Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions
Abstract
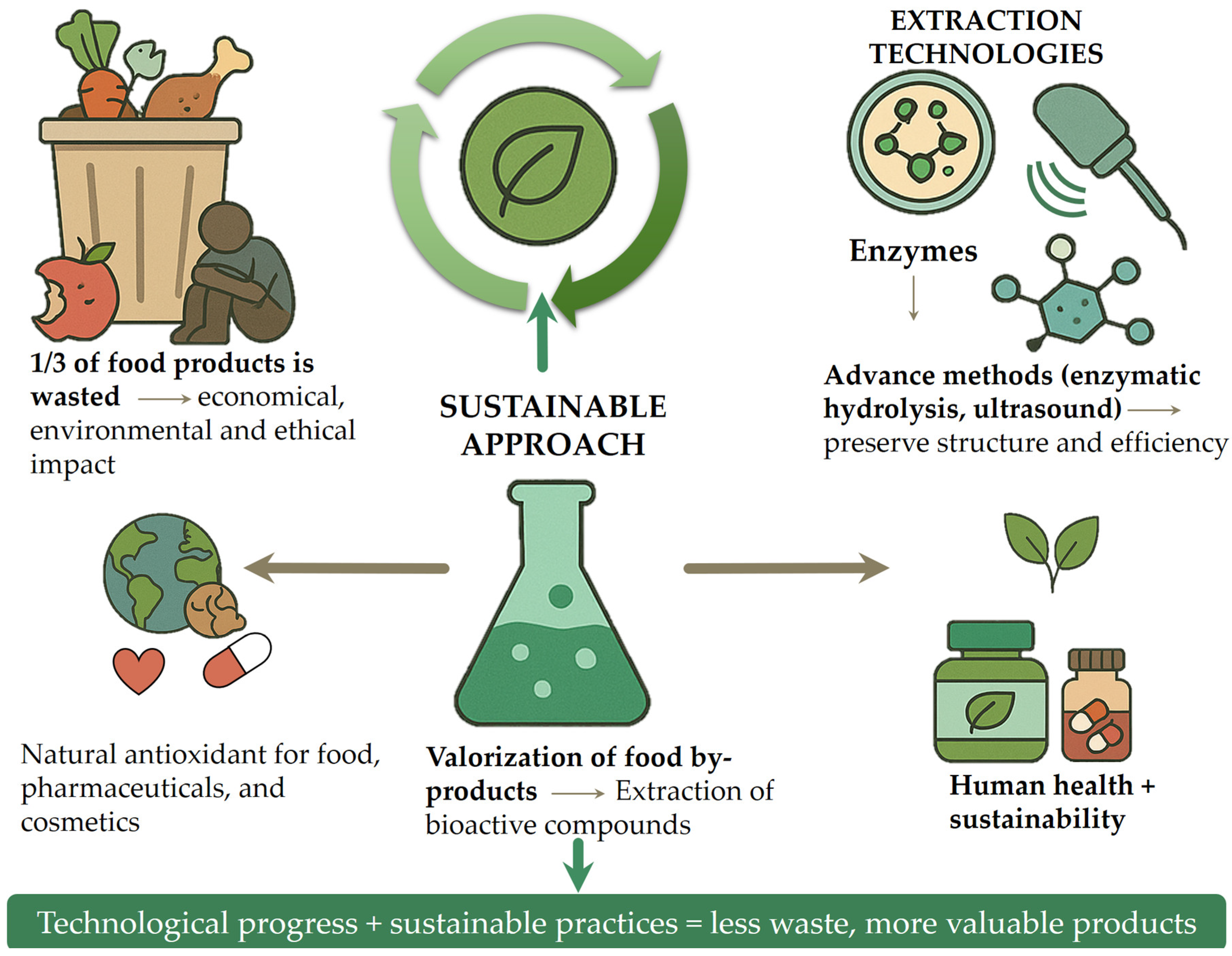
1. Introduction
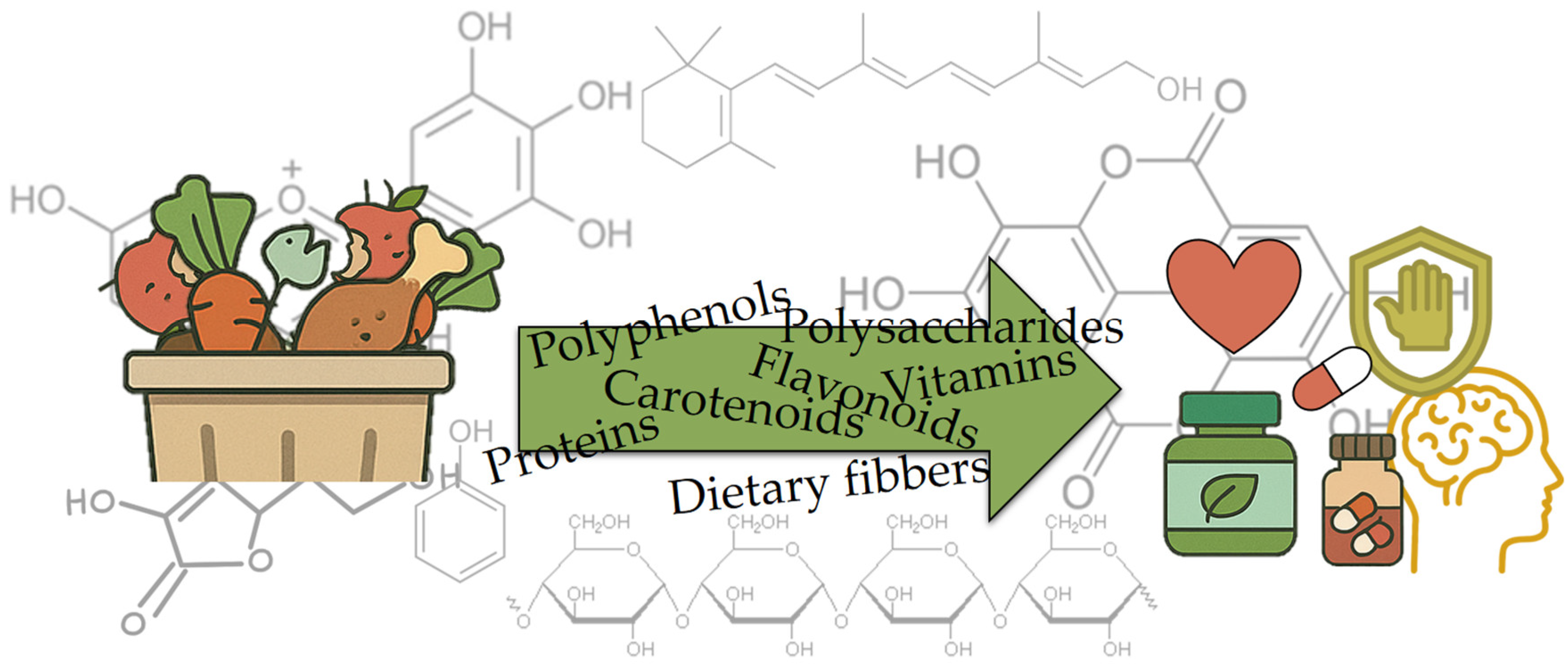
2. Bioactive Compounds in Food Waste and Their Health Benefits
2.1. Techniques Used for the Bioactives Extraction
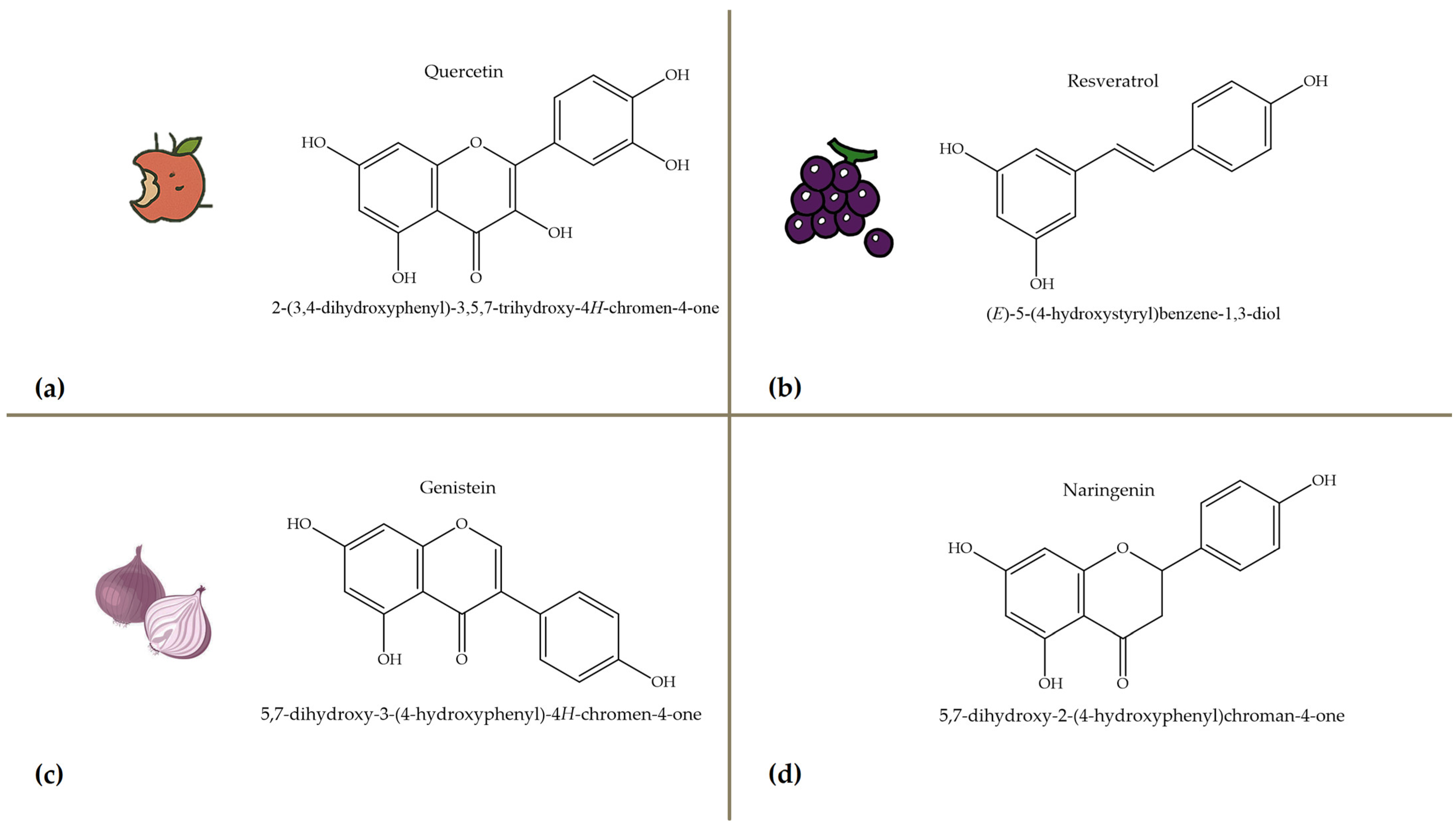
2.2. Polyphenols
2.3. Carotenoids
2.4. Dietary Fibers
2.5. Vitamins
2.6. Polysaccharides
2.7. Essential Oils
2.8. Other Compounds
3. Valorization the Food Waste in the Food Industry
4. The Valorization of Food Waste into Nutraceuticals and Dietary Supplements
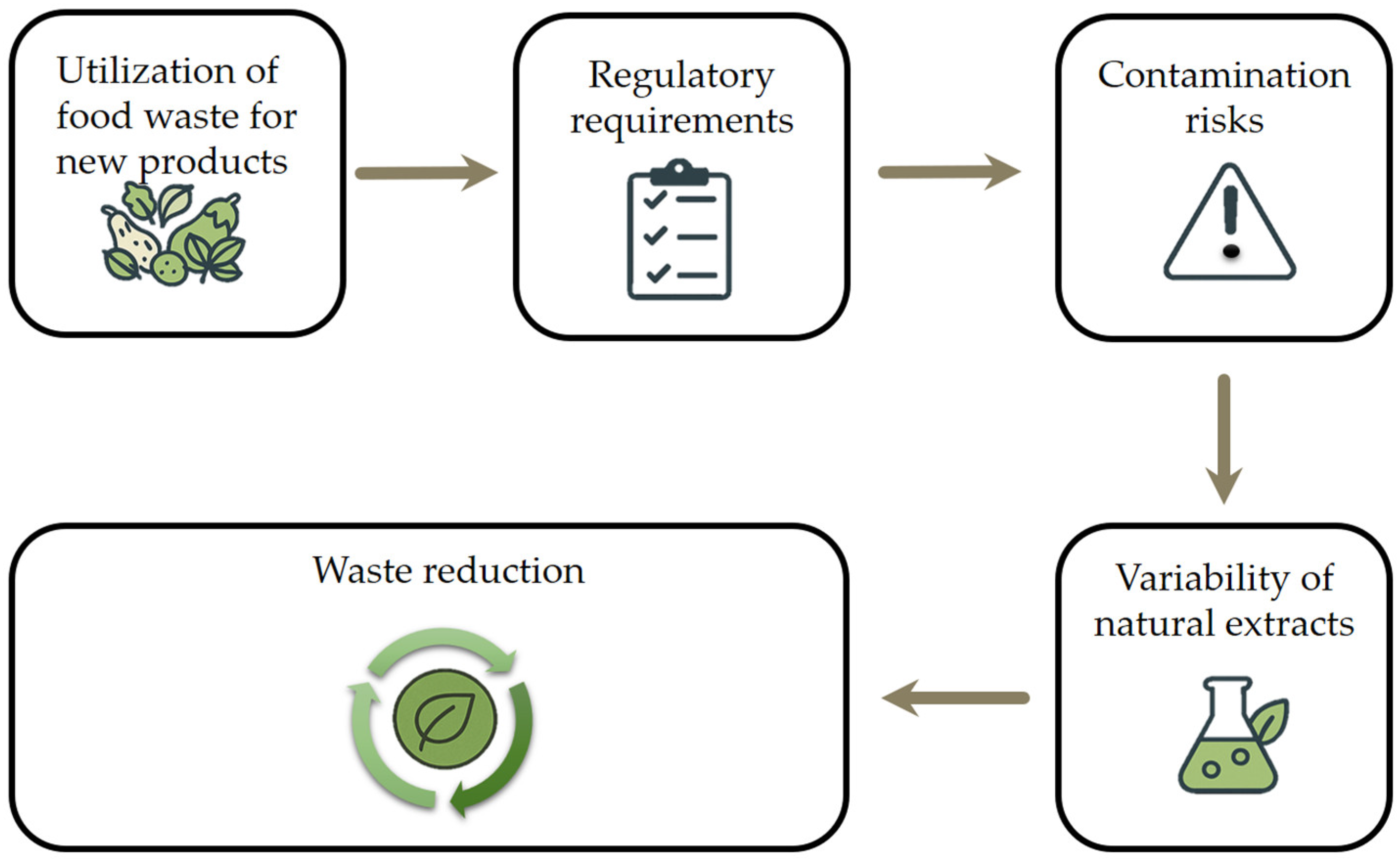
5. Challenges and Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moraes, N.V.; Lermen, F.H.; Echeveste, M.E.S. A systematic literature review on food waste/loss prevention and minimization methods. J. Environ. Manag. 2021, 286, 112268. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.B.; Gorski, I.; Neff, R.A. Understanding and addressing waste of food in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 1633–1648. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Tamasiga, P.; Ouassou, E.H.; Onyeaka, H.; Bakwena, M.; Happonen, A.; Molala, M. Forecasting disruptions in global food value chains to tackle food insecurity: The role of AI and big data analytics—A bibliometric and scientometric analysis. J. Agric. Food Res. 2023, 14, 100819. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming plant-based waste and by-products into valuable products using various “Food Industry 4.0” enabling technologies: A literature review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining upcycled food: The dual role of upcycling in reducing food loss and waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Thorsen, M.; Skeaff, S.; Goodman-Smith, F.; Thong, B.; Bremer, P.; Mirosa, M. Upcycled foods: A nudge toward nutrition. Front. Nutr. 2022, 9, 1071829. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Dietary Antioxidants and Chronic Diseases. Antioxidants 2023, 12, 362. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fruit and vegetable processing by-products as functional meat product ingredients -a chance to improve the nutritional value. LWT 2023, 189, 115442. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; Scarafoni, A.; Pilu, R.; Scaglia, B.; De Nisi, P.; Adani, F. The recovery from agro-industrial wastes provides different profiles of anti-inflammatory polyphenols for tailored applications. Front. Sustain. Food Syst. 2022, 6, 996562. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar]
- Pereira, J.A.M.; Berenguer, C.V.; Andrade, C.F.P.; Câmara, J.S. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Appl. Sci. 2022, 12, 2747. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. [Google Scholar] [CrossRef]
- Silletta, A.; Mancuso, A.; d’Avanzo, N.; Cristiano, M.C.; Paolino, D. Antimicrobial Compounds from Food Waste in Cosmetics. Cosmetics 2024, 11, 151. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive compounds from pomegranate peels—Biological properties, structure–function relationships, health benefits and food applications—A comprehensive review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef]
- El Oihabi, M.; Soultana, M.; El Fellah, I.; Fakih Lanjri, H.; Ben Allal, L.; Ammari, M.; Fakih Lanjri, A. Optimized extraction of phenolic compounds and antioxidant activity from cannabis Co-products via a combination of solvent-ultrasound-assisted extraction, response surface methodology, and sensitivity analysis. Case Stud. Chem. Environ. Eng. 2024, 10, 100906. [Google Scholar] [CrossRef]
- Sheibani, S.; Jafarzadeh, S.; Qazanfarzadeh, Z.; Osadee Wijekoon, M.M.J.; Mohd Rozalli, N.H.; Mohammadi Nafchi, A. Sustainable strategies for using natural extracts in smart food packaging. Int. J. Biol. Macromol. 2024, 267, 131537. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Mulligan, C.N.; Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery by-products: Extraction Techniques and their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Sah, P.; Rai, A.K.; Syiem, D.; Garay, R.P. Sirtuin activators as an anti-aging intervention for longevity. Explor Drug Sci. 2025, 3, 100881. [Google Scholar] [CrossRef]
- Samota, M.K.; Kaur, M.; Sharma, M.; Sarita; Krishnan, V.; Thakur, J.; Rawat, M.; Phogat, B.; Guru, P.N. Hesperidin from citrus peel waste: Extraction and its health implications. Qual. Assur. Saf. Crop. Foods 2023, 15, 71–99. [Google Scholar] [CrossRef]
- Shah, M.A.; Tariq, S.; Abuzar, S.M.; Ilyas, K.; Qadees, I.; Alsharif, I.; Anam, K.; Almutairi, R.T.; Al-Regaiey, K.A.; Babalghith, A.O.; et al. Peel waste of citrus fruits: A valuable and renewable source of polyphenols for the treatment of diabesity. Curr. Res. Biotechnol. 2024, 7, 100204. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yip, Y.M. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Futur. Pharmacol. 2023, 3, 14–37. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Azman, N.F.I.N.; Azlan, A.; Khoo, H.E.; Razman, M.R. Antioxidant properties of fresh and frozen peels of citrus species. Curr. Res. Nutr. Food Sci. 2019, 7, 331–339. [Google Scholar] [CrossRef]
- Chernukha, I.; Kupaeva, N.; Kotenkova, E.; Khvostov, D. Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties. Antioxidants 2022, 11, 1243. [Google Scholar] [CrossRef]
- Ashraf, H.; Iahtisham-Ul-Haq; Butt, M.S.; Nayik, G.A.; Ramniwas, S.; Damto, T.; Ali Alharbi, S.; Ansari, M.J. Phytochemical and antioxidant profile of citrus peel extracts in relation to different extraction parameters. Int. J. Food Prop. 2024, 27, 286–299. [Google Scholar] [CrossRef]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of phenolic compounds with antioxidant activity from olive pomace using natural deep eutectic solvents: Modelling and optimization by response surface methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Brahmi, F.; Mateos-Aparicio, I.; Garcia-Alonso, A.; Abaci, N.; Saoudi, S.; Smail-Benazzouz, L.; Guemghar-Haddadi, H.; Madani, K.; Boulekbache-Makhlouf, L. Optimization of Conventional Extraction Parameters for Recovering Phenolic Compounds from Potato (Solanum tuberosum L.) Peels and Their Application as an Antioxidant in Yogurt Formulation. Antioxidants 2022, 11, 1401. [Google Scholar] [CrossRef]
- Aquino, G.; Basilicata, M.G.; Crescenzi, C.; Vestuto, V.; Salviati, E.; Cerrato, M.; Ciaglia, T.; Sansone, F.; Pepe, G.; Campiglia, P. Optimization of microwave-assisted extraction of antioxidant compounds from spring onion leaves using Box–Behnken design. Sci. Rep. 2023, 13, 14923. [Google Scholar] [CrossRef] [PubMed]
- Godoy, R.L.d.O.; Cabral, L.M.C.; Gomes, F.D.S.; Silva, L.O.M.; Beres, C.; Pagani, M.M.; Brígida, A.I.S.; Santiago, M.C.P.d.A.; Pacheco, S. Processing tomato waste as a potential bioactive compounds source: Phenolic compounds, antioxidant capacity and bioacessibility studies. Ciência Rural 2021, 52, e20201070. [Google Scholar]
