Developing a Feeding Module with a Blend of Garlic Oil and Cinnamon Bark for Enhancing Antioxidant Status and Immunity of Murrah Buffalo (Bubalus bubalis) with an Improvement in Feed Efficiency and Reduced Methane Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Blend of Garlic Oil and Cinnamon Bark
2.2. Experimental Animals, Diet and Management
2.3. Recording of Feed Intake and Body Weight Changes
2.4. Haemato-Biochemical Studies
2.5. Erythrocytic Antioxidant Status
2.6. Immunity Assessment
2.7. Digestion Trial and Sampling Protocol
2.8. Enteric Methane Production Measurement
2.9. Chemical Analyses
2.10. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, S.S.; Dey, A. Nutrition in health and immune function of ruminants. Indian J. Anim. Sci. 2015, 85, 103–112. [Google Scholar] [CrossRef]
- Niu, X.; Ding, Y.; Chen, S.; Gooneratne, R.; Ju, X. Effect of immune stress on growth performance and immune functions of livestock: Mechanisms and prevention. Animals 2022, 12, 909. [Google Scholar] [CrossRef]
- Klasing, K.C. Nutrition and the immune system. Br. Poult. Sci. 2007, 48, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between microbes and host intestinal health: Modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, H.; Sharan, K. Food and nutrition as modifiers of the immune system: A mechanistic overview. Trends Food Sci. Technol. 2022, 123, 393–403. [Google Scholar] [CrossRef]
- Getabalew, M.; Alemneh, T.; Akeberegn, D. Methane production in ruminant animals: Implication for their impact on climate change. Concepts Dairy Vet. Sci. 2019, 2, 2637–4749. [Google Scholar]
- Firkins, J.L.; Mackie, R.I. Ruminal protein breakdown and ammonia assimilation. In Improving Rumen Function; Burleigh Dodds Science Publishing: Sawston, UK, 2020; pp. 383–420. [Google Scholar]
- Sheoran, S.; Dey, A.; Sindhu, S. Reduction of methane and nitrogen emission and improvement of feed efficiency, rumen fermentation, and milk production through strategic supplementation of eucalyptus (Eucalyptus citriodora) leaf meal in the diet of lactating buffalo (Bubalus bubalis). Environ. Sci. Pollut. Res. 2023, 30, 125510–125525. [Google Scholar] [CrossRef]
- Kholif, A.E. The Impact of Varying Levels of Laurus nobilis Leaves as a Sustainable Feed Additive on Ruminal Fermentation: In Vitro Gas Production, Methane and Carbon Dioxide Emissions, and Ruminal Degradability of a Conventional Diet for Ruminants. Fermentation 2024, 10, 387. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Amer, S.A.; Abdel-Wareth, A.A.A.; Gouda, A.; Saleh, G.K.; Nassar, A.H.; Sherief, W.R.I.A.; Albogami, S.; Shalaby, S.I.; Abdelazim, A.M.; Abomughaid, M.M. Impact of dietary lavender essential oil on the growth and fatty acid profile of breast muscles, antioxidant activity, and inflammatory responses in broiler chickens. Antioxidants 2022, 11, 1798. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- DAHD. 20th Livestock Census-2019 All India Report; Ministry of Fisheries, Animal Husbandry and Dairying, Department of Animal Husbandry and Dairying, Government of New Delhi: New Delhi, India, 2019; pp. 1–42. [Google Scholar]
- ICAR. Nutrient Requirement of Animals-Buffalo; Indian Council of Agricultural Research: New Delhi, India, 2024; pp. 1–40. ISBN 978-81-954201-9-3. [Google Scholar]
