Abstract
Food waste is a major economic, environmental, and ethical challenge, as around a third of the edible food produced worldwide is lost or wasted. This inefficiency not only increases food insecurity but also results in resource depletion and environmental degradation. Dealing with food waste through sustainable management strategies, such as upcycling food by-products, has proven to be a promising approach to optimize resource use and support the circular economy. Valorization of food waste enables the extraction of valuable bioactive compounds with strong antioxidant properties. These natural antioxidants play a crucial role in mitigating diseases caused by oxidative stress, including cardiovascular diseases, neurodegenerative diseases, and diabetes. Utilizing food-derived polysaccharides as functional ingredients in the food, pharmaceutical, and cosmetics industries represents an environmentally friendly alternative to synthetic additives and is in line with global sustainability goals. Various extraction techniques, including enzymatic hydrolysis and ultrasound-assisted methods, enhance the recovery of these bioactives while preserving their structural integrity and efficacy. By integrating technological advances and sustainable practices, the food industry can significantly reduce waste while developing high-value products that contribute to human health and environmental protection. This review underscores the significance of food by-product valorization, aiming to bridge the gap between fundamental research and practical applications for a more sustainable future. The literature was selected based on scientific relevance, methodological quality, and applicability to the food, pharmaceutical, or cosmetic sectors. Studies lacking empirical data, not addressing the extraction or application of bioactives, or published in languages other than English were excluded.
1. Introduction
Food waste is a significant global issue with economic, environmental, and ethical implications. While food is discarded in many parts of the world, hunger persists elsewhere, highlighting a moral paradox. An estimated one-third of all food produced for human consumption, approximately 1.3 billion tons annually, is either lost or wasted. In calorie terms, one in four calories intended for human consumption is never eaten. In 2018 alone, around 821.6 million people suffered from hunger [1]. Beyond the economic costs, food waste represents a misuse of valuable resources, such as water, land, energy, labor, and capital, and contributes to environmental degradation through greenhouse gas emissions, soil depletion, excessive water use, and biodiversity loss [2]. While some losses in food systems are inevitable, identifying an economically optimal level of loss is challenging due to trade-offs and the difficulty of valuing non-market goods, such as clean air or biodiversity [3]. Addressing these inefficiencies is a complex yet urgent task for policymakers, researchers, and stakeholders across the agri-food sector [4].
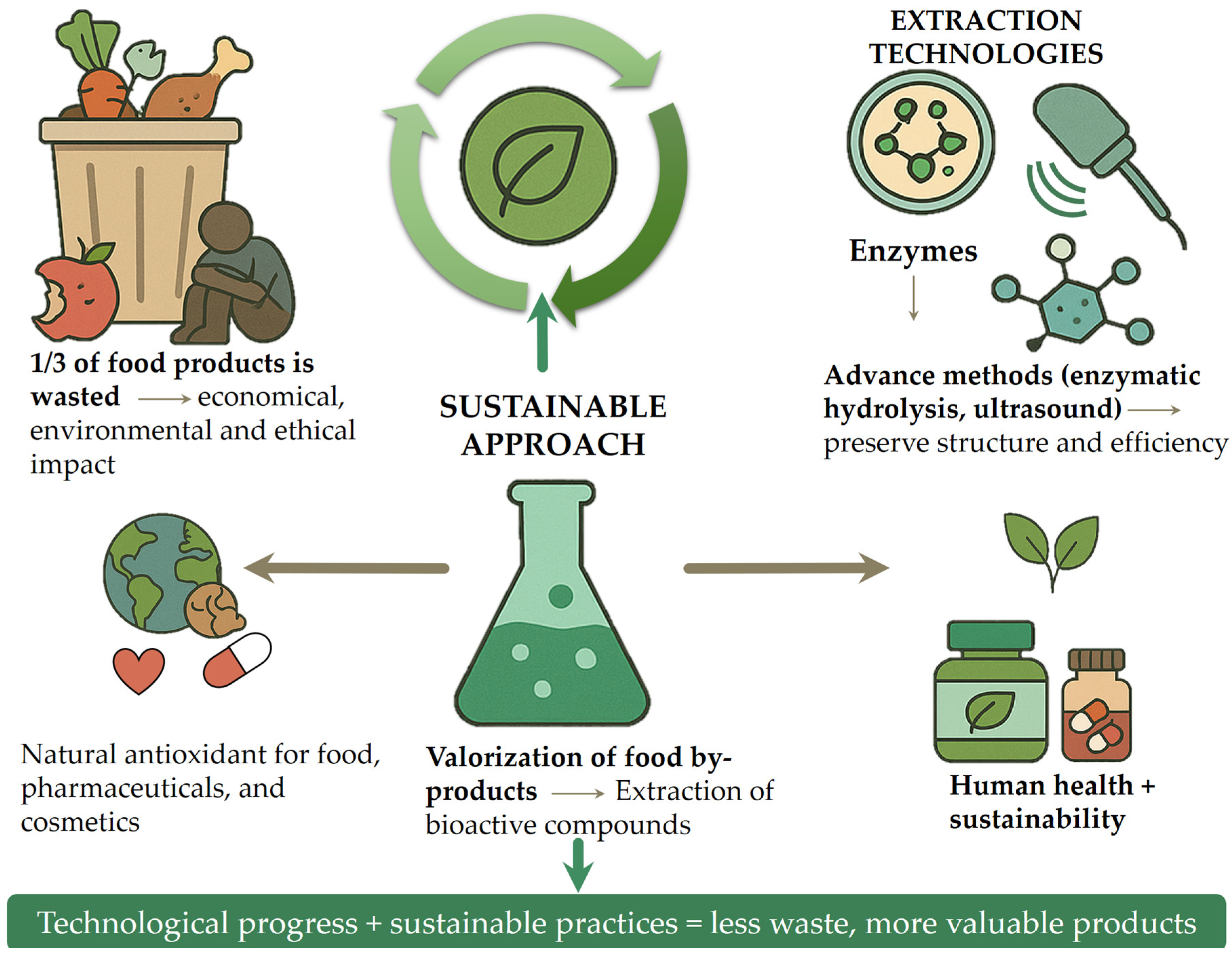
Global trends such as urbanization, population growth, and pandemics are increasing pressure on food systems. Reducing and repurposing food waste is widely recognized as a key strategy to improve resource efficiency, enhance food security, and support the transition to a sustainable, circular economy. The circular economy model in food systems promotes waste minimization, reuse of by-products, and lifecycle extension of food-derived materials through innovative processing and upcycling. These efforts align with the United Nations Sustainable Development Goals, particularly Goal 12.3, which aims to halve global per capita food waste by 2030 [5]. Amid rising food prices and widespread food insecurity, the loss of nutrient-rich materials further exposes systemic inefficiencies. Developing sustainable food waste management strategies, such as bioconversion, enzymatic extraction, and the production of high-value compounds, can reduce waste while supporting environmental and social goals (Figure 1) [6]. The image contrasts conventional food waste management methods, such as animal feeding, composting, and landfill disposal, with the more sustainable approach of food waste valorization. It highlights how by-products can be transformed into value-added products like foods, pharmaceuticals, and packaging materials, promoting a circular economy.

Figure 1.
Food waste management.
Upcycling food by-products presents a promising solution by transforming waste into valuable ingredients for industrial use. The food industry generates substantial volumes of by-products, including fruit and vegetable peels, seeds, stems, and pulp, many of which are discarded despite being rich in bioactive compounds such as antioxidants, fiber, proteins, and essential fatty acids. Utilizing these by-products reduces the environmental impact, conserves resources, and creates innovative products for the food, pharmaceutical, and cosmetic sectors [7]. A key advantage of upcycled by-products is their high antioxidant content. Peels, seeds, and leaves of fruits and vegetables contain phenolic compounds, flavonoids, carotenoids, and vitamins C and E, which are effective in neutralizing free radicals and reducing oxidative stress. These compounds are known to help prevent chronic diseases, such as cardiovascular conditions, cancer, and neurodegenerative disorders [8,9,10]. Extracting natural antioxidants from food waste offers a clean-label, sustainable alternative to synthetic additives for use in dietary supplements and functional foods. These extracts can enhance product shelf life, stability, and consumer health benefits [11,12].
Although food waste valorization has been widely recognized as a promising strategy, the existing literature often lacks a focused and up-to-date synthesis that connects recent technological advances with their practical applications in industry. This review addresses that gap by consolidating peer-reviewed research published primarily between 2010 and 2024, with an emphasis on sustainable extraction techniques and the recovery of high-value bioactive compounds, particularly natural antioxidants and polysaccharides, from food by-products. A key contribution of this work is its multidisciplinary perspective, linking bioactive compound recovery not only to their chemical properties but also to their cross-sector applications in the food, pharmaceutical, and cosmetic industries. Unlike broader or purely theoretical discussions, this review frames food waste valorization within the context of the circular economy and the United Nations’ Sustainable Development Goal 12.3, offering a policy-relevant and application-oriented synthesis. Special attention is given to bioactive-rich materials, such as peels, seeds, and pulp, which can serve as sustainable, clean-label alternatives to synthetic additives in health-promoting products. By evaluating both the scientific foundations and industrial-scale implementation of these strategies, the review aims to bridge the gap between research and practice, demonstrating how food waste can be transformed into valuable resources that support both environmental sustainability and human health. Figure 2 illustrates a sustainable strategy for food by-product valorization, highlighting the integration of advanced extraction technologies designed to maximize the recovery of functional compounds. The literature was selected based on scientific relevance, methodological rigor, and applicability to target industries; studies lacking empirical data, those not addressing bioactive extraction or application, and non-English publications were excluded.
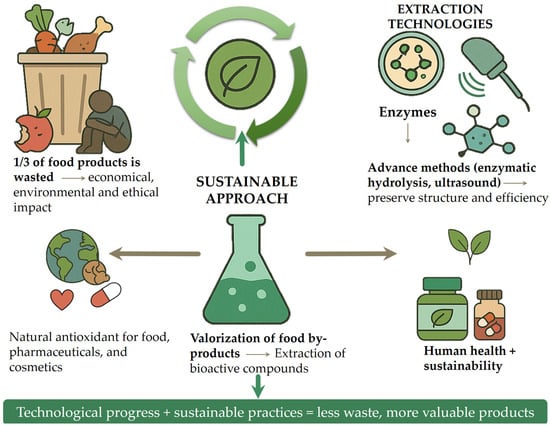
Figure 2.
Sustainable approach for the valorization of food by-products through advanced extraction technologies.
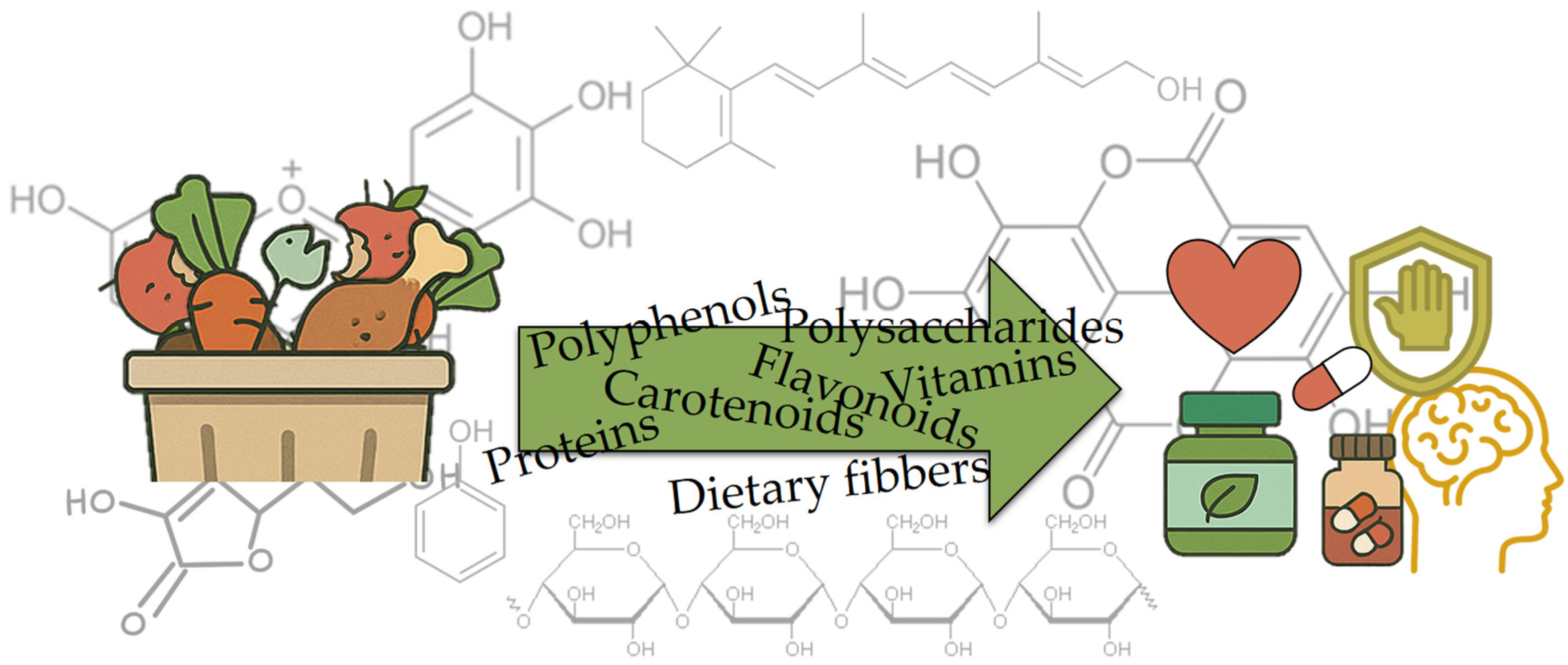
2. Bioactive Compounds in Food Waste and Their Health Benefits
Recently, the shift toward sustainable development has led to the valorization of waste products from the food industry as a source of high-value “functional ingredients“ [13]. Bioactive compounds in food waste are gaining increasing attention due to their potential health benefits, as well as their ability to reduce environmental pollution and increase food sustainability. Food waste, especially from fruits and vegetables, is often rich in bioactive compounds such as polyphenols, carotenoids, vitamins, and dietary fibers [11,12,14,15]. Figure 3 depicts the transformation of food waste biomass into a spectrum of high-value bioactives, polyphenols, polysaccharides, carotenoids, flavonoids, vitamins, proteins, and dietary fibers, symbolized by the green arrow overlaying the molecular structures. These recovered compounds are then linked to health-oriented applications, as shown by icons representing nutraceutical supplements, cardiovascular and immune support, and cognitive benefits. These compounds exhibit antioxidant, anti-inflammatory, antimicrobial, and anti-cancer properties that contribute to various health benefits [16,17,18,19,20]. Utilizing bioactive compounds from food waste not only increases the value of discarded food but also represents a sustainable method of resource recovery.