- Uğurlu, Ş.; Günan Yücel, H.; Aksu, Z. Valorization of food wastes with a sequential two-step process for microbial β-carotene production: A zero waste approach. J. Environ. Manag. 2023, 340, 118003. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef] [PubMed]
- Jayesree, N.; Hang, P.K.; Priyangaa, A.; Krishnamurthy, N.P.; Ramanan, R.N.; Turki, M.S.A.; Charis, M.G.; Ooi, C.W. Valorisation of carrot peel waste by water-induced hydrocolloidal complexation for extraction of carotene and pectin. Chemosphere 2021, 272, 129919. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Athanasiadis, V.; Kalompatsios, D.; Lalas, S.I. Optimization of Carotenoids and Other Antioxidant Compounds Extraction from Carrot Peels Using Response Surface Methodology. Biomass 2024, 5, 3. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. [Google Scholar] [CrossRef]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.G.; Ravishankar, K.V. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Meral, H.; Demirdöven, A. Recovery of carotenoids as bioactive compounds from peach pomace by an eco-friendly ultrasound-assisted enzymatic extraction. J. Food Sci. Technol. 2024, 61, 2354–2366. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Sportiello, L.; Marchesi, E.; Tolve, R.; Favati, F. Green Extraction of Carotenoids from Pumpkin By-Products Using Natural Hydrophobic Deep Eutectic Solvents: Preliminary Insights. Molecules 2025, 30, 548. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Ospino, A.; Rita Jesus, A.; Paiva, A.; Esteve, M.J.; Frígola, A.; Blesa, J.; López-Malo, D. Comparison of green solvents for the revalorization of orange by-products: Carotenoid extraction and in vitro antioxidant activity. Food Chem. 2024, 442, 138530. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Improving carotenoid extraction, stability, and antioxidant activity from Citrus sinensis peels using green solvents. Eur. Food Res. Technol. 2023, 249, 2349–2361. [Google Scholar] [CrossRef]
- Gavril, R.N.; Stoica, F.; Lipșa, F.D.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Pumpkin and Pumpkin By-Products: A Comprehensive Overview of Phytochemicals, Extraction, Health Benefits, and Food Applications. Foods 2024, 13, 2694. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Khorasaniha, R.; Olof, H.; Voisin, A.; Armstrong, K.; Wine, E.; Vasanthan, T.; Armstrong, H. Diversity of fibers in common foods: Key to advancing dietary research. Food Hydrocoll. 2023, 139, 108495. [Google Scholar] [CrossRef]
- Suresh, A.; Shobna; Salaria, M.; Morya, S.; Khalid, W.; Afzal, F.A.; Khan, A.A.; Safdar, S.; Khalid, M.Z.; Mukonzo Kasongo, E.L. Dietary fiber: An unmatched food component for sustainable health. Food Agric. Immunol. 2024, 35, 2384420. [Google Scholar] [CrossRef]
- Buljeta, I.; Šubarić, D.; Babić, J.; Pichler, A.; Šimunović, J.; Kopjar, M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Appl. Sci. 2023, 13, 9309. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Wagner, C.E.; Richter, J.K.; Ikuse, M.; Ganjyal, G.M. Classification of select functional dietary fiber ingredients based on quantitative properties and latent qualitative criteria. J. Food Sci. 2024, 89, 6098–6112. [Google Scholar] [CrossRef]
- Fatima, I.; Gamage, I.; De Almeida, R.J.R.; Cabandugama, P.; Kamath, G. Current Understanding of Dietary Fiber and Its Role in Chronic Diseases. Mo. Med. 2023, 120, 381. [Google Scholar]
- Deng, M.; Dan, L.; Ye, S.; Chen, X.; Wang, X.; Tian, L.; Chen, J. Higher Fiber Intake is Associated with Reduced Risk of Related Surgery among Individuals with Inflammatory Bowel Disease in a Prospective Cohort Study. J. Nutr. 2023, 153, 2274–2282. [Google Scholar] [CrossRef]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef]
- Xie, Y.; Gou, L.; Peng, M.; Zheng, J.; Chen, L. Effects of soluble fiber supplementation on glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 1800–1810. [Google Scholar] [CrossRef]
- Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Calhoun, J.; Hanus, A.; Payne-Foster, P.; Stout, R.; Sherman, B.W. Increased dietary fiber is associated with weight loss among Full Plate Living program participants. Front. Nutr. 2023, 10, 1110748. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, L.A. Dietary fiber influence on overall health, with an emphasis on CVD, diabetes, obesity, colon cancer, and inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, Y.; Yang, N.; Xu, Y.; Wang, G. Longitudinal associations of dietary fiber and its source with 48-week weight loss maintenance, cardiometabolic risk factors and glycemic status under metformin or acarbose treatment: A secondary analysis of the March randomized trial. Nutr. Diabetes 2024, 14, 81. [Google Scholar] [CrossRef]
- Brennan, A.; Browne, S.; Kristo, S.; Sikalidis, A.K. Food Waste and Nutrition Quality in the Context of Public Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 5379. [Google Scholar] [CrossRef]
- Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients 2023, 15, 3594. [Google Scholar] [CrossRef]
- Hodge, C.; Taylor, C. Vitamin A Deficiency. Clin. Exp. Optom. 2023, 77, 76–77. [Google Scholar]
- Spiker, M.L.; Hiza, H.A.B.; Siddiqi, S.M.; Neff, R.A. Wasted Food, Wasted Nutrients: Nutrient Loss from Wasted Food in the United States and Comparison to Gaps in Dietary Intake. J. Acad. Nutr. Diet 2017, 117, 1031–1040.e22. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Cannas, C.; Lostia, G.; Serra, P.A.; Peana, A.T.; Migheli, R. Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease? Antioxidants 2024, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Mattas, K.; Gurinovic, M.; Gkotzamani, A.; Koukounaras, A. Revealing the power of green leafy vegetables: Cultivating diversity for health, environmental benefits, and sustainability. Glob. Food Secur. 2024, 43, 100816. [Google Scholar] [CrossRef]
- Siitonen, A.; Nieminen, F.; Kallio, V.; Tuccillo, F.; Kantanen, K.; Ramos-Diaz, J.M.; Jouppila, K.; Piironen, V.; Kariluoto, S.; Edelmann, M. B Vitamins in Legume Ingredients and Their Retention in High Moisture Extrusion. Foods 2024, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; He, H.; Wang, Y.; Wang, J.; Liu, H. Toward Achieving Rapid Estimation of Vitamin C in Citrus Peels by NIR Spectra Coupled with a Linear Algorithm. Molecules 2023, 28, 1681. [Google Scholar] [CrossRef]
- Benny, N.; Shams, R.; Dash, K.K.; Pandey, V.K.; Bashir, O. Recent trends in utilization of citrus fruits in production of eco-enzyme. J. Agric. Food Res. 2023, 13, 100657. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M. Synthetic or Food-Derived Vitamin C—Are They Equally Bioavailable? Nutrients 2013, 5, 4284. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Int. 2022, 3, 100158. [Google Scholar] [CrossRef]
