby inhibiting tumor cell invasion, proliferation, and survival [386]. In HER2-negative breast cancer patients, adjuvant therapy combining NOV-002 with doxorubicin, cyclophosphamide, and docetaxel chemotherapy improved clinical outcomes with reduced treatment-related toxicity compared to chemotherapy alone [397]. Poly(ADP-ribose) polymerase (PARP), an enzyme frequently overexpressed in diverse malignancies, plays a pivotal role in preserving genomic integrity and mediating the cellular response to oxidative stress. PARP inhibitors, when combined with platinum-based chemotherapeutics, disrupt cancer cells’ adaptive responses to oxidative stress by impairing DNA repair capacity [398]. Phase II clinical trials demonstrated that triple-negative breast cancer (TNBC) patients derived significant benefit from the combination of the PARP inhibitor veliparib and carboplatin, exhibiting improved progression-free survival [399,400]. Similarly, preclinical studies in Brca1/2-deficient breast cancer mouse models showed that olaparib, a selective PARP inhibitor, enhanced the anti-tumor efficacy of platinum-based therapies, leading to profound tumor growth suppression [401,402]. Furthermore, PARP inhibition has been shown to sensitize NSCLC cells to cisplatin, representing a promising strategy for overcoming chemoresistance in this tumor type [403].
Cancer therapies targeting oxidative stress exert effects beyond direct cytotoxicity toward cancer cells, as they can profoundly influence tumor progression and metastatic potential by modulating stromal remodeling. The nature and subcellular localization of ROS exhibit intricate associations with tumor survival and metastatic dissemination across diverse cancer subtypes, highlighting that single-target interventions tested in simplified experimental settings, while valuable for mechanistic dissection, may fail to recapitulate therapeutic responses in the context of the complex TME. Cancer-associated fibroblasts (CAFs), localized at tumor-stromal interfaces or infiltrating neoplastic tissues, are critical drivers of cancer initiation, progression, and metastasis [404,405]. High CAF density within tumors correlates with adverse clinical outcomes, enhanced infiltration of tumor-associated macrophages, and promotion of EMT [404,405]. Long-term radiotherapy or chemotherapy promotes the activation of CAFs within the TME by enhancing mitochondrial ROS production [406,407]. Chemotherapy-induced ROS or antioxidant depletion may further promote the immunosuppressive polarization of tumor-associated macrophages, thereby fostering an immunosuppressive niche [406,407]. These findings underscore the necessity of integrating both the direct cytotoxic effects of ROS on cancer cells and the indirect pro-tumorigenic impacts mediated by ROS in stromal components during therapeutic strategy design. Notably, oxidative stress-driven mechanisms exhibit context-dependent effects across tumor types: preclinical evidence demonstrates that ROS suppress metastasis in lung cancer models while facilitating metastatic progression in pancreatic cancer models [408,409]. These contextual discrepancies likely arise from inherent variations in ROS biology, encompassing ROS species, subcellular localization, cellular redox responsiveness, and the microenvironmental presence of ferroptosis-regulatory pathways [26,410]. Mechanistic studies demonstrate that mitochondrial ROS act as well-characterized inducers of regulated cell death, whereas nitrogen oxide derived ROS species are increasingly implicated in promoting cell proliferation and migratory phenotypes [7,321]. These findings highlight the need for caution in deploying ROS-modulating therapies, as inappropriate use may not only abrogate therapeutic efficacy but also potentiate malignant progression. Notably, cancer cells exhibit distinct deviations from normal cellular ROS homeostasis, including differential exposure thresholds and stress response dynamics. Continued mechanistic dissection of these context-dependent complexities will be pivotal for developing precision-targeted interventions that leverage ROS signaling or its regulatory networks to selectively address specific cancer vulnerabilities.
5.2.2. Inflammation-Based Cancer Intervention Strategies
Inflammatory cells and their secreted mediators, including cytokines and chemokines, constitute a critical component of the TME, orchestrating processes central to tumor survival, immune cell infiltration, and evasion of immunosurveillance [271]. Modulating inflammatory and immune signaling has thus emerged as a pivotal dimension of cancer prevention and therapeutic strategies (Table 3). Substantial evidence supports the chemopreventive effects of non-steroidal anti-inflammatory drugs (NSAIDs) across multiple cancer types, including colorectal, breast, and esophageal malignancies. Both epidemiological observations and randomized controlled trials demonstrate that regular aspirin use confers significant cancer risk reduction, most pronounced for CRC, while improving clinical outcomes in diagnosed patients by lowering 5-year mortality and disease recurrence rates [17,358]. Combination therapy with aspirin and esomeprazole has been shown to significantly improve clinical outcomes in patients with Barrett’s esophagus while mitigating the risk of esophageal cancer development [411]. NSAIDs exert their chemopreventive effects primarily by inhibiting COX-2 activity, thereby reducing prostaglandin biosynthesis. Prostaglandins mediate pro-inflammatory signaling cascades and promote tumor cell survival, angiogenesis, and immune evasion, which are key processes driving malignant progression. The FDA has approved celecoxib, a selective COX-2 inhibitor, for the treatment of familial adenomatous polyposis [340]. Clinical trials further demonstrate that celecoxib combined with cytotoxic chemotherapy (5-fluorouracil, leucovorin, irinotecan) constitutes a safe and efficacious synergistic regimen for metastatic CRC. Compared with monotherapy, this combinatorial approach improves progression-free survival and one-year overall survival rates, highlighting the potential of inflammatory pathway modulation in enhancing anticancer efficacy [412]. Celecoxib in combination with paclitaxel enhances dendritic cell maturation, thereby triggering T-cell-dependent anti-tumor immune responses and augmenting immune-mediated rejection of TNBC. This synergistic interaction leads to suppressed tumor recurrence and reduced lung metastasis in preclinical models [413]. Clinically, COX-2-high tumor patients derive greater benefit from celecoxib-based combination therapies, as evidenced by significantly improved prognostic outcomes compared to COX-2-low counterparts [414]. A phase III clinical trial in resected stage III CRC patients found no overall statistically significant improvement in disease-free survival (DFS) with celecoxib monotherapy [415]. However, stratified analysis by PIK3CA mutation status revealed a notable subgroup effect: patients harboring PIK3CA gain-of-function mutations experienced prolonged DFS when treated with celecoxib, whereas those with wild-type PIK3CA derived no such benefit [415]. An adjuvant therapy trial evaluating celecoxib in ERBB2-negative breast cancer patients found no significant improvement in DFS following two years of treatment as an add-on to standard therapy [416]. Furthermore, preclinical and clinical evidence suggests that adjuvant celecoxib added to chemotherapy regimens may exert detrimental effects on clinical outcomes in breast cancer subsets, particularly in tumors with low prostaglandin-endoper oxide synthase 2 (PTGS2) expression [417]. These divergent findings underscore the intricate interplay between anti-inflammatory pathways and tumor-specific biological contexts, highlighting the challenges posed by mechanistic complexity and interpatient heterogeneity in translating COX-2-targeted strategies into clinical benefit.
Tumor-associated macrophages represent a dominant immunocyte population within the TME, playing a pivotal role in promoting tumor progression and mediating multidrug resistance [418]. Therapeutic strategies targeting TAMs primarily involve two complementary approaches: direct induction of macrophage apoptosis or inhibition of their recruitment to the tumor niche, and reprogramming their functional polarization to elicit a cytotoxic anti-tumor phenotype (Table 3) [419]. The CCL2/CCR2 chemokine axis is central to orchestrating the recruitment, survival, and expansion of inflammatory monocytes, TAMs, and MDSCs [420]. While preclinical and early clinical data show that CCR2 inhibitors as single agents exhibit modest therapeutic efficacy, their combinatorial use with chemotherapy or immune checkpoint inhibitors has emerged as a promising strategy, leveraging synergistic interactions to enhance anti-tumor immunity and overcome resistance. Monoclonal antibodies targeting CCL2 have been shown to effectively reduce macrophage accumulation in preclinical tumor models and enhance anti-tumor efficacy when combined with conventional chemotherapy [421,422]. A Phase I clinical trial demonstrated that PF-04136309, a small-molecule CCR2 inhibitor, was well-tolerated when integrated into FOLFIRINOX chemotherapy regimens for patients with borderline resectable or locally advanced pancreatic cancer, establishing the safety and feasibility for combinatorial approaches [423]. In pancreatic ductal adenocarcinoma (PDAC) mouse models, the dual CCR2/5 inhibitor BMS-687681, when combined with αPD-1 immunotherapy and radiation, reprogrammed the TME by augmenting intratumoral infiltration of effector T cells and memory T cells while reducing accumulation of Tregs, M2-polarized TAMs, and MDSCs. This multimodal strategy exhibited significantly enhanced anti-tumor activity compared to single-agent or dual-combination regimens in preclinical testing [138]. Bisphosphonates, which exert cytotoxic effects on macrophages while modulating bone remodeling, are clinically utilized for treating postmenopausal osteoporosis and managing bone metastases in cancer patients [424]. In breast and prostate cancer populations, bisphosphonate monotherapy or combination with hormone therapy has been shown to significantly reduce disease recurrence rates and improve overall survival [424]. Trabectedin, a marine-derived alkaloid with selective cytotoxicity toward monocyte-derived macrophages, exhibits multifunctional anti-tumor activities: it inhibits pro-inflammatory mediator secretion, reduces TAMs density within the TME, and suppresses angiogenesis. This agent has been approved by regulatory authorities for the treatment of advanced soft tissue sarcoma and ovarian cancer [425]. Additionally, therapeutic strategies aimed at reprogramming TAMs from an immunosuppressive M2 phenotype to a cytotoxic M1 phenotype represent a promising frontier in cancer immunotherapy, leveraging macrophage plasticity to enhance anti-tumor immune responses. Preclinical studies have shown that the agonistic anti-CD40 antibody CP-870,893 reprograms TAMs into M1-like effector cells with enhanced antigen-presenting capacity, thereby restoring tumor immune surveillance and promoting tumor regression in murine models [426]. Phase I clinical trials demonstrated that combination therapy with anti-CD40 antibody and gemcitabine provided clinical benefit to select patients with advanced pancreatic cancer, highlighting the feasibility of TAMs-targeted immune modulation [427]. The immune checkpoint axis formed by SIRPα, expressed on phagocytes including TAMs, and CD47 on tumor cells inhibits intracellular signaling cascades, thereby negatively regulating phagocytic activity and enabling tumor cells to evade immune elimination. Monoclonal antibodies targeting CD47 have been shown to block this immunosuppressive interaction, successfully inducing antibody-dependent cellular phagocytosis of tumor cells in diverse preclinical tumor models [428,429]. However, these findings underscore the therapeutic potential of CD47 blockade, clinical translation remains contingent on robust validation through ongoing and future clinical trials.
Proinflammatory cytokines, such as tumor necrosis factor (TNF), IL-6, and IL-1β, typically drive tumor progression by enhancing tumor cell proliferation, survival, and invasive potential, as well as promoting angiogenesis [430]. Current therapeutic strategies targeting pro-tumorigenic cytokine signaling largely depend on antibody-based interventions (Table 3). However, monotherapeutic approaches using anti-cytokine antibodies have generally demonstrated suboptimal efficacy, characterized by limited clinical responses and treatment-associated toxicities [138]. Even in multiple myeloma, where IL-6 is recognized as a critical growth factor driving tumor cell proliferation and survival, the clinical efficacy of anti-IL-6 mAbs has been disappointing—despite anecdotal reports of transient benefits in select patient subgroups [208]. This suboptimal response necessitates the integration of these biologics with conventional chemotherapies or targeted agents. For instance, clinical investigations have demonstrated that anti-IL-1β mAbs enhance the therapeutic efficacy of PD-1 checkpoint inhibitors in breast cancer patients [431]. Preclinical studies using renal cell carcinoma mouse models further revealed that combinations of IL-1β inhibitors with anti-PD-1 antibodies or tyrosine kinase inhibitors yielded significantly enhanced anti-tumor responses compared to monotherapeutic approaches [432]. Siltuximab, a chimeric monoclonal antibody targeting IL-6, exhibited acceptable safety profiles and improved clinical outcomes when combined with mitoxantrone/prednisone (M/P) in patients with castration-resistant prostate cancer (CRPC) with prior chemotherapy exposure [433]. Infliximab, a monoclonal antibody against TNF-α, in combination with oxaliplatin has been shown to partially reverse oxaliplatin resistance in CRC, potentially through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity mechanisms [434]. Additionally, interferon-α (IFN-α), an FDA-approved adjuvant therapy for melanoma, has been shown to improve DFS and OS in patients who underwent surgical resection [435]. However, select clinical trials have reported suboptimal outcomes, potentially compromising patient health and quality of life [436]. Indeed, cytokine-based anti-tumor therapies remain in the translational development phase, necessitating continued preclinical and clinical investigations to enhance therapeutic windows and mitigate treatment-related toxicities.

Table 3.
Anticancer treatments according to their role in regulating inflammation.
Table 3.