- Patra, A.K. Trends and projected estimates of GHG emissions from Indian livestock in comparisons with GHG emissions from world and developing countries. Asian-Australas. J. Anim. Sci. 2014, 27, 592. [Google Scholar] [CrossRef]
- Dey, A.; Paul, S.S.; Lailer, P.C.; Dahiya, S.S. Reducing enteric methane production from buffalo (Bubalus bubalis) by garlic oil supplementation in in vitro rumen fermentation system. SN Appl. Sci. 2021, 3, 187. [Google Scholar] [CrossRef]
- Sari, N.F.; Ray, P.; Rymer, C.; Kliem, K.E.; Stergiadis, S. Garlic and its bioactive compounds: Implications for methane emissions and ruminant nutrition. Animals 2022, 12, 2998. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.; Dunshea, F.R. Cinnamon: A natural feed additive for poultry health and production—A review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 2021, 8, 628445. [Google Scholar] [CrossRef]
- Oh, J.; Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Varga, G.A.; Pate, J.; Walusimbi, S.; Brzezicka, E.; Toyokawa, K.; et al. Immune and production responses of dairy cows to postruminal supplementation with phytonutrients. J. Dairy Sci. 2013, 96, 7830–7843. [Google Scholar] [CrossRef]
- Imbabi, T.; Hassan, T.M.; Osman, A.; El Aziz, A.H.A.; Tantawi, A.A.; Nasr, M.A. Impacts of thyme and/or garlic oils on growth, immunity, antioxidant and net farm income in Damascus goats. Sci. Rep. 2024, 14, 13173. [Google Scholar] [CrossRef]
- Chaves, A.V.; Dugan, M.E.R.; Stanford, K.; Gibson, L.L.; Bystrom, J.M.; McAllister, T.A.; Van Herk, F.; Benchaar, C. A dose-response of cinnamaldehyde supplementation on intake, ruminal fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest. Sci. 2011, 141, 213–220. [Google Scholar] [CrossRef]
- Singh, R.K.; Dey, A.; Paul, S.S.; Singh, M.; Dahiya, S.S.; Punia, B.S. Associative effects of plant secondary metabolites in modulating in vitro methanogenesis, volatile fatty acids production and fermentation of feed in buffalo (Bubalus bubalis). Agrofor. Syst. 2020, 94, 1555–1566. [Google Scholar] [CrossRef]
- Kumar, K. Effects of Feed Additives Rich in Essential Oils on Rumen Fermentation, Methanogenesis and Nutrient Utilization in Buffalo. Master’s Thesis, Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar, Haryana, India, 2017. [Google Scholar]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.K.; Loos, J.A. Biochemical Methods in Red Cell Genetics; Academic Press: New York, NY, USA, 1969; pp. 127–129. [Google Scholar]
- Bergmeyer, H. UV method of catalase assay. In Methods of Enzymatic Analysis; Deerfield Beach, Florida, Bansal: Weinheim, Germany, 1983. [Google Scholar]
- Madesh, M.; Balasubramanian, K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J. Biochem. Biophys. 1998, 35, 184–188. [Google Scholar]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Richateich, R. Clinical Chemistry Theory and Practice; Academic Press: New York, NY, USA; London, UK, 1969. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2014. [Google Scholar]
- AOAC. Association of Official Analytical Chemistry–AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- SPSS, Version 20.0; Statistical Packages for Social Sciences; SPSS Inc.: Chicago, IL, USA, 2011.