- Chakrabartty, I.; Mohanta, Y.K.; Nongbet, A.; Mohanta, T.K.; Mahanta, S.; Das, N.; Saravanan, M.; Sharma, N. Exploration of Lamiaceae in Cardio Vascular Diseases and Functional Foods: Medicine as Food and Food as Medicine. Front. Pharmacol. 2022, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.-M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Pereira, C.; Barros, L.; Allegra, M.; Bai, L.; et al. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.L.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Chen, S.; Teng, B.; Veeraperumal, S.; Zhong, S.; Tan, K. Oligosaccharides as Potential Regulators of Gut Microbiota and Intestinal Health in Post-COVID-19 Management. Pharmaceuticals 2023, 16, 860. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Patrick Manzi, H.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: A review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef] [PubMed]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Grover, S.; Aggarwal, P.; Kumar, A.; Kaur, S.; Yadav, R.; Babbar, N. Utilizing citrus peel waste: A review of essential oil extraction, characterization, and food-industry potential. Biomass Convers. Biorefin. 2024, 15, 5043–5064. [Google Scholar] [CrossRef]
- Rajput, S.; Kaur, S.; Panesar, P.S.; Thakur, A. Supercritical fluid extraction of essential oils from Citrus reticulata peels: Optimization and characterization studies. Biomass Convers. Biorefin. 2023, 13, 14605–14614. [Google Scholar] [CrossRef]
- Tunç, M.T.; Odabaş, H.İ. Single-step recovery of pectin and essential oil from lemon waste by ohmic heating assisted extraction/hydrodistillation: A multi-response optimization study. Innov. Food Sci. Emerg. Technol. 2021, 74, 102850. [Google Scholar] [CrossRef]
- Sandhu, H.K.; Sinha, P.; Emanuel, N.; Kumar, N.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Effect of Ultrasound-Assisted Pretreatment on Extraction Efficiency of Essential Oil and Bioactive Compounds from Citrus Waste By-Products. Separations 2021, 8, 244. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A step closer to circular bioeconomy for citrus peel waste: A review of yields and technologies for sustainable management of essential oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Pahmeyer, M.J.; Assadpour, E.; Jafari, S.M. Extraction and purification of d-limonene from orange peel wastes: Recent advances. Ind. Crops Prod. 2022, 177, 114484. [Google Scholar] [CrossRef]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef]
- Santonocito, D.; Delli Carri, M.; Campisi, A.; Sposito, G.; Pellitteri, R.; Raciti, G.; Cardullo, N.; Aquino, G.; Basilicata, M.G.; Pepe, G.; et al. Steroidal Alkaloids from Food Waste of Tomato Processing Inhibit Neuroblastoma Cell Viability. Int. J. Mol. Sci. 2023, 24, 16915. [Google Scholar] [CrossRef]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef]
- Gade, A.; Pinapati, K.K.; Verma, V.; Akula, S.J.; Sharma, A.; Pullapanthula, R.; Srivastava, N.; Gade, A.; Pinapati, K.K.; Verma, V.; et al. Evaluation of Antibiofilm Activity of Alkaloids Extracted from Capsicum annuum Stalk: A Preliminary Study of Phytochemical Screening in Vegetable Waste. WBioV 2024, 15, 233–250. [Google Scholar] [CrossRef]
- 1Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Saeed, F.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Food Processing Waste: A Potential Source for Bioactive Compounds. In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Cham, Switzerland, 2020; pp. 625–649. [Google Scholar]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium cepa L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Food waste and by-product valorization as an integrated approach with zero waste: Future challenges. In Future Foods Global Trends, Opportunities, and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2022; pp. 569–596. [Google Scholar]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An Overview on the Natural and Synthetic Types. Eur. Chem. Bull. 2017, 6, 365. [Google Scholar] [CrossRef]
- Uzombah, T.A.; Uzombah, T.A. The Implications of Replacing Synthetic Antioxidants with Natural Ones in the Food Systems. In Natural Food Additives; IntechOpen: London, UK, 2022. [Google Scholar]
- Sadiq, M.; Adil, M.; Paul, J. Organic food consumption and contextual factors: An attitude–behavior–context perspective. Bus. Strateg. Environ. 2023, 32, 3383–3397. [Google Scholar] [CrossRef]
- Caponio, F.; Piga, A.; Poiana, M. Valorization of Food Processing By-Products. Foods 2022, 11, 3246. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-food wastes utilization by Blakeslea trispora for carotenoids production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef]
- Carpenter, M.; Savage, A.M. Nutrient availability in urban food waste: Carbohydrate bias in the Philadelphia–Camden urban matrix. J. Urban Ecol. 2021, 7, juab012. [Google Scholar] [CrossRef]
- Moonsamy, T.A.; Rajauria, G.; Priyadarshini, A.; Jansen, M.A.K. Food waste: Analysis of the complex and variable composition of a promising feedstock for valorisation. Food Bioprod. Process. 2024, 148, 31–42. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- Mohammed, M.; Ozbay, I.; Karademir, A.; Isleyen, M. Pre-treatment and utilization of food waste as energy source by bio-drying process. Energy Procedia 2017, 128, 100–107. [Google Scholar] [CrossRef]
- Sumaiyah; Leisyah, B.M. The effect of antioxidant of grapeseed oil as skin anti-aging in nanoemulsion and emulsion preparations. Rasayan J. Chem. 2019, 12, 1185–1194. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-De la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xu, B.C.; Lei, H.M.; Luo, X.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Liang, R. Effects of grape seed extract on meat color and premature browning of meat patties in high-oxygen packaging. J. Integr. Agric. 2022, 21, 2445–2455. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Costa, M.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Use of Grape By-Products to Enhance Meat Quality and Nutritional Value in Monogastrics. Foods 2022, 11, 2754. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843. [Google Scholar] [CrossRef]
- Katsimbri, P.; Korakas, E.; Kountouri, A.; Ikonomidis, I.; Tsougos, E.; Vlachos, D.; Papadavid, E.; Raptis, A.; Lambadiari, V. The Effect of Antioxidant and Anti-Inflammatory Capacity of Diet on Psoriasis and Psoriatic Arthritis Phenotype: Nutrition as Therapeutic Tool? Antioxidants 2021, 10, 157. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 11786388211058560. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ. Technol. Innov. 2018, 10, 62–70. [Google Scholar] [CrossRef]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and waste water can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.B.S.; Heinonen, M.; Frankel, E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998, 61, 71–75. [Google Scholar] [CrossRef]
- Jain, P.; Bhuiyan, M.H.; Hossain, K.R.; Bachar, S.C. Antibacterial and antioxidant activities of local seeded banana fruits. African J. Pharm. Pharmacol. 2011, 5, 1398–1403. [Google Scholar] [CrossRef]