Anticancer treatments according to their role in regulating inflammation.
| Name | Mechanism of Action; Effects on Inflammation | Effect on Tumors | References |
|---|---|---|---|
| NSAIDs | Inhibit COX-2 activity, reduce prostaglandin biosynthesis | Reduce the incidence of cancers; inhibit tumor cell survival, angiogenesis, and immune evasion; improve clinical outcomes | [17,358] |
| CCR2 inhibitors (PF-04136309, BMS-687681) | Cause remodeling of immune cells within the tumor | Enhance anti-tumor immunity and overcome resistance | [138] |
| Bisphosphonates | Cytotoxic effects on macrophages | Reduce breast and prostate cancer patients’ recurrence rates and improve overall survival | [424] |
| Trabectedin | Cytotoxicity toward monocyte-derived macrophages; inhibits pro-inflammatory mediator secretion | Inhibit advanced soft tissue sarcoma and ovarian cancer | [425] |
| CP-870,893 | Reprograms TAMs into M1-like effector cells | Facilitate the depletion of tumor stroma; inhibit tumor growth | [426] |
| Anti-IL-1β mAbs | A monoclonal antibody against IL-1β | Combined treatment enhances the efficacy of chemotherapy drugs | [431,432] |
| Siltuximab | A chimeric monoclonal antibody targeting IL-6 | Combined therapy enhances the efficacy of chemotherapy drugs and improves their safety | [433] |
| Infliximab | A monoclonal antibody against TNF-α | Reverse oxaliplatin resistance in CRC | [434] |
| Interferon-α | Regulate the differentiation and infiltration of immune cells | Improve DFS and OS in patients | [435] |
6. Conclusions
Oxidative stress and inflammation serve as dual drivers of tumorigenesis, functioning synergistically through intricate regulatory networks to promote tumor initiation, clonal expansion, invasive phenotype acquisition, and therapeutic resistance. Oxidative stress disrupts cellular redox homeostasis via the excessive accumulation of ROS, thereby inducing DNA adduct formation, genetic mutations, and epigenetic reprogramming. These molecular perturbations activate pro-oncogenic signaling cascades while concurrently stimulating the secretion of proinflammatory cytokines, thereby establishing a self-reinforcing “oxidative stress–inflammation” positive feedback loop. Proinflammatory cytokines further promote tumor cell survival, angiogenesis, and formation of immunosuppressive TME, thereby driving progressive tumor malignancy. During cancer therapy, oxidative stress and inflammation exhibit bifunctional roles: on one hand, many chemotherapeutic and radiotherapeutic modalities depend on elevating ROS levels to induce tumor cell death. Conversely, cancer cells frequently acquire resistance by upregulating intrinsic antioxidant defense mechanisms, leading to progressive therapy tolerance. In the context of immunotherapy, oxidative stress and the inflammatory TME exert profound effects on effector immune cell functionality and tumor immune evasion capacity. Concurrently, emerging therapeutic strategies aim to modulate the oxidative stress–inflammation axis, encompassing antioxidant supplementation, immunomodulatory pharmacotherapies, targeted inhibition of proinflammatory signaling pathways, and combined intervention. These approaches offer diverse translational opportunities, including cancer risk reduction, enhancement of chemotherapeutic sensitivity, and improvement of clinical prognoses. Preliminary findings from select clinical trials have demonstrated encouraging efficacy, presenting novel therapeutic prospects for patients. Nevertheless, existing therapeutic challenges primarily arise from the intricate nature of oxidative stress- and inflammation-driven tumorigenic mechanisms, coupled with the pleiotropic effects of therapeutic targets. Given the suboptimal outcomes of numerous current approaches, there is an imperative need for in-depth investigations to elucidate these complex pathways and optimize targeted therapeutic strategies.
In conclusion, the development of optimized therapeutic strategies targeting oxidative stress and inflammation in oncology poses significant future challenges. First, there is a critical need for precise elucidation of the spatiotemporal-specific crosstalk mechanisms between oxidative stress and inflammation to inform precision-targeted interventions. Second, ongoing efforts must focus on optimizing existing treatment regimens to achieve balanced modulation of these interconnected pathways, while mitigating potential adverse effects, such as impairment of host immune defenses and diminished resistance to infections, that arise from dysregulated redox-inflammatory homeostasis. Third, intensifying biomarker discovery efforts is essential to establish a scientific basis for personalized therapeutic approaches. Notwithstanding these challenges, breakthroughs in multi-omics methodologies now afford comprehensive mechanistic insights into the dynamic crosstalk between oxidative stress and inflammation within the TME [437]. Concurrently, innovative nanomaterial-based drug delivery platforms show substantial promise in surmounting target specificity limitations, enabling site-specific high-concentration drug release, and enhancing treatment efficacy [438]. Ultimately, the elucidation of the synergistic mechanisms underpinning oxidative stress and inflammation presents novel therapeutic targets for oncological interventions. Nevertheless, realizing precision and personalized cancer prevention and treatment strategies necessitates a fundamental paradigm shift from traditional “single-agent inhibition” towards “systemic regulation” approaches. This transformative shift is predicated on three key pillars: in-depth mechanistic understanding, precision molecular subtyping, and technological advancements. Such an integrative approach will be pivotal for maximizing therapeutic efficacy and improving patient outcomes in cancer management.
Author Contributions
Conceptualization, D.W.; methodology, M.W. and Y.X.; formal analysis, M.W. and Y.X.; investigation, J.M., M.L. and X.Z.; data curation, L.Z. and X.S.; writing—original draft preparation, M.W. and Y.X.; writing—review and editing, L.Z. and X.S.; visualization, J.M., M.L. and X.Z.; supervision, J.W. and H.L.; project administration, D.W. and B.X.; funding acquisition, D.W. and B.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82100586 and 32301201), the Guangdong basic and applied basic research foundation (2023A1515140175), and the Shenzhen Science and Technology Program (JCYJ20220530165205012).
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript. TME, Tumor microenvironment; ROS, Reactive oxygen species; RNS, Reactive nitrogen species; MAPK, Mitogen-activated protein kinase; HIFs, Hypoxia-inducible factors; TNF-α, Tumor necrosis factor α; IL-6, Interleukin-6; Tregs, Regulatory T cells; MDSC, Myeloid-derived suppressor cells; EMT, Epithelial–mesenchymal transition; IL-1β, Interleukin-1β; ALRS, AIM2-like receptors; NADPH, Nicotinamide adenine dinucleotide phosphate; CRC, Colorectal cancer; COX-2, Cyclooxygenase-2; NAC, N-acetylcysteine; SODs, Superoxide dismutases; PDGF, Platelet-derived growth factor; EGF, Epidermal growth factor; TGFβ, Transforming growth factor β; TCA, Tricarboxylic acid; DNMT, DNA methyltransferase; TSG, Tumor suppressor gene; 5-LO, 5-lipoxygenase; NOX4, NADPH oxidase 4; PTEN, Phosphatase and tensin homolog; CCS, Copper chaperone for superoxide dismutase; ECM, Extracellular matrix; ccRCC, Clear cell renal cell carcinoma; MMP2, Matrix metalloproteinase-2; AURKA, Aberrant expression of serine-threonine kinase A; OSCC, Oral squamous cell carcinoma; E-cadherin, Epithelial cadherin; GSH, Glutathione; TrxR, Thioredoxin reductase; GST, Glutathione S-transferase; AREs, Antioxidant response elements; Keap1, Kelch-like ECH-associated protein 1; GST, Glutathione S-transferase; HO-1, Heme oxygenase-1; AhR, Aryl hydrocarbon receptor; C300, Cysteine at position 300; PTG, Glycogen-targeting protein; PPP, Pentose phosphate pathway; ABC, ATP-binding cassette; MDR, Multidrug resistance; ER, Endoplasmic reticulum; IP3R, Inositol 1,4,5-trisphosphate receptors; GBM, Glioblastoma; HGSOC, High-grade serous ovarian cancer; MIF, Macrophage migration inhibitory factor; TAMs, Tumor-associated macrophages; TANs, Tumor-associated neutrophils; NETs, Neutrophil extracellular traps; HNSCC, Head and neck squamous cell carcinoma; ADCC, Antibody-dependent cell-mediated cytotoxicity; IL-1, Interleukin-1; TNFR2, TNF receptor 2; IFN-I, Type I interferons; IBD, Inflammatory bowel disease; DAMPs, Damage-associated molecular patterns; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; CRP, C-reactive protein; BER, Base excision repair; HCC, Hepatocellular carcinoma; MDA, Malondialdehyde; 4-HNE, 4-hydroxynonenal; PUFAs, Polyunsaturated fatty acids; CL, Cardiolipin; ACC1, acetyl-CoA carboxylase 1; PUFA, Polyunsaturated fatty acid; NSCLC, Non-small cell lung cancer; ICIs, Immune checkpoint inhibitors; SOD, Superoxide dismutase; GPx, Glutathione peroxidase; CSC, Cancer stem cells; CRP/ALB, C-reactive protein/albumin; PGs, Prostaglandins; PGE2, Prostaglandin E2; FAP, Familial adenomatous polyposis; NSCLC, Non-small cell lung cancer; HDACs, Histone deacetylases; DCA, Deoxycholic acid; FXR, Farnesoid X receptor; LCA, Lithocholic acid; 4-HPR, N-(4-hydroxyphenyl)-retinamide; TrxR, Thioredoxin reductase; BCL-2, B-cell lymphoma-2; GSSG, Glutathione disulfide; TNBC, Triple-negative breast cancer; CAFs, Cancer-associated fibroblasts; NSAIDs, Non-steroidal anti-inflammatory drugs; DFS, Disease-free survival; PTGS2, Prostaglandin-endoper oxide synthase 2; PDAC, Pancreatic ductal adenocarcinoma; TNF, Tumor necrosis factor; M/P, Mitoxantrone/prednisone; CRPC, Castration-resistant prostate cancer; IFN-α, Interferon-α; M1, M1-type macrophages; M2, M2-type macrophages; N1, N1-type neutrophils; N2, N2-type neutrophils; MDSC, Myeloid-derived suppressor cells; DC, Dendritic cell; O2•−, Superoxide anion; OH•, Hydroxyl radical; NO2•, Nitrogen dioxide; RO•/ROO•, Alkoxyl/alkyl peroxyl, H2O2, Hydrogen peroxide; NO, Nitric oxide; HOCl, Hypochlorous acid.
References
- Arafeh, R.; Shibue, T.; Dempster, J.M.; Hahn, W.C.; Vazquez, F. The present and future of the Cancer Dependency Map. Nat. Rev. Cancer 2025, 25, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Singh, M.K.; Han, S.; Ranbhise, J.; Ha, J.; Choe, W.; Yoon, K.S.; Yeo, S.G.; Kim, S.S.; Kang, I. Oxidative Stress and Cancer Therapy: Controlling Cancer Cells Using Reactive Oxygen Species. Int. J. Mol. Sci. 2024, 25, 12387. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in cancer: Therapeutic opportunities from new insights. Mol. Cancer 2025, 24, 51. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Wang, J.; Guan, R.; Sun, J.; Jin, P.; Shen, J. Role of Oxidative Stress in the Occurrence, Development, and Treatment of Breast Cancer. Antioxidants 2025, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Yang, X.; Yan, L.; Shi, Z. Oxidative stress is two-sided in the treatment of acute myeloid leukemia. Cancer Med. 2024, 13, e6806. [Google Scholar] [CrossRef]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Shahgoli, V.K.; Noorolyai, S.; Ahmadpour Youshanlui, M.; Saeidi, H.; Nasiri, H.; Mansoori, B.; Holmskov, U.; Baradaran, B. Inflammatory bowel disease, colitis, and cancer: Unmasking the chronic inflammation link. Int. J. Color. Dis. 2024, 39, 173. [Google Scholar] [CrossRef]
- Kapoor, G.; Prakash, S.; Jaiswal, V.; Singh, A.K. Chronic Inflammation and Cancer: Key Pathways and Targeted Therapies. Cancer Investig. 2025, 43, 1–23. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Chen, A.; Huang, H.; Fang, S.; Hang, Q. ROS: A “booster” for chronic inflammation and tumor metastasis. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189175. [Google Scholar] [CrossRef] [PubMed]
- Fiorilla, I.; Martinotti, S.; Todesco, A.M.; Bonsignore, G.; Cavaletto, M.; Patrone, M.; Ranzato, E.; Audrito, V. Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma. Cells 2023, 12, 2048. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Bhardwaj, A.; Singh, S.; Srivastava, S.K.; McClellan, S.; Nirodi, C.S.; Piazza, G.A.; Grizzle, W.E.; Owen, L.B.; Singh, A.P. An undesired effect of chemotherapy: Gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J. Biol. Chem. 2013, 288, 21197–21207. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhang, H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 5126048. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Azmanova, M.; Pitto-Barry, A. Oxidative Stress in Cancer Therapy: Friend or Enemy? ChemBioChem 2022, 23, e202100641. [Google Scholar] [CrossRef]
- Laila, U.E.; Zhao, Z.L.; Liu, H.; Xu, Z.X. Aspirin in Cancer Therapy: Pharmacology and Nanotechnology Advances. Int. J. Nanomed. 2025, 20, 2327–2365. [Google Scholar] [CrossRef]