- Snedecor, G.; Cochran, W. Statistical Methods, 8th ed.; East West Press Pvt. Ltd.: New Delhi, India, 1994; p. 313. [Google Scholar]
- Lawson, L.D. The Composition and Chemistry of Garlic Cloves and Processed Garlic; Koch, H.P., Lawson, L.D., Eds.; Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species; Williams and Wilkins: Baltimore, MD, USA, 2006. [Google Scholar]
- Ozma, M.A.; Abbasi, A.; Rezaee, M.A.; Hosseini, H.; Sabahi, N.H.S.; Noori, S.M.A.; Sepordeh, S.; Khodadadi, E.; Lahouty, M.; Kafil, H.S.; et al. A critical review on the nutritional and medicinal profiles of garlic’s (Allium sativum L.) bioactive compounds. Food Rev. Int. 2023, 39, 6324–6361. [Google Scholar] [CrossRef]
- Mirunalini, S.; Dhamodharan, G.; Karthishwaran, K. A natural wonder drug helps to prevent cancer: Garlic oil. Not. Sci. Biol. 2010, 2, 14–19. [Google Scholar] [CrossRef]
- Ding, H.; Ao, C.; Zhang, X. Potential use of garlic products in ruminant feeding: A review. Anim. Nutr. 2023, 14, 343–355. [Google Scholar] [CrossRef]
- Borzoei, A.; Rafraf, M.; Niromanesh, S.; Farzadi, L.; Narimani, F.; Doostan, F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J. Tradit. Complement. Med. 2018, 8, 128–133. [Google Scholar] [CrossRef]
- Jamroz, D.; Wertelecki, T.; Houszka, M.; Kamel, C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006, 90, 255–268. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, R.; Lopez, S.; Fernandez, M.; Bodas, R.; Gonzalez, J.S. Screening the activity of plants and spices for decreasing ruminal methane production in vitro. Anim. Feed. Sci. Technol. 2008, 147, 36–52. [Google Scholar] [CrossRef]
- Luo, Q.; Li, N.; Zheng, Z.; Chen, L.; Mu, S.; Chen, L.; Liu, Z.; Yan, J.; Sun, C. Dietary cinnamaldehyde supplementation improves the growth performance, oxidative stability, immune function, and meat quality in finishing pigs. Livest. Sci. 2020, 240, 104221. [Google Scholar] [CrossRef]