- Ramli, S.; Alkarkhi, A.F.M.; Shin Yong, Y.; Min-Tze, L.; Easa, A.M. Effect of banana pulp and peel flour on physicochemical properties and in vitro starch digestibility of yellow alkaline noodles. Int. J. Food Sci. Nutr. 2009, 60, 326–340. [Google Scholar] [CrossRef]
- Bhavani, M.; Sonia, M.; Deepika, S.; Awuchi, C.G. Bioactive, antioxidant, industrial, and nutraceutical applications of banana peel. Int. J. Food Prop. 2023, 26, 1277–1289. [Google Scholar] [CrossRef]
- Haslinda, W.H.; Cheng, L.H.; Chong, L.C.; Aziah, A.A.N. Chemical composition and physicochemical properties of green banana (Musa acuminata × balbisiana Colla cv. Awak) flour. Int. J. Food Sci. Nutr. 2009, 60, 232–239. [Google Scholar] [CrossRef]
- Hussein, M.; Yonis, A.; Abd El—Mageed, H. Effect of Adding Carrot Powder on the Rheological and Sensory Properties of Pan Bread. J. Food Dairy Sci. 2013, 4, 281–289. [Google Scholar] [CrossRef]
- Tiţa, O.; Constantinescu, M.A.; Tiţa, M.A.; Bătuşaru, C.; Mironescu, I. Sensory, textural, physico-chemical and enzymatic characterization of melted cheese with added potato and carrot peels. Front. Nutr. 2023, 10, 1260076. [Google Scholar] [CrossRef]
- Kaur, P.; Subramanian, J.; Singh, A. Green extraction of bioactive components from carrot industry waste and evaluation of spent residue as an energy source. Sci. Rep. 2022, 12, 16607. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT Food Sci. Technol. 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dalei, G.; Jena, M.; Jena, D.; Pattanaik, C.; Das, B.R.; Das, S. Fabrication of Cucumber Peel Extract-Imbued Dragon Fruit Peel Pectin Hydrogen Packaging Films: Assessment in Preservation of Chicken Meat. Poly. Eng. Sci. 2024, 64, 1391–1403. [Google Scholar] [CrossRef]
- Esparvarini, Z.; Bazargani-Gilani, B.; Pajohi-Alamoti, M.; Nourian, A. Gelatin-starch composite coating containing cucumber peel extract and cumin essential oil: Shelf life improvement of a cheese model. Food Sci. Nutr. 2022, 10, 964–978. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, S.; Lin, J.; Yang, J.; Zhang, Y.; Shen, Q.; Zhong, F.; Hou, D.; Zhou, S. Valorization of barley (Hordeum vulgare L.) brans from the sustainable perspective: A comprehensive review of bioactive compounds and health benefits with emphasis on their potential applications. Food Chem. 2024, 460, 140772. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Ramírez-Maganda, J.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; García-Magaña, M.d.L.; Bello-Pérez, L.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Nutritional properties and phenolic content of a bakery product substituted with a mango (Mangifera indica) “Ataulfo” processing by-product. Food Res. Int. 2015, 73, 117–123. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar]
- Elsawy, H.A.; Alessa, F.M.; El-Kholany, E.A. Production and Evaluation of Peanut Butter Prepared with Peanut Shells. Curr. Nutr. Food Sci. 2023, 20, 1019–1027. [Google Scholar] [CrossRef]
- Bertagnolli, S.M.M.; Silveira, M.L.R.; Fogaça, A.d.O.; Umann, L.; Penna, N.G. Bioactive compounds and acceptance of cookies made with Guava peel flour. Food Sci. Technol. 2014, 34, 303–308. [Google Scholar] [CrossRef]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of bioactive components from avocado peels using microwave-assisted extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.d.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M.P.L.V.O. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of Apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Lazzaroli, C.; Sordini, B.; Daidone, L.; Veneziani, G.; Esposto, S.; Urbani, S.; Selvaggini, R.; Servili, M.; Taticchi, A. Recovery and valorization of food industry by-products through the application of Olea europaea L. leaves in kombucha tea manufacturing. Food Biosci. 2023, 53, 102551. [Google Scholar] [CrossRef]
- Radočaj, O.; Dimić, E.; Diosady, L.L.; Vujasinović, V. Optimizing the texture attributes of a fat-based spread using instrumental measurements. J. Texture Stud. 2011, 42, 394–403. [Google Scholar] [CrossRef]
- Bansal, S.; Sudha, M.L. Nutritional, microstructural, rheological and quality characteristics of biscuits using processed wheat germ. Int. J. Food Sci. Nutr. 2011, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Ghiasi, F.; Eskandari, M.H.; Farahnaky, A. Roasted Wheat Germ: A Natural Plant Product in Development of Nutritious Milk Pudding; Physicochemical and Nutritional Properties. Foods 2022, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products--a promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; López-Bascón, M.A.; Sánchez-González, C.; Xiao, J.; Sumalla-Cano, S.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L. In Vitro and In Vivo Insights into a Broccoli Byproduct as a Healthy Ingredient for the Management of Alzheimer’s Disease and Aging through Redox Biology. J. Agric. Food Chem. 2024, 72, 5197–5211. [Google Scholar] [CrossRef]
- Liu, X.Q.; Yonekura, M.; Tsutsumi, M.; Sano, Y. Physicochemical Properties of Aggregates of Globin Hydrolysates. J. Agric. Food Chem. 1996, 44, 2957–2961. [Google Scholar] [CrossRef]
- Devadason, I.P.; Anjaneyulu, A.S.R.; Babji, Y. Effect of Different Binders on the Physico-Chemical, Textural, Histological, and Sensory Qualities of Retort Pouched Buffalo Meat Nuggets. J. Food Sci. 2010, 75, S31–S35. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Abrigo, C.; Cipolla, G. High-performance liquid chromatography of food colours and its relevance in forensic chemistry. J. Chromatogr. A 1994, 674, 281–299. [Google Scholar] [CrossRef]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Olt, V.; Báez, J.; Curbelo, R.; Boido, E.; Amarillo, M.; Gámbaro, A.; Alborés, S.; Gerez García, N.; Cesio, M.V.; Heinzen, H.; et al. Tannat grape pomace as an ingredient for potential functional biscuits: Bioactive compound identification, in vitro bioactivity, food safety, and sensory evaluation. Front. Nutr. 2023, 10, 1241105. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, P.; Hoffmann, M. Valorization of bio-waste eggshell as a viable source of dietary calcium for confectionery products. J. Sci. Food Agric. 2022, 102, 3193–3203. [Google Scholar] [CrossRef]
- Arnold, M.; Rajagukguk, Y.V.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Suliburska, J.; Wawrzyniak, N.; Gramza-Michałowska, A. Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread. Int. J. Environ. Res. Public Health 2022, 19, 4195. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Diep, T.; Hudson, H.A.; Hincke, M.T. High value applications and current commercial market for eggshell membranes and derived bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of antioxidant-rich edible active films and coatings incorporated with de-oiled ethanolic green algae extract: A candidate for prolonging the shelf life of fresh produce. RSC Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef]
- Besediuk, V.; Yatskov, M.; Korchyk, N.; Kucherova, A.; Maletskyi, Z. Whey—From waste to a valuable resource. J. Agric. Food Res. 2024, 18, 101280. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