- Shah, M.; Parmar, R.; Patel, K.; Nagani, A. Indole-based COX-2 inhibitors: A decade of advances in inflammation, cancer, and Alzheimer’s therapy. Bioorg Chem. 2024, 153, 107931. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Liang, J.; Hansch, G.M.; Hubner, K.; Samstag, Y. Sulforaphane as anticancer agent: A double-edged sword? Tricky balance between effects on tumor cells and immune cells. Adv. Biol. Regul. 2019, 71, 79–87. [Google Scholar] [CrossRef]
- Baird, L.; Kensler, T.W.; Yamamoto, M. Novel NRF2-activated cancer treatments utilizing synthetic lethality. IUBMB Life 2022, 74, 1209–1231. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, H.; Tan, S.; Dong, Q.; Fan, X.; Wang, Y.; Zhang, H.; He, J. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp. Hematol. Oncol. 2022, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- O’Malley, J.; Kumar, R.; Inigo, J.; Yadava, N.; Chandra, D. Mitochondrial Stress Response and Cancer. Trends Cancer 2020, 6, 688–701. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.X.Y.; Chia, K.; Ong, D.S.T. Epigenetic plasticity and redox regulation of neural stem cell state and fate. Free Radic. Biol. Med. 2021, 170, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Trevino, S.; Rosas-Murrieta, N.H.; Millan-Perez-Pena, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondrial complex III: An essential component of universal oxygen sensing machinery? Respir. Physiol. Neurobiol. 2010, 174, 175–181. [Google Scholar] [CrossRef]
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, S.J.; So, B.R.; Kim, C.K.; Kim, K.M.; Jung, S.K. NADPH Oxidase and Epidermal Growth Factor Receptor Are Promising Targets of Phytochemicals for Ultraviolet-Induced Skin Carcinogenesis. Antioxidants 2021, 10, 1909. [Google Scholar] [CrossRef]
- Wang, L.; Kuang, Z.; Zhang, D.; Gao, Y.; Ying, M.; Wang, T. Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother. 2021, 133, 110978. [Google Scholar] [CrossRef]
- Liang, S.; Ma, H.Y.; Zhong, Z.; Dhar, D.; Liu, X.; Xu, J.; Koyama, Y.; Nishio, T.; Karin, D.; Karin, G.; et al. NADPH Oxidase 1 in Liver Macrophages Promotes Inflammation and Tumor Development in Mice. Gastroenterology 2019, 156, 1156–1172.e6. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef]
- Maya-Mendoza, A.; Ostrakova, J.; Kosar, M.; Hall, A.; Duskova, P.; Mistrik, M.; Merchut-Maya, J.M.; Hodny, Z.; Bartkova, J.; Christensen, C.; et al. Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol. Oncol. 2015, 9, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takenaga, K.; Akimoto, M.; Koshikawa, N.; Yamaguchi, A.; Imanishi, H.; Nakada, K.; Honma, Y.; Hayashi, J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 2008, 320, 661–664. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Garg, M.; Braunstein, G.; Koeffler, H.P. LAMC2 as a therapeutic target for cancers. Expert Opin. Ther. Targets 2014, 18, 979–982. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef]
- Moore, J.M.; Correa, R.; Rosenberg, S.M.; Hastings, P.J. Persistent damaged bases in DNA allow mutagenic break repair in Escherichia coli. PLoS Genet. 2017, 13, e1006733. [Google Scholar] [CrossRef]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magri, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef]
- Messina, S.; De Simone, G.; Ascenzi, P. Cysteine-based regulation of redox-sensitive Ras small GTPases. Redox Biol. 2019, 26, 101282. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Doppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid Redox Signal 2022, 36, 423–440. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Kim, K.C.; Na, S.Y.; Chang, W.Y.; Kim, G.Y.; Kim, H.S.; Hyun, J.W. Oxidative stress causes epigenetic alteration of CDX1 expression in colorectal cancer cells. Gene 2013, 524, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ni, X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr. Drug Targets 2015, 16, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Z.; Zou, Z.; Xiao, G.; Luo, G.; Yang, H. Clinicopathological significance of RUNX3 gene hypermethylation in hepatocellular carcinoma. Tumour Biol. 2014, 35, 10333–10340. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Du, W.; Jiang, P.; Mancuso, A.; Stonestrom, A.; Brewer, M.D.; Minn, A.J.; Mak, T.W.; Wu, M.; Yang, X. TAp73 enhances the pentose phosphate pathway and supports cell proliferation. Nat. Cell Biol. 2013, 15, 991–1000. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Hong, P.; Vaquero, E.C.; Lee, J.K.; Fischer, L.; Friess, H.; Buchler, M.W.; Lerch, M.M.; Pandol, S.J.; Gukovskaya, A.S. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G1137–G1147. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, H.; Dinavahi, R.; Li, F.; Xiang, Y.; Raman, V.; Bhujwalla, Z.M.; Felsher, D.W.; Cheng, L.; Pevsner, J.; et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 2007, 12, 230–238. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Shen, Z.; Yang, Q.; Sui, F.; Pu, J.; Ma, J.; Ma, S.; Yao, D.; Ji, M.; Hou, P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics 2019, 9, 4461–4473. [Google Scholar] [CrossRef] [PubMed]
- Pani, G.; Galeotti, T.; Chiarugi, P. Metastasis: Cancer cell’s escape from oxidative stress. Cancer Metastasis Rev. 2010, 29, 351–378. [Google Scholar] [CrossRef]
- Zuo, J.; Zhao, M.; Liu, B.; Han, X.; Li, Y.; Wang, W.; Zhang, Q.; Lv, P.; Xing, L.; Shen, H.; et al. TNF-alpha-mediated upregulation of SOD-2 contributes to cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Oncol. Rep. 2019, 42, 1497–1506. [Google Scholar]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 Controls Breast Cancer Cell Migration by Regulating Hydrogen Peroxide Transport and Its Downstream Cell Signaling. Mol. Cell Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Muthuramalingam, K.; Cho, M. Redox Regulation of NOX Isoforms on FAK(Y397)/SRC(Y416) Phosphorylation Driven Epithelial-to-Mesenchymal Transition in Malignant Cervical Epithelial Cells. Cells 2020, 9, 1555. [Google Scholar] [CrossRef]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.W.; Kim, J.R. Reactive oxygen species regulate urokinase plasminogen activator expression and cell invasion via mitogen-activated protein kinase pathways after treatment with hepatocyte growth factor in stomach cancer cells. J. Exp. Clin. Cancer Res. 2009, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Q.; Yang, Z.; Ni, Y.; Agbana, Y.L.; Bai, H.; Yi, Z.; Yi, X.; Kuang, Y.; Zhu, Y. G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROS-MAPK axis pathway. Int. J. Oncol. 2020, 57, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Xi, C.; Hu, Y.; Buckhaults, P.; Moskophidis, D.; Mivechi, N.F. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J. Biol. Chem. 2012, 287, 35646–35657. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, L.; Han, L.; Xu, Q.; Ma, Q. Superoxide dismutase promotes the epithelial-mesenchymal transition of pancreatic cancer cells via activation of the H2O2/ERK/NF-kappaB axis. Int. J. Oncol. 2015, 46, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 2020, 37, 168–182 e164. [Google Scholar] [CrossRef]
- Wu, B.; Cheng, Y.; Li, L.; Du, Z.; Liu, Q.; Tan, X.; Li, X.; Zhao, G.; Li, E. Role of the sulfur-containing amino acid-ROS axis in cancer chemotherapeutic drug resistance. Drug Resist. Updat. 2025, 81, 101238. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, J.; Ling, Z.; Zhang, C.; Zhou, Y.; Wang, D.; Zhou, L.; Wang, Z.; Sun, N.; Wang, X.; et al. Aryl hydrocarbon receptor sulfenylation promotes glycogenolysis and rescues cancer chemoresistance. J. Clin. Investig. 2023, 133, e170753. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R., Jr.; Yang, D.H.; Chen, Z.S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/beta-catenin signalings activate HIF-1alpha-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.Y.; Kim, J.H.; Cho, S.G. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers 2010, 2, 859–884. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, K.; Chen, Y.; Chen, H.; Nice, E.C.; Huang, C. Redox regulation in tumor cell epithelial-mesenchymal transition: Molecular basis and therapeutic strategy. Signal Transduct. Target. Ther. 2017, 2, 17036. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef]
- Zhang, J.; Ali, M.Y.; Chong, H.B.; Tien, P.C.; Woods, J.; Noble, C.; Vornbaumen, T.; Ordulu, Z.; Possemato, A.P.; Harry, S.; et al. Oxidation of retromer complex controls mitochondrial translation. Nature 2025, 641, 1048–1058. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Zhan, Y.; Zou, M.; Wu, L.; Zhang, C.; Chen, B.; Zeng, H.; Yang, R.; Hu, T.; et al. Role of the USP family in autophagy regulation and cancer progression. Apoptosis 2025, 30, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Luo, Y.; Liu, Y.; Yi, Y.; Li, J.; Pan, Y.; Li, W.; You, W.; Hu, Q.; et al. USP5-Beclin 1 axis overrides p53-dependent senescence and drives Kras-induced tumorigenicity. Nat. Commun. 2022, 13, 7799. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Santarcangelo, C.; Khan, H.; Daglia, M. Improvement of Oxidative Stress and Mitochondrial Dysfunction by beta-Caryophyllene: A Focus on the Nervous System. Antioxidants 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- AlBasher, G.; AlKahtane, A.A.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; Abdel-Daim, M.M.; Al-Sultan, N.K.; Al-Qahtani, A.A.; Ali, H.; et al. Methotrexate-induced apoptosis in human ovarian adenocarcinoma SKOV-3 cells via ROS-mediated bax/bcl-2-cyt-c release cascading. Onco Targets Ther. 2019, 12, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cui, L.; Ye, J.; Yang, G.; Lu, G.; Fang, X.; Zeng, Z.; Zhou, J. Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int. J. Biol. Sci. 2020, 16, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J. Cytochrome c in cancer therapy and prognosis. Biosci. Rep. 2022, 42, BSR20222171. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. Biomed. Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.Y.; Yu, S.N.; Park, S.G.; Yu, H.S.; Seo, Y.K.; Ahn, S.C. Monensin Induces PC-3 Prostate Cancer Cell Apoptosis via ROS Production and Ca2+ Homeostasis Disruption. Anticancer. Res. 2016, 36, 5835–5843. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Patwardhan, R.S.; Jayakumar, S.; Pachpatil, P.; Das, D.; Panigrahi, G.C.; Gota, V.; Patwardhan, S.; Sandur, S.K. Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung cancer cells to radiation-induced killing via mitochondrial ROS-dependent ferroptosis. Acta Pharmacol. Sin. 2024, 45, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, Y.; Li, X.; Zhou, J.; Yang, W.; Wang, X.; Jiao, S.; Zuo, W.; You, Z.; Ying, W.; et al. O-GlcNAcylation regulates the stability of transferrin receptor (TFRC) to control the ferroptosis in hepatocellular carcinoma cells. Redox Biol. 2024, 73, 103182. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tavana, O.; Chu, B.; Erber, L.; Chen, Y.; Baer, R.; Gu, W. NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol. Cell 2017, 68, 224–232.e4. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, J.; Lv, Y.; Bai, B.; Shan, L.; Chen, K.; Dai, S.; Zhu, H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med. Oncol. 2021, 38, 21. [Google Scholar] [CrossRef]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Coats, S.; Flanagan, W.M.; Nourse, J.; Roberts, J.M. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 1996, 272, 877–880. [Google Scholar] [CrossRef]
- Nevins, J.R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998, 9, 585–593. [Google Scholar] [PubMed]
- Brown, K.; Xie, S.; Qiu, X.; Mohrin, M.; Shin, J.; Liu, Y.; Zhang, D.; Scadden, D.T.; Chen, D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013, 3, 319–327. [Google Scholar] [CrossRef]
- Kaul, S.C.; Aida, S.; Yaguchi, T.; Kaur, K.; Wadhwa, R. Activation of wild type p53 function by its mortalin-binding, cytoplasmically localizing carboxyl terminus peptides. J. Biol. Chem. 2005, 280, 39373–39379. [Google Scholar] [CrossRef]
- Trinh, D.L.N.; Elwi, A.N.; Kim, S.W. Direct interaction between p53 and Tid1 proteins affects p53 mitochondrial localization and apoptosis. Oncotarget 2010, 1, 396–404. [Google Scholar] [CrossRef]
- Arena, G.; Cisse, M.Y.; Pyrdziak, S.; Chatre, L.; Riscal, R.; Fuentes, M.; Arnold, J.J.; Kastner, M.; Gayte, L.; Bertrand-Gaday, C.; et al. Mitochondrial MDM2 Regulates Respiratory Complex I Activity Independently of p53. Mol. Cell 2018, 69, 594–609.e8. [Google Scholar] [CrossRef]
- Fasano, C.; Disciglio, V.; Bertora, S.; Lepore Signorile, M.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef]
- Hu, C.; Ni, Z.; Li, B.S.; Yong, X.; Yang, X.; Zhang, J.W.; Zhang, D.; Qin, Y.; Jie, M.M.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017, 66, 31–42. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Wu, C.; Wang, C.; Zhang, R.; He, J.; Xia, Z.J.; Joshi, N.; Karp, J.M.; Kuai, R. Precise modulation and use of reactive oxygen species for immunotherapy. Sci. Adv. 2024, 10, eadl0479. [Google Scholar] [CrossRef]