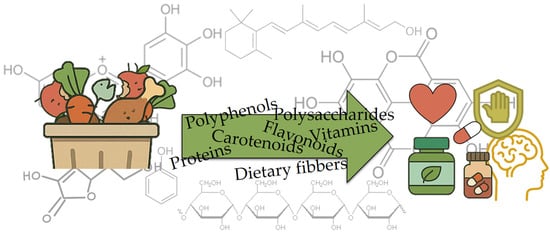
Figure 3.
Bioactive compounds in food waste.
2.1. Techniques Used for the Bioactives Extraction
Innovative extraction methods utilizing green solvents for the recovery of bioactive compounds from food waste have received considerable attention in recent years. Conventional extraction techniques, such as maceration, Soxhlet extraction, and solvent extraction with petroleum-based solvents, are often inefficient, consume a lot of energy, take a long time, and are harmful to the environment. In contrast, innovative extraction techniques, such as supercritical fluid extraction (SFE), subcritical water extraction (SWE), ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), and deep eutectic solvent (DES) extraction, offer numerous advantages in terms of efficiency, sustainability, and selectivity [21,22]. Table 1 provides a comparative overview of the conventional and recent extraction techniques used for recovering bioactive compounds from food waste, highlighting their principles, efficiencies, and sustainability aspects.

Table 1.
Comparison of extraction techniques for recovery of bioactive compounds from food waste [21,22,23,24,25].
One of the most promising methods, supercritical fluid extraction (SFE), especially using supercritical CO2, offers a non-toxic and environmentally friendly alternative for extracting bioactive compounds. The tunable properties of supercritical CO2 allow for selective extraction, reducing the need for additional purification steps. This technique works at relatively low temperatures and preserves the integrity of heat-sensitive bioactive compounds such as polyphenols, flavonoids, and carotenoids. Additionally, CO2 is easily recyclable, making this method highly sustainable compared to classic solvent-based techniques. Subcritical water extraction (SWE) is another environmentally friendly alternative in which water is used at elevated temperatures and pressures to modify its dielectric properties and thereby improve its solvating ability for a wide range of bioactives. In contrast to classical extraction methods based on organic solvents, SWE avoids toxicity issues and offers rapid extraction with high yields. This method is particularly effective for extracting phenolic compounds from food waste, like fruit peels and vegetable residues, which are often discarded despite their high antioxidant content. For example, Lachos-Perez et al. [23] demonstrated that subcritical water extraction (SWE) from defatted orange peel yielded 31.70 mg GAE/g of polyphenols, significantly higher than Soxhlet extraction (7.75 mg GAE/g), shaker extraction using ethanol (4.42 mg GAE/g), and ultrasound-assisted extraction with ethanol (5.83 mg GAE/g). Ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) have also proven to be efficient and sustainable alternatives. In UAE, the cell walls are broken up with ultrasonic waves, which facilitates the release of intracellular bioactives. This process is faster, requires less solvent, and enhances mass transfer compared to classic maceration. Similarly, MAE utilizes microwave energy to rapidly heat solvents and plant matrices, significantly reducing the extraction time and improving bioactive yields while consuming less energy than conventional methods. Overall, these innovative extraction methods outperform classical techniques by offering higher efficiency, improved selectivity, lower energy consumption, and reduced environmental impact [24,25]. They align with the principles of green chemistry and the circular economy and are, therefore, ideal for valorizing food waste to high-value bioactive compounds for nutraceutical, pharmaceutical, and cosmetic applications.
2.2. Polyphenols
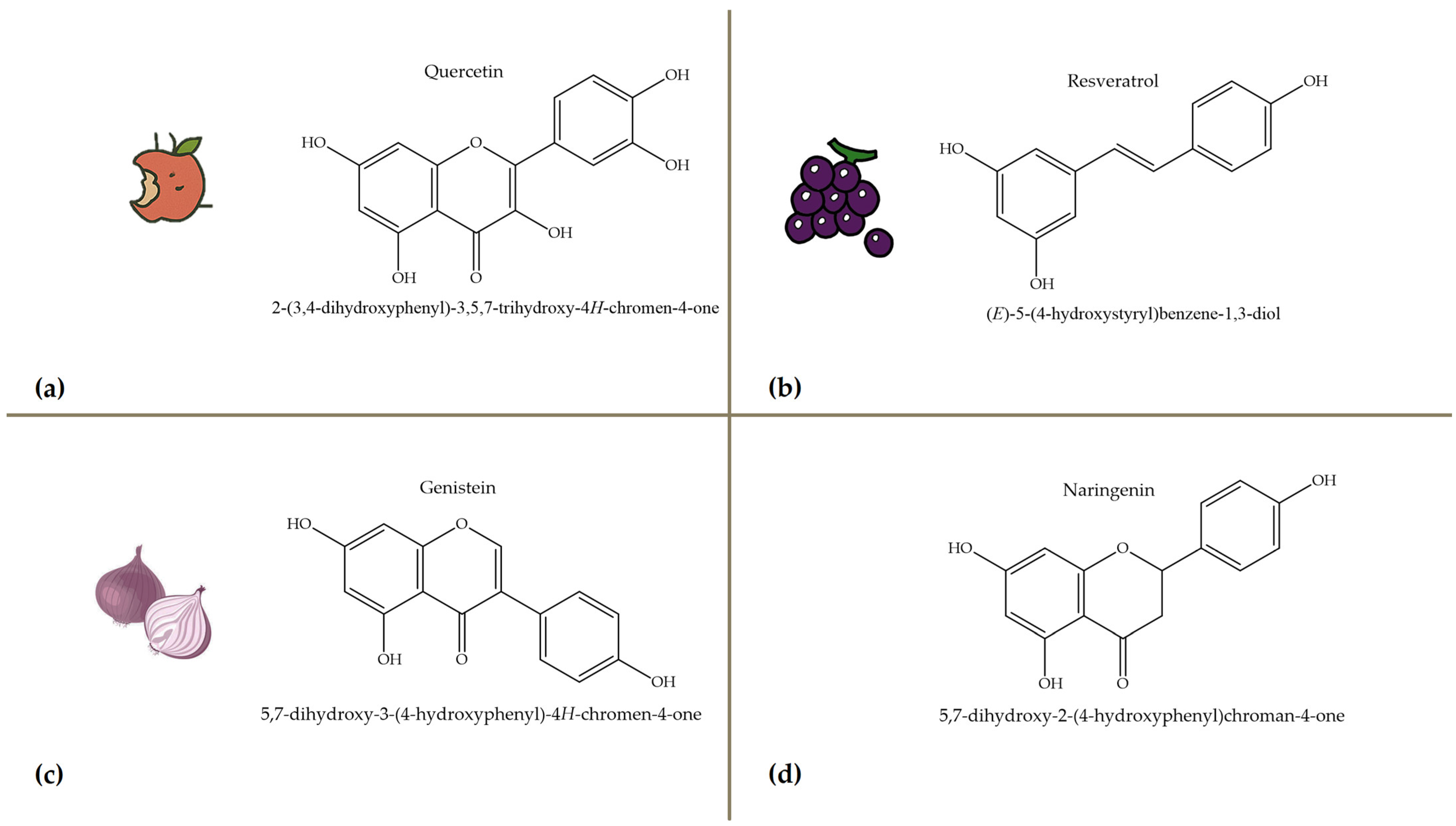
Polyphenols, bioactive compounds present in fruit and vegetable waste, are known for their strong antioxidant properties [26]. These compounds play a crucial role in reducing oxidative stress, fighting inflammation, and preventing chronic diseases, making the recovery of these compounds from food waste an important strategy for both human health and sustainability. Apple peels, for example, which are often discarded during processing, are particularly rich in quercetin, a flavonoid known for its anti-inflammatory, anti-proliferative, and cardioprotective effects [27]. Quercetin is associated with a lower risk of heart disease, cancer, and neurodegenerative disorders due to its ability to neutralize free radicals and modulate cellular signaling pathways. Apple peels also contain other polyphenols such as catechins and chlorogenic acid, which contribute to their high antioxidant capacity. Another important polyphenol, resveratrol, is found in grape skins and pomace, which are often discarded during wine production [28]. Figure 4 focuses on selected polyphenols, including quercetin, resveratrol, genistein, and naringenin, derived from fruits and vegetables, while Figure 5 depicts representative carotenoids, such as lycopene, β-carotene, and lutein, which are found in commonly consumed plant sources. Resveratrol has been extensively studied for its cardioprotective properties [29], as it helps to lower LDL (bad cholesterol), reduce blood clotting, and improve endothelial function. It also has anti-aging effects by activating sirtuins [30], which are proteins that play a key role in cell repair and longevity.
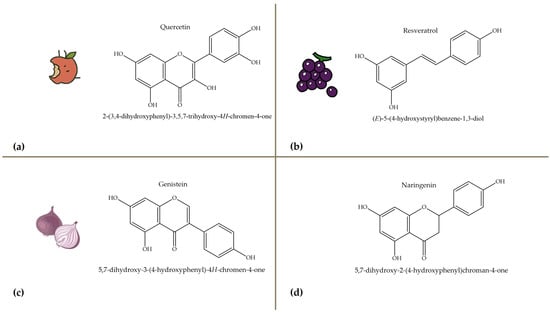
Figure 4.
Chemical structures and natural sources of selected polyphenolic compounds. (a) Quercetine (from apples); (b) resveratrol (from grapes); (c) genistein (from onions); (d) naringenin (from citrus fruits).
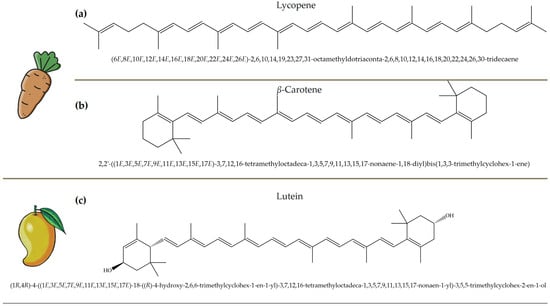
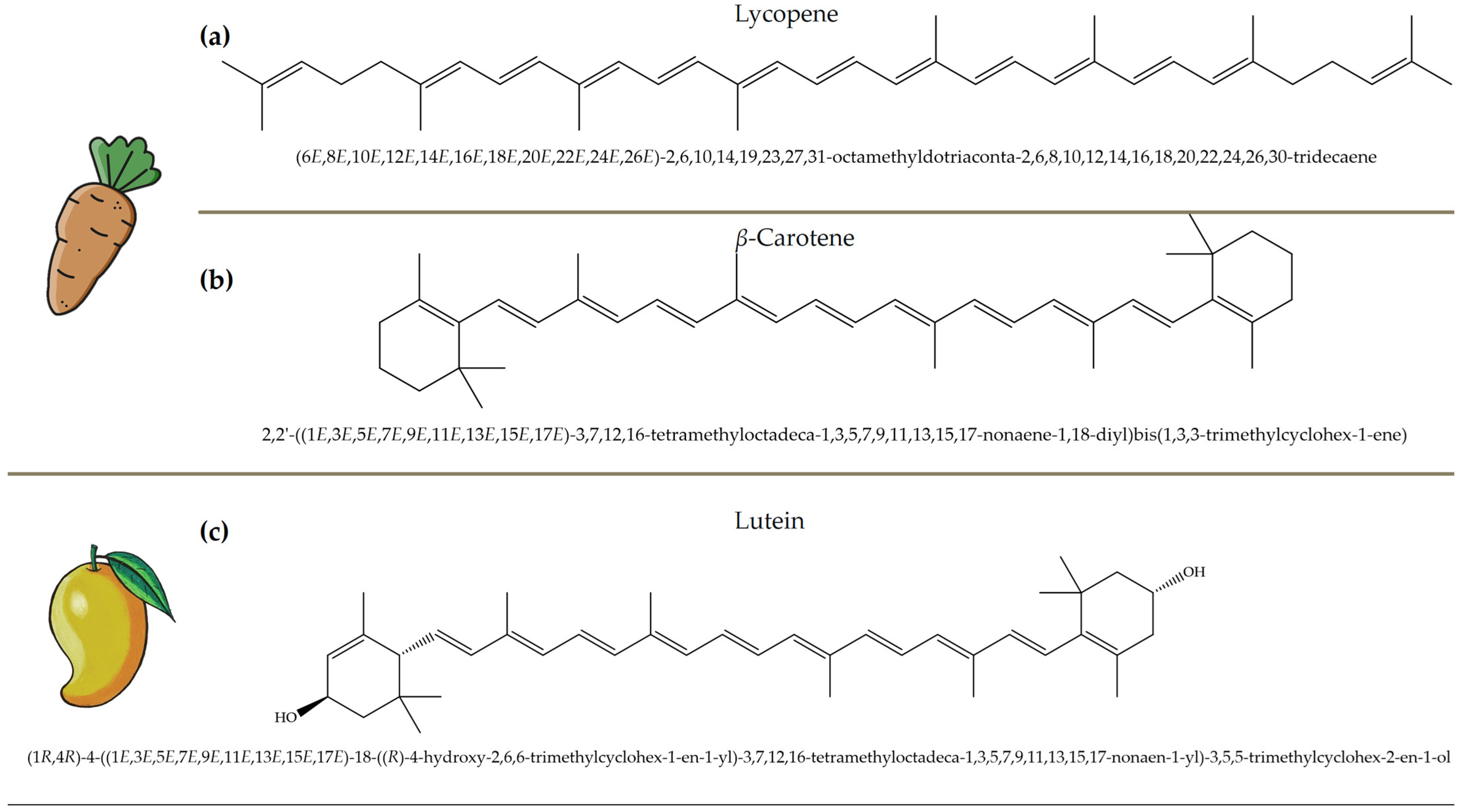
Figure 5.
Chemical structures and natural sources of selected carotenoids. (a) lycopene (commonly found in carrots and tomatoes); (b) β-Carotene (from carrots and other orange vegetables); (c) lutein (abundant in mangoes and leafy greens).