- Jakhmola, R.C.; Sahoo, A.; Tripathi, M.K.; Sharma, T. Phytochemicals in Animal Nutrition. In Proceedings of the 1st Conference of Indian Academy of Veterinary Nutrition and Animal Welfare, Chhattisgarh, India, 11–12 September 2011; College of Veterinary Science and Animal Husbandry (IGKV): Durg Chhattisgarh, India, 2011; pp. 24–25. [Google Scholar]
- Ramdani, D.; Yuniarti, E.; Jayanegara, A.; Chaudhry, A.S. Roles of essential oils, polyphenols, and saponins of medicinal plants as natural additives and anthelmintics in ruminant diets: A systematic review. Animals 2023, 13, 767. [Google Scholar] [CrossRef]
- Wang, F.; Xu, R.; Zheng, F.; Liu, H. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp. Anim. Tokyo 2018, 67, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Venskutonis, P.R.; Gruzdien, A.; Tirzite, D.; Tirzitis, G. Assessment of antioxidant activity of plant extracts by different methods. Acta Hortic. 2005, 677, 99–107. [Google Scholar] [CrossRef]
- Droge, W. Aging-related changes in the thiol/disulfide redox state: Implications for the use of thiol antioxidants. Exp. Gerontol 2002, 37, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.D.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Kumar, K.; Dey, A.; Rose, M.K.; Dahiya, S.S. Impact of dietary phytogenic composite feed additives on immune response, antioxidant status, methane production, growth performance and nutrient utilization of buffalo (Bubalus bubalis) calves. Antioxidants 2022, 11, 325. [Google Scholar] [CrossRef]
- El-Naggar, S.; Ibrahim, E.M. Impact of incorporating garlic or cumin powder in lambs ration on nutrients digestibility, blood constituents and growth performance. Egypt. J. Nutr. Feed. 2018, 21, 355–364. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, S.; Kim, M.J.; Han, S.G.; Lee, H.G. Dietary supplementation with combined extracts from garlic (Allium sativum), brown seaweed (Undaria pinnatifida), and pinecone (Pinus koraiensis) improves milk production in Holstein cows under heat stress conditions. Asian-Australas. J. Anim. Sci. 2020, 33, 111–119. [Google Scholar] [CrossRef]