- Hui, C.; Marquez, C.; Lau, B.; Das, M.; Myall, N.J.; Roy, M.; Wakelee, H.A.; Neal, J.W.; Kovalchuk, N.; Chin, A.; et al. Patient Selection and Outcomes for Hypofractionated Accelerated Radiation and Concurrent Chemotherapy for Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2024, 25, e92–e100.e4. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, S.; Shen, S.; Gu, Z.; Chen, J.; Song, D.; Sun, P.; Wang, C.; Guo, Z.; Xiao, Y.; et al. Radiotherapy-triggered reduction of platinum-based chemotherapeutic prodrugs in tumours. Nat. Biomed. Eng. 2024, 8, 1425–1435. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, L.; Ren, E.; Chen, H.; Gao, X.; Cheng, H.; An, Y.; Chu, C.; Liu, G. Ruthenium-Based Metal-Organic Nanoradiosensitizers Enhance Radiotherapy by Combining ROS Generation and CO Gas Release. Angew. Chem. Int. Ed. Engl. 2022, 61, e202211674. [Google Scholar] [CrossRef]
- Guo, S.; Burcus, N.I.; Scott, M.; Jing, Y.; Semenov, I. The role of reactive oxygen species in the immunity induced by nano-pulse stimulation. Sci. Rep. 2021, 11, 23745. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, S.; Liao, T.; Yuan, M.; Qian, W.; Chen, Q.; Liang, W.; Cheng, X.; Zhou, Q.; Ju, Z.; et al. Integrative single-cell metabolomics and phenotypic profiling reveals metabolic heterogeneity of cellular oxidation and senescence. Nat. Commun. 2025, 16, 2740. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, M.; Zhang, F.; Chen, G.; Shu, Z.; Li, L.; Zhang, F.; Wang, Y.; Wang, Y.; Duan, X.; et al. A Bimetallic Electro-Sensitizer Improves ROS Therapy by Relieving Autophagy-Induced ROS Tolerance and Immune Suppression. Small 2024, 20, e2402312. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Gu, J.; Xue, C.; Zhang, L.; Chen, J.; Shen, L. Relaxed 3D genome conformation facilitates the pluripotent to totipotent-like state transition in embryonic stem cells. Nucleic Acids Res. 2021, 49, 12167–12177. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Yang, S.; Fei, W.; Qin, J.; Lu, W.; Xu, J. FXN targeting induces cell death in ovarian cancer stem-like cells through PRDX3-Mediated oxidative stress. iScience 2024, 27, 110506. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ye, Y.Z.; Huang, Y.H.; Tzeng, Y.M.; Gurbanov, R.; Wang, W.L.; Chang, W.W. Ovatodiolide inhibits endometrial cancer stemness via reactive oxygen species-mediated DNA damage and cell cycle arrest. Chem. Biol. Interact. 2024, 403, 111244. [Google Scholar] [CrossRef]
- Liu, S.; Qiu, Y.; Xiang, R.; Huang, P. Characterization of H2O2-Induced Alterations in Global Transcription of mRNA and lncRNA. Antioxidants 2022, 11, 495. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, X.; Qiu, J. Emerging role of NRF2 in ROS-mediated tumor chemoresistance. Biomed. Pharmacother. 2020, 131, 110676. [Google Scholar] [CrossRef]
- Cabrera-Serrano, A.J.; Sanchez-Maldonado, J.M.; Gonzalez-Olmedo, C.; Carretero-Fernandez, M.; Diaz-Beltran, L.; Gutierrez-Bautista, J.F.; Garcia-Verdejo, F.J.; Galvez-Montosa, F.; Lopez-Lopez, J.A.; Garcia-Martin, P.; et al. Crosstalk Between Autophagy and Oxidative Stress in Hematological Malignancies: Mechanisms, Implications, and Therapeutic Potential. Antioxidants 2025, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef]
- Han, R.; Zhao, M.; Wang, Z.; Liu, H.; Zhu, S.; Huang, L.; Wang, Y.; Wang, L.; Hong, Y.; Sha, Y.; et al. Super-efficient in Vivo Two-Photon Photodynamic Therapy with a Gold Nanocluster as a Type I Photosensitizer. ACS Nano 2020, 14, 9532–9544. [Google Scholar] [CrossRef]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Y.; Liang, X.; Liu, J.; Luo, Y.; Zhang, Y.; Li, T.; Liu, C.; Luo, X.; Chen, J.; et al. Sonodynamic Therapy of NRP2 Monoclonal Antibody-Guided MOFs@COF Targeted Disruption of Mitochondrial and Endoplasmic Reticulum Homeostasis to Induce Autophagy-Dependent Ferroptosis. Adv. Sci. 2023, 10, e2303872. [Google Scholar] [CrossRef]
- Yuan, J.; Khan, S.U.; Yan, J.; Lu, J.; Yang, C.; Tong, Q. Baicalin enhances the efficacy of 5-Fluorouracil in gastric cancer by promoting ROS-mediated ferroptosis. Biomed. Pharmacother. 2023, 164, 114986. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Cheng, F.; Cao, W.; Geng, Y.; Chen, Z.; Wei, W.; Zhang, L. Xanthatin induce DDP-resistance lung cancer cells apoptosis through regulation of GLUT1 mediated ROS accumulation. Drug Dev. Res. 2023, 84, 1266–1278. [Google Scholar] [CrossRef]
- Dilenko, H.; Barton Tomankova, K.; Valkova, L.; Hosikova, B.; Kolarikova, M.; Malina, L.; Bajgar, R.; Kolarova, H. Graphene-Based Photodynamic Therapy and Overcoming Cancer Resistance Mechanisms: A Comprehensive Review. Int. J. Nanomed. 2024, 19, 5637–5680. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Xuan, C.; Li, X.; Tan, Y.; Yang, M.; Cao, M.; Chen, C.; Huang, X.; Hu, R. DPP9 regulates NQO1 and ROS to promote resistance to chemotherapy in liver cancer cells. Redox Biol. 2024, 75, 103292. [Google Scholar] [CrossRef]
- Kopecka, J.; Trouillas, P.; Gasparovic, A.C.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug Resist. Updat. 2020, 49, 100670. [Google Scholar] [CrossRef]
- Jin, P.; Jiang, J.; Zhou, L.; Huang, Z.; Nice, E.C.; Huang, C.; Fu, L. Mitochondrial adaptation in cancer drug resistance: Prevalence, mechanisms, and management. J. Hematol. Oncol. 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chang, L.; Tang, L.; Jiang, H.; Xu, X.; Zhang, X.; Chen, J.; Dong, L.; Xu, Q.; Cao, R.; et al. Long noncoding RNA GDIL acts as a scaffold for CHAC1 and XRN2 to promote platinum resistance of colorectal cancer through inhibition of glutathione degradation. Cell Death Dis. 2025, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Zhao, K.; Wang, X.; Li, H.; Yu, M.; He, M.; Xue, X.; Zhu, Y.; Zhang, C.; Cheng, Y.; et al. iASPP Is an Antioxidative Factor and Drives Cancer Growth and Drug Resistance by Competing with Nrf2 for Keap1 Binding. Cancer Cell 2017, 32, 561–573.e6. [Google Scholar] [CrossRef] [PubMed]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hutter, J.C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021, 596, 576–582. [Google Scholar] [CrossRef]
- Hu, A.; Pu, Y.; Xu, N.; Yang, H.; Hu, X.; Sun, R.; Jin, R.; Nie, Y. Hierarchically decorated magnetic nanoparticles amplify the oxidative stress and promote the chemodynamic/magnetic hyperthermia/immune therapy. Acta Biomater. 2024, 173, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ai, X.; Yang, K.; Yang, Z.; Fei, F.; Liao, X.; Qiu, Z.; Gimple, R.C.; Yuan, H.; Huang, H.; et al. Targeting Microglial Metabolic Rewiring Synergizes with Immune-Checkpoint Blockade Therapy for Glioblastoma. Cancer Discov. 2023, 13, 974–1001. [Google Scholar] [CrossRef]
- Duan, G.; Huang, C.; Zhao, J.; Zhang, Y.; Zhao, W.; Dai, H. Investigating subtypes of lung adenocarcinoma by oxidative stress and immunotherapy related genes. Sci. Rep. 2023, 13, 20930. [Google Scholar] [CrossRef]
- Yang, T.; Wang, G.; Zhang, M.; Hu, X.; Li, Q.; Yun, F.; Xing, Y.; Song, X.; Zhang, H.; Hu, G.; et al. Triggering endogenous Z-RNA sensing for anti-tumor therapy through ZBP1-dependent necroptosis. Cell Rep. 2023, 42, 113377. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, S.; He, X.; Yuan, Y.; Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 2024, 17, 13. [Google Scholar] [CrossRef]
- Nihira, N.T.; Kudo, R.; Ohta, T. Inflammation and tumor immune escape in response to DNA damage. Semin. Cancer Biol. 2025, 110, 36–45. [Google Scholar] [CrossRef]
- Krugliak Cleveland, N.; Torres, J.; Rubin, D.T. What Does Disease Progression Look Like in Ulcerative Colitis, and How Might It Be Prevented? Gastroenterology 2022, 162, 1396–1408. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Song, C.; Jiang, L.; Dai, J.; Lin, Y.; Xu, X.; Yu, C.; Ge, Z.; Ding, Y.; Wen, Y.; et al. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int. J. Cancer 2020, 147, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Ohnishi, S.; Ma, N.; Hiraku, Y.; Murata, M. Crosstalk between DNA Damage and Inflammation in the Multiple Steps of Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1808. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Dash, P.; Suklabaidya, S.; Murmu, K.C.; Sasmal, P.K.; Jogdand, G.M.; Parida, D.; Sethi, M.; Das, B.; Mohapatra, D.; et al. MIF confers survival advantage to pancreatic CAFs by suppressing interferon pathway-induced p53-dependent apoptosis. FASEB J. 2022, 36, e22449. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Li, L.; Yu, R.; Cai, T.; Chen, Z.; Lan, M.; Zou, T.; Wang, B.; Wang, Q.; Zhao, Y.; Cai, Y. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int. Immunopharmacol. 2020, 88, 106939. [Google Scholar] [CrossRef]
- Wu, M.; Ma, M.; Tan, Z.; Zheng, H.; Liu, X. Neutrophil: A New Player in Metastatic Cancers. Front. Immunol. 2020, 11, 565165. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and Role of a Subset of Tumor-Associated Neutrophils with Antigen-Presenting Cell Features in Early-Stage Human Lung Cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Eruslanov, E.; Michaeli, J.; Levy, L.; Zolotarov, L.; Singhal, S.; Albelda, S.M.; Granot, Z.; Fridlender, Z.G. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—A new mechanism of impaired antitumor immunity. Int. J. Cancer 2014, 135, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.L.; Zhou, Z.J.; Hu, Z.Q.; Huang, X.W.; Wang, Z.; Chen, E.B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U. Neutrophil Extracellular Traps (NETs) in Cancer Invasion, Evasion and Metastasis. Cancers 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, F.; Chen, L.; Fang, C.; Li, S.; Yuan, S.; Qian, X.; Yin, Y.; Yu, B.; Fu, B.; et al. Neutrophil Extracellular Traps (NETs) Promote Non-Small Cell Lung Cancer Metastasis by Suppressing lncRNA MIR503HG to Activate the NF-kappaB/NLRP3 Inflammasome Pathway. Front. Immunol. 2022, 13, 867516. [Google Scholar]
- Deng, J.; Kang, Y.; Cheng, C.C.; Li, X.; Dai, B.; Katz, M.H.; Men, T.; Kim, M.P.; Koay, E.A.; Huang, H.; et al. DDR1-induced neutrophil extracellular traps drive pancreatic cancer metastasis. JCI Insight. 2021, 6, e146133. [Google Scholar] [CrossRef]
- Khan, U.; Chowdhury, S.; Billah, M.M.; Islam, K.M.D.; Thorlacius, H.; Rahman, M. Neutrophil Extracellular Traps in Colorectal Cancer Progression and Metastasis. Int. J. Mol. Sci. 2021, 22, 7260. [Google Scholar] [CrossRef]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e7. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Li, L.; Zhang, Z.; Jin, X.; Wu, P.; Sun, S.; Pan, J.; Su, K.; Jia, F.; et al. Aged neutrophils form mitochondria-dependent vital NETs to promote breast cancer lung metastasis. J. Immunother. Cancer 2021, 9, e002875. [Google Scholar] [CrossRef]
- Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Papagoras, C.; Miltiades, P.; Angelidou, I.; Mitsios, A.; Kotsianidis, I.; Skendros, P.; Sivridis, E.; et al. Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth. PLoS ONE 2016, 11, e0154484. [Google Scholar] [CrossRef]
- Millrud, C.R.; Kagedal, A.; Kumlien Georen, S.; Winqvist, O.; Uddman, R.; Razavi, R.; Munck-Wikland, E.; Cardell, L.O. NET-producing CD16high CD62Ldim neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int. J. Cancer 2017, 140, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Schedel, F.; Mayer-Hain, S.; Pappelbaum, K.I.; Metze, D.; Stock, M.; Goerge, T.; Loser, K.; Sunderkotter, C.; Luger, T.A.; Weishaupt, C. Evidence and impact of neutrophil extracellular traps in malignant melanoma. Pigment. Cell Melanoma Res. 2020, 33, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mackaness, G.B. Cellular resistance to infection. J. Exp. Med. 1962, 116, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Eum, H.H.; Kwon, M.; Ryu, D.; Jo, A.; Chung, W.; Kim, N.; Hong, Y.; Son, D.S.; Kim, S.T.; Lee, J.; et al. Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Exp. Mol. Med. 2020, 52, 1976–1988. [Google Scholar] [CrossRef]
- Bernsmeier, C.; van der Merwe, S.; Perianin, A. Innate immune cells in cirrhosis. J. Hepatol. 2020, 73, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Bruns, H.; Buttner, M.; Fabri, M.; Mougiakakos, D.; Bittenbring, J.T.; Hoffmann, M.H.; Beier, F.; Pasemann, S.; Jitschin, R.; Hofmann, A.D.; et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci. Transl. Med. 2015, 7, 282ra247. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers 2021, 13, 3253. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.B.; Severn, C.E.; Twomey, C.; Greenhough, A.; Cash, J.; Toye, A.M.; Mellor, H.; Martin, P. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J. 2018, 37, e97786. [Google Scholar] [CrossRef]