Similarly, citrus by-products, including orange peel and pulp residues, are an important source of flavonoids, particularly hesperidin and naringenin. These bioactive compounds have been extensively studied for their ability to lower cholesterol, improve blood circulation, and protect against cardiovascular disease [31,32]. In particular, research has demonstrated that the antioxidant activity in citrus peel is significantly higher than in the fruit itself, highlighting its potential as a valuable but underutilized resource for functional food and nutraceutical applications [33,34,35]. For example, Azman et al. [36] investigated the antioxidant properties, such as phenolic and flavonoid content, of fresh and frozen citrus peels. Frozen citrus peels had a significantly higher antioxidant content compared to fresh peels. Frozen lemon peels had a 54.7% higher total flavonoid content than fresh lemon peels. A possible reason for this could be the deactivation of oxidative and hydrolytic enzymes during the freezing process. The obtained results indicate that frozen citrus peels can be used for the production of food supplements due to their exceptional antioxidant activity. Furthermore, the onion (Allium cepa L.) is one of the most widely used vegetables in the world, and its processing generates significant amounts of by-products, especially from the skins. Although the skins are often treated as waste due to their intense odor, research has proven that they contain high concentrations of bioactive compounds. Chernukha et al. [37] investigated the different antioxidant profiles of husk waste obtained from red, yellow, and white varieties of onions. Isoflavones, flavonols, flavanonols, and flavonoid-O-glycosides were detected in the skins of red and yellow onions, while the skins of white onions contained lower amounts of antioxidants. Beyond their health-promoting properties, polyphenols extracted from food waste have promising applications in the food, pharmaceutical, and cosmetic industries. Their strong antioxidant and antimicrobial activities make them ideal natural preservatives that extend the shelf life of perishable goods while reducing reliance on synthetic additives. Additionally, polyphenol-rich extracts from fruit and vegetable waste are being incorporated into dietary supplements, skincare formulations, and biodegradable packaging materials, further strengthening their role in sustainability and circular economy practices [22]. By utilizing the full potential of polyphenols from food by-products, the industry can not only contribute to waste reduction but also create value-added products that support human health and environmental protection. Examples of the antioxidant activity of some polyphenols and flavonoids extracted from food waste are given in Table 2.

Table 2.
Antioxidant activity of the polyphenols and flavonoids extracts from food waste. Antioxidant activity was assessed using widely recognized methods, including DPPH, ABTS, and FRAP assays, to provide a comprehensive evaluation of the antioxidant potential.
2.3. Carotenoids
Carotenoids, which are abundant in food waste, such as carrot peels, tomato skins, and mango peels, are a valuable class of bioactive compounds with significant health benefits [43]. These naturally occurring pigments have strong antioxidant properties and help to neutralize free radicals, reduce oxidative stress, and protect against chronic diseases. Lycopene, an important carotenoid in tomato skin, has been associated with a reduced risk of prostate and breast cancer due to its strong antioxidant and anti-inflammatory activity [44,45,46]. Studies have shown that lycopene can effectively inhibit lipid peroxidation and DNA damage, making it an important compound for disease prevention and overall health. Similarly, β-carotene is found in high concentrations in carrot peels [47,48]. It serves as a precursor to vitamin A, which is essential for vision, immune function, and skin health. Research indicates that the β-carotene content in carrot peels is about 50% higher than in the pulp, emphasizing the untapped nutritional potential of food waste. This compound not only supports eye health by preventing age-related macular degeneration, but also enhances skin repair and immune response [49]. Figure 5 depicts representative carotenoids, including lycopene, β-carotene, and lutein, found in commonly consumed plant sources.
Mango peels, another rich source of carotenoids such as lutein and zeaxanthin, offer additional health benefits [50,51]. These compounds are particularly beneficial for eye health, as they accumulate in the retina and help protect against harmful blue light and oxidative stress, reducing the risk of cataracts and age-related macular degeneration. Their powerful antioxidant activity also contributes to cardiovascular health by lowering oxidative damage to lipids and improving blood vessel function. Beyond their health-promoting properties, carotenoids are also used industrially in food, cosmetics, and pharmaceuticals. Their natural pigmentation makes them valuable colorants in food and offers a sustainable alternative to synthetic dyes. Their ability to prevent oxidative degradation also makes them ideal natural preservatives that extend the shelf life of food and cosmetic products. Examples of the antioxidant activity of some carotenoids from food waste are given in Table 3.

Table 3.
Antioxidant activity of carotenoid extracts from food waste.
2.4. Dietary Fibers
Dietary fibers, the indigestible parts of plants, are widely recognized for their crucial role in maintaining human health [62,63]. Although commonly associated with whole grains, fruits, and vegetables, a significant amount of these valuable nutrients is often discarded as food waste [64,65]. This waste, consisting of peels, seeds, pulps, and other by-products of food processing and consumption, presents a unique opportunity to recover and utilize these dietary fibers, not only reducing waste but also contributing to improved public health. Dietary fibers are broadly divided into two categories based on their solubility in water: soluble and insoluble [66]. Soluble fibers, such as pectins, gums, and beta-glucans, dissolve in water and form a gel-like substance. Thanks to this property, they can slow down digestion, regulate blood sugar levels, and lower cholesterol levels by hindering the absorption of cholesterol in the intestine. Insoluble fibers, including cellulose and hemicellulose, do not dissolve in water and are primarily used to bulk up stool, promote regular bowel movements, and prevent constipation. Both types of fiber play different but complementary roles in maintaining digestive health and overall well-being.
The health benefits extend beyond their role in digestion. There is much evidence to suggest that an adequate intake of dietary fiber is associated with a lower risk of several chronic diseases [67,68]. For example, the ability of soluble fiber to lower cholesterol levels contributes to improved cardiovascular health and reduces the risk of heart disease, stroke, and other related conditions. Furthermore, the regulation of blood sugar levels by soluble fiber is particularly beneficial for people who have or are at risk of developing type 2 diabetes. By slowing down the absorption of sugar, they help to prevent a rise in blood glucose levels, improve insulin sensitivity, and reduce stress on the pancreas. Insoluble fibers make an equally important contribution to digestive health [69,70]. By adding bulk to the stool, it facilitates the passage of stool through the digestive tract, prevents constipation, and reduces the risk of associated complications, such as hemorrhoids and diverticulosis. Moreover, a high-fiber diet is associated with a lower risk of colorectal cancer [71], possibly due to the shorter transit time of stool through the colon, which minimizes exposure to potential carcinogens. In addition to these well-known benefits, new research suggests that dietary fibers may also play a role in weight control [72,73,74]. High-fiber foods tend to be more filling, which promotes satiety and reduces the likelihood of overeating. Additionally, fermentation of some dietary fibers in the gut produces short-chain fatty acids, which have been shown to have beneficial effects on metabolism and appetite regulation.
Given the abundance of dietary fiber in food waste, recovering and recycling it is a sustainable approach to increasing fiber intake and promoting public health. Various methods exist to extract and purify these fibers from food waste, including physical, chemical, and enzymatic processes [64]. The extracted fibers can then be incorporated into a variety of food products, such as baked goods, cereals, and beverages, enhancing their nutritional value and thus contributing to a more balanced diet.
2.5. Vitamins
Although often overlooked, food waste can be a surprisingly rich source of various vitamins. It offers a potential pathway for nutrient recovery and contributes to improved public health and a more sustainable food system [75]. This exploration will delve into the presence of vitamins in food waste, emphasizing their individual health benefits and the potential for their recovery and utilization. Vitamins are organic compounds that are required in small amounts for various physiological functions. They are broadly classified into two categories: fat-soluble vitamins (A, D, E, and K) and water-soluble vitamins (B vitamins and vitamin C). Each vitamin plays a unique and crucial role in maintaining human health, and their deficiency can lead to a range of health problems. Food waste, i.e., peels, seeds, pulps, and other by-products of food processing and consumption, often contains significant concentrations of these vital nutrients. A short overview of the health benefits of the vitamins extracted from food waste is given in Table 4.

Table 4.
Overview of vitamins in food waste and their health benefits.
The potential for recovering vitamins from food waste is significant. Various extraction methods, including solvent extraction, supercritical fluid extraction, and enzymatic methods, can be used to isolate and concentrate these valuable nutrients. The recovered vitamins can then be incorporated into food supplements or even animal feed, increasing nutritional value and reducing waste. The recovery of vitamins from food waste offers several advantages. It provides a sustainable approach to increasing vitamin intake and reduces reliance on synthetic vitamin supplements. Furthermore, the vitamins contained in food waste can be in a form that is more bioavailable than synthetic vitamins, meaning that they can be more easily absorbed and utilized by the body [88].
2.6. Polysaccharides
Polysaccharides, including pectins, hemicelluloses, and dietary fibers, are abundant in food waste such as fruit peels, vegetable residues, and cereal by-products [89]. Various extraction techniques, such as hot water extraction, enzymatic hydrolysis, ultrasound-assisted extraction, and microwave-assisted extraction, are used to efficiently recover these bioactive compounds. The choice of extraction method has an impact on the yield, structural integrity, and bioactivity of the polysaccharides. Among these methods, green and environmentally friendly extraction approaches, such as enzyme-assisted and ultrasound-assisted techniques, are preferred, as they minimize the use of harsh chemicals and maintain the functional properties of the polysaccharides.
One of the most significant properties of polysaccharides extracted from food waste is their potent antioxidant activity. These polysaccharides exhibit antioxidant potential based on mechanisms such as free radical scavenging, metal ion chelation, and inhibition of lipid peroxidation [90]. The presence of hydroxyl groups and other functional moieties in their structure contributes to their electron-donating ability, effectively neutralizing reactive oxygen species. Studies have shown that polysaccharides derived from food waste, such as citrus peels, apple pomace, and mushroom residues, possess high antioxidant capacity comparable to conventional antioxidants such as vitamin C and tocopherols [90,91,92,93]. The molecular weight, degree of branching, and monosaccharide composition significantly influence their antioxidant activity. Furthermore, structural modifications such as sulfation, acetylation, and carboxymethylation can enhance their bioactivity, making them more effective in oxidative stress management. These bioactive compounds contribute to reducing oxidative stress-related diseases, including cardiovascular disease, neurodegenerative diseases, diabetes, and certain cancers. By scavenging free radicals and reducing inflammation, polysaccharides help protect cells from oxidative damage, which is a key factor in aging and chronic disease progression [92,93]. For instance, pectins extracted from fruit peels have been shown to lower cholesterol levels, improve gut health, and modulate the immune system. Similarly, β-glucans from cereal bran and fungal waste enhance immune function by stimulating macrophage activity and cytokine production. Moreover, polysaccharides contribute to gut health by acting as prebiotics, promoting the growth of beneficial gut microbiota [94,95,96,97]. They serve as fermentable substrates for probiotic bacteria, such as Lactobacillus and Bifidobacterium, leading to the production of short-chain fatty acids (SCFAs) such as butyrate, which play a crucial role in maintaining gut integrity and reducing inflammation. This gut-modulating effect is particularly beneficial for preventing metabolic disorders such as obesity and type 2 diabetes. Additionally, polysaccharides have anti-diabetic properties by regulating blood glucose levels, enhancing insulin sensitivity, and inhibiting carbohydrate-digesting enzymes.
2.7. Essential Oils
Plants produce essential oils (EOs), which are volatile aromatic compounds historically used as flavoring agents in food, medicine, and cosmetics. Essential oils are often found in the parts of plants that are considered food waste, such as peels, making these by-products valuable sources for extracting aromatic and bioactive compounds [85]. A good example is citrus fruit peels, whose EOs are primarily composed of a mixture of volatile compounds, including terpenes and oxygenated derivatives, such as aldehydes (like citral), alcohols, and esters [98]. For example, according to the Grover et al. [99], citrus essential oil (EO) is an eco-friendly, inexpensive, and natural alternative to artificial antioxidants and preservatives, with wide applications in enhancing the shelf life of foods like fruits, vegetables, dairy, bakery, and meat products, as well as in the pharmaceutical and cosmetic industries. Conventional methods for extracting essential oils (EOs), such as cold pressing, solvent extraction, and distillation, often require high energy, long processing times, and high temperatures that can degrade heat-sensitive compounds, and sometimes involve toxic solvents unsuitable for food use. As a result, greener technologies are emerging, offering eco-friendly, energy-efficient, and low-emission alternatives for EO extraction [99]. Methods such as supercritical fluid extraction (SFE) [100], Ohmic heating-assisted extraction/hydrodistillation [101], ultrasound-assisted extraction [102], and green and solvent-free extraction processes using ultrasound and microwave techniques [103] have been optimized and developed for EO extraction from citrus peels. According to the review article by Teigiserova et al. [104], solvent-free microwave extraction is the most effective method, which provides a high yield in a short extraction time.
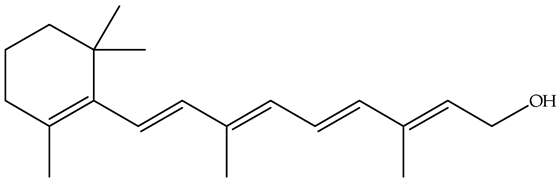
EOs are high in terpenoid compounds, which may also be directly extracted from food waste. For example, the main constituent of orange and other citrus fruit essential oils is d-limonene or (+)-limonene, comprising 3.8–5.3% of waste orange peel and 90–98% of orange essential oil [105]. It is commonly used as a flavoring in food, cosmetics, and medicines, as well as a fragrance in perfumes, with potential medical applications like gallstone dissolution and cancer prevention, and industrial uses in adhesives, solvents, electronics, and lipid extraction from foods [105]. In addition to limonene, orange waste, pine substrates, and apple pomace waste may serve as valuable sources of terpenes, such as camphene, myrcene, p-cymene, terpinolene, cadinene, sabinene, longifolene, germacrene, linalool, terpinene, β-phellandrene, 3-carene, spathulenol, and α-farnesene [18]. According to the review article by Siddiqui et al. [105], for terpenoid extraction, in addition to substituting the conventional solvent hexane with certain bio-based solvents, a range of techniques have been developed. These include enhanced solvent extraction processes through temperature and pressure intensification or ultrasound, improved distillation methods, most commonly using various microwave-based techniques, as well as enzymes and supercritical CO2 extraction.
2.8. Other Compounds
Cocoa bean shells are industry by-products rich in bioactive compounds, including alkaloids that can be effectively extracted. For instance, cocoa bean shells may serve as a source of theobromine and caffeine, which can be successfully extracted using deep eutectic solvents in combination with microwave-assisted extraction [106]. According to Santonocito et al. [107], food waste of tomato processing may be a source of steroidal alkaloids, which inhibit neuroblastoma cell viability. Also, potato peel waste is a source of steroidal alkaloids, which Hossain et al. [108] successfully extracted by ultrasound-assisted extractions. Sweet pepper stalks, a common food waste, contain alkaloids that exhibit antioxidant and antimicrobial properties [109].
Glucosinolates are sulfur-containing compounds found in cruciferous vegetables that act as precursors to bioactive compounds, such as isothiocyanates and indoles, known for their potential health benefits, including antioxidant, anti-inflammatory, anticancer, and detoxifying properties. For example, Amofa-Diatuo et al. [110] successfully extracted isothiocyanates from cauliflower food waste (leaves and stems) using ultrasound-assisted extraction, and subsequently used these extracts to create new functional apple beverages enriched with isothiocyanates. Glucosinolates are also present in broccoli, cabbage, and other cruciferous vegetable waste, such as leaves and stalks, making them valuable sources of glucosinolates (reviewed by Shinali et al.).