- Kewan, K.Z.; Ali, M.M.; Ahmed, B.M.; El-Kolty, S.A.; Nayel, U.A. The effect of yeast (Saccharomyces cerevisiae), garlic (Allium sativum) and their combination as feed additives in finishing diets on the performance, ruminal fermentation, and immune status of lambs. Egypt. J. Nutr. Feed. 2021, 24, 55–76. [Google Scholar] [CrossRef]
- El-Azrak, K.E.D.M.; Morsy, A.S.; Soltan, Y.A.; Hashem, N.M.; Sallam, S.M.A. Impact of specific essential oils blend on milk production, serum biochemical parameters and kid performance of goats. Anim. Biotechnol. 2022, 33, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Chen, Y.; Min, B.J.; Kim, H.J.; Kwon, O.S.; Shon, K.S.; Kim, I.-S.; Kim, S.J.; Asamer, A. Effects of essential oils supplementation on growth performance, IgG concentration and fecal noxious gas concentration of weaned pigs. Asian-Aust. J. Anim. Sci. 2006, 19, 80–85. [Google Scholar]
- Mir, P.S.; Mears, G.J.; Okine, E.K.; Entz, T.; Ross, C.M.; Husar, S.D.; Mir, Z. Effects of increasing dietary grain on viscosity of duodenal digesta and plasma hormone, glucose and amino acid concentrations in steers. Can. J. Anim. Sci. 2000, 80, 703–712. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Ma, T.; Chen, D.; Tu, Y.; Zhang, N.; Si, B.; Deng, K.; Diao, Q. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. J. Anim. Sci. Biotechnol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Abdou, A.R.; El-Gendy, M.H. Impact of Anise, Clove, and Thyme essential oils as feed supplements on the productive performance and digestion of Barki ewes. Aust. J. Basic Appl. Sci. 2019, 13, 1–13. [Google Scholar]
- Wallace, R.J.; McEwan, N.R.; McIntosh, F.M.; Teferedegne, B.; Newbold, C.J. Natural products as manipulators of rumen fermentation. Asian-Aust. J. Anim. Sci. 2002, 15, 1458–1468. [Google Scholar] [CrossRef]