- Mabeau, S.; Baty-Julien, C.; Hélias, A.B.; Chodosas, O.; Surbled, M.; Metra, P.; Durand, D.; Morice, G.; Chesné, C.; Mekideche, K. Antioxidant activity of artichoke extracts and by-products. Acta Hortic. 2007, 730, 491–496. [Google Scholar] [CrossRef]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of Antioxidant Activity of Tomato Juice Upon Functionalisation with Vegetable Byproduct Extracts. LWT—Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Górecka, D.; Pachołek, B.; Dziedzic, K.; Górecka, M. Raspberry pomace as a potential fiber source for cookies enrichment. Acta Sci. Pol. Technol. Aliment. 2010, 9, 451–461. [Google Scholar]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2015, 53, 1140. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Čechovičienė, I.; Jukniūtė, K.; Šlepetienė, A.; Paulauskienė, A. Qualitative properties of cookies enriched with berries pomace. Food Sci. Technol. 2021, 41, 474–481. [Google Scholar] [CrossRef]
- Laccourreye, O.; Maisonneuve, H. French scientific medical journals confronted by developments in medical writing and the transformation of the medical press. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 475–480. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoǧlu, E.; Ibanoǧlu, Ş. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J. Food Eng. 2008, 87, 554–563. [Google Scholar] [CrossRef]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q.; et al. Potential of Fruit Wastes as Natural Resources of Bioactive Compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Gałkowska, D.; Długosz, M.; Juszczak, L.; Takamine, K.; Abe, J.; Shimono, K.; Sameshima, Y.; Morimura, S.; Kida, K.; Sriamornsak, P. Chemistry of Pectin and Its Pharmaceutical Uses: A Review. Silpakorn Univ. Open J. Syst. 2003, 3, 206–228. [Google Scholar]
- Kang, H.J.; Chawla, S.P.; Jo, C.; Kwon, J.H.; Byun, M.W. Studies on the development of functional powder from citrus peel. Bioresour. Technol. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus peel as a renewable bioresource: Transforming waste to food additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, W.S.; Oh, S.W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T.; Cappadone, C.; Malucelli, E. Nutrients and Nutraceuticals from Vitis vinifera L. Pomace: Biological Activities, Valorization, and Potential Applications. Nutrients 2025, 17, 583. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate Juice, Total Pomegranate Ellagitannins, and Punicalagin Suppress Inflammatory Cell Signaling in Colon Cancer Cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ayodele, O.M. Effect of Adding Pomegranate (Punica granatum) Juice and Seed Extract on the Physicochemical, Rheological and Sensory Properties of Ice Cream. J. Adv. Food Sci. Technol. 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2013, 4, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Lu, Z.; Yang, C. Enhanced Morris method for global sensitivity analysis: Good proxy of Sobol’ index. Struct. Multidiscip. Optim. 2019, 59, 373–387. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Jridi, M.; Khemakhem, I.; Bouaziz, M.; Attia, H. Storage stability of traditional Tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem. 2017, 233, 476–482. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Joshi, A.; Sethi, S.; Arora, B.; Azizi, A.F.; Thippeswamy, B. Potato Peel Composition and Utilization. In Potato Nutrition and Food Security; Springer: Cham, Switzerland, 2020; pp. 229–245. [Google Scholar]
- Arora, A.; Camire, M.E. Performance of potato peels in muffins and cookies. Food Res. Int. 1994, 27, 15–22. [Google Scholar] [CrossRef]
- Franco, D.; Pateiro, M.; Rodríguez Amado, I.; López Pedrouso, M.; Zapata, C.; Vázquez, J.A.; Lorenzo, J.M. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 1891–1902. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.-H.; Al-sayed, H.; Yasin, N.; Afifi, E. Effect of Different Extraction Methods on Stablity of Anthocyanins Extracted from Red Onion peels (Allium cepa) and Its Uses as Food Colorants. Bull. Natl. Nutr. Inst. Arab Repub. Egypt 2016, 47, 1–24. [Google Scholar] [CrossRef][Green Version]
- Vichare, S.A.; Morya, S. Exploring waste utilization potential: Nutritional, functional and medicinal properties of oilseed cakes. Front. Food Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Ahmad, T.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Abdullah; Xu, Y.; Zisheng, L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020, 99, 21–33. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Ermosh, L.G.; Prisuhina, N.V.; Koch, D.A.; Eremina, E.V. The use of oilseed cake for supplementation of bakery products. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 022090. [Google Scholar] [CrossRef]
- Abedini, A.; Alizadeh, A.M.; Mahdavi, A.; Golzan, S.A.; Salimi, M.; Tajdar-Oranj, B.; Hosseini, H. Oilseed Cakes in the Food Industry; A Review on Applications, Challenges, and Future Perspectives. Curr. Nutr. Food Sci. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- Tsakali, E.; Petrotos, K.; D’Alessandr, A.G.; Goulas, P. A review on whey composition and the methods used for its utilization for food and pharmaceutical products. In Proceedings of the 6th International Conference on Simulation and Modelling in the Food and Bio-Industry, Braganca, Portugal, 24–26 June 2010; pp. 195–201. [Google Scholar]
- Soltani, M.; Say, D.; Guzeler, N. Functional Properties and Nutritional Quality of Whey Proteins. J. Int. Environ. Appl. Sci. 2017, 12, 334–338. [Google Scholar]
- Arshad, U.E.T.; Hassan, A.; Ahmad, T.; Naeem, M.; Chaudhary, M.T.; Abbas, S.Q.; Randhawa, M.A.; Pimentel, T.C.; da Cruz, A.G.; Aadil, R.M. A recent glance on the valorisation of cheese whey for industrial prerogative: High-value-added products development and integrated reutilising strategies. Int. J. Food Sci. Technol. 2023, 58, 2001–2013. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, İ.A. Use of coffee silverskin to improve the functional properties of cookies. J. Food Sci. Technol. 2019, 56, 2979–2988. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; Del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; Castillo, M.D. Del Use of coffee silverskin and stevia to improve the formulation of biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee Silverskin Extract Protects against Accelerated Aging Caused by Oxidative Agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef]
- Karmakar, R.; Aggarwal, S.; Kathuria, D.; Singh, N.; Tripathi, V.; Sharma, P.K.; Mitra, S.; Bhattacharya, S. Valorization of food waste stream by harnessing bioactive compounds: A comprehensive review on the process, challenges and solutions. Food Biosci. 2025, 69, 106833. [Google Scholar] [CrossRef]
- United Nations. The 17 Goals: Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 28 May 2025).