Food waste may also contain other compounds whose potential utilization is uncertain, requiring further research to assess their toxicity and explore their possible pharmaceutical applications. For example, oxygen heterocyclic compounds (OHCs), including coumarins, furocoumarins, and polymethoxyflavones, are specialized plant metabolites that play a vital role in plant defense by protecting against infections and supporting growth. Recent research has extensively documented their presence in various foods and food waste, particularly in citrus fruits, cinnamon, carrots, parsley, and similar items. While the beneficial effects of polymethoxyflavones on human health are well-established, concerns have been raised regarding the potential risks associated with furocoumarins and coumarin, and their presence in extracts has to be monitored. In general, when extracting bioactive compounds from food waste, it is crucial to consider the safety profile, as the waste may contain undesirable compounds that could also be present in the extracts. These unwanted substances could pose potential health risks, highlighting the importance of thorough purification and safety assessments during the extraction process.
3. Valorization the Food Waste in the Food Industry
As previously mentioned, food waste and by-products are abundant in numerous bioactive compounds and dietary fibers, which could be used in improving the nutritive value of existing and new food products [25,111,112]. The term “by-product” is increasingly becoming the focus of discussions on the valorization of residues from the food industry. This shift reflects the growing awareness of the potential to utilize these residues as substrates for the production of functional compounds and the development of new products with additional market value. The overarching goal of the industry is, therefore, to reduce food waste, valorize by-products, and improve waste management [113]. Nowadays, most of the food waste that is being valorized is used for food supplements [114]. The substitution of synthetic supplements and food additives with natural alternatives is a growing trend, driven by increasing consumer awareness of product safety and health concerns [115]. Synthetic antioxidants and preservatives have been favored in the food industry due to their purity, availability, consistent activity, and lack of negative impacts on the sensory and physical properties of the final products [116]. However, their artificial origin has led to increased consumer aversion, as many associate them with harmful chemicals. In addition, some synthetic additives can have toxic effects when used in high concentrations, which raises additional health concerns [117]. In the current market, there is a clear trend towards green formulations, where natural preservatives and antioxidants are used not only in food but also in personal care, cosmetics, and pharmaceuticals [118]. Given the serious and growing environmental problem that food waste poses, it is essential to develop sustainable methods for its valorization, such as using food waste as a source of bioactive compounds to create high-value products, whether in the form of entirely new products used as dietary supplements or by adding such food waste to extend the shelf life and improve the nutritional value of existing products [119]. Food waste is often abundant in biologically active compounds, including polyphenols [120], carotenoids [121], dietary fibers [89], carbohydrates [122,123], and proteins [124], each of which has significant potential. Polyphenols are potent antioxidants that help prevent lipid oxidation, thereby extending shelf life and enhancing product stability. Carotenoids offer both antioxidant activity and natural coloring properties, aligning with the clean-label trend. Dietary fibers contribute to gut health and can improve the texture and moisture retention in baked goods. Carbohydrates, particularly indigestible ones, support prebiotic functions and contribute to energy metabolism. Proteins from food waste, such as whey or oilseed cakes, can serve as valuable nutritional enhancers and functional agents (e.g., emulsifiers or foaming agents). Generally, these compounds reflect their multifunctionality, applicability across various food matrices, and strong consumer interest in natural, health-promoting ingredients. In order to reuse food waste efficiently and sustainably, new technologies for the preservation of these compounds need to be explored. Nowadays, food waste is usually used for the production of animal feed, cosmetics, and pharmaceuticals. Stabilization processes are crucial for the successful reuse of food waste, as its high moisture content can lead to the spoilage and degradation of bioactive compounds [125]. Drying, freeze-drying, or dehydration are common methods to reduce the moisture content and preserve the functional properties of the compounds [126]. These processes ensure that the by-products can be stored for longer, and their nutritional and functional integrity is maintained when they are added to food. Examples of some applications of food waste in food products are given in Table 5.

Table 5.
Overview of some applications of food waste in food products.
The main food waste of interest is waste from fruit processing. By-products of the fruit-processing industry, such as peels, seeds, and pulp, contain valuable compounds like pectin, flavonoids, carotenoids, fibers, and polyphenols [191,192]. These by-products, typically considered waste, have the potential to be repurposed either by being added to other food products after undergoing stabilization processes, as they often have a high moisture content [193], or by serving as substrates for the extraction of bioactive compounds [194], which can then be used as functional additives in food [195]. Pectin is widely used in the food industry as a gelling agent, particularly in jams and jellies, and also has prebiotic properties that support gut health [196]. Apple pomace and citrus peels are the primary plant materials used for the extraction of pectin, which has a wide range of applications [197,198]. It is commonly used in the production of jams, jellies, and confectionery, but its applications extend beyond food. Pectin’s ability to form gels in acidic environments also makes it useful in pharmaceuticals and cosmetics, where it can serve as an emulsifier or stabilizer [199]. Another use of fruit peels, specifically citrus peels, which are rich in flavonoids and vitamin C, can be processed into powders or extracts and used as natural preservatives or flavor enhancers in a variety of food applications [200,201]. Significant quantities of citrus by-products are promising for the development of health-promoting foods by incorporating them as functional ingredients. Their high content of bioactive compounds, including flavonoids and indigestible carbohydrates such as dietary fibers, makes them ideal for enhancing foods with additional health benefits. In addition to the peels, the seeds are also a promising source of bioactive compounds [202]. Extracts from grapefruit seeds are marketed as a natural and environmentally friendly pesticide in organic farming and have proven their effectiveness against various pathogens. In a study by Kim et al. [203], the electrostatic coating of alginate–chitosan with grapefruit seed extract was tested on shrimps (Litopenaeus vannamei). This showed that the shelf life can be extended when stored for 15 days at 4 °C. This highlights the application of citrus seed extracts, not only in crop protection but also in the food industry, particularly in enhancing the quality and safety of seafood products [203]. In addition to grapefruit seed extract, another oil from grape seeds, usually a by-product of wine production, is also used as a cosmetic ingredient, as well as in marinades, infusions, and dressings in cooking [204].
Recent efforts have focused on incorporating phenolic compounds into commercial products. The peel and skin of the pomegranate have been identified as a source of ellagitannins and punicalagins, which have been patented for their commercial use as antioxidants in food and cosmetic products [205]. Microencapsulated polyphenols from pomegranate peel have been used to enrich ice cream with natural antioxidants [206], and bread has been fortified with pomegranate peel powder [207]. Tomato pomace is most commonly used in powdered form as a nutritious and antioxidant additive in various foods, including wheat flour products, meat products, dairy and oil products, and confectioneries [208]. Abid et al. [209] enriched Tunisian butter with an extract from tomato processing by-products. The storage stability of the enriched butter during 60 days of storage at 4 °C significantly improved due to the strong antioxidant activity, which inhibited the formation of peroxides and conjugated dienes, as well as the degradation of unsaturated fatty acids to oxidation products. The addition of tomato by-products not only improves the nutritional profile of dairy products but also contributes to their sensory qualities by giving them a unique flavor and color [210]. Large quantities of potatoes are processed for human consumption, resulting in significant amounts of waste in the form of potato peels. These peels can serve as a potential source of fiber in processed products [211]. Along with fiber, the peels are rich in antioxidants and essential nutrients, with a potential for implementation in various formulations. Potato peel powder in baked goods can enhance their nutritional value while improving texture and moisture retention [212]. Franco et al. [213] found that antioxidant protection and inhibition of hexanal production in soybean oil increased with higher concentrations of potato peel extract. Phenolic acids are the most important compounds found in potato peels, with chlorogenic acid (49–61%) being the most abundant, followed by caffeic acid (2.3–19.9%), gallic acid (7.8%), and protocatechuic acid (0.21%) [214].
Anthocyanins are utilized in the food industry as a natural alternative to synthetic dyes [215]. In a study by Ali et al. [216], natural anthocyanins extracted from red onion skins were successfully applied as colorants in confectioneries, especially in hard candies and glazed jellies. The products containing 0.3% anthocyanins in hard candies and 0.25% in glazed jelly received high ratings for color and overall acceptability, comparable to those achieved with synthetic dyes. Additionally, the use of natural colorants meets the growing consumer demand for clean label products, which focus on transparency and natural ingredients [214].
Oilseed by-products, such as sunflower seeds, soybean seeds, soybean oil waste, soybean-processing wastewater, olive pomace, and olive oil-processing wastewater, also contain significant amounts of bioactive compounds [217]. These include phytosterols, polyphenols, and proteins [218]. The by-products resulting from the various oil productions are rich in proteins and fibers and contain numerous other bioactive compounds, which can be incorporated into the production of functional bakery products [219]. The addition of oilseed cakes to bakery products increases the sustainability of production. Oilseed cakes, especially those leftover after the production of cold-pressed and virgin oils, have a high nutritional value (flavonoids, proanthocyanins, and phenolic acids) [220]. By adding up to 15% of these meals to bakery products, functional products with greater added value and good sensory and technological properties can be obtained [221]. Due to the high oil content, special attention should be paid to the storage conditions and shelf life of the meals and the products [222].
Whey, a by-product of cheese production, is an important waste product in the dairy industry. For every 10 kg of cheese produced, up to 9 kg of whey is generated [223]. It is generally used as fertilizer, animal feed, and for human consumption. Whey contains approximately 55% of the nutrients in milk and represents about 20% of the total protein content, which corresponds to 85 to 95% of the original milk volume. Whey is the serum phase of milk, the liquid remaining after the removal of fats and casein, and is composed mainly of soluble components such as lactose, soluble salts, and globular proteins. Liquid whey is characterized by its greenish-yellow hue, which is attributed to the presence of riboflavin (vitamin B2) [224]. The nutritional profile and technological properties of whey make it a valuable ingredient in various food products and nutraceutical applications. Studies have highlighted its potential health benefits, including its role as a source of high-quality protein and bioactive compounds that may enhance muscle recovery and support immune function [225]. Furthermore, the rich chemical composition of whey presents opportunities for its utilization in functional foods, helping to reduce and promote sustainability in the dairy sector [226].
Considering the nutritional and chemical properties, antioxidant activity, and present BACs, the coffee bean silver skin that is left over after roasting appears promising for use in the food industry [227]. Due to its fiber-rich composition, silver skin has been incorporated into baked goods as a source of dietary fiber [228] and used in the production of a novel beverage for weight control [229]. Moreover, extracts from silver skin could serve as a natural colorant and fiber source in biscuits, which could improve the quality, shelf life, and sensory properties of biscuits [230]. The bioactive potential of coffee silver skin also makes it a viable alternative to synthetic chemicals in cosmetic formulations. Its rich content of phenolic compounds, melanoidins, and caffeine contributes to its strong antioxidant capacity, which can be used against aging and shows resistance to oxidative stress induced by tert-butyl hydroperoxide in human keratinocytes [231]. The valorization of food waste as a source of bioactive compounds and functional ingredients represents a significant shift toward sustainable food systems. By repurposing waste such as fruit peels, pomace, and dairy by-products, the food industry can reduce environmental burdens while enhancing product nutrition and shelf life. However, the practical application of food waste in food products faces several challenges. First, variability in the composition and quality of by-products complicates standardization and consistency in formulations. Moreover, many of these waste streams are highly perishable, requiring energy-intensive stabilization methods like drying or freeze-drying, which can be economically and environmentally costly. Regulatory hurdles and consumer perceptions also limit widespread adoption. While natural additives are appealing, acceptance may waver if products are linked to waste. Despite these obstacles, evidence supports the efficacy of food waste-derived ingredients, such as polyphenols from fruit peels or proteins from whey, for improving antioxidant activity and nutritional profiles. Advancements in processing technologies and clear labeling practices will be crucial to overcoming skepticism and achieving commercial viability. Ultimately, while promising, the integration of food waste into food products demands a careful balance between functionality, safety, economic feasibility, and consumer trust.
4. The Valorization of Food Waste into Nutraceuticals and Dietary Supplements
The valorization of food waste into nutraceuticals and dietary supplements has garnered increasing global attention as a promising solution to address both environmental sustainability and public health goals [232]. This innovative strategy not only supports the principles of a circular economy by turning waste into value-added products but also aligns with the United Nations Sustainable Development Goals (SDGs), particularly those targeting responsible consumption and production (SDG 12), good health and well-being (SDG 3), and climate action (SDG 13) [233]. In recent years, a growing body of research has investigated the bioactive potential of food waste streams. For example, grape pomace, a major by-product of the winemaking industry, is a rich source of resveratrol and quercetin, two powerful polyphenolic compounds. Resveratrol has been extensively studied for its cardioprotective, neuroprotective, and anti-aging effects, while quercetin exhibits potent antioxidant and anti-inflammatory activity [234]. Likewise, citrus peels, commonly discarded during juice production, contain high concentrations of hesperidin and naringin, flavonoids known to support vascular health and reduce oxidative damage [235]. Another illustrative case is banana peels, which are rich in catechins and anthocyanins. These compounds have shown potential in mitigating oxidative stress and regulating glucose metabolism, making them candidates for managing conditions such as diabetes and metabolic syndrome [236]. Similarly, apple peels and other fruit skin residues are abundant in quercetin glycosides and chlorogenic acid, with antioxidant activities comparable to that of synthetic antioxidants like ascorbic acid (vitamin C) [237]. These examples underscore how food waste, often discarded at an industrial scale, can be reimagined as a sustainable source of health-promoting ingredients.
Vegetable waste, including peels and trimmings from carrots, beets, tomatoes, and leafy greens, has also shown promise. Tomato skins, for instance, contain lycopene, a carotenoid with strong antioxidant and anti-proliferative properties, which is now being incorporated into capsules and supplements aimed at reducing prostate cancer risk and improving skin health [238]. Carrot waste is similarly valued for its β-carotene content, a precursor of vitamin A that plays a vital role in immune function and vision.