- Walker, N.D.; Newbold, C.J.; Wallace, R.J. Nitrogen metabolism in the rumen. In Nitrogen and Phosphorus Nutrition of Cattle: Reducing the Environmental Impact of Cattle Operations; CABI Publishing: Wallingford, UK, 2005; pp. 71–115. [Google Scholar]
- Lambo, M.T.; Ma, H.; Liu, R.; Dai, B.; Zhang, Y.; Li, Y. Mechanism, effectiveness, and the prospects of medicinal plants and their bioactive compounds in lowering ruminants’ enteric methane emission. Animal 2024, 18, 101134. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 62, 1303–1317. [Google Scholar] [CrossRef]
- Mbiriri, D.; Cho, S.; Mamvura, C.; Choi, N. Assessment of rumen microbial adaptation to garlic oil, carvacrol and thymol using the consecutive batch culture system. J. Vet. Sci. Anim. Husb. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J. Appl. Microbiol. 2015, 119, 127–138. [Google Scholar] [CrossRef] [PubMed]


| Attributes | Concentrate Mixture 1 (n = 8) | Wheat Straw (n = 8) | Oats Green (n = 8) |
|---|---|---|---|
| Organic matter | 93.16 | 92.17 | 92.62 |
| Crude protein | 20.22 | 4.24 | 7.48 |
| Ether extract | 5.35 | 1.31 | 2.27 |
| Total ash | 6.84 | 7.83 | 7.38 |
| Neutral detergent fibre | 39.53 | 81.08 | 64.31 |
| Acid detergent fibre | 10.12 | 52.80 | 37.50 |
| Attributes | Treatments | SEM | p Value | |
|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | |||
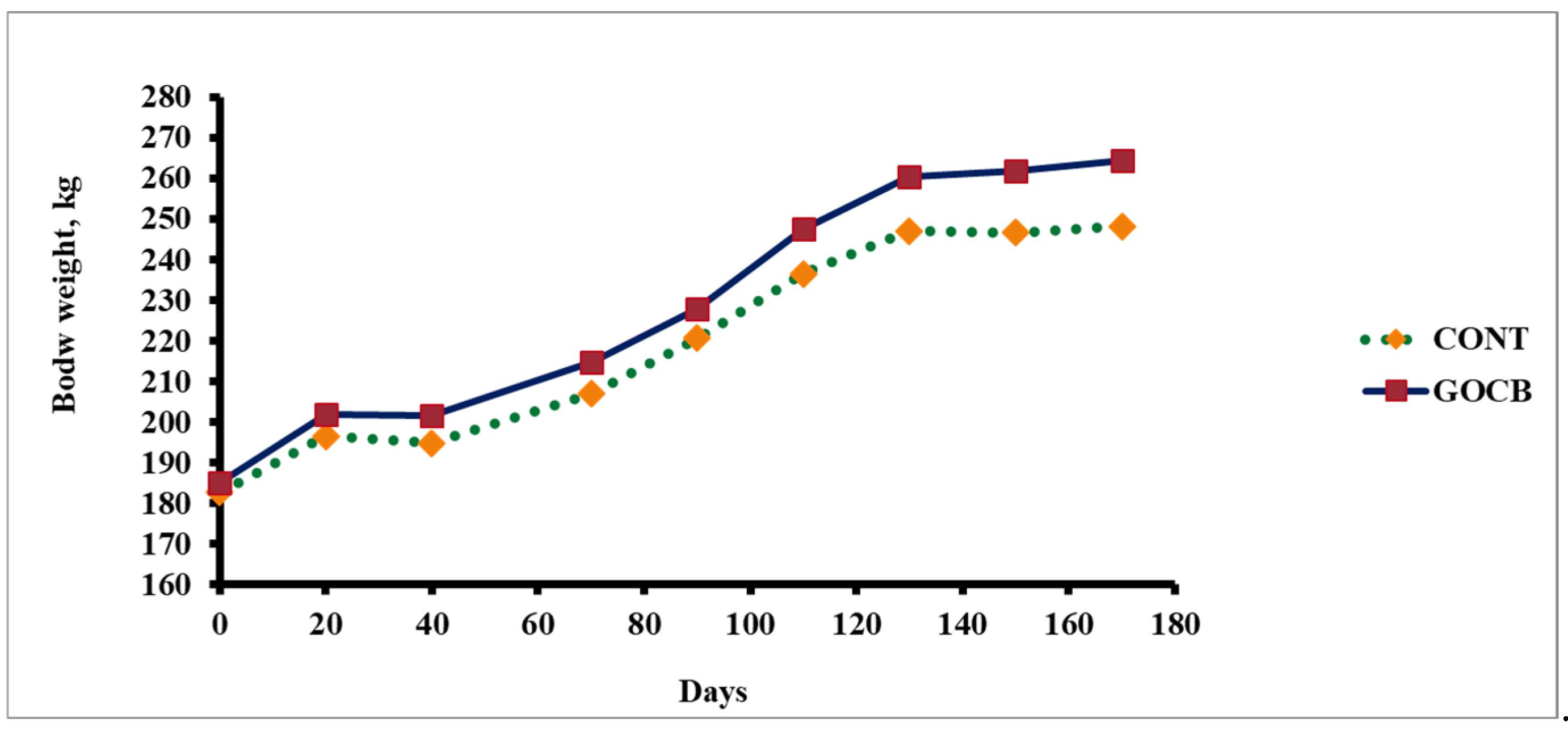
| Body Weight (kg) | ||||
| Initial | 182.70 | 185.04 | 12.90 | 0.935 |
| Final | 248.20 | 264.54 | 14.14 | 0.594 |
| Total Gain δ | 65.50 a | 79.50 b | 3.07 | 0.01 |
| ADG (g) | 385.08 a | 467.66 b | 18.05 | 0.01 |
| Dry Matter Intake (kg/d) | ||||
| Wheat Straw | 1.61 | 1.73 | 0.184 | 0.763 |
| Conc. Mix | 1.82 | 1.82 | 0 | 1.00 |
| Oats Green Fodder | 1.16 | 1.16 | 0 | 1.00 |
| Total | 4.59 | 4.72 | 0.184 | 0.762 |