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Xu, Y.; He, P.; He, B.; Chen, Z. Bioactive flavonoids metabolites in citrus species: Their potential health benefits and medical potentials. Front Pharmacol. 2025, 16, 1552171. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Sallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Bolaño, D.C.; Insuasty, D.; Rodríguez Macías, J.D.; Grande-Tovar, C.D. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules 2024, 29, 3079. [Google Scholar] [CrossRef]
- Hlatshwayo, S.; Thembane, N.; Krishna, S.B.N.; Gqaleni, N.; Ngcobo, M. Extraction and Processing of Bioactive Phytoconstituents from Widely Used South African Medicinal Plants for the Preparation of Effective Traditional Herbal Medicine Products: A Narrative Review. Plants 2025, 14, 206. [Google Scholar] [CrossRef]
- Mititelu, M.; Neacșu, S.M.; Busnatu, Ș.S.; Scafa-Udriște, A.; Andronic, O.; Lăcraru, A.-E.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Negrei, C.; Olteanu, G. Assessing Heavy Metal Contamination in Food: Implications for Human Health and Environmental Safety. Toxics 2025, 13, 333. [Google Scholar] [CrossRef]
- Almansour, M.; Akrami, M. Towards Zero Waste: An In-Depth Analysis of National Policies, Strategies, and Case Studies in Waste Minimisation. Sustainability 2024, 16, 10105. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghian, H.; Bolton, K.; Rousta, K. Challenges for Upcycled Foods: Definition, Inclusion in the Food Waste Management Hierarchy and Public Acceptability. Foods 2021, 10, 2874. [Google Scholar] [CrossRef]
- Despoudi, S.; Bucatariu, C.; Otles, S.; Kartal, C. Food waste management, valorization, and sustainability in the food industry. In Food Waste Recovery, Processing Technologies, Industrial Techniques, and Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 3–19. [Google Scholar]
- Pleissner, D. Recycling and reuse of food waste. Curr. Opin. Green Sustain. Chem. 2018, 13, 39–43. [Google Scholar] [CrossRef]
- Garske, B.; Heyl, K.; Ekardt, F.; Weber, L.M.; Gradzka, W. Challenges of Food Waste Governance: An Assessment of European Legislation on Food Waste and Recommendations for Improvement by Economic Instruments. Land 2020, 9, 231. [Google Scholar] [CrossRef]
- Faishal, A. Suprapto Laws and Regulations Regarding Food Waste Management as a Function of Environmental Protection in a Developing Nation. Int. J. Crim. Justice Sci. 2022, 17, 223–237. [Google Scholar]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Albizzati, P.F.; Tonini, D.; Astrup, T.F. A Quantitative Sustainability Assessment of Food Waste Management in the European Union. Environ. Sci. Technol. 2021, 55, 16099–16109. [Google Scholar] [CrossRef]
- Bhat, R. Sustainability challenges in the valorization of agri-food wastes and by-products. In Valorization of Agri-Food Wastes and By-Products, Recent Trends, Innovations and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2021; pp. 1–27. [Google Scholar]
- Pinela, J.; Añibarro-Ortega, M.; Barros, L. Food Waste Biotransformation into Food Ingredients: A Brief Overview of Challenges and Opportunities. Foods 2024, 13, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Stojakowska, A. Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference? Foods 2020, 10, 59. [Google Scholar] [CrossRef]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Targeted metabolic profiling indicates differences in primary and secondary metabolites in Kinnow mandarin (C. nobilis × C. deliciosa) from different climatic conditions. J. Food Compos. Anal. 2019, 83, 103278. [Google Scholar] [CrossRef]
- Serrano-García, I.; Hurtado-Fernández, E.; Gonzalez-Fernandez, J.J.; Hormaza, J.I.; Pedreschi, R.; Reboredo-Rodríguez, P.; Figueiredo-González, M.; Olmo-García, L.; Carrasco-Pancorbo, A. Prolonged on-tree maturation vs. cold storage of Hass avocado fruit: Changes in metabolites of bioactive interest at edible ripeness. Food Chem. 2022, 394, 133447. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Sasse, A.; Colindres, P.; Brewer, M.S. Effect of Natural and Synthetic Antioxidants on the Oxidative Stability of Cooked, Frozen Pork Patties. J. Food Sci. 2009, 74, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Hamdo, H.H.; Khayata, W.; Al-Assaf, Z. Synergistic effect of combined some natural and synthetic antioxidants to increase oxidative stability using DPPH test. Int. J. ChemTech Res. 2014, 6, 2539–2545. [Google Scholar]
- O’Connor, J.; Mickan, B.S.; Siddique, K.H.M.; Rinklebe, J.; Kirkham, M.B.; Bolan, N.S. Physical, chemical, and microbial contaminants in food waste management for soil application: A review. Environ. Pollut. 2022, 300, 118860. [Google Scholar] [CrossRef]
- Nicastro, R.; Papale, M.; Fusco, G.M.; Capone, A.; Morrone, B.; Carillo, P. Legal Barriers in Sustainable Agriculture: Valorization of Agri-Food Waste and Pesticide Use Reduction. Sustainability 2024, 16, 8677. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Su, Y.; Xie, B. Occurrence, influence and removal strategies of mycotoxins, antibiotics and microplastics in anaerobic digestion treating food waste and co-digestive biosolids: A critical review. Bioresour. Technol. 2021, 330, 124987. [Google Scholar] [CrossRef]
- Ma, J.; Wu, S.; Shekhar, N.V.R.; Biswas, S.; Sahu, A.K. Determination of Physicochemical Parameters and Levels of Heavy Metals in Food Waste Water with Environmental Effects. Bioinorg. Chem. Appl. 2020, 2020, 8886093. [Google Scholar] [CrossRef]
- Viscardi, S.; Colicchia, C. A classification of food products to enhance circular economy and reduce waste: A systematic literature review. Resour. Conserv. Recycl. Adv. 2024, 23, 200229. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022, 424, 127432. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Conidi, C.; Cassano, A. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit. Rev. Food Sci. Nutr. 2019, 59, 2927–2948. [Google Scholar] [CrossRef]
- Redlingshöfer, B.; Barles, S.; Weisz, H. Are waste hierarchies effective in reducing environmental impacts from food waste? A systematic review for OECD countries. Resour. Conserv. Recycl. 2020, 156, 104723. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Dutta, S.; He, M.; Xiong, X.; Tsang, D.C.W. Sustainable management and recycling of food waste anaerobic digestate: A review. Bioresour. Technol. 2021, 341, 125915. [Google Scholar] [CrossRef]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Flora, C.B. Food security in the context of energy and resource depletion: Sustainable agriculture in developing countries. Renew. Agric. Food Syst. 2010, 25, 118–128. [Google Scholar] [CrossRef]
- Wunderlich, S.M.; Martinez, N.M. Conserving natural resources through food loss reduction: Production and consumption stages of the food supply chain. Int. Soil Water Conserv. Res. 2018, 6, 331–339. [Google Scholar] [CrossRef]
- Tomlinson, I. Doubling food production to feed the 9 billion: A critical perspective on a key discourse of food security in the UK. J. Rural Stud. 2013, 29, 81–90. [Google Scholar] [CrossRef]
- Gusmerotti, N.M.; Corsini, F.; Borghini, A.; Frey, M. Assessing the role of preparation for reuse in waste-prevention strategies by analytical hierarchical process: Suggestions for an optimal implementation in waste management supply chain. Environ. Dev. Sustain. 2019, 21, 2773–2792. [Google Scholar] [CrossRef]
- Awino, F.B.; Apitz, S.E. Solid waste management in the context of the waste hierarchy and circular economy frameworks: An international critical review. Integr. Environ. Assess. Manag. 2024, 20, 9–35. [Google Scholar] [CrossRef] [PubMed]
- Peano, C.; Merlino, V.M.; Sottile, F.; Borra, D.; Massaglia, S. Sustainability for Food Consumers: Which Perception? Sustainability 2019, 11, 5955. [Google Scholar] [CrossRef]