Technological advancements in extraction and processing have been pivotal in realizing the full potential of these by-products. Despite these advancements, several challenges hinder the wide-scale application of food waste-derived nutraceuticals. One primary concern is the variability in the composition of food waste, which depends on several factors, including plant variety, geographic origin, harvesting conditions, and post-harvest handling. This variability can affect the consistency and reproducibility of the bioactive compounds extracted, posing challenges for both quality control and regulatory approval [239]. Additionally, the presence of contaminants such as pesticides, heavy metals, or microbial pathogens in food waste raises safety concerns [240]. It is critical to implement robust decontamination and purification steps and adhere to strict food safety guidelines to ensure that the end products are safe for human consumption. Furthermore, the regulatory framework for nutraceuticals varies significantly across regions, complicating the commercialization process. In the EU, for instance, compounds classified as novel foods must undergo a rigorous approval process, whereas in the US, the FDA requires compliance with the Dietary Supplement Health and Education Act (DSHEA) but does not mandate pre-market approval unless new dietary ingredients are used. Another limitation lies in consumer acceptance. Although the concept of food waste-derived products is sustainable and scientifically sound, some consumers may harbor negative perceptions or misconceptions about consuming supplements sourced from waste [241]. Clear labelling, transparent communication, and public education campaigns are essential to increase awareness and acceptance of these products. From a scientific standpoint, further research is needed to fully understand the bioavailability, metabolism, and long-term health effects of food waste-derived bioactives. While in vitro and animal studies have demonstrated promising results, there is a scarcity of well-designed clinical trials that confirm their efficacy and safety in humans. Moreover, synergistic effects among bioactive compounds are often overlooked, despite growing evidence that combinations of phytochemicals can exert greater therapeutic effects than isolated constituents [242].
The integration of food by-product valorization into nutraceutical development also has significant economic and environmental benefits. By reducing waste disposal costs and generating new revenue streams, food processors can improve profitability. Additionally, repurposing waste into valuable supplements contributes to greenhouse gas reduction, decreased reliance on synthetic additives, and improved resource efficiency [243]. To accelerate progress in this area, collaborative efforts between academia, industry, and regulatory bodies are essential. Research institutions can lead the way in identifying new bioactives and optimizing extraction processes, while industry partners can scale up production and manage supply chains. Policymakers, meanwhile, can foster innovation by providing clear regulatory pathways and financial incentives for sustainable practices.
5. Challenges and Future Perspective
Food waste from the processing of fruits, vegetables, grains, and other agricultural raw materials, such as peels, skins, seeds, stems, and leaves, is usually considered inedible [244], but the nutritional value of these by-products demonstrate that industry has an opportunity to rethink the use of food waste [245]. These materials can be harnessed to develop new and functional food products, dietary supplements, or to enhance the nutritional profile of existing products [246]. Repurposing these materials for human consumption can contribute to reducing food waste while simultaneously providing the market with novel, health-promoting ingredients. However, in order to use these by-products and successfully introduce such products to market, it is essential to align with regulatory and legislative policies [247,248] to ensure that they meet food safety standards, proper labeling requirements, and environmental sustainability criteria [249]. Regulatory bodies such as the European Food Safety Authority (EFSA) and the Food and Drug Administration (FDA) require extensive testing and validation to demonstrate that food waste, when incorporated into food products, is safe for consumption [250,251]. This includes assessing the presence of any potential contaminants, allergens, or other harmful substances that could arise from the processing of a by-product [252].
The food waste that can be used as an additive or supplement and is rich in natural antioxidants often presents a complex mixture of secondary metabolites, many of which may not be fully identified [253]. This complexity poses a challenge in terms of understanding their complete composition and functionality once digested. One of the key issues with natural extracts is the variability in both quality and composition. They can be influenced by plant variety [254], climatic conditions [255], and the degree of ripeness at the time of harvest [256]. As a result, it becomes essential to analyze the composition of each extract batch to ensure consistency and, if necessary, adjust the quantity added to the final product [257]. The problem of variability in natural extracts highlights the need for advanced quality control and standardization practices. Without such measures, the effectiveness of antioxidants can fluctuate, leading to inconsistent product properties [258,259,260]. It may also influence sensory properties, depending on the form of waste and the product in which it is used [261]. To reduce the negative impact of natural extract variability, a fair compromise is to combine natural and synthetic antioxidants to achieve a synergistic effect [262].
Alongside the inconsistent chemical composition, utilizing BACs from food waste in the industry is significantly limited due to high contamination risks [263], including pesticide residues [264], mycotoxins [265], microbial contaminants, and heavy metals [266]. Thus, safety assessments are essential to ensure that potentially reusable food waste and derived extracts are suitable for bioactive molecule extraction. To address these contamination risks, food safety protocols require comprehensive testing at various stages, from raw material collection to the final extract [267].
The processes for reducing these antinutrients, while effective, can significantly increase operational costs, energy consumption, and processing time, particularly in large-scale production [268]. A promising alternative for industrial applications for removing contaminants from wastewater streams derived from processing raw materials is the use of membrane processes [269]. These methods are particularly valuable for recovering, separating, and fractionating specific bioactive compounds [270]. Their application has seen particular success in the valorization of food by-products and the treatment of wastewater in the food sector, making them an increasingly adopted solution for industries seeking to integrate sustainable practices and maximize resource recovery.
Due to the heterogeneity and complex biochemical structure of food waste, it is a challenge to utilize it in a single process [271]. The difficulties associated with using a single production method to maximize the value of food waste frequently lead to the development of integrated production systems, which enable the generation of multiple products through a targeted valorization strategy [272]. To promote the utilization of agro-industrial food waste for the development of new products, several steps must be optimized, including preprocessing, fermentation processes or extraction, and subsequent treatment [273].
Hodges et al. [274] highlighted the significant challenges that lie ahead in feeding the projected global population of nine billion people by 2050 [275]. Meeting future demand will be difficult due to the compounded effects of climate change and the depletion of natural resources [276]. These factors restrict agricultural growth and limit food production, creating significant obstacles for food safety [277]. Additionally, the inefficiencies in the food supply chain, particularly food thrown away, contribute to the problem. Tomlinson [277] estimated that the food waste could be sufficient to feed 1 billion people annually. A shift toward circular economy principles, where food by-products and waste are repurposed for human consumption or industrial use, presents a promising way of reducing the food waste created by industry. Figure 6 outlines key considerations in the utilization of food waste for new product development. It emphasizes the importance of meeting regulatory requirements and addressing contamination risks and the variability of natural extracts, which are critical challenges in ensuring product safety and consistency. Despite these hurdles, the approach ultimately contributes to significant waste reduction and supports sustainable innovation.
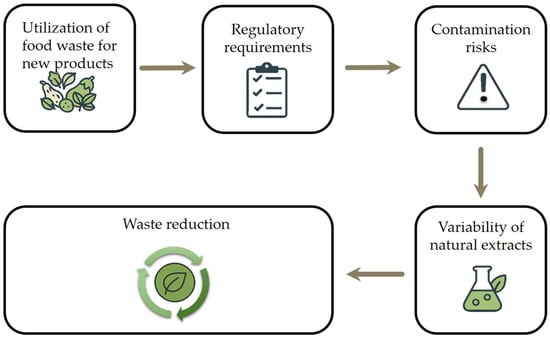
Figure 6.
Challenges and future perspectives in waste management.
In the hierarchy of waste management, waste prevention and minimization remain the priorities [278], followed closely by the utilization of by-products, especially for human consumption [279]. Achieving zero waste in the food industry remains a challenge, requiring efforts from industry, governmental institutions, academia, individuals, and organizations [280,281,282].
6. Conclusions
The valorization of food waste represents a strategic and sustainable approach to mitigating the global challenges of food loss, environmental degradation, and resource inefficiency. By reclassifying food by-products as sources of valuable bioactive compounds, particularly natural antioxidants and functional polysaccharides, the food and allied industries can simultaneously reduce waste and develop products with added nutritional and functional value. These bioactives have demonstrated significant potential in reducing oxidative stress and preventing chronic conditions, such as cardiovascular and neurodegenerative diseases. The application of green and efficient extraction techniques, such as enzymatic hydrolysis and ultrasound-assisted extraction, further enhances the recovery of these compounds while preserving their structural integrity and bioactivity. As such, food waste valorization not only aligns with circular economy principles but also contributes to the advancement of clean-label, health-promoting ingredients. This review underscores the importance of integrating scientific innovation with sustainability goals to foster a resilient and health-oriented food system. Continued research into optimized extraction methods and the biological efficacy of recovered compounds is essential to fully realize the potential of food by-product utilization. In this context, food waste management becomes not merely a challenge but also a valuable opportunity to support human health and environmental protection through the development of natural antioxidant-rich formulations.
Author Contributions
Conceptualization, A.J.T.; methodology, A.J.T., D.Š. and A.Š.; software, M.B., T.J. and D.V.; formal analysis, N.B., K.K. and A.S.; investigation, N.B., K.K. and A.S.; writing—original draft preparation, N.B., K.K. and A.S.; writing—review and editing, D.Š., A.Š., M.B., T.J., J.G.K., D.V. and A.J.T.; visualization, D.Š., A.Š. and M.B.; supervision, J.G.K. and A.J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Moraes, N.V.; Lermen, F.H.; Echeveste, M.E.S. A systematic literature review on food waste/loss prevention and minimization methods. J. Environ. Manag. 2021, 286, 112268. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.B.; Gorski, I.; Neff, R.A. Understanding and addressing waste of food in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 1633–1648. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Tamasiga, P.; Ouassou, E.H.; Onyeaka, H.; Bakwena, M.; Happonen, A.; Molala, M. Forecasting disruptions in global food value chains to tackle food insecurity: The role of AI and big data analytics—A bibliometric and scientometric analysis. J. Agric. Food Res. 2023, 14, 100819. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming plant-based waste and by-products into valuable products using various “Food Industry 4.0” enabling technologies: A literature review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Asioli, D.; Banovic, M.; Perito, M.A.; Peschel, A.O.; Stancu, V. Defining upcycled food: The dual role of upcycling in reducing food loss and waste. Trends Food Sci. Technol. 2023, 132, 132–137. [Google Scholar] [CrossRef]
- Thorsen, M.; Skeaff, S.; Goodman-Smith, F.; Thong, B.; Bremer, P.; Mirosa, M. Upcycled foods: A nudge toward nutrition. Front. Nutr. 2022, 9, 1071829. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Zujko, M.E.; Witkowska, A.M. Dietary Antioxidants and Chronic Diseases. Antioxidants 2023, 12, 362. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fruit and vegetable processing by-products as functional meat product ingredients -a chance to improve the nutritional value. LWT 2023, 189, 115442. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; Scarafoni, A.; Pilu, R.; Scaglia, B.; De Nisi, P.; Adani, F. The recovery from agro-industrial wastes provides different profiles of anti-inflammatory polyphenols for tailored applications. Front. Sustain. Food Syst. 2022, 6, 996562. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar]
- Pereira, J.A.M.; Berenguer, C.V.; Andrade, C.F.P.; Câmara, J.S. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Appl. Sci. 2022, 12, 2747. [Google Scholar] [CrossRef]
- Habib, H.M.; El-Gendi, H.; El-Fakharany, E.M.; El-Ziney, M.G.; El-Yazbi, A.F.; Al Meqbaali, F.T.; Ibrahim, W.H. Antioxidant, Anti-Inflammatory, Antimicrobial, and Anticancer Activities of Pomegranate Juice Concentrate. Nutrients 2023, 15, 2709. [Google Scholar] [CrossRef]
- Silletta, A.; Mancuso, A.; d’Avanzo, N.; Cristiano, M.C.; Paolino, D. Antimicrobial Compounds from Food Waste in Cosmetics. Cosmetics 2024, 11, 151. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Nayik, G.A. Bioactive compounds from pomegranate peels—Biological properties, structure–function relationships, health benefits and food applications—A comprehensive review. J. Funct. Foods 2024, 116, 106132. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Sar, T.; Kiraz, P.; Braho, V.; Harirchi, S.; Akbas, M.Y. Novel Perspectives on Food-Based Natural Antimicrobials: A Review of Recent Findings Published since 2020. Microorganisms 2023, 11, 2234. [Google Scholar] [CrossRef]