- Ramirez-Pedraza, M.; Fernandez, M. Interplay Between Macrophages and Angiogenesis: A Double-Edged Sword in Liver Disease. Front. Immunol. 2019, 10, 2882. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Garstka, M.A.; Li, Z.F. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol. Immunol. 2020, 117, 201–215. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, Y.; Guo, N.; Wang, S. MDSCs: Key Criminals of Tumor Pre-metastatic Niche Formation. Front. Immunol. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Chen, M.L.; Pittet, M.J.; Gorelik, L.; Flavell, R.A.; Weissleder, R.; von Boehmer, H.; Khazaie, K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 419–424. [Google Scholar] [CrossRef]
- Khalaf, K.; Hana, D.; Chou, J.T.; Singh, C.; Mackiewicz, A.; Kaczmarek, M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front. Immunol. 2021, 12, 656364. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Lou, D.F.; Li, D.Y.; Jiang, W.; Dong, J.Y.; Gao, W.; Chen, H.C. Elevated levels of IL-17A and IL-35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non-small cell lung cancer. Oncol. Lett. 2020, 20, 611–622. [Google Scholar] [CrossRef]
- Timperi, E.; Pacella, I.; Schinzari, V.; Focaccetti, C.; Sacco, L.; Farelli, F.; Caronna, R.; Del Bene, G.; Longo, F.; Ciardi, A.; et al. Regulatory T cells with multiple suppressive and potentially pro-tumor activities accumulate in human colorectal cancer. Oncoimmunology 2016, 5, e1175800. [Google Scholar] [CrossRef] [PubMed]
- Shaopeng, Z.; Yang, Z.; Yuan, F.; Chen, H.; Zhengjun, Q. Regulation of regulatory T cells and tumor-associated macrophages in gastric cancer tumor microenvironment. Cancer Med. 2024, 13, e6959. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Li, J.; Zhu, J.; Zuo, S.; Wang, X.; Liu, Y.; Liu, J.; Liu, X.; Wang, P.; Chen, S. Hydrogen Sulfide Creates a Favorable Immune Microenvironment for Colon Cancer. Cancer Res. 2023, 83, 595–612. [Google Scholar] [CrossRef]
- Li, F.; Guo, Z.; Lizee, G.; Yu, H.; Wang, H.; Si, T. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin. Chem. Lab. Med. 2014, 52, 1357–1365. [Google Scholar] [CrossRef]
- O’Carroll, S.J.; Kho, D.T.; Wiltshire, R.; Nelson, V.; Rotimi, O.; Johnson, R.; Angel, C.E.; Graham, E.S. Pro-inflammatory TNFalpha and IL-1beta differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J. Neuroinflammation 2015, 12, 131. [Google Scholar] [CrossRef]
- Hillyer, P.; Mordelet, E.; Flynn, G.; Male, D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: Discriminating the tissue-specific functions that affect leucocyte migration. Clin. Exp. Immunol. 2003, 134, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Cui, J.; Barnes, L.; Cheresh, D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J. Cell Biol. 2004, 167, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Burdick, M.M.; Henson, K.A.; Delgadillo, L.F.; Choi, Y.E.; Goetz, D.J.; Tees, D.F.; Benencia, F. Expression of E-selectin ligands on circulating tumor cells: Cross-regulation with cancer stem cell regulatory pathways? Front. Oncol. 2012, 2, 103. [Google Scholar] [CrossRef] [PubMed]
- Hauselmann, I.; Roblek, M.; Protsyuk, D.; Huck, V.; Knopfova, L.; Grassle, S.; Bauer, A.T.; Schneider, S.W.; Borsig, L. Monocyte Induction of E-Selectin-Mediated Endothelial Activation Releases VE-Cadherin Junctions to Promote Tumor Cell Extravasation in the Metastasis Cascade. Cancer Res. 2016, 76, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Goel, S.; Kamoun, W.S.; Maru, Y.; Fukumura, D.; Duda, D.G.; Jain, R.K. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc. Natl. Acad. Sci. USA 2011, 108, 3725–3730. [Google Scholar] [CrossRef]
- Kang, S.A.; Blache, C.A.; Bajana, S.; Hasan, N.; Kamal, M.; Morita, Y.; Gupta, V.; Tsolmon, B.; Suh, K.S.; Gorenstein, D.G.; et al. The effect of soluble E-selectin on tumor progression and metastasis. BMC Cancer 2016, 16, 331. [Google Scholar]
- Shea, D.J.; Li, Y.W.; Stebe, K.J.; Konstantopoulos, K. E-selectin-mediated rolling facilitates pancreatic cancer cell adhesion to hyaluronic acid. FASEB J. 2017, 31, 5078–5086. [Google Scholar] [CrossRef]
- Tichet, M.; Prod’Homme, V.; Fenouille, N.; Ambrosetti, D.; Mallavialle, A.; Cerezo, M.; Ohanna, M.; Audebert, S.; Rocchi, S.; Giacchero, D.; et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat. Commun. 2015, 6, 6993. [Google Scholar] [CrossRef]
- Zamarron, B.F.; Chen, W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef]
- Amin, M.N.; Siddiqui, S.A.; Ibrahim, M.; Hakim, M.L.; Ahammed, M.S.; Kabir, A.; Sultana, F. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 2020, 8, 2050312120965752. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Mei, Y.; Lei, L.; Binte Hanafi, Z.; Jin, Z.; Liu, Y.; Song, Y.; Zhang, Y.; Hu, B.; Liu, C.; et al. Immune suppressive function of IL-1alpha release in the tumor microenvironment regulated by calpain 1. Oncoimmunology 2022, 11, 2088467. [Google Scholar] [CrossRef]
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The role of IL-1beta in the early tumor cell-induced angiogenic response. J. Immunol. 2013, 190, 3500–3509. [Google Scholar] [CrossRef]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5, 114. [Google Scholar] [CrossRef]
- Haabeth, O.A.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 2016, 5, e1039763. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1beta Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, Z.; He, J.; Yan, C.; Ding, S. IL-6 Inhibits Starvation-induced Autophagy via the STAT3/Bcl-2 Signaling Pathway. Sci. Rep. 2015, 5, 15701. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Chen, M.; Litifu, B. Serum interleukin-6 and survivin levels predict clinical response to etanercept treatment in patients with established rheumatoid arthritis. Mod. Rheumatol. 2018, 28, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londono-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal 2017, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Sapochnik, M.; Haedo, M.R.; Fuertes, M.; Ajler, P.; Carrizo, G.; Cervio, A.; Sevlever, G.; Stalla, G.K.; Arzt, E. Autocrine IL-6 mediates pituitary tumor senescence. Oncotarget 2017, 8, 4690–4702. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Ujvari, B.; Ramana, V.; Donald, J. Cross-talk between EGFR and IL-6 drives oncogenic signaling and offers therapeutic opportunities in cancer. Cytokine Growth Factor. Rev. 2018, 41, 18–27. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Das, A.K.; Mandal, M. Differential expression of IL-6/IL-6R and MAO-A regulates invasion/angiogenesis in breast cancer. Br. J. Cancer 2018, 118, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, J.; Wu, X.; Liang, Z. PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2018, 108, 618–624. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Bai, F.; Ding, L.; Huang, Y.; Lu, C.; Chen, S.; Li, C.; Yue, X.; Liang, X.; et al. IL-6 promotes metastasis of non-small-cell lung cancer by up-regulating TIM-4 via NF-kappaB. Cell Prolif. 2020, 53, e12776. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef]
- Sawant, D.V.; Yano, H.; Chikina, M.; Zhang, Q.; Liao, M.; Liu, C.; Callahan, D.J.; Sun, Z.; Sun, T.; Tabib, T.; et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 2019, 20, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; De Martino, M.; Venturutti, L.; Rivas, M.A.; Proietti, C.J.; Inurrigarro, G.; Frahm, I.; Allemand, D.H.; Deza, E.G.; Ares, S.; et al. TNFalpha-Induced Mucin 4 Expression Elicits Trastuzumab Resistance in HER2-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Tilkin-Mariame, A.F.; Touriol, C.; Rochaix, P.; Lajoie-Mazenc, I.; Andrieu-Abadie, N.; Levade, T.; et al. Blocking Tumor Necrosis Factor alpha Enhances CD8 T-cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015, 75, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor Necrosis Factor alpha and Regulatory T Cells in Oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal 2017, 10, eaaf8608. [Google Scholar] [CrossRef]
- Batlle, E.; Massague, J. Transforming Growth Factor-beta Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Hisamatsu, T.; Haemmerle, M.; Cho, M.S.; Pradeep, S.; Rupaimoole, R.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Wong, S.T.C.; Sood, A.K.; et al. Role of Platelet-Derived Tgfbeta1 in the Progression of Ovarian Cancer. Clin. Cancer Res. 2017, 23, 5611–5621. [Google Scholar] [CrossRef]
- Melzer, C.; Hass, R.; von der Ohe, J.; Lehnert, H.; Ungefroren, H. The role of TGF-beta and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun. Signal 2017, 15, 19. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Otterbein, H.; Ungefroren, H.; Hass, R. Changes in uPA, PAI-1, and TGF-beta Production during Breast Cancer Cell Interaction with Human Mesenchymal Stroma/Stem-Like Cells (MSC). Int. J. Mol. Sci. 2019, 20, 2630. [Google Scholar] [CrossRef]
- Esquivel-Velazquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFbeta-mediated immune evasion in cancer. Nat. Rev. Cancer 2022, 22, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.; Labiad, O.; Hernandez-Vargas, H.; This, S.; Sanlaville, A.; Leon, S.; Dalle, S.; Sheppard, D.; Travis, M.A.; Paidassi, H.; et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat. Commun. 2021, 12, 6228. [Google Scholar] [CrossRef] [PubMed]
- Gato-Canas, M.; Zuazo, M.; Arasanz, H.; Ibanez-Vea, M.; Lorenzo, L.; Fernandez-Hinojal, G.; Vera, R.; Smerdou, C.; Martisova, E.; Arozarena, I.; et al. PDL1 Signals through Conserved Sequence Motifs to Overcome Interferon-Mediated Cytotoxicity. Cell Rep. 2017, 20, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Donnelly, C.R.; Heath, B.R.; Bellile, E.; Donnelly, L.A.; Taner, H.F.; Broses, L.; Brenner, J.C.; Chinn, S.B.; Ji, R.R.; et al. Cancer-specific type-I interferon receptor signaling promotes cancer stemness and effector CD8+ T-cell exhaustion. Oncoimmunology 2021, 10, 1997385. [Google Scholar] [CrossRef]
- Pidugu, V.K.; Wu, M.M.; Yen, A.H.; Pidugu, H.B.; Chang, K.W.; Liu, C.J.; Lee, T.C. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling. Oncogene 2019, 38, 3232–3247. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Y.; Markelc, B.; Kaeppler, J.; Vermeer, J.A.; Muschel, R.J. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J. Clin. Investig. 2019, 129, 4224–4238. [Google Scholar] [CrossRef]
- Cunningham, C.R.; Champhekar, A.; Tullius, M.V.; Dillon, B.J.; Zhen, A.; de la Fuente, J.R.; Herskovitz, J.; Elsaesser, H.; Snell, L.M.; Wilson, E.B.; et al. Type I and Type II Interferon Coordinately Regulate Suppressive Dendritic Cell Fate and Function during Viral Persistence. PLoS Pathog. 2016, 12, e1005356. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, B.; Oh, G.T.; Kim, Y.J. OASL1 inhibits translation of the type I interferon-regulating transcription factor IRF7. Nat. Immunol. 2013, 14, 346–355. [Google Scholar] [CrossRef]
- Boukhaled, G.M.; Harding, S.; Brooks, D.G. Opposing Roles of Type I Interferons in Cancer Immunity. Annu. Rev. Pathol. 2021, 16, 167–198. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, S.; Osswald, L.; Saller, B.S.; Unger, S.; De Feo, D.; Vinnakota, J.M.; Konantz, M.; Uhl, F.M.; Becker, H.; Lubbert, M.; et al. Oncogenic KrasG12D causes myeloproliferation via NLRP3 inflammasome activation. Nat. Commun. 2020, 11, 1659. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Tu, Y.; Long, Z.; Liu, J.; Kong, D.; Peng, J.; Wu, H.; Zheng, G.; Zhao, J.; Chen, Y.; et al. Reactive Oxygen Species Bridge the Gap between Chronic Inflammation and Tumor Development. Oxid. Med. Cell Longev. 2022, 2022, 2606928. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm (2020) 2024, 5, e519. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Qi, L.; Dong, Y.M.; Chao, H.; Zhao, P.; Ma, S.L.; Li, G. Glyphosate based-herbicide disrupts energy metabolism and activates inflammatory response through oxidative stress in mice liver. Chemosphere 2023, 315, 137751. [Google Scholar] [CrossRef]
- Sun, Q.; Gong, L.; Qi, R.; Qing, W.; Zou, M.; Ke, Q.; Zhang, L.; Tang, X.; Nie, Q.; Yang, Y.; et al. Oxidative stress-induced KLF4 activates inflammatory response through IL17RA and its downstream targets in retinal pigment epithelial cells. Free Radic. Biol. Med. 2020, 147, 271–281. [Google Scholar] [CrossRef]
- Hussain, S.P.; Harris, C.C. Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer 2007, 121, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Peng, J.; Tang, H.; Wang, S.; Peng, S.; Ye, F.; Wang, J.; Ouyang, K.; Li, J.; et al. Inhibition of NF-kappaB signaling unveils novel strategies to overcome drug resistance in cancers. Drug Resist. Updat. 2024, 73, 101042. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; De Rosa, M.; Sala, M.; Pacelli, R.; Campiglia, P.; Trimarco, B.; Iaccarino, G.; et al. A Novel Small Peptide Inhibitor of NFkappaB, RH10, Blocks Oxidative Stress-Dependent Phenotypes in Cancer. Oxid. Med. Cell Longev. 2018, 2018, 5801807. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Fiordelisi, A.; Santulli, G.; Ciccarelli, M.; Cerasuolo, F.A.; Sala, M.; Sommella, E.; Campiglia, P.; Illario, M.; Iaccarino, G.; et al. Exploiting GRK2 Inhibition as a Therapeutic Option in Experimental Cancer Treatment: Role of p53-Induced Mitochondrial Apoptosis. Cancers 2020, 12, 3530. [Google Scholar] [CrossRef]