- Friedmann, H. Scaling up: Bringing public institutions and food service corporations into the project for a local, sustainable food system in Ontario. Agric. Hum. Values 2007, 24, 389–398. [Google Scholar] [CrossRef]
- Boccia, F.; Covino, D. Innovation and sustainability in agri-food companies: The role of quality. Riv. Studi Sulla Sostenibilita 2016, 2016, 131–141. [Google Scholar] [CrossRef]


| Extraction Method | Principle | Solvent Use | Time and Energy Efficiency | Selectivity/Yield | Preservation of Bioactivity | Environmental Impact | Key Advantages | Key Limitations |
|---|---|---|---|---|---|---|---|---|
| Conventional Solvent Extraction (CSE) | Organic solvents extract compounds via diffusion | High (organic solvents, e.g., ethanol, methanol) | Low; long time, high energy | Moderate; depends on polarity and solvent used | May degrade heat-sensitive compounds | High; solvent toxicity, waste issues | Low cost, simple, well-established | Low efficiency; high solvent consumption; not eco-friendly |
| Ultrasound-Assisted Extraction (UAE) | Cavitation bubbles enhance cell disruption and mass transfer | Moderate (often ethanol-water mixtures) | High; short extraction time | High; enhances extraction of phenolics and flavonoids | Good if temperature is controlled | Lower than CSE; greener solvents | Cost-effective, scalable, gentle on bioactives | Equipment scale-up challenges; needs parameter optimization |
| Microwave-Assisted Extraction (MAE) | Dielectric heating causes rapid internal heating and cell rupture | Moderate to low | Very high; rapid heating | High for polar compounds | Good under controlled exposure | Low, especially with water or green solvents | Fast, solvent-saving, suitable for thermolabile compounds | Risk of degradation at high temps; special equipment required |
| Subcritical Water Extraction (SWE) | Water at 100–374 °C under pressure acts as polar–nonpolar solvent | Water only | Moderate to high; pressure/temperature dependent | High; extracts wide range of bioactives | High for heat-stable compounds | Very low; no solvent residues | Green process, non-toxic, broad solubility range | High-pressure setup; limited to heat-stable compounds |
| Supercritical CO2 Extraction (SC-CO2) | CO2 above critical point acts as a solvent (gas-like diffusivity, liquid-like solvency) | CO2 (non-toxic, recyclable); may use co-solvents (e.g., ethanol) | High; rapid mass transfer | High for non-polar bioactives (e.g., lipids, carotenoids) | Excellent for thermolabile compounds | Very low; no toxic residues | Non-toxic, solvent-free products, selective extraction | High equipment cost; limited for polar compounds unless co-solvents used |
| Pulsed Electric Fields (PEF) | Short pulses of high-voltage electric fields permeabilize cell membranes | Aqueous medium | Very high as pre-treatment | Enhances extraction when combined with other methods | Excellent for sensitive bioactives | Low; no chemicals used | Non-thermal, preserves nutrients, reduces solvent use | Not a stand-alone extraction; needs coupling with other methods |
| Deep Eutectic Solvents (DESs) | Natural compounds (e.g., sugars, acids) form liquid mixtures for selective solubilization | Low toxicity; biodegradable | High; depends on DES formulation | High selectivity; good for phenolics, flavonoids | Very good; mild conditions | Very low; customizable and biodegradable | Tunable, green solvents; excellent for polyphenol extraction | Post-extraction separation/purification can be complex |
| Food Waste | Extraction Method | Extraction Solvent | Polyphenols/Flavonoids Concentration Antioxidant Activity | Reference |
|---|---|---|---|---|
| Moroccan cannabis stem  | Ultrasound-assisted extraction | Ethanol, methanol, acetone, and water | Acetone: TPC = 99 ± 22.82 mg GAE/g, TFC = 117.33 ± 24.82 mg QE/g, DPPH-IC50 = 22.13 ± 4.32 µg/mL Ethanol: TPC = 95.34 ± 21.14 mg GAE/g, TFC = 98.57 ± 23.17 mg QE/g, DPPH-IC50 = 19.62 ± 3.56 µg/mL Methanol: TPC = 86.32 ± 19.32 mg GAE/g, TFC = 89.65 ± 19.87 mg QE/g, DPPH-IC50 = 26.00 ± 3.24 µg/mL Water: TPC = 66.07 ± 1.23 mg GAE/g, TFC = 62.88 ± 4.64 mg QE/g, DPPH-IC50 = 45.09 ± 3.24 µg/mL | [21] |
Orange peel | Classic solid–liquid extraction | Ethanol, methanol, and water | Ethanol: TPC = 1.84 ± 0.05 mg GAE/g, TFC = 74.01 ± 5.1 mg QE/g, DPPH-IC50 = 55.5 ± 2.15%, FRAP = 9.6 ± 0.3 mg TE/g, ABTS = 6.5 ± 0.25 µmol TE/g Methanol: TPC = 1.89 ± 0.05 mg GAE/g, TFC = 80.05 ± 5.2 mg QE/g, DPPH-IC50 = 58.5 ± 2.5%, FRAP = 11.9 ± 2.3 mg TE/g, ABTS = 7.2 ± 0.35 µmol TE/g Water: TPC = 1.56 ± 0.04 mg GAE/g, TFC = 50.5 ± 4.2 mg QE/g, DPPH-IC50 = 42.8 ± 1.85%, FRAP = 8.9 ± 0.2 mg TE/g, ABTS = 5.45 ± 0.1 µmol TE/g | [38] |
Olive pomace | Classic solid–liquid extraction | Natural deep eutectic solvents | TPC = 15.56 mg GAE/g dw, FRAP = 178.14 mol FSE/g dw, DPPH = 72.75 mol TE/g dw | [39] |
Potato peel | Classic solid–liquid extraction | Ethanol | TPC = 2.04 ± 0.87 mg GAE/g dw, TFC = 0.21 ± 0.008 mg QE/g dw DPPH = 179.75 ± 3.18 μg/mL | [40] |
Spring onion leaves | Microwave-assisted extraction | Ethanol | TPC = 1.35 mg GAE/g dw FRAP = 14.02 mmol Fe(II)/g dw | [41] |
Tomato waste | Ultrasound assisted extraction | 70% (v/v) ethanol–water | TPC = 4.63 ± 0.016 mg GAE/g, TFC = 0.956 ± 0.07 mg RUE/100 g, ABTS = 27.90 ± 0.10 µmol TE/g | [42] |
| Food Waste | Extraction Method | Extraction Solvent | Antioxidant Activity | Reference |
|---|---|---|---|---|
Peach pomace | Ultrasound-assisted enzymatic extraction | Hexane/acetone/ethanol; 50/25/25 v/v | ABTS = 1933.33 mg Trolox/L FRAP = 52.66 µmol Trolox/L | [52] |
Carrot pomace | Ultrasonication and high shear dispersion techniques | Flaxseed oil | ABTS = 1596.04 ± 69.45 μg Trolox/mL DPPH = 380.21 ± 39.62 μg Trolox/mL FRAP = 941.20 ± 19.91 μM Trolox/mL | [53] |
Passion fruit peel | Ultrasound-assisted extraction | Olive oil and sunflower oil | Inhibition of DPPH = 35.2 ± 1.4% | [54] |
Carrot juice | Microwave-assisted extraction | Flaxseed oil | Inhibition of DPPH = 70.67 ± 0.85% | [55] |
Tomato waste | Conventional solid–liquid extraction at room temperature | Hydrophobic deep eutectic solvents composed of menthol and fatty acids | AAR = 63.7 ± 4 µmol AAE/g dw PR = 26.7 ± 1.8 µmol AAE/g dw | [56] |
15 food waste matrices | Supercritical fluid extraction | Supercritical CO2 and ethanol as co-solvent | SPF = 36.6 ± 2.0%, SPP = 20.7 ± 1.8%, TMF = 30.4 ± 1.7%, TMP = 87.9 ± 1.6%, APF = 39.2 ± 2.2%, APP = 51.9 ± 2.2%, PKF = 42.3 ± 1.1%, PKP = 77.1 ± 1.0%, PCF = 7.0 ± 5.1%, PCP = 34.1 ± 3.1%, GPF = 17.5 ± 4.6%, YPF = 49.7 ± 0.9%, RPF = 46.5 ± 2.7%, XPW = 19.0 ± 3.4%, MIX = 57.7 ± 2.5% | [57] |
Orange peel | Ultrasound-assisted extraction | Organic solvent, vegetable oils, fatty acids, and deep eutectic solvents | Hexane: DPPH = 944.3 ± 13.1 µM TE/mL, ABTS = 355.1 ± 4.5 µM TE/mL, FRAP = 854.2 ± 8.5 µM TE/mL Olive oil: DPPH = 1790.6 ± 17.7 µM TE/mL, ABTS = 661.2 ± 4.8 µM TE/mL, FRAP = 990.6 ± 11.4 µM TE/mL Octanoic acid: DPPH = 2438.8 ± 21.2 µM TE/mL, ABTS = 463.1 ± 6.5 µM TE/mL, FRAP = 743.41 ± 2.1 µM TE/mL Octanoic: L-Proline DES; DPPH = 1340.4 ± 18.3 µM TE/mL, ABTS = 1057.3 ± 18.3 µM TE/mL, FRAP = 1456.4 ± 12.2 µM TE/mL | [58] |
Citrus sinensis peels | Ultrasound-assisted extraction | Hydrophobic deep eutectic solvents | Methanol: Eucalytol DES; DPPH ≈ 2500.1 ± 5.3 µM TE/mL | [59] |
Pumpkin peel | Ultrasonic-assisted extraction | 3:1 v/v n-hexane/acetone solvent mixture | DPPH = 7.25 µM TE/g dw | [60] |
Cantaloupe waste | Ultrasound-assisted extraction | Hexane/acetone mixture | IC50 = 7.33 ± 0.22 μg/mL | [61] |
| Solubility | Vitamin | Structure | Properties |
|---|---|---|---|