- El Oihabi, M.; Soultana, M.; El Fellah, I.; Fakih Lanjri, H.; Ben Allal, L.; Ammari, M.; Fakih Lanjri, A. Optimized extraction of phenolic compounds and antioxidant activity from cannabis Co-products via a combination of solvent-ultrasound-assisted extraction, response surface methodology, and sensitivity analysis. Case Stud. Chem. Environ. Eng. 2024, 10, 100906. [Google Scholar] [CrossRef]
- Sheibani, S.; Jafarzadeh, S.; Qazanfarzadeh, Z.; Osadee Wijekoon, M.M.J.; Mohd Rozalli, N.H.; Mohammadi Nafchi, A. Sustainable strategies for using natural extracts in smart food packaging. Int. J. Biol. Macromol. 2024, 267, 131537. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Mulligan, C.N.; Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery by-products: Extraction Techniques and their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Sah, P.; Rai, A.K.; Syiem, D.; Garay, R.P. Sirtuin activators as an anti-aging intervention for longevity. Explor Drug Sci. 2025, 3, 100881. [Google Scholar] [CrossRef]
- Samota, M.K.; Kaur, M.; Sharma, M.; Sarita; Krishnan, V.; Thakur, J.; Rawat, M.; Phogat, B.; Guru, P.N. Hesperidin from citrus peel waste: Extraction and its health implications. Qual. Assur. Saf. Crop. Foods 2023, 15, 71–99. [Google Scholar] [CrossRef]
- Shah, M.A.; Tariq, S.; Abuzar, S.M.; Ilyas, K.; Qadees, I.; Alsharif, I.; Anam, K.; Almutairi, R.T.; Al-Regaiey, K.A.; Babalghith, A.O.; et al. Peel waste of citrus fruits: A valuable and renewable source of polyphenols for the treatment of diabesity. Curr. Res. Biotechnol. 2024, 7, 100204. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yip, Y.M. Therapeutic Potential of Bioactive Flavonoids from Citrus Fruit Peels toward Obesity and Diabetes Mellitus. Futur. Pharmacol. 2023, 3, 14–37. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Azman, N.F.I.N.; Azlan, A.; Khoo, H.E.; Razman, M.R. Antioxidant properties of fresh and frozen peels of citrus species. Curr. Res. Nutr. Food Sci. 2019, 7, 331–339. [Google Scholar] [CrossRef]
- Chernukha, I.; Kupaeva, N.; Kotenkova, E.; Khvostov, D. Differences in Antioxidant Potential of Allium cepa Husk of Red, Yellow, and White Varieties. Antioxidants 2022, 11, 1243. [Google Scholar] [CrossRef]
- Ashraf, H.; Iahtisham-Ul-Haq; Butt, M.S.; Nayik, G.A.; Ramniwas, S.; Damto, T.; Ali Alharbi, S.; Ansari, M.J. Phytochemical and antioxidant profile of citrus peel extracts in relation to different extraction parameters. Int. J. Food Prop. 2024, 27, 286–299. [Google Scholar] [CrossRef]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of phenolic compounds with antioxidant activity from olive pomace using natural deep eutectic solvents: Modelling and optimization by response surface methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Brahmi, F.; Mateos-Aparicio, I.; Garcia-Alonso, A.; Abaci, N.; Saoudi, S.; Smail-Benazzouz, L.; Guemghar-Haddadi, H.; Madani, K.; Boulekbache-Makhlouf, L. Optimization of Conventional Extraction Parameters for Recovering Phenolic Compounds from Potato (Solanum tuberosum L.) Peels and Their Application as an Antioxidant in Yogurt Formulation. Antioxidants 2022, 11, 1401. [Google Scholar] [CrossRef]
- Aquino, G.; Basilicata, M.G.; Crescenzi, C.; Vestuto, V.; Salviati, E.; Cerrato, M.; Ciaglia, T.; Sansone, F.; Pepe, G.; Campiglia, P. Optimization of microwave-assisted extraction of antioxidant compounds from spring onion leaves using Box–Behnken design. Sci. Rep. 2023, 13, 14923. [Google Scholar] [CrossRef] [PubMed]
- Godoy, R.L.d.O.; Cabral, L.M.C.; Gomes, F.D.S.; Silva, L.O.M.; Beres, C.; Pagani, M.M.; Brígida, A.I.S.; Santiago, M.C.P.d.A.; Pacheco, S. Processing tomato waste as a potential bioactive compounds source: Phenolic compounds, antioxidant capacity and bioacessibility studies. Ciência Rural 2021, 52, e20201070. [Google Scholar]
- Uğurlu, Ş.; Günan Yücel, H.; Aksu, Z. Valorization of food wastes with a sequential two-step process for microbial β-carotene production: A zero waste approach. J. Environ. Manag. 2023, 340, 118003. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef] [PubMed]
- Jayesree, N.; Hang, P.K.; Priyangaa, A.; Krishnamurthy, N.P.; Ramanan, R.N.; Turki, M.S.A.; Charis, M.G.; Ooi, C.W. Valorisation of carrot peel waste by water-induced hydrocolloidal complexation for extraction of carotene and pectin. Chemosphere 2021, 272, 129919. [Google Scholar] [CrossRef]
- Mantiniotou, M.; Athanasiadis, V.; Kalompatsios, D.; Lalas, S.I. Optimization of Carotenoids and Other Antioxidant Compounds Extraction from Carrot Peels Using Response Surface Methodology. Biomass 2024, 5, 3. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. [Google Scholar] [CrossRef]
- Ranganath, K.G.; Shivashankara, K.S.; Roy, T.K.; Dinesh, M.R.; Geetha, G.A.; Pavithra, K.C.G.; Ravishankar, K.V. Profiling of anthocyanins and carotenoids in fruit peel of different colored mango cultivars. J. Food Sci. Technol. 2018, 55, 4566. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Meral, H.; Demirdöven, A. Recovery of carotenoids as bioactive compounds from peach pomace by an eco-friendly ultrasound-assisted enzymatic extraction. J. Food Sci. Technol. 2024, 61, 2354–2366. [Google Scholar] [CrossRef]
- Tiwari, S.; Upadhyay, N.; Singh, A.K.; Meena, G.S.; Arora, S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56, 4678–4687. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-assisted extraction of carotenoids from carrot juice processing waste using flaxseed oil as a solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Sportiello, L.; Marchesi, E.; Tolve, R.; Favati, F. Green Extraction of Carotenoids from Pumpkin By-Products Using Natural Hydrophobic Deep Eutectic Solvents: Preliminary Insights. Molecules 2025, 30, 548. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Viñas-Ospino, A.; Rita Jesus, A.; Paiva, A.; Esteve, M.J.; Frígola, A.; Blesa, J.; López-Malo, D. Comparison of green solvents for the revalorization of orange by-products: Carotenoid extraction and in vitro antioxidant activity. Food Chem. 2024, 442, 138530. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Improving carotenoid extraction, stability, and antioxidant activity from Citrus sinensis peels using green solvents. Eur. Food Res. Technol. 2023, 249, 2349–2361. [Google Scholar] [CrossRef]
- Gavril, R.N.; Stoica, F.; Lipșa, F.D.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Pumpkin and Pumpkin By-Products: A Comprehensive Overview of Phytochemicals, Extraction, Health Benefits, and Food Applications. Foods 2024, 13, 2694. [Google Scholar] [CrossRef]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Khorasaniha, R.; Olof, H.; Voisin, A.; Armstrong, K.; Wine, E.; Vasanthan, T.; Armstrong, H. Diversity of fibers in common foods: Key to advancing dietary research. Food Hydrocoll. 2023, 139, 108495. [Google Scholar] [CrossRef]
- Suresh, A.; Shobna; Salaria, M.; Morya, S.; Khalid, W.; Afzal, F.A.; Khan, A.A.; Safdar, S.; Khalid, M.Z.; Mukonzo Kasongo, E.L. Dietary fiber: An unmatched food component for sustainable health. Food Agric. Immunol. 2024, 35, 2384420. [Google Scholar] [CrossRef]
- Buljeta, I.; Šubarić, D.; Babić, J.; Pichler, A.; Šimunović, J.; Kopjar, M. Extraction of Dietary Fibers from Plant-Based Industry Waste: A Comprehensive Review. Appl. Sci. 2023, 13, 9309. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Wagner, C.E.; Richter, J.K.; Ikuse, M.; Ganjyal, G.M. Classification of select functional dietary fiber ingredients based on quantitative properties and latent qualitative criteria. J. Food Sci. 2024, 89, 6098–6112. [Google Scholar] [CrossRef]
- Fatima, I.; Gamage, I.; De Almeida, R.J.R.; Cabandugama, P.; Kamath, G. Current Understanding of Dietary Fiber and Its Role in Chronic Diseases. Mo. Med. 2023, 120, 381. [Google Scholar]
- Deng, M.; Dan, L.; Ye, S.; Chen, X.; Wang, X.; Tian, L.; Chen, J. Higher Fiber Intake is Associated with Reduced Risk of Related Surgery among Individuals with Inflammatory Bowel Disease in a Prospective Cohort Study. J. Nutr. 2023, 153, 2274–2282. [Google Scholar] [CrossRef]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef]
- Xie, Y.; Gou, L.; Peng, M.; Zheng, J.; Chen, L. Effects of soluble fiber supplementation on glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 1800–1810. [Google Scholar] [CrossRef]
- Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal Cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Calhoun, J.; Hanus, A.; Payne-Foster, P.; Stout, R.; Sherman, B.W. Increased dietary fiber is associated with weight loss among Full Plate Living program participants. Front. Nutr. 2023, 10, 1110748. [Google Scholar] [CrossRef] [PubMed]
- Alahmari, L.A. Dietary fiber influence on overall health, with an emphasis on CVD, diabetes, obesity, colon cancer, and inflammation. Front. Nutr. 2024, 11, 1510564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, Y.; Yang, N.; Xu, Y.; Wang, G. Longitudinal associations of dietary fiber and its source with 48-week weight loss maintenance, cardiometabolic risk factors and glycemic status under metformin or acarbose treatment: A secondary analysis of the March randomized trial. Nutr. Diabetes 2024, 14, 81. [Google Scholar] [CrossRef]
- Brennan, A.; Browne, S.; Kristo, S.; Sikalidis, A.K. Food Waste and Nutrition Quality in the Context of Public Health: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 5379. [Google Scholar] [CrossRef]
- Moskwa, J.; Bronikowska, M.; Socha, K.; Markiewicz-Żukowska, R. Vegetable as a Source of Bioactive Compounds with Photoprotective Properties: Implication in the Aging Process. Nutrients 2023, 15, 3594. [Google Scholar] [CrossRef]
- Hodge, C.; Taylor, C. Vitamin A Deficiency. Clin. Exp. Optom. 2023, 77, 76–77. [Google Scholar]
- Spiker, M.L.; Hiza, H.A.B.; Siddiqi, S.M.; Neff, R.A. Wasted Food, Wasted Nutrients: Nutrient Loss from Wasted Food in the United States and Comparison to Gaps in Dietary Intake. J. Acad. Nutr. Diet 2017, 117, 1031–1040.e22. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Cannas, C.; Lostia, G.; Serra, P.A.; Peana, A.T.; Migheli, R. Food and Food Waste Antioxidants: Could They Be a Potent Defence against Parkinson’s Disease? Antioxidants 2024, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Mattas, K.; Gurinovic, M.; Gkotzamani, A.; Koukounaras, A. Revealing the power of green leafy vegetables: Cultivating diversity for health, environmental benefits, and sustainability. Glob. Food Secur. 2024, 43, 100816. [Google Scholar] [CrossRef]
- Siitonen, A.; Nieminen, F.; Kallio, V.; Tuccillo, F.; Kantanen, K.; Ramos-Diaz, J.M.; Jouppila, K.; Piironen, V.; Kariluoto, S.; Edelmann, M. B Vitamins in Legume Ingredients and Their Retention in High Moisture Extrusion. Foods 2024, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; He, H.; Wang, Y.; Wang, J.; Liu, H. Toward Achieving Rapid Estimation of Vitamin C in Citrus Peels by NIR Spectra Coupled with a Linear Algorithm. Molecules 2023, 28, 1681. [Google Scholar] [CrossRef]
- Benny, N.; Shams, R.; Dash, K.K.; Pandey, V.K.; Bashir, O. Recent trends in utilization of citrus fruits in production of eco-enzyme. J. Agric. Food Res. 2023, 13, 100657. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M. Synthetic or Food-Derived Vitamin C—Are They Equally Bioavailable? Nutrients 2013, 5, 4284. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Int. 2022, 3, 100158. [Google Scholar] [CrossRef]
- Chakrabartty, I.; Mohanta, Y.K.; Nongbet, A.; Mohanta, T.K.; Mahanta, S.; Das, N.; Saravanan, M.; Sharma, N. Exploration of Lamiaceae in Cardio Vascular Diseases and Functional Foods: Medicine as Food and Food as Medicine. Front. Pharmacol. 2022, 13, 2041. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Bai, L.; Xu, D.; Zhou, Y.-M.; Zhang, Y.-B.; Zhang, H.; Chen, Y.-B.; Pereira, C.; Barros, L.; Allegra, M.; Bai, L.; et al. Antioxidant Activities of Natural Polysaccharides and Their Derivatives for Biomedical and Medicinal Applications. Antioxidants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.L.; Teng, B. Immunostimulatory Effects of Polysaccharides from Spirulina platensis In Vivo and Vitro and Their Activation Mechanism on RAW246.7 Macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Chen, S.; Teng, B.; Veeraperumal, S.; Zhong, S.; Tan, K. Oligosaccharides as Potential Regulators of Gut Microbiota and Intestinal Health in Post-COVID-19 Management. Pharmaceuticals 2023, 16, 860. [Google Scholar] [CrossRef]
- Zhao, Q.; Jiang, Y.; Zhao, Q.; Patrick Manzi, H.; Su, L.; Liu, D.; Huang, X.; Long, D.; Tang, Z.; Zhang, Y. The benefits of edible mushroom polysaccharides for health and their influence on gut microbiota: A review. Front. Nutr. 2023, 10, 1213010. [Google Scholar] [CrossRef] [PubMed]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Grover, S.; Aggarwal, P.; Kumar, A.; Kaur, S.; Yadav, R.; Babbar, N. Utilizing citrus peel waste: A review of essential oil extraction, characterization, and food-industry potential. Biomass Convers. Biorefin. 2024, 15, 5043–5064. [Google Scholar] [CrossRef]
- Rajput, S.; Kaur, S.; Panesar, P.S.; Thakur, A. Supercritical fluid extraction of essential oils from Citrus reticulata peels: Optimization and characterization studies. Biomass Convers. Biorefin. 2023, 13, 14605–14614. [Google Scholar] [CrossRef]
- Tunç, M.T.; Odabaş, H.İ. Single-step recovery of pectin and essential oil from lemon waste by ohmic heating assisted extraction/hydrodistillation: A multi-response optimization study. Innov. Food Sci. Emerg. Technol. 2021, 74, 102850. [Google Scholar] [CrossRef]
- Sandhu, H.K.; Sinha, P.; Emanuel, N.; Kumar, N.; Sami, R.; Khojah, E.; Al-Mushhin, A.A.M. Effect of Ultrasound-Assisted Pretreatment on Extraction Efficiency of Essential Oil and Bioactive Compounds from Citrus Waste By-Products. Separations 2021, 8, 244. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-refinery of orange peels waste: A new concept based on integrated green and solvent free extraction processes using ultrasound and microwave techniques to obtain essential oil, polyphenols and pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A step closer to circular bioeconomy for citrus peel waste: A review of yields and technologies for sustainable management of essential oils. J. Environ. Manag. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Pahmeyer, M.J.; Assadpour, E.; Jafari, S.M. Extraction and purification of d-limonene from orange peel wastes: Recent advances. Ind. Crops Prod. 2022, 177, 114484. [Google Scholar] [CrossRef]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef]
- Santonocito, D.; Delli Carri, M.; Campisi, A.; Sposito, G.; Pellitteri, R.; Raciti, G.; Cardullo, N.; Aquino, G.; Basilicata, M.G.; Pepe, G.; et al. Steroidal Alkaloids from Food Waste of Tomato Processing Inhibit Neuroblastoma Cell Viability. Int. J. Mol. Sci. 2023, 24, 16915. [Google Scholar] [CrossRef]
- Hossain, M.B.; Tiwari, B.K.; Gangopadhyay, N.; O’Donnell, C.P.; Brunton, N.P.; Rai, D.K. Ultrasonic extraction of steroidal alkaloids from potato peel waste. Ultrason. Sonochem. 2014, 21, 1470–1476. [Google Scholar] [CrossRef]