| FCR | 11.97 b | 10.11 a | 0.438 | 0.022 |
| FE (%) | 8.40 a | 10.08 b | 0.386 | 0.018 |
| Parameters | Day | Treatments | SEM | p Value | |
|---|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | ||||
| Hb (g/dL) | 0 | 10.56 | 10.89 | 0.82 | 0.670 |
| 90 | 10.92 | 11.05 | 0.77 | 0.751 | |
| 170 | 11.03 | 11.32 | 0.82 | 0.432 | |
| PCV (%) | 0 | 35.62 | 35.472 | 1.48 | 0.547 |
| 90 | 34.59 | 34.92 | 1.23 | 0.234 | |
| 170 | 35.64 | 34.79 | 1.85 | 0.502 | |
| Total Protein (g/dL) | 0 | 7.86 | 7.52 | 0.29 | 0.162 |
| 90 | 8.54 | 8.20 | 0.34 | 0.372 | |
| 170 | 8.20 | 8.34 | 0.41 | 0.421 | |
| Albumin (g/dL) | 0 | 3.17 | 3.64 | 0.06 | 0.371 |
| 90 | 3.23 | 3.15 | 0.03 | 0.431 | |
| 170 | 3.74 | 3.52 | 0.08 | 0.785 | |
| Globulin (g/dL) | 0 | 4.69 | 3.88 | 0.43 | 0.132 |
| 90 | 5.31 | 5.05 | 0.34 | 0.569 | |
| 170 | 4.46 | 4.62 | 0.39 | 0.328 | |
| A: G Ratio | 0 | 0.68 | 0.94 | 0.06 | 0.425 |
| 90 | 0.61 | 0.62 | 0.05 | 0.823 | |
| 170 | 0.84 | 0.73 | 0.03 | 0.164 | |
| AST (U/L) | 0 | 110.32 | 112.58 | 6.35 | 0.432 |
| 90 | 114.68 | 116.58 | 4.61 | 0.579 | |
| 170 | 115.69 | 117.54 | 7.72 | 0.620 | |
| ALT (U/L) | 0 | 66.23 | 75.48 | 8.97 | 0.720 |
| 90 | 67.58 | 72.59 | 7.58 | 0.354 | |
| 170 | 70.61 | 76.28 | 6.59 | 0.679 | |
| Urea (mg/dL) | 0 | 27.86 | 29.74 | 1.95 | 0.450 |
| 90 | 33.23 | 32.32 | 1.74 | 0.112 | |
| 170 | 36.56 b | 32.71 a | 1.68 | 0.042 | |
| Attributes | Treatments | SEM | p Value | |
|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | |||
| T-SH (μmol mg−1 Hb) | 188.31 a | 285.47 b | 35.48 | 0.006 |
| GSH (μmol mg−1 Hb) | 14.25 a | 28.49 b | 2.57 | 0.002 |
| Catalase (mmol mg−1 Hb) | 1.01 a | 6.12 b | 0.39 | 0.003 |
| SOD (mmol MTT formazon formed mg−1 Hb) | 0.13 a | 0.28 b | 0.04 | 0.006 |
| LPO (nmol MDA mg−1 Hb) | 10.28 b | 7.6 a | 1.56 | 0.009 |
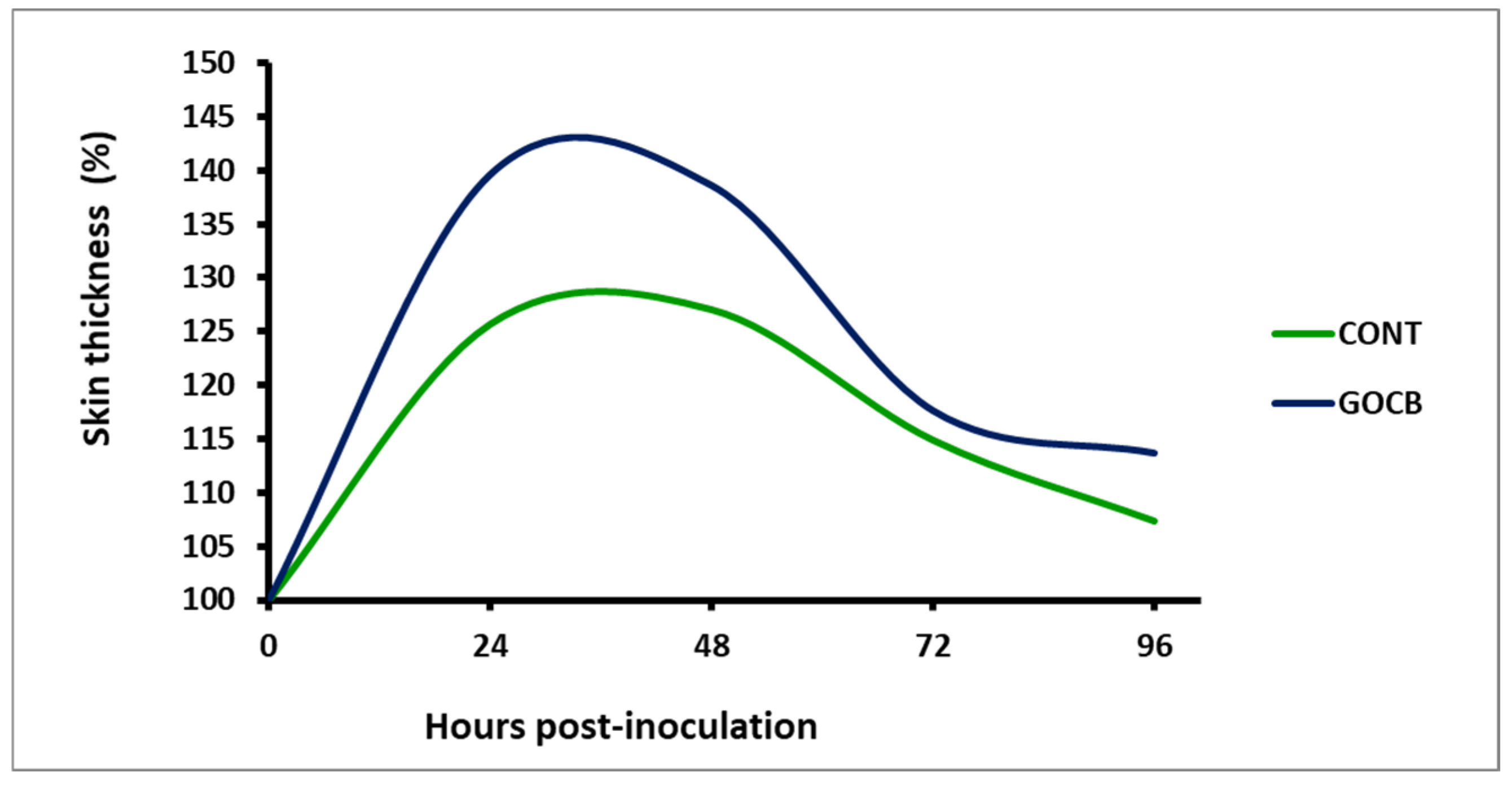
| Hours (Post-Injection) | Treatments | SEM | p Value | Period