- Soprano, M.; Sorriento, D.; Rusciano, M.R.; Maione, A.S.; Limite, G.; Forestieri, P.; D’Angelo, D.; D’Alessio, M.; Campiglia, P.; Formisano, P.; et al. Oxidative Stress Mediates the Antiproliferative Effects of Nelfinavir in Breast Cancer Cells. PLoS ONE 2016, 11, e0155970. [Google Scholar] [CrossRef]
- Sorriento, D.; Campanile, A.; Santulli, G.; Leggiero, E.; Pastore, L.; Trimarco, B.; Iaccarino, G. A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFkappaB activity. Mol. Cancer 2009, 8, 97. [Google Scholar] [CrossRef]
- Xu, P.; Xue, Y.N.; Ji, H.H.; Tan, C.; Guo, S. H2O2-induced oxidative stress disrupts mitochondrial functions and impairs migratory potential of human epidermal melanocytes. Exp. Dermatol. 2020, 29, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, Z.; Lu, D.; Li, H. Oxidative stress-mediated tetrabromobisphenol A disrupts mitochondrial function in HepG2 cells and activates ferroptosis signalling to induce apoptosis. J. Environ. Manag. 2025, 382, 125360. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Wang, Z.; Ding, H.; Tao, X.; Zhang, J.; Dai, K.; Li, X.; Shen, H.; Li, H.; Chen, Z.; et al. Microglial mitochondrial DNA release contributes to neuroinflammation after intracerebral hemorrhage through activating AIM2 inflammasome. Exp. Neurol. 2024, 382, 114950. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Hood, L. Structure and evolution of transplantation antigens: Partial amino-acid sequences of H-2K and H-2D alloantigens. Proc. Natl. Acad. Sci. USA 1976, 73, 599–603. [Google Scholar] [CrossRef]
- Bardelcikova, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef]
- Zheng, S.; Song, Q.; Zhang, P. Metabolic Modifications, Inflammation, and Cancer Immunotherapy. Front. Oncol. 2021, 11, 703681. [Google Scholar] [CrossRef]
- Colarusso, C.; Terlizzi, M.; Di Caprio, S.; Falanga, A.; D’Andria, E.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R. Conventional Chemotherapy and Inflammation: What Is the Role of the Inflammasome in the Tumor Microenvironment? Biomedicines 2025, 13, 203. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Karol, A.B.; Joshi, H.; Reford, E.; Izadmehr, S.; Doroshow, D.B.; Galsky, M.D. C-reactive protein (CRP) as a prognostic biomarker in patients with urothelial carcinoma: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2024, 197, 104352. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Knupfer, H.; Preiss, R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int. J. Color. Dis. 2010, 25, 135–140. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P. Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 2012, 46, 382–419. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.B.; Lackey, L.; Carpenter, M.A.; Rathore, A.; Land, A.M.; Leonard, B.; Refsland, E.W.; Kotandeniya, D.; Tretyakova, N.; Nikas, J.B.; et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature 2013, 494, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, C.; Wang, S.; Xiang, Z.; Dou, R.; Lin, Z.; Zheng, J.; Xiong, B. SDC2 and TFPI2 Methylation in Stool Samples as an Integrated Biomarker for Early Detection of Colorectal Cancer. Cancer Manag. Res. 2021, 13, 3601–3617. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, S.; Park, S.; Nam, S.; Chung, J.W.; Kim, K.O.; An, J.; Kim, J.H. Significance of 8-OHdG Expression as a Predictor of Survival in Colorectal Cancer. Cancers 2023, 15, 4613. [Google Scholar] [CrossRef]
- Ai, R.; Tao, Y.; Hao, Y.; Jiang, L.; Dan, H.; Ji, N.; Zeng, X.; Zhou, Y.; Chen, Q. Microenvironmental regulation of the progression of oral potentially malignant disorders towards malignancy. Oncotarget 2017, 8, 81617–81635. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Wang, C.Y.; Werb, Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015, 44–46, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, K.; Kaprio, T.; Beilmann-Lehtonen, I.; Stenman, U.H.; Bockelman, C.; Haglund, C. TATI, TAT-2, and CRP as Prognostic Factors in Colorectal Cancer. Oncology 2022, 100, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.R.; Li, X.; Han, C.; Chang, Y.; Fu, Y.; Feng, G.C.; Lei, Y.; Li, H.Y.; Tang, P.M.; Ji, S.R.; et al. C-Reactive Protein Induces Immunosuppression by Activating FcgammaR2B in Pulmonary Macrophages to Promote Lung Metastasis. Cancer Res. 2024, 84, 4184–4198. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, Z.; Wang, J.; Chen, M.; Wan, Y.; Huang, H.; Liu, Z.; Wang, J.; Hou, J. C-reactive protein impairs immune response of CD8+ T cells via FcgammaRIIb-p38MAPK-ROS axis in multiple myeloma. J. Immunother. Cancer 2023, 11, e007593. [Google Scholar] [CrossRef]
- Nurmi, A.M.; Mustonen, H.K.; Stenman, U.H.; Seppanen, H.E.; Haglund, C.H. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci. Rep. 2021, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin. Chem. Lab. Med. 2023, 61, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, K.; Hou, Y.; You, Z.; Shu, C.; Wei, X.; Wu, T.; Shi, N.; Zhang, G.; Wu, J.; et al. YY1 complex in M2 macrophage promotes prostate cancer progression by upregulating IL-6. J. Immunother. Cancer 2023, 11, e006020. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Rupert, J.E.; Narasimhan, A.; Jengelley, D.H.A.; Jiang, Y.; Liu, J.; Au, E.; Silverman, L.M.; Sandusky, G.; Bonetto, A.; Cao, S.; et al. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J. Exp. Med. 2021, 218, e20190450. [Google Scholar] [CrossRef]
- Soler, M.F.; Abaurrea, A.; Azcoaga, P.; Araujo, A.M.; Caffarel, M.M. New perspectives in cancer immunotherapy: Targeting IL-6 cytokine family. J. Immunother. Cancer 2023, 11, e007530. [Google Scholar] [CrossRef]
- Jalilian, E.; Abolhasani-Zadeh, F.; Afgar, A.; Samoudi, A.; Zeinalynezhad, H.; Langroudi, L. Neutralizing tumor-related inflammation and reprogramming of cancer-associated fibroblasts by Curcumin in breast cancer therapy. Sci. Rep. 2023, 13, 20770. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, S.S.; Raj, R.; Nagar, A.; Hawthorne, S.; Paiva-Santos, A.C.; Kamal, M.A.; El-Daly, M.M.; Azhar, E.I.; Sharma, A. Chronic Inflammation’s Transformation to Cancer: A Nanotherapeutic Paradigm. Molecules 2023, 28, 4413. [Google Scholar] [CrossRef]
- Stamatakis, K.; Torres-Gerica, P.; Jimenez-Segovia, A.; Ramos-Munoz, E.; Crespo-Toro, L.; Fuentes, P.; Toribio, M.L.; Callejas-Hernandez, F.; Carrato, A.; Garcia Bermejo, M.L.; et al. Cyclooxygenase 2 Effector Genes as Potential Inflammation-Related Biomarkers for Colorectal Cancer Circulating Tumor Cells Detection by Liquid Biopsy. Front. Pharmacol. 2021, 12, 806395. [Google Scholar] [CrossRef]
- Wang, D.; Cabalag, C.S.; Clemons, N.J.; DuBois, R.N. Cyclooxygenases and Prostaglandins in Tumor Immunology and Microenvironment of Gastrointestinal Cancer. Gastroenterology 2021, 161, 1813–1829. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.; Wang, Y.; Xuan, Y.; Shen, M.; Hu, X.; Li, Y.; Guo, Y.; Wang, J.; Tan, F. Thiostrepton induces oxidative stress, mitochondrial dysfunction and ferroptosis in HaCaT cells. Cell Signal 2024, 121, 111285. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Molon, M.; Biesiadecki, M.; Mokrzynska, A.; Balawender, K. Oxidative Stress Markers in Urine and Serum of Patients with Bladder Cancer. Antioxidants 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Brassea-Perez, E.; Hernandez-Camacho, C.J.; Labrada-Martagon, V.; Vazquez-Medina, J.P.; Gaxiola-Robles, R.; Zenteno-Savin, T. Oxidative stress induced by phthalates in mammals: State of the art and potential biomarkers. Environ. Res. 2022, 206, 112636. [Google Scholar] [CrossRef]
- Marino, M.; Rendine, M.; Venturi, S.; Porrini, M.; Gardana, C.; Klimis-Zacas, D.; Riso, P.; Del Bo, C. Red raspberry (Rubus idaeus) preserves intestinal barrier integrity and reduces oxidative stress in Caco-2 cells exposed to a proinflammatory stimulus. Food Funct. 2024, 15, 6943–6954. [Google Scholar] [CrossRef]
- Cherkas, A.; Zarkovic, N. 4-Hydroxynonenal in Redox Homeostasis of Gastrointestinal Mucosa: Implications for the Stomach in Health and Diseases. Antioxidants 2018, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef]
- Gall Troselj, K.; Tomljanovic, M.; Jaganjac, M.; Matijevic Glavan, T.; Cipak Gasparovic, A.; Milkovic, L.; Borovic Sunjic, S.; Buttari, B.; Profumo, E.; Saha, S.; et al. Oxidative Stress and Cancer Heterogeneity Orchestrate NRF2 Roles Relevant for Therapy Response. Molecules 2022, 27, 1468. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.X.; Wang, Y.B.; Wu, J.; Ding, G.S.; Wu, Y.; Wei, L.H.; Wang, F.; Zhang, Z.M. Clinical significance of serum oxidative stress and serum uric acid levels before surgery for hepatitis B-related liver cancer. World J. Gastrointest. Surg. 2023, 15, 1995–2002. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Brzozowa-Zasada, M.; Ianaro, A.; Piecuch, A.; Michalski, M.; Matysiak, N.; Steplewska, K. Immunohistochemical Expression of Glutathione Peroxidase-2 (Gpx-2) and Its Clinical Relevance in Colon Adenocarcinoma Patients. Int. J. Mol. Sci. 2023, 24, 14650. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liao, W.; Wang, S. The clinical significance of glutathione peroxidase 2 in glioblastoma multiforme. Transl. Neurosci. 2021, 12, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lawinski, M.; Zadka, K.; Ksepka, N.; Matin, M.; Wysocki, K.; Karkocha, D.; Gradowska, A.; Atanasov, A.G.; Slodkowski, M.; Wierzbicka, A.; et al. Does Resveratrol Impact Oxidative Stress Markers in Patients with Head and Neck Cancer Receiving Home Enteral Nutrition? Nutrients 2025, 17, 504. [Google Scholar] [CrossRef]
- de Oliveira, S.T.; Bessani, M.P.; Scandolara, T.B.; Silva, J.C.; Kawassaki, A.C.B.; Fagotti, P.A.F.; Maito, V.T.; de Souza, J.A.; Rech, D.; Panis, C. Systemic lipid peroxidation profile from patients with breast cancer changes according to the lymph nodal metastasis status. Oncoscience 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Yang, W.; Wang, C.; Guo, T.; Yang, J.; Shao, Z.; Cai, G.; Cai, S.; Zhang, L.; et al. Molecular Profiling Provides Clinical Insights Into Targeted and Immunotherapies as Well as Colorectal Cancer Prognosis. Gastroenterology 2023, 165, 414–428.e7. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The role of lipids in cancer progression and metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef]
- Zhang, H.L.; Hu, B.X.; Li, Z.L.; Du, T.; Shan, J.L.; Ye, Z.P.; Peng, X.D.; Li, X.; Huang, Y.; Zhu, X.Y.; et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 2022, 24, 88–98. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Yang, M.; Cui, W.; Lv, X.; Xiong, G.; Sun, C.; Xuan, H.; Ma, W.; Cui, X.; Cheng, Y.; Han, L.; et al. S100P is a ferroptosis suppressor to facilitate hepatocellular carcinoma development by rewiring lipid metabolism. Nat. Commun. 2025, 16, 509. [Google Scholar] [CrossRef]
- Hu, D.; Li, Y.; Li, R.; Wang, M.; Zhou, K.; He, C.; Wei, Q.; Qian, Z. Recent advances in reactive oxygen species (ROS)-responsive drug delivery systems for photodynamic therapy of cancer. Acta Pharm. Sin. B 2024, 14, 5106–5131. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.S.; Witzel, H.R.; Weinmann, A.; Bartsch, F.; Schindeldecker, M.; Galle, P.R.; Lang, H.; Roth, W.; Ridder, D.A.; Straub, B.K. Reduced Lipid Peroxidation Predicts Unfavorable Prognosis in Hepatocellular Carcinoma, but Not Intrahepatic Cholangiocarcinoma. Biomedicines 2023, 11, 2471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Gong, J.; Liu, W.; Zhao, H.; Xue, W.; Ren, Z.; Bao, J.; Lin, Z. Development of a breast cancer invasion score to predict tumor aggressiveness and prognosis via PI3K/AKT/mTOR pathway analysis. Cell Death Discov. 2025, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Li, Y.; Jin, M.; Liu, J.; Wu, Z.; Zhu, F.; Zhao, L.; Fan, Y.; Xu, L.; Ji, J. Downregulation of 4-HNE and FOXO4 collaboratively promotes NSCLC cell migration and tumor growth. Cell Death Dis. 2024, 15, 546. [Google Scholar] [CrossRef]
- Panda, B.; Tripathy, A.; Patra, S.; Kullu, B.; Tabrez, S.; Jena, M. Imperative connotation of SODs in cancer: Emerging targets and multifactorial role of action. IUBMB Life 2024, 76, 592–613. [Google Scholar] [CrossRef]
- Vilchis-Landeros, M.M.; Vazquez-Meza, H.; Vazquez-Carrada, M.; Uribe-Ramirez, D.; Matuz-Mares, D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5675. [Google Scholar] [CrossRef]