- Gade, A.; Pinapati, K.K.; Verma, V.; Akula, S.J.; Sharma, A.; Pullapanthula, R.; Srivastava, N.; Gade, A.; Pinapati, K.K.; Verma, V.; et al. Evaluation of Antibiofilm Activity of Alkaloids Extracted from Capsicum annuum Stalk: A Preliminary Study of Phytochemical Screening in Vegetable Waste. WBioV 2024, 15, 233–250. [Google Scholar] [CrossRef]
- 1Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Ain, H.B.U.; Saeed, F.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Food Processing Waste: A Potential Source for Bioactive Compounds. In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Cham, Switzerland, 2020; pp. 625–649. [Google Scholar]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of Industrial Onion Wastes (Allium cepa L.): Dietary Fibre and Bioactive Compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Food waste and by-product valorization as an integrated approach with zero waste: Future challenges. In Future Foods Global Trends, Opportunities, and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2022; pp. 569–596. [Google Scholar]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural bioactive compounds from food waste: Toxicity and safety concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An Overview on the Natural and Synthetic Types. Eur. Chem. Bull. 2017, 6, 365. [Google Scholar] [CrossRef]
- Uzombah, T.A.; Uzombah, T.A. The Implications of Replacing Synthetic Antioxidants with Natural Ones in the Food Systems. In Natural Food Additives; IntechOpen: London, UK, 2022. [Google Scholar]
- Sadiq, M.; Adil, M.; Paul, J. Organic food consumption and contextual factors: An attitude–behavior–context perspective. Bus. Strateg. Environ. 2023, 32, 3383–3397. [Google Scholar] [CrossRef]
- Caponio, F.; Piga, A.; Poiana, M. Valorization of Food Processing By-Products. Foods 2022, 11, 3246. [Google Scholar] [CrossRef]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M. Agro-food wastes utilization by Blakeslea trispora for carotenoids production. Acta Biochim. Pol. 2012, 59, 151–153. [Google Scholar] [CrossRef]
- Carpenter, M.; Savage, A.M. Nutrient availability in urban food waste: Carbohydrate bias in the Philadelphia–Camden urban matrix. J. Urban Ecol. 2021, 7, juab012. [Google Scholar] [CrossRef]
- Moonsamy, T.A.; Rajauria, G.; Priyadarshini, A.; Jansen, M.A.K. Food waste: Analysis of the complex and variable composition of a promising feedstock for valorisation. Food Bioprod. Process. 2024, 148, 31–42. [Google Scholar] [CrossRef]
- Prandi, B.; Faccini, A.; Lambertini, F.; Bencivenni, M.; Jorba, M.; Van Droogenbroek, B.; Bruggeman, G.; Schöber, J.; Petrusan, J.; Elst, K.; et al. Food wastes from agrifood industry as possible sources of proteins: A detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem. 2019, 286, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.K.; Chiu, S.L.H.; Lo, I.M.C. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- Mohammed, M.; Ozbay, I.; Karademir, A.; Isleyen, M. Pre-treatment and utilization of food waste as energy source by bio-drying process. Energy Procedia 2017, 128, 100–107. [Google Scholar] [CrossRef]
- Sumaiyah; Leisyah, B.M. The effect of antioxidant of grapeseed oil as skin anti-aging in nanoemulsion and emulsion preparations. Rasayan J. Chem. 2019, 12, 1185–1194. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-De la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Yang, X.Y.; Xu, B.C.; Lei, H.M.; Luo, X.; Zhu, L.X.; Zhang, Y.M.; Mao, Y.W.; Liang, R. Effects of grape seed extract on meat color and premature browning of meat patties in high-oxygen packaging. J. Integr. Agric. 2022, 21, 2445–2455. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Costa, M.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Use of Grape By-Products to Enhance Meat Quality and Nutritional Value in Monogastrics. Foods 2022, 11, 2754. [Google Scholar] [CrossRef] [PubMed]
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843. [Google Scholar] [CrossRef]
- Katsimbri, P.; Korakas, E.; Kountouri, A.; Ikonomidis, I.; Tsougos, E.; Vlachos, D.; Papadavid, E.; Raptis, A.; Lambadiari, V. The Effect of Antioxidant and Anti-Inflammatory Capacity of Diet on Psoriasis and Psoriatic Arthritis Phenotype: Nutrition as Therapeutic Tool? Antioxidants 2021, 10, 157. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 11786388211058560. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ. Technol. Innov. 2018, 10, 62–70. [Google Scholar] [CrossRef]
- Albini, A.; Albini, F.; Corradino, P.; Dugo, L.; Calabrone, L.; Noonan, D.M. From antiquity to contemporary times: How olive oil by-products and waste water can contribute to health. Front. Nutr. 2023, 10, 1254947. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.B.S.; Heinonen, M.; Frankel, E.N. Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 1998, 61, 71–75. [Google Scholar] [CrossRef]
- Jain, P.; Bhuiyan, M.H.; Hossain, K.R.; Bachar, S.C. Antibacterial and antioxidant activities of local seeded banana fruits. African J. Pharm. Pharmacol. 2011, 5, 1398–1403. [Google Scholar] [CrossRef]
- Ramli, S.; Alkarkhi, A.F.M.; Shin Yong, Y.; Min-Tze, L.; Easa, A.M. Effect of banana pulp and peel flour on physicochemical properties and in vitro starch digestibility of yellow alkaline noodles. Int. J. Food Sci. Nutr. 2009, 60, 326–340. [Google Scholar] [CrossRef]
- Bhavani, M.; Sonia, M.; Deepika, S.; Awuchi, C.G. Bioactive, antioxidant, industrial, and nutraceutical applications of banana peel. Int. J. Food Prop. 2023, 26, 1277–1289. [Google Scholar] [CrossRef]
- Haslinda, W.H.; Cheng, L.H.; Chong, L.C.; Aziah, A.A.N. Chemical composition and physicochemical properties of green banana (Musa acuminata × balbisiana Colla cv. Awak) flour. Int. J. Food Sci. Nutr. 2009, 60, 232–239. [Google Scholar] [CrossRef]
- Hussein, M.; Yonis, A.; Abd El—Mageed, H. Effect of Adding Carrot Powder on the Rheological and Sensory Properties of Pan Bread. J. Food Dairy Sci. 2013, 4, 281–289. [Google Scholar] [CrossRef]
- Tiţa, O.; Constantinescu, M.A.; Tiţa, M.A.; Bătuşaru, C.; Mironescu, I. Sensory, textural, physico-chemical and enzymatic characterization of melted cheese with added potato and carrot peels. Front. Nutr. 2023, 10, 1260076. [Google Scholar] [CrossRef]
- Kaur, P.; Subramanian, J.; Singh, A. Green extraction of bioactive components from carrot industry waste and evaluation of spent residue as an energy source. Sci. Rep. 2022, 12, 16607. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.; Torresi, P.A.; Molli, J.S.; Cardell, D.; Mammarella, E.; Yori, J.C. Novel method for valorization of by-products from carrot discards. LWT Food Sci. Technol. 2019, 100, 374–380. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and therapeutic potential of cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dalei, G.; Jena, M.; Jena, D.; Pattanaik, C.; Das, B.R.; Das, S. Fabrication of Cucumber Peel Extract-Imbued Dragon Fruit Peel Pectin Hydrogen Packaging Films: Assessment in Preservation of Chicken Meat. Poly. Eng. Sci. 2024, 64, 1391–1403. [Google Scholar] [CrossRef]
- Esparvarini, Z.; Bazargani-Gilani, B.; Pajohi-Alamoti, M.; Nourian, A. Gelatin-starch composite coating containing cucumber peel extract and cumin essential oil: Shelf life improvement of a cheese model. Food Sci. Nutr. 2022, 10, 964–978. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, S.; Lin, J.; Yang, J.; Zhang, Y.; Shen, Q.; Zhong, F.; Hou, D.; Zhou, S. Valorization of barley (Hordeum vulgare L.) brans from the sustainable perspective: A comprehensive review of bioactive compounds and health benefits with emphasis on their potential applications. Food Chem. 2024, 460, 140772. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- O’Shea, N.; Arendt, E.K.; Gallagher, E. Dietary fibre and phytochemical characteristics of fruit and vegetable by-products and their recent applications as novel ingredients in food products. Innov. Food Sci. Emerg. Technol. 2012, 16, 1–10. [Google Scholar] [CrossRef]
- Ramírez-Maganda, J.; Blancas-Benítez, F.J.; Zamora-Gasga, V.M.; García-Magaña, M.d.L.; Bello-Pérez, L.A.; Tovar, J.; Sáyago-Ayerdi, S.G. Nutritional properties and phenolic content of a bakery product substituted with a mango (Mangifera indica) “Ataulfo” processing by-product. Food Res. Int. 2015, 73, 117–123. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of waste mango peels for extraction of polyphenolic antioxidants by ultrasound-assisted natural deep eutectic solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar]
- Elsawy, H.A.; Alessa, F.M.; El-Kholany, E.A. Production and Evaluation of Peanut Butter Prepared with Peanut Shells. Curr. Nutr. Food Sci. 2023, 20, 1019–1027. [Google Scholar] [CrossRef]
- Bertagnolli, S.M.M.; Silveira, M.L.R.; Fogaça, A.d.O.; Umann, L.; Penna, N.G. Bioactive compounds and acceptance of cookies made with Guava peel flour. Food Sci. Technol. 2014, 34, 303–308. [Google Scholar] [CrossRef]
- Araujo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of bioactive components from avocado peels using microwave-assisted extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.d.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Sobral, M.M.C.; Viegas, O.; Cunha, S.C.; Alarcón-Enos, J.; Pinho, O.; Ferreira, I.M.P.L.V.O. Incorporation of avocado peel extract to reduce cooking-induced hazards in beef and soy burgers: A clean label ingredient. Food Res. Int. 2021, 147, 110434. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M.M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. Preserv. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of Apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Lazzaroli, C.; Sordini, B.; Daidone, L.; Veneziani, G.; Esposto, S.; Urbani, S.; Selvaggini, R.; Servili, M.; Taticchi, A. Recovery and valorization of food industry by-products through the application of Olea europaea L. leaves in kombucha tea manufacturing. Food Biosci. 2023, 53, 102551. [Google Scholar] [CrossRef]
- Radočaj, O.; Dimić, E.; Diosady, L.L.; Vujasinović, V. Optimizing the texture attributes of a fat-based spread using instrumental measurements. J. Texture Stud. 2011, 42, 394–403. [Google Scholar] [CrossRef]
- Bansal, S.; Sudha, M.L. Nutritional, microstructural, rheological and quality characteristics of biscuits using processed wheat germ. Int. J. Food Sci. Nutr. 2011, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Ghiasi, F.; Eskandari, M.H.; Farahnaky, A. Roasted Wheat Germ: A Natural Plant Product in Development of Nutritious Milk Pudding; Physicochemical and Nutritional Properties. Foods 2022, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products--a promising source of bioactive ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; López-Bascón, M.A.; Sánchez-González, C.; Xiao, J.; Sumalla-Cano, S.; Battino, M.; Forbes-Hernández, T.Y.; Quiles, J.L. In Vitro and In Vivo Insights into a Broccoli Byproduct as a Healthy Ingredient for the Management of Alzheimer’s Disease and Aging through Redox Biology. J. Agric. Food Chem. 2024, 72, 5197–5211. [Google Scholar] [CrossRef]
- Liu, X.Q.; Yonekura, M.; Tsutsumi, M.; Sano, Y. Physicochemical Properties of Aggregates of Globin Hydrolysates. J. Agric. Food Chem. 1996, 44, 2957–2961. [Google Scholar] [CrossRef]
- Devadason, I.P.; Anjaneyulu, A.S.R.; Babji, Y. Effect of Different Binders on the Physico-Chemical, Textural, Histological, and Sensory Qualities of Retort Pouched Buffalo Meat Nuggets. J. Food Sci. 2010, 75, S31–S35. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Abrigo, C.; Cipolla, G. High-performance liquid chromatography of food colours and its relevance in forensic chemistry. J. Chromatogr. A 1994, 674, 281–299. [Google Scholar] [CrossRef]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Olt, V.; Báez, J.; Curbelo, R.; Boido, E.; Amarillo, M.; Gámbaro, A.; Alborés, S.; Gerez García, N.; Cesio, M.V.; Heinzen, H.; et al. Tannat grape pomace as an ingredient for potential functional biscuits: Bioactive compound identification, in vitro bioactivity, food safety, and sensory evaluation. Front. Nutr. 2023, 10, 1241105. [Google Scholar] [CrossRef] [PubMed]
- Pokorski, P.; Hoffmann, M. Valorization of bio-waste eggshell as a viable source of dietary calcium for confectionery products. J. Sci. Food Agric. 2022, 102, 3193–3203. [Google Scholar] [CrossRef]
- Arnold, M.; Rajagukguk, Y.V.; Sidor, A.; Kulczyński, B.; Brzozowska, A.; Suliburska, J.; Wawrzyniak, N.; Gramza-Michałowska, A. Innovative Application of Chicken Eggshell Calcium to Improve the Functional Value of Gingerbread. Int. J. Environ. Res. Public Health 2022, 19, 4195. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Diep, T.; Hudson, H.A.; Hincke, M.T. High value applications and current commercial market for eggshell membranes and derived bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef]
- Mondal, K.; Bhattacharjee, S.K.; Mudenur, C.; Ghosh, T.; Goud, V.V.; Katiyar, V. Development of antioxidant-rich edible active films and coatings incorporated with de-oiled ethanolic green algae extract: A candidate for prolonging the shelf life of fresh produce. RSC Adv. 2022, 12, 13295–13313. [Google Scholar] [CrossRef]
- Besediuk, V.; Yatskov, M.; Korchyk, N.; Kucherova, A.; Maletskyi, Z. Whey—From waste to a valuable resource. J. Agric. Food Res. 2024, 18, 101280. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef]
- Mabeau, S.; Baty-Julien, C.; Hélias, A.B.; Chodosas, O.; Surbled, M.; Metra, P.; Durand, D.; Morice, G.; Chesné, C.; Mekideche, K. Antioxidant activity of artichoke extracts and by-products. Acta Hortic. 2007, 730, 491–496. [Google Scholar] [CrossRef]
- Larrosa, M.; Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A. Increase of Antioxidant Activity of Tomato Juice Upon Functionalisation with Vegetable Byproduct Extracts. LWT—Food Sci. Technol. 2002, 35, 532–542. [Google Scholar] [CrossRef]
- Górecka, D.; Pachołek, B.; Dziedzic, K.; Górecka, M. Raspberry pomace as a potential fiber source for cookies enrichment. Acta Sci. Pol. Technol. Aliment. 2010, 9, 451–461. [Google Scholar]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2015, 53, 1140. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Čechovičienė, I.; Jukniūtė, K.; Šlepetienė, A.; Paulauskienė, A. Qualitative properties of cookies enriched with berries pomace. Food Sci. Technol. 2021, 41, 474–481. [Google Scholar] [CrossRef]
- Laccourreye, O.; Maisonneuve, H. French scientific medical journals confronted by developments in medical writing and the transformation of the medical press. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 475–480. [Google Scholar] [CrossRef]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanoǧlu, E.; Ibanoǧlu, Ş. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J. Food Eng. 2008, 87, 554–563. [Google Scholar] [CrossRef]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q.; et al. Potential of Fruit Wastes as Natural Resources of Bioactive Compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural Pectin Polysaccharides as Edible Coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Gałkowska, D.; Długosz, M.; Juszczak, L.; Takamine, K.; Abe, J.; Shimono, K.; Sameshima, Y.; Morimura, S.; Kida, K.; Sriamornsak, P. Chemistry of Pectin and Its Pharmaceutical Uses: A Review. Silpakorn Univ. Open J. Syst. 2003, 3, 206–228. [Google Scholar]