| Fat-soluble vitamins | A | 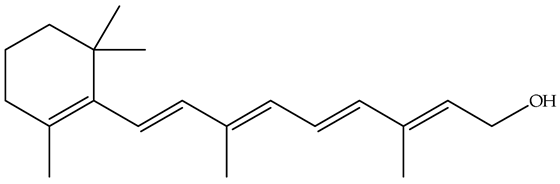 | Vitamin A, which is abundant in vegetable peels and scrapes such as carrot tops and pumpkin seeds, is crucial for vision, immune function, cell growth, and differentiation [60,76]. Its deficiency can lead to night blindness, impaired immune response, and even blindness [77]. The carotenoids present in these food wastes can be converted into vitamin A in the body and are, therefore, a valuable source of this essential nutrient. |
| D |  | Although vitamin D is primarily synthesized in the skin through sun exposure, it can also be obtained from food. Some food waste, particularly from the processing of fatty fish, may contain vitamin D [78]. This vitamin is important for calcium absorption, bone health, and immune function. Vitamin D deficiency is linked to rickets in children and osteoporosis in adults, as well as an increased risk of infections and other health problems [79]. | |
| E | 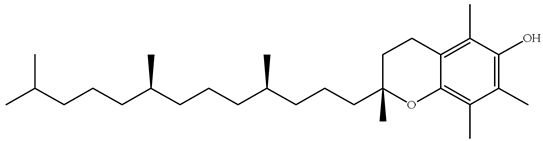 | Vitamin E is found in various food wastes, including seed husks and vegetable oils, and acts as a powerful antioxidant that protects cells from free radical damage. It also plays a role in immune function and blood clotting [80,81]. A deficiency is relatively rare but can lead to neurological problems. | |
| K | 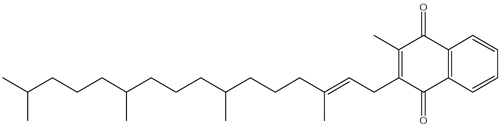 | Vitamin K is found in the waste of green leafy vegetables and some fruit peels [82] and is essential for blood clotting and bone health. It also plays a role in regulating calcium metabolism. A deficiency of vitamin K can lead to bleeding disorders and impaired bone health. | |
| Water-soluble vitamins | B |  | This group of vitamins plays a crucial role in energy metabolism, DNA synthesis, and nerve function. Various B vitamins are found in different food wastes [83,84]. For example, B vitamins such as folate are found in vegetable leaves. B vitamins are important for converting food into energy, supporting nerve function, and the formation of red blood cells. A deficiency of B vitamins can lead to a number of health problems, including fatigue, anemia, and neurological problems. |
| C |  | Vitamin C is abundant in citrus fruit peels and other fruit and vegetable waste and is a powerful antioxidant that supports immune function, collagen synthesis, and wound healing [85,86,87]. It also promotes the absorption of iron. A deficiency of vitamin C can lead to scurvy, which is characterized by fatigue, weakness, and bleeding gums. |
| Food Waste/Source | Bioactive Compounds | Application | Positive Effect | References |
|---|---|---|---|---|
| Grape seed oil | Proanthocyanidins Phenolic compounds Vitamin E isomers (ɣ-tocotrienol) | Culinary oil Addition to meat products | Enhancing lipid stability of meats Protection against oxidative brain damage Antimicrobial activity | [127,128,129,130,131] |
| Rice husk and bran | δ-Tocopherols, ɣ-oryzanol, | Supplement | Reduction in serum levels of hs-CRP and IL-6 (anti-inflammatory effect) | [132,133,134] |
| Olive oil wastewater | Hydroxytyrosol Verbascoside Oleuropein Ligstroside | Nutraceutical Supplement Stock feed | Tumor cell inhibition in vitro and in vivo Natural cheese antioxidants by adding to sheep feed | [135,136] |
| Banana peel | Dietary fiber Galleocatehin Anthocyanins | Herbal medicine Implementation in food products (noodles) Supplementation | Reducing blood sugar Bacteriostatic or fungistatic | [137,138,139,140,141] |
| Carrot peel | Carotenoids Xantophylls | Addition to jams Implementation in bakery products | Increasing antioxidant activity of products Increasing the amount of dietary fibers | [142,143,144,145] |
| Cucumber peel | Cucurbitacins Cucumegastigmanes I and II Cucumerin A and B | Development of food and snack films | Shelf-life extension of cheese, more stable pH, moisture level, and lipid oxidation, sensory improvements | [146,147,148] |
| Barley bran | Beta glucan Arabinoxylan | Edible films | Lowering serum cholesterol Acceleration of gastrointestinal transit time | [149,150] |
| Mango peel | Carotenoids Dietary fiber | Mango peel powder incorporation | Increase in dietary fiber and polyphenols (0.46 to 1.80 mg/g in macaroni) | [151,152,153,154] |
| Peanut shell | Luteolin Isosaponeratin | Addition to peanut butter | Increasing fiber levels and antioxidants in peanut butter | [155] |
| Guava peel | Lycopene Beta carotenes | Flour | Replacing wheat flour in cookie preparation—decreasing fat and carbohydrate levels | [156] |
| Avocado peel | 5-O-caffeoylquinic acid Catechin Epicatechin | Extract | Prevention of oxidation in meat Antimicrobial activity against Listeria innocou and E. coli | [157,158,159] |
| Apple peel | Cyanidin 3-glucoside | Extract | Inhibition of HepG2 human liver cancer cells Strawberry preservation with chitosan-based coating Improvement of oxidative stability of mayonnaise | [160,161,162] |
| Olive leaves | Oleuropein Tyrosol Hydroxytyrosol Quercetin Rutin | Substrate for fermentation | Production of kombucha with infused olive leaves—increased antioxidant activity and sensory properties | [163] |
| Pumpkin flour (cold-pressed cake) | Beta-carotene | Addition to products | Fortification of sponge cake | [164] |
| Wheat germ | Carotenoids y-oryzanol biogenic amides | Addition to products | Improvement of sensory and antioxidative properties | [165,166] |
| Broccoli waste | Glucosinolates Isothiocyanates Vitamin C | Novel green tea-based beverage with added nutritional value | [167,168] | |
| Animal blood | Hemoglobin Thrombin ACE-inhibiting bioactive peptides | Stabilizer Color additive Emulsifier Protein and iron supplement | [169,170,171,172] | |
| Fish oil | Omega-3 fatty acids | Extract | Food supplements | [173,174] |
| Wine pomace | Anthocyanins | Extract Flour | Colorant food additive in yogurt and salad dressings Antioxidative activity Development of functional foods | [119,175,176,177] |
| Eggshells | Calcium | Development of functional foods | [178,179,180] | |
| Algae | Alginate Omega-3 fatty acids | Residual biomass | Implementation in active edible packaging | [161,181] |
| Whey | Protein | Supplements Addition to products | [182,183] | |
| Artichoke by-product | Chlorogenic acid Cynarin Narirutin | Extract | Increase in antioxidant activity and shelf life in tomato juice | [184,185] |
| Raspberry pomace | Gallic acid Caffeic acid Ellagic acid Fiber | Dried pomace | Addition to cookies, increase in fibers and organoleptic properties | [186,187,188] |
| Coconut skin milk | Protein | Coconut protein powder | Emulsifying properties | [189] |
| Cauliflower trimmings | Dietary fibers Carotenoids Vitamin E | Ready-to-eat extrudes | Increased protein content and water absorption index | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekavac, N.; Krog, K.; Stanić, A.; Šamec, D.; Šalić, A.; Benković, M.; Jurina, T.; Gajdoš Kljusurić, J.; Valinger, D.; Jurinjak Tušek, A. Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions. Antioxidants 2025, 14, 714. https://doi.org/10.3390/antiox14060714
Bekavac N, Krog K, Stanić A, Šamec D, Šalić A, Benković M, Jurina T, Gajdoš Kljusurić J, Valinger D, Jurinjak Tušek A. Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions. Antioxidants. 2025; 14(6):714. https://doi.org/10.3390/antiox14060714
Chicago/Turabian StyleBekavac, Nikša, Korina Krog, Ana Stanić, Dunja Šamec, Anita Šalić, Maja Benković, Tamara Jurina, Jasenka Gajdoš Kljusurić, Davor Valinger, and Ana Jurinjak Tušek. 2025. "Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions" Antioxidants 14, no. 6: 714. https://doi.org/10.3390/antiox14060714
APA StyleBekavac, N., Krog, K., Stanić, A., Šamec, D., Šalić, A., Benković, M., Jurina, T., Gajdoš Kljusurić, J., Valinger, D., & Jurinjak Tušek, A. (2025). Valorization of Food Waste: Extracting Bioactive Compounds for Sustainable Health and Environmental Solutions. Antioxidants, 14(6), 714. https://doi.org/10.3390/antiox14060714