- Strycharz-Dudziak, M.; Kielczykowska, M.; Drop, B.; Swiatek, L.; Kliszczewska, E.; Musik, I.; Polz-Dacewicz, M. Total Antioxidant Status (TAS), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) in Oropharyngeal Cancer Associated with EBV Infection. Oxid. Med. Cell Longev. 2019, 2019, 5832410. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Kutikhin, A.G. Inherited variations in the SOD and GPX gene families and cancer risk. Free Radic. Res. 2012, 46, 581–599. [Google Scholar] [CrossRef]
- Abd Al Moaty, M.N.; El Ashry, E.S.H.; Awad, L.F.; Mostafa, A.; Abu-Serie, M.M.; Teleb, M. Harnessing ROS-Induced Oxidative Stress for Halting Colorectal Cancer via Thiazolidinedione-Based SOD Inhibitors. ACS Omega 2022, 7, 21267–21279. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Li, H.H.; Lin, J.H.; Chiang, M.C.; Hsu, T.W.; Li, A.F.; Yen, D.H.; Hsu, H.S.; Hung, S.C. Esophageal Cancer Stem-like Cells Resist Ferroptosis-Induced Cell Death by Active Hsp27-GPX4 Pathway. Biomolecules 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zheng, Y.; He, H.; Yang, L.; Yang, J.; Li, M.; Dai, W.; Huang, H. FBXO31 sensitizes cancer stem cells-like cells to cisplatin by promoting ferroptosis and facilitating proteasomal degradation of GPX4 in cholangiocarcinoma. Liver Int. 2022, 42, 2871–2888. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryc, E.; Surowska, O.; Heryc, R.; Serwin, N.; Napiontek-Balinska, S.; Dolegowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, Y.; Duan, Y.; Zhang, X.; Yao, J.; Yang, D.; Wei, Z.; Zhu, Z.; Xu, J.; Hu, Z.; et al. Superoxide dismutase promotes gastric tumorigenesis mediated by Helicobacter pylori and enhances resistance to 5-fluorouracil in gastric cancer. iScience 2025, 28, 111553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, W.; Yang, L.; Wang, T.; Li, C.; Yu, J.; Zhang, P.; Yin, Y.; Li, R.; Tao, K. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci. Rep. 2023, 13, 14359. [Google Scholar] [CrossRef]
- Iglesias-Matesanz, P.; Lacalle-Gonzalez, C.; Lopez-Blazquez, C.; Ochieng’ Otieno, M.; Garcia-Foncillas, J.; Martinez-Useros, J. Glutathione Peroxidases: An Emerging and Promising Therapeutic Target for Pancreatic Cancer Treatment. Antioxidants 2024, 13, 1405. [Google Scholar] [CrossRef]
- Wu, H.; Liao, X.; Huang, W.; Hu, H.; Lan, L.; Yang, Q.; An, Y. Examining the prognostic and clinicopathological significance of GPX4 in human cancers: A meta-analysis. Free Radic. Res. 2025, 59, 239–249. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, P.J.; Lin, S.H.; Yeh, K.T.; Tsao, T.C.; Chen, Y.E.; Lin, S.H.; Yang, S.F. Association between EGFR Gene Mutation and Antioxidant Gene Polymorphism of Non-Small-Cell Lung Cancer. Diagnostics 2020, 10, 692. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Monson, S.; Chen, P.J.; Gangi, A.; Waters, K.; Billet, S.; Hendifar, A.; Lu, S.; Zell, J.A.; Gong, J. Chemopreventive strategies for sporadic colorectal cancer: A narrative review. Transl. Gastroenterol. Hepatol. 2025, 10, 11. [Google Scholar] [CrossRef]
- Wang, Q.; Bin, C.; Xue, Q.; Gao, Q.; Huang, A.; Wang, K.; Tang, N. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Iborra, M.; Moret, I.; Rausell, F.; Bastida, G.; Aguas, M.; Cerrillo, E.; Nos, P.; Beltran, B. Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem. Soc. Trans. 2011, 39, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gupta, N.; Khan, R.; Kumar, R.; Sharma, M.; Kumar, L.; Sharma, A. Interrelationship between angiogenesis, inflammation and oxidative stress in Indian patients with multiple myeloma. Clin. Transl. Oncol. 2016, 18, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, H.; Whelan, K.A.; Tanaka, K.; Natsuizaka, M.; Long, A.; Guo, A.; Chang, S.; Kagawa, S.; Srinivasan, S.; Guha, M.; et al. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene 2015, 34, 5229–5239. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.D.; Debeljak, M.; Riel, S.; Millena, A.C.; Eshleman, J.R.; Paller, C.J.; Odero-Marah, V. Val16A SOD2 Polymorphism Promotes Epithelial-Mesenchymal Transition Antagonized by Muscadine Grape Skin Extract in Prostate Cancer Cells. Antioxidants 2021, 10, 213. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, S.Y.; Moon, A. C-Reactive Protein Signaling Pathways in Tumor Progression. Biomol. Ther. 2023, 31, 473–483. [Google Scholar] [CrossRef]
- Sawada, N.; Iwasaki, M.; Yamaji, T.; Goto, A.; Shimazu, T.; Inoue, M.; Tanno, K.; Sakata, K.; Yamagishi, K.; Iso, H.; et al. The Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT): Study Design and Participants. J. Epidemiol. 2020, 30, 46–54. [Google Scholar] [CrossRef]
- Hart, P.C.; Rajab, I.M.; Alebraheem, M.; Potempa, L.A. C-Reactive Protein and Cancer-Diagnostic and Therapeutic Insights. Front. Immunol. 2020, 11, 595835. [Google Scholar] [CrossRef]
- Kostner, A.H.; Kersten, C.; Lowenmark, T.; Ydsten, K.A.; Peltonen, R.; Isoniemi, H.; Haglund, C.; Gunnarsson, U.; Isaksson, B. The prognostic role of systemic inflammation in patients undergoing resection of colorectal liver metastases: C-reactive protein (CRP) is a strong negative prognostic biomarker. J. Surg. Oncol. 2016, 114, 895–899. [Google Scholar] [CrossRef]
- Yang, M.; Lin, S.Q.; Liu, X.Y.; Tang, M.; Hu, C.L.; Wang, Z.W.; Zhang, Q.; Zhang, X.; Song, M.M.; Ruan, G.T.; et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front. Immunol. 2023, 14, 1131496. [Google Scholar] [CrossRef]
- Chen, D.; Ma, Y.; Li, J.; Wen, L.; Liu, L.; Su, J.; Wu, J.; Wang, P.; Zhang, G.; Huang, C.; et al. Prognostic and clinicopathological significance of C-reactive protein-albumin-lymphocyte(CALLY) in patients with digestive system neoplasms: A systematic review and meta-analysis. World J. Surg. Oncol. 2025, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Tajdari, M.; Peyrovinasab, A.; Bayanati, M.; Ismail Mahboubi Rabbani, M.; Abdolghaffari, A.H.; Zarghi, A. Dual COX-2/TNF-alpha Inhibitors as Promising Anti-inflammatory and Cancer Chemopreventive Agents: A Review. Iran. J. Pharm. Res. 2024, 23, e151312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Sun, W.; Cao, J.; Ma, Z. COX-2 in lung cancer: Mechanisms, development, and targeted therapies. Chronic Dis. Transl. Med. 2024, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, A.; Yin, B.; Wu, D.; Han, S.; Zhang, W.; Liu, J.; Sun, K. SND1 promotes the proliferation of osteosarcoma cells by upregulating COX-2/PGE2 expression via activation of NF-kappaB. Oncol. Rep. 2019, 41, 579–589. [Google Scholar]
- Abe, A.; Fukui, H.; Fujii, S.; Fujita, M.; Mukawa, K.; Ichikawa, K.; Tomita, S.; Ono, Y.; Imai, Y.; Imura, J.; et al. Involvement of cyclooxygenase-2 and vascular endothelial growth factor in vascularization and lymph node metastasis of colorectal cancers with submucosal invasion. J. Gastroenterol. Hepatol. 2007, 22, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Kosumi, K.; Hamada, T.; Zhang, S.; Liu, L.; da Silva, A.; Koh, H.; Twombly, T.S.; Mima, K.; Morikawa, T.; Song, M.; et al. Prognostic association of PTGS2 (COX-2) over-expression according to BRAF mutation status in colorectal cancer: Results from two prospective cohorts and CALGB 89803 (Alliance) trial. Eur. J. Cancer 2019, 111, 82–93. [Google Scholar] [CrossRef]
- Mahboubi-Rabbani, M.; Abdolghaffari, A.H.; Ghesmati, M.; Amini, A.; Zarghi, A. Selective COX-2 inhibitors as anticancer agents: A patent review (2018–2023). Expert Opin. Ther. Pat. 2024, 34, 733–757. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Sheth, H.; Elliott, F.; Reed, L.; Macrae, F.; Mecklin, J.P.; Moslein, G.; McRonald, F.E.; Bertario, L.; Evans, D.G.; et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: A double-blind, randomised, placebo-controlled trial. Lancet 2020, 395, 1855–1863. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-kappaB to the COX-2 promoter. Aging 2020, 12, 611–627. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Xu, Y.; Liu, Y.; Li, W.; Cai, J.; Zhang, Y. Enalapril overcomes chemoresistance and potentiates antitumor efficacy of 5-FU in colorectal cancer by suppressing proliferation, angiogenesis, and NF-kappaB/STAT3-regulated proteins. Cell Death Dis. 2020, 11, 477. [Google Scholar] [CrossRef]
- Ramisetti, S.V.; Patra, T.; Munirathnam, V.; Sainath, J.V.; Veeraiyan, D.; Namani, A. NRF2 Signaling Pathway in Chemo/Radio/Immuno-Therapy Resistance of Lung Cancer: Looking Beyond the Tip of the Iceberg. Arch. Bronconeumol. 2024, 60 (Suppl. S2), S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, Y.; Li, D.; Wei, J.; Chen, K.; Zhang, E.; Liu, G.; Chu, X.; Liu, X.; Liu, W.; et al. Cancer associated fibroblasts and metabolic reprogramming: Unraveling the intricate crosstalk in tumor evolution. J. Hematol. Oncol. 2024, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Galasso, L.; Termite, F.; Mignini, I.; Esposto, G.; Borriello, R.; Vitale, F.; Nicoletti, A.; Paratore, M.; Ainora, M.E.; Gasbarrini, A.; et al. Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights. Cancers 2025, 17, 368. [Google Scholar] [CrossRef]
- Hong, B.Y.; Chhaya, A.; Robles, A.; Cervantes, J.; Tiwari, S. The role of Fusobacterium nucleatum in the pathogenesis of colon cancer. J. Investig. Med. 2024, 72, 819–827. [Google Scholar] [CrossRef]
- Dadgar-Zankbar, L.; Elahi, Z.; Shariati, A.; Khaledi, A.; Razavi, S.; Khoshbayan, A. Exploring the role of Fusobacterium nucleatum in colorectal cancer: Implications for tumor proliferation and chemoresistance. Cell Commun. Signal 2024, 22, 547. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, H.; Li, H.; Zheng, M.; Zuo, X.; Wang, W.; Wang, S.; Lu, Y.; Wang, J.; Li, Y.; et al. Intratumor microbiome-derived butyrate promotes lung cancer metastasis. Cell Rep. Med. 2024, 5, 101488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Lin, Y.; Cao, C.; Chen, D.; Huang, X.; Li, C.; Xu, H.; Lai, H.; Chen, H.; et al. Roles of the gut microbiota in hepatocellular carcinoma: From the gut dysbiosis to the intratumoral microbiota. Cell Death Discov. 2025, 11, 140. [Google Scholar] [CrossRef]
- Zitvogel, L.; Daillere, R.; Roberti, M.P.; Routy, B.; Kroemer, G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017, 15, 465–478. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Huang, J.; Shen, L.; Xiong, Y. Precise targeting of lipid metabolism in the era of immuno-oncology and the latest advances in nano-based drug delivery systems for cancer therapy. Acta Pharm. Sin. B 2024, 14, 4717–4737. [Google Scholar] [CrossRef]
- Hecht, F.; Zocchi, M.; Alimohammadi, F.; Harris, I.S. Regulation of antioxidants in cancer. Mol. Cell 2024, 84, 23–33. [Google Scholar] [CrossRef]
- Woldeselassie, M.; Tamene, A. Therapeutic controversies over use of antioxidant supplements during cancer treatment: A scoping review. Front. Nutr. 2024, 11, 1480780. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.B.M.; Abdelhameed, K.M.A.; Sobhy, S.E.; Konper, H.M.A.; Hassanein, Z.A.E.; Saleh, A.A.; Jamal, M.T.; Hafez, E.E. Antioxidant activity, antibacterial behavior, and anticancer impact of Egyptian propolis. Open Vet. J. 2025, 15, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef]
- Blot, W.J.; Li, J.Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.Q.; Yang, C.S.; Zheng, S.F.; Gail, M.; Li, G.Y.; et al. Nutrition intervention trials in Linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993, 85, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.L.; Dawsey, S.M.; Kamangar, F.; Fan, J.H.; Abnet, C.C.; Sun, X.D.; Johnson, L.L.; Gail, M.H.; Dong, Z.W.; Yu, B.; et al. Total and cancer mortality after supplementation with vitamins and minerals: Follow-up of the Linxian General Population Nutrition Intervention Trial. J. Natl. Cancer Inst. 2009, 101, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shu, X.O.; Li, H.; Yang, G.; Cai, H.; Ji, B.T.; Gao, J.; Gao, Y.T.; Zheng, W.; Xiang, Y.B. Vitamin intake and liver cancer risk: A report from two cohort studies in China. J. Natl. Cancer Inst. 2012, 104, 1173–1181. [Google Scholar] [CrossRef]
- Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar]
- Bates, C.A.; Vincent, M.J.; Buerger, A.N.; Santamaria, A.B.; Maier, A.; Jack, M. Investigating the relationship between beta-carotene intake from diet and supplements, smoking, and lung cancer risk. Food Chem. Toxicol. 2024, 194, 115104. [Google Scholar] [CrossRef]