- Kang, H.J.; Chawla, S.P.; Jo, C.; Kwon, J.H.; Byun, M.W. Studies on the development of functional powder from citrus peel. Bioresour. Technol. 2006, 97, 614–620. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus peel as a renewable bioresource: Transforming waste to food additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, W.S.; Oh, S.W. Effect of layer-by-layer antimicrobial edible coating of alginate and chitosan with grapefruit seed extract for shelf-life extension of shrimp (Litopenaeus vannamei) stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef]
- Prata, C.; Zalambani, C.; Rossi, F.; Rossello, S.; Cerchiara, T.; Cappadone, C.; Malucelli, E. Nutrients and Nutraceuticals from Vitis vinifera L. Pomace: Biological Activities, Valorization, and Potential Applications. Nutrients 2025, 17, 583. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate Juice, Total Pomegranate Ellagitannins, and Punicalagin Suppress Inflammatory Cell Signaling in Colon Cancer Cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ayodele, O.M. Effect of Adding Pomegranate (Punica granatum) Juice and Seed Extract on the Physicochemical, Rheological and Sensory Properties of Ice Cream. J. Adv. Food Sci. Technol. 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Antioxidant capacity versus chemical safety of wheat bread enriched with pomegranate peel powder. Food Funct. 2013, 4, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Lu, Z.; Yang, C. Enhanced Morris method for global sensitivity analysis: Good proxy of Sobol’ index. Struct. Multidiscip. Optim. 2019, 59, 373–387. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Jridi, M.; Khemakhem, I.; Bouaziz, M.; Attia, H. Storage stability of traditional Tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem. 2017, 233, 476–482. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Joshi, A.; Sethi, S.; Arora, B.; Azizi, A.F.; Thippeswamy, B. Potato Peel Composition and Utilization. In Potato Nutrition and Food Security; Springer: Cham, Switzerland, 2020; pp. 229–245. [Google Scholar]
- Arora, A.; Camire, M.E. Performance of potato peels in muffins and cookies. Food Res. Int. 1994, 27, 15–22. [Google Scholar] [CrossRef]
- Franco, D.; Pateiro, M.; Rodríguez Amado, I.; López Pedrouso, M.; Zapata, C.; Vázquez, J.A.; Lorenzo, J.M. Antioxidant ability of potato (Solanum tuberosum) peel extracts to inhibit soybean oil oxidation. Eur. J. Lipid Sci. Technol. 2016, 118, 1891–1902. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.-H.; Al-sayed, H.; Yasin, N.; Afifi, E. Effect of Different Extraction Methods on Stablity of Anthocyanins Extracted from Red Onion peels (Allium cepa) and Its Uses as Food Colorants. Bull. Natl. Nutr. Inst. Arab Repub. Egypt 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Vichare, S.A.; Morya, S. Exploring waste utilization potential: Nutritional, functional and medicinal properties of oilseed cakes. Front. Food Sci. Technol. 2024, 4, 1441029. [Google Scholar] [CrossRef]
- Ahmad, T.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Abdullah; Xu, Y.; Zisheng, L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food Sci. Technol. 2020, 99, 21–33. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Food industry by-products used as functional ingredients of bakery products. Trends Food Sci. Technol. 2017, 67, 106–128. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Ermosh, L.G.; Prisuhina, N.V.; Koch, D.A.; Eremina, E.V. The use of oilseed cake for supplementation of bakery products. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 022090. [Google Scholar] [CrossRef]
- Abedini, A.; Alizadeh, A.M.; Mahdavi, A.; Golzan, S.A.; Salimi, M.; Tajdar-Oranj, B.; Hosseini, H. Oilseed Cakes in the Food Industry; A Review on Applications, Challenges, and Future Perspectives. Curr. Nutr. Food Sci. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- Tsakali, E.; Petrotos, K.; D’Alessandr, A.G.; Goulas, P. A review on whey composition and the methods used for its utilization for food and pharmaceutical products. In Proceedings of the 6th International Conference on Simulation and Modelling in the Food and Bio-Industry, Braganca, Portugal, 24–26 June 2010; pp. 195–201. [Google Scholar]
- Soltani, M.; Say, D.; Guzeler, N. Functional Properties and Nutritional Quality of Whey Proteins. J. Int. Environ. Appl. Sci. 2017, 12, 334–338. [Google Scholar]
- Arshad, U.E.T.; Hassan, A.; Ahmad, T.; Naeem, M.; Chaudhary, M.T.; Abbas, S.Q.; Randhawa, M.A.; Pimentel, T.C.; da Cruz, A.G.; Aadil, R.M. A recent glance on the valorisation of cheese whey for industrial prerogative: High-value-added products development and integrated reutilising strategies. Int. J. Food Sci. Technol. 2023, 58, 2001–2013. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Gocmen, D.; Sahan, Y.; Yildiz, E.; Coskun, M.; Aroufai, İ.A. Use of coffee silverskin to improve the functional properties of cookies. J. Food Sci. Technol. 2019, 56, 2979–2988. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; Del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silverskin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; Castillo, M.D. Del Use of coffee silverskin and stevia to improve the formulation of biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee Silverskin Extract Protects against Accelerated Aging Caused by Oxidative Agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef]
- Karmakar, R.; Aggarwal, S.; Kathuria, D.; Singh, N.; Tripathi, V.; Sharma, P.K.; Mitra, S.; Bhattacharya, S. Valorization of food waste stream by harnessing bioactive compounds: A comprehensive review on the process, challenges and solutions. Food Biosci. 2025, 69, 106833. [Google Scholar] [CrossRef]
- United Nations. The 17 Goals: Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 28 May 2025).
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Xu, Y.; He, P.; He, B.; Chen, Z. Bioactive flavonoids metabolites in citrus species: Their potential health benefits and medical potentials. Front Pharmacol. 2025, 16, 1552171. [Google Scholar] [CrossRef]
- Zaini, H.M.; Roslan, J.; Sallah, S.; Munsu, E.; Sulaiman, N.S.; Pindi, W. Banana peels as a bioactive ingredient and its potential application in the food industry. J. Funct. Foods 2022, 92, 105054. [Google Scholar] [CrossRef]
- Asma, U.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Apples and Apple By-Products: Antioxidant Properties and Food Applications. Antioxidants 2023, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Bolaño, D.C.; Insuasty, D.; Rodríguez Macías, J.D.; Grande-Tovar, C.D. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules 2024, 29, 3079. [Google Scholar] [CrossRef]
- Hlatshwayo, S.; Thembane, N.; Krishna, S.B.N.; Gqaleni, N.; Ngcobo, M. Extraction and Processing of Bioactive Phytoconstituents from Widely Used South African Medicinal Plants for the Preparation of Effective Traditional Herbal Medicine Products: A Narrative Review. Plants 2025, 14, 206. [Google Scholar] [CrossRef]
- Mititelu, M.; Neacșu, S.M.; Busnatu, Ș.S.; Scafa-Udriște, A.; Andronic, O.; Lăcraru, A.-E.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Negrei, C.; Olteanu, G. Assessing Heavy Metal Contamination in Food: Implications for Human Health and Environmental Safety. Toxics 2025, 13, 333. [Google Scholar] [CrossRef]
- Almansour, M.; Akrami, M. Towards Zero Waste: An In-Depth Analysis of National Policies, Strategies, and Case Studies in Waste Minimisation. Sustainability 2024, 16, 10105. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghian, H.; Bolton, K.; Rousta, K. Challenges for Upcycled Foods: Definition, Inclusion in the Food Waste Management Hierarchy and Public Acceptability. Foods 2021, 10, 2874. [Google Scholar] [CrossRef]
- Despoudi, S.; Bucatariu, C.; Otles, S.; Kartal, C. Food waste management, valorization, and sustainability in the food industry. In Food Waste Recovery, Processing Technologies, Industrial Techniques, and Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 3–19. [Google Scholar]
- Pleissner, D. Recycling and reuse of food waste. Curr. Opin. Green Sustain. Chem. 2018, 13, 39–43. [Google Scholar] [CrossRef]
- Garske, B.; Heyl, K.; Ekardt, F.; Weber, L.M.; Gradzka, W. Challenges of Food Waste Governance: An Assessment of European Legislation on Food Waste and Recommendations for Improvement by Economic Instruments. Land 2020, 9, 231. [Google Scholar] [CrossRef]
- Faishal, A. Suprapto Laws and Regulations Regarding Food Waste Management as a Function of Environmental Protection in a Developing Nation. Int. J. Crim. Justice Sci. 2022, 17, 223–237. [Google Scholar]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Albizzati, P.F.; Tonini, D.; Astrup, T.F. A Quantitative Sustainability Assessment of Food Waste Management in the European Union. Environ. Sci. Technol. 2021, 55, 16099–16109. [Google Scholar] [CrossRef]
- Bhat, R. Sustainability challenges in the valorization of agri-food wastes and by-products. In Valorization of Agri-Food Wastes and By-Products, Recent Trends, Innovations and Sustainability Challenges; Academic Press: Cambridge, MA, USA, 2021; pp. 1–27. [Google Scholar]
- Pinela, J.; Añibarro-Ortega, M.; Barros, L. Food Waste Biotransformation into Food Ingredients: A Brief Overview of Challenges and Opportunities. Foods 2024, 13, 3389. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Stojakowska, A. Stem Lettuce and Its Metabolites: Does the Variety Make Any Difference? Foods 2020, 10, 59. [Google Scholar] [CrossRef]
- Saini, M.K.; Capalash, N.; Kaur, C.; Singh, S.P. Targeted metabolic profiling indicates differences in primary and secondary metabolites in Kinnow mandarin (C. nobilis × C. deliciosa) from different climatic conditions. J. Food Compos. Anal. 2019, 83, 103278. [Google Scholar] [CrossRef]
- Serrano-García, I.; Hurtado-Fernández, E.; Gonzalez-Fernandez, J.J.; Hormaza, J.I.; Pedreschi, R.; Reboredo-Rodríguez, P.; Figueiredo-González, M.; Olmo-García, L.; Carrasco-Pancorbo, A. Prolonged on-tree maturation vs. cold storage of Hass avocado fruit: Changes in metabolites of bioactive interest at edible ripeness. Food Chem. 2022, 394, 133447. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential Anticancer Properties of Grape Antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef]
- Sasse, A.; Colindres, P.; Brewer, M.S. Effect of Natural and Synthetic Antioxidants on the Oxidative Stability of Cooked, Frozen Pork Patties. J. Food Sci. 2009, 74, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Hamdo, H.H.; Khayata, W.; Al-Assaf, Z. Synergistic effect of combined some natural and synthetic antioxidants to increase oxidative stability using DPPH test. Int. J. ChemTech Res. 2014, 6, 2539–2545. [Google Scholar]
- O’Connor, J.; Mickan, B.S.; Siddique, K.H.M.; Rinklebe, J.; Kirkham, M.B.; Bolan, N.S. Physical, chemical, and microbial contaminants in food waste management for soil application: A review. Environ. Pollut. 2022, 300, 118860. [Google Scholar] [CrossRef]
- Nicastro, R.; Papale, M.; Fusco, G.M.; Capone, A.; Morrone, B.; Carillo, P. Legal Barriers in Sustainable Agriculture: Valorization of Agri-Food Waste and Pesticide Use Reduction. Sustainability 2024, 16, 8677. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Su, Y.; Xie, B. Occurrence, influence and removal strategies of mycotoxins, antibiotics and microplastics in anaerobic digestion treating food waste and co-digestive biosolids: A critical review. Bioresour. Technol. 2021, 330, 124987. [Google Scholar] [CrossRef]
- Ma, J.; Wu, S.; Shekhar, N.V.R.; Biswas, S.; Sahu, A.K. Determination of Physicochemical Parameters and Levels of Heavy Metals in Food Waste Water with Environmental Effects. Bioinorg. Chem. Appl. 2020, 2020, 8886093. [Google Scholar] [CrossRef]
- Viscardi, S.; Colicchia, C. A classification of food products to enhance circular economy and reduce waste: A systematic literature review. Resour. Conserv. Recycl. Adv. 2024, 23, 200229. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022, 424, 127432. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Conidi, C.; Cassano, A. Membrane-based technologies for meeting the recovery of biologically active compounds from foods and their by-products. Crit. Rev. Food Sci. Nutr. 2019, 59, 2927–2948. [Google Scholar] [CrossRef]
- Redlingshöfer, B.; Barles, S.; Weisz, H. Are waste hierarchies effective in reducing environmental impacts from food waste? A systematic review for OECD countries. Resour. Conserv. Recycl. 2020, 156, 104723. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Dutta, S.; He, M.; Xiong, X.; Tsang, D.C.W. Sustainable management and recycling of food waste anaerobic digestate: A review. Bioresour. Technol. 2021, 341, 125915. [Google Scholar] [CrossRef]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Flora, C.B. Food security in the context of energy and resource depletion: Sustainable agriculture in developing countries. Renew. Agric. Food Syst. 2010, 25, 118–128. [Google Scholar] [CrossRef]
- Wunderlich, S.M.; Martinez, N.M. Conserving natural resources through food loss reduction: Production and consumption stages of the food supply chain. Int. Soil Water Conserv. Res. 2018, 6, 331–339. [Google Scholar] [CrossRef]
- Tomlinson, I. Doubling food production to feed the 9 billion: A critical perspective on a key discourse of food security in the UK. J. Rural Stud. 2013, 29, 81–90. [Google Scholar] [CrossRef]
- Gusmerotti, N.M.; Corsini, F.; Borghini, A.; Frey, M. Assessing the role of preparation for reuse in waste-prevention strategies by analytical hierarchical process: Suggestions for an optimal implementation in waste management supply chain. Environ. Dev. Sustain. 2019, 21, 2773–2792. [Google Scholar] [CrossRef]
- Awino, F.B.; Apitz, S.E. Solid waste management in the context of the waste hierarchy and circular economy frameworks: An international critical review. Integr. Environ. Assess. Manag. 2024, 20, 9–35. [Google Scholar] [CrossRef] [PubMed]
- Peano, C.; Merlino, V.M.; Sottile, F.; Borra, D.; Massaglia, S. Sustainability for Food Consumers: Which Perception? Sustainability 2019, 11, 5955. [Google Scholar] [CrossRef]
- Friedmann, H. Scaling up: Bringing public institutions and food service corporations into the project for a local, sustainable food system in Ontario. Agric. Hum. Values 2007, 24, 389–398. [Google Scholar] [CrossRef]
- Boccia, F.; Covino, D. Innovation and sustainability in agri-food companies: The role of quality. Riv. Studi Sulla Sostenibilita 2016, 2016, 131–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).