- Krannich, F.; Mucke, R.; Buntzel, J.; Schomburg, L.; Micke, O.; Hubner, J.; Dorfler, J. A systematic review of Selenium as a complementary treatment in cancer patients. Complement. Ther. Med. 2024, 86, 103095. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Luo, M.; Li, L. Association Between Vitamin Intake and Colorectal Cancer: Evidence from NHANES Data. J. Gastrointest. Cancer 2024, 55, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Seraphin, G.; Rieger, S.; Hewison, M.; Capobianco, E.; Lisse, T.S. The impact of vitamin D on cancer: A mini review. J. Steroid Biochem. Mol. Biol. 2023, 231, 106308. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, S.D.; Bin-Melaih, H.H.; Atiya, E.M.; Fahmy, U.A.; Binmahfouz, L.S.; Neamatallah, T.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Self-Nanoemulsifying Drug Delivery System of 2-Methoxyestradiol Exhibits Enhanced Anti-Proliferative and Pro-Apoptotic Activities in MCF-7 Breast Cancer Cells. Life 2022, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, R.R.; Ye, Q.; Qiu, F.; Xu, H.Y.; Wei, F.G.; Zhang, H. 4-Hydroxyphenyl Retinamide Preferentially Targets FLT3 Mutated Acute Myeloid Leukemia via ROS Induction and NF-kappaB Inhibition. Curr. Med. Sci. 2020, 40, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; He, L.; Hutchens, S.; Garrett, T.E.; Pazoles, C.J.; Tew, K.D. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008, 68, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Kumbul, Y.C.; Naziroglu, M. Paclitaxel Promotes Oxidative Stress-Mediated Human Laryngeal Squamous Tumor Cell Death through the Stimulation of Calcium and Zinc Signaling Pathways: No Synergic Action of Melatonin. Biol. Trace Elem. Res. 2022, 200, 2084–2098. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef]
- Ozalp, G.R.; Ustuner, B.; Avci, G.; Bari, O.; Yilmaz, M.M.; Denk, B.; Aktar, A. Vincristine-associated total antioxidant and oxidant status of ovaries and in vitro nuclear oocyte maturation in dogs with canine transmissible venereal tumor. Anim. Reprod. Sci. 2023, 253, 107260. [Google Scholar] [CrossRef]
- Mendez, N.; Alarcon, P.; Millan, C.; Burgos, R.A.; Morera, F.J.; Ojeda, J. Vincristine, carboplatin and cisplatin increase oxidative burst induced by PAF in canine neutrophils. Vet. Immunol. Immunopathol. 2020, 221, 110011. [Google Scholar] [CrossRef]
- Varricchi, G.; Ameri, P.; Cadeddu, C.; Ghigo, A.; Madonna, R.; Marone, G.; Mercurio, V.; Monte, I.; Novo, G.; Parrella, P.; et al. Antineoplastic Drug-Induced Cardiotoxicity: A Redox Perspective. Front. Physiol. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Simunek, T.; Sterba, M.; Popelova, O.; Adamcova, M.; Hrdina, R.; Gersl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.T.; Chung, Y.M.; Kim, J.J.; Chung, J.S.; Kim, B.S.; Kim, H.J.; Kim, J.S.; Yoo, Y.D. Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem. Biophys. Res. Commun. 2007, 359, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Joo, S.H. Modulation of Reactive Oxygen Species to Overcome 5-Fluorouracil Resistance. Biomol. Ther. 2022, 30, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Yu, J.; Pan, Y.; Zhang, N.; Qi, Y.; Huang, Y. Recent Advances of Platinum-Based Anticancer Complexes in Combinational Multimodal Therapy. Adv. Healthc. Mater. 2023, 12, e2300253. [Google Scholar] [CrossRef]
- Ahmad, S.; Isab, A.A.; Al-Arfaj, A.R. Antitumor potential of platinum(II) complexes of selenium donor ligands. Metallomics 2023, 15, mfad020. [Google Scholar] [CrossRef]
- Montero, A.J.; Diaz-Montero, C.M.; Deutsch, Y.E.; Hurley, J.; Koniaris, L.G.; Rumboldt, T.; Yasir, S.; Jorda, M.; Garret-Mayer, E.; Avisar, E.; et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res. Treat. 2012, 132, 215–223. [Google Scholar] [CrossRef]
- Teyssonneau, D.; Dariane, C.; Barret, E.; Beauval, J.B.; Brureau, L.; Fiard, G.; Fromont, G.; Crehange, G.; Gauthe, M.; Ruffion, A.; et al. PARP inhibitors in prostate cancers, is it time for combinations? Ther. Adv. Med. Oncol. 2024, 16, 17588359241242959. [Google Scholar] [CrossRef]
- Kummar, S.; Chen, A.; Parchment, R.E.; Kinders, R.J.; Ji, J.; Tomaszewski, J.E.; Doroshow, J.H. Advances in using PARP inhibitors to treat cancer. BMC Med. 2012, 10, 25. [Google Scholar] [CrossRef]
- Morganti, S.; Marra, A.; De Angelis, C.; Toss, A.; Licata, L.; Giugliano, F.; Taurelli Salimbeni, B.; Berton Giachetti, P.P.M.; Esposito, A.; Giordano, A.; et al. PARP Inhibitors for Breast Cancer Treatment: A Review. JAMA Oncol. 2024, 10, 658–670. [Google Scholar] [CrossRef]
- Evers, B.; Drost, R.; Schut, E.; de Bruin, M.; van der Burg, E.; Derksen, P.W.; Holstege, H.; Liu, X.; van Drunen, E.; Beverloo, H.B.; et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin. Cancer Res. 2008, 14, 3916–3925. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Vitale, I.; Galluzzi, L.; Adam, J.; Olaussen, K.A.; Kepp, O.; Senovilla, L.; Talhaoui, I.; Guegan, J.; Enot, D.P.; et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 2013, 73, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Xu, J. Cancer-associated fibroblasts: A versatile mediator in tumor progression, metastasis, and targeted therapy. Cancer Metastasis Rev. 2024, 43, 1095–1116. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Z.; Gu, M.; Wei, P.; Wang, X.; Li, W. Cancer-associated fibroblasts: Heterogeneity and their role in the tumor immune response. Clin. Exp. Med. 2024, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Yu, R.; Eriksson, J.E.; Tsai, H.I.; Zhu, H. Cancer-associated fibroblasts in pancreatic ductal adenocarcinoma therapy: Challenges and opportunities. Cancer Lett. 2024, 591, 216859. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, W.; Ke, Y.; Hu, X.; Yang, C.; Zhang, R.; Wu, H.; Liu, D.; Yu, H.; Wu, C. Cancer-associated fibroblasts: A pivotal regulator of tumor microenvironment in the context of radiotherapy. Cell Commun. Signal 2025, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Pauklin, S. ROS and TGFbeta: From pancreatic tumour growth to metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 152. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, H.; Wu, J.; Guo, H.; Chen, X. Dissecting the pleiotropic roles of reactive oxygen species (ROS) in lung cancer: From carcinogenesis toward therapy. Med. Res. Rev. 2024, 44, 1566–1595. [Google Scholar] [CrossRef]
- Ren, H.; Wang, M.; Ma, X.; An, L.; Guo, Y.; Ma, H. METTL3 in cancer-associated fibroblasts-derived exosomes promotes the proliferation and metastasis and suppresses ferroptosis in colorectal cancer by eliciting ACSL3 m6A modification. Biol. Direct 2024, 19, 68. [Google Scholar] [CrossRef]
- Jankowski, J.A.Z.; de Caestecker, J.; Love, S.B.; Reilly, G.; Watson, P.; Sanders, S.; Ang, Y.; Morris, D.; Bhandari, P.; Brooks, C.; et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): A randomised factorial trial. Lancet 2018, 392, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, T.M.; Alm El-Din, M.A.; Rashdan, A.R. Celecoxib as an adjuvant to chemotherapy for patients with metastatic colorectal cancer: A randomized controlled clinical study. Saudi Med. J. 2022, 43, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yang, H.; Ye, Z.; Gao, B.; Qian, Z.; Ding, Y.; Mao, Z.; Du, Y.; Wang, W. Celecoxib Augments Paclitaxel-Induced Immunogenic Cell Death in Triple-Negative Breast Cancer. ACS Nano 2024, 18, 15864–15877. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Q.; Wang, J.; Liu, M.; Wang, Y.; Chen, Z.; Ye, Y.; Guan, Q.; Zhou, Y. A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study. Medicine 2019, 98, e16234. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.A.; Twombly, T.; Ma, C.; Shi, Q.; Haruki, K.; Fujiyoshi, K.; Vayrynen, J.; Zhao, M.; Knight, J.; Mane, S.; et al. Improved Survival With Adjuvant Cyclooxygenase 2 Inhibition in PIK3CA-Activated Stage III Colon Cancer: CALGB/SWOG 80702 (Alliance). J. Clin. Oncol. 2024, 42, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Shi, Q.; Fuchs, C.S.; Meyer, J.; Niedzwiecki, D.; Zemla, T.; Kumthekar, P.; Guthrie, K.A.; Couture, F.; Kuebler, P.; et al. Effect of Celecoxib vs Placebo Added to Standard Adjuvant Therapy on Disease-Free Survival Among Patients With Stage III Colon Cancer: The CALGB/SWOG 80702 (Alliance) Randomized Clinical Trial. JAMA 2021, 325, 1277–1286. [Google Scholar] [CrossRef]
- Hamy, A.S.; Tury, S.; Wang, X.; Gao, J.; Pierga, J.Y.; Giacchetti, S.; Brain, E.; Pistilli, B.; Marty, M.; Espie, M.; et al. Celecoxib With Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial. J. Clin. Oncol. 2019, 37, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, Y.; Lv, C. Decoding the spatiotemporal heterogeneity of tumor-associated macrophages. Mol. Cancer 2024, 23, 150. [Google Scholar] [CrossRef]
- Marelli, G.; Sica, A.; Vannucci, L.; Allavena, P. Inflammation as target in cancer therapy. Curr. Opin. Pharmacol. 2017, 35, 57–65. [Google Scholar] [CrossRef]
- Pozzi, S.; Satchi-Fainaro, R. The role of CCL2/CCR2 axis in cancer and inflammation: The next frontier in nanomedicine. Adv. Drug Deliv. Rev. 2024, 209, 115318. [Google Scholar] [CrossRef]
- Loberg, R.D.; Ying, C.; Craig, M.; Day, L.L.; Sargent, E.; Neeley, C.; Wojno, K.; Snyder, L.A.; Yan, L.; Pienta, K.J. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007, 67, 9417–9424. [Google Scholar] [CrossRef] [PubMed]
- Moisan, F.; Francisco, E.B.; Brozovic, A.; Duran, G.E.; Wang, Y.C.; Chaturvedi, S.; Seetharam, S.; Snyder, L.A.; Doshi, P.; Sikic, B.I. Enhancement of paclitaxel and carboplatin therapies by CCL2 blockade in ovarian cancers. Mol. Oncol. 2014, 8, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Nywening, T.M.; Wang-Gillam, A.; Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Cusworth, B.M.; Toriola, A.T.; Nieman, R.K.; Worley, L.A.; Yano, M.; et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: A single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Veeraballi, S.; Bandaru, S.S.; Kiwan, C.; Chan, K.H.; Shaaban, H.S. A multifaceted role of bisphosphonates from palliative care to anti-cancer therapy in solid tumors. J. Oncol. Pharm. Pr. 2025, 31, 107–118. [Google Scholar] [CrossRef]
- Boccia, S.M.; Sassu, C.M.; Ergasti, R.; Vertechy, L.; Apostol, A.I.; Palluzzi, E.; Fagotti, A.; Scambia, G.; Marchetti, C. Focus on Trabectedin in Ovarian Cancer: What Do We Still Need to Know? Drug Des. Devel Ther. 2024, 18, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Chiorean, E.G.; Fishman, M.P.; Saboury, B.; Teitelbaum, U.R.; Sun, W.; Huhn, R.D.; Song, W.; Li, D.; Sharp, L.L.; et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011, 331, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Torigian, D.A.; Chiorean, E.G.; Saboury, B.; Brothers, A.; Alavi, A.; Troxel, A.B.; Sun, W.; Teitelbaum, U.R.; Vonderheide, R.H.; et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2013, 19, 6286–6295. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Terabe, M.; Ghosh, A.; Ridnour, L.A.; DeGraff, W.G.; Wink, D.A.; Berzofsky, J.A.; Roberts, D.D. CD47 in the tumor microenvironment limits cooperation between antitumor T-cell immunity and radiotherapy. Cancer Res. 2014, 74, 6771–6783. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef]
- Kureshi, C.T.; Dougan, S.K. Cytokines in cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef]
- Kaplanov, I.; Carmi, Y.; Kornetsky, R.; Shemesh, A.; Shurin, G.V.; Shurin, M.R.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. USA 2019, 116, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Aggen, D.H.; Ager, C.R.; Obradovic, A.Z.; Chowdhury, N.; Ghasemzadeh, A.; Mao, W.; Chaimowitz, M.G.; Lopez-Bujanda, Z.A.; Spina, C.S.; Hawley, J.E.; et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin. Cancer Res. 2021, 27, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; De Bono, J.S.; Flechon, A.; Heidenreich, A.; Voog, E.; Davis, N.B.; Qi, M.; Bandekar, R.; Vermeulen, J.T.; Cornfeld, M.; et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 85–93. [Google Scholar] [CrossRef]
- Huang, D.; Xue, J.; Li, S.; Yang, D. Oxaliplatin and infliximab synergize to induce regression of colon cancer. Oncol. Lett. 2018, 15, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T.K.; Gutzmer, R.; Hauschild, A.; Heinzerling, L.; Schadendorf, D.; Nashan, D.; Holzle, E.; Kiecker, F.; Becker, J.; Sunderkotter, C.; et al. Adjuvant treatment with pegylated interferon alpha-2a versus low-dose interferon alpha-2a in patients with high-risk melanoma: A randomized phase III DeCOG trial. Ann. Oncol. 2016, 27, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, A.; Coens, C.; Suciu, S.; Santinami, M.; Kruit, W.; Testori, A.; Marsden, J.; Punt, C.; Sales, F.; Gore, M.; et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma: A phase III randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group. J. Clin. Oncol. 2009, 27, 2916–2923. [Google Scholar]
- Sabit, H.; Arneth, B.; Pawlik, T.M.; Abdel-Ghany, S.; Ghazy, A.; Abdelazeem, R.M.; Alqosaibi, A.; Al-Dhuayan, I.S.; Almulhim, J.; Alrabiah, N.A.; et al. Leveraging Single-Cell Multi-Omics to Decode Tumor Microenvironment Diversity and Therapeutic Resistance. Pharmaceuticals 2025, 18, 75. [Google Scholar] [CrossRef]
- Peng, X.; Fang, J.; Lou, C.; Yang, L.; Shan, S.; Wang, Z.; Chen, Y.; Li, H.; Li, X. Engineered nanoparticles for precise targeted drug delivery and enhanced therapeutic efficacy in cancer immunotherapy. Acta Pharm. Sin. B 2024, 14, 3432–3456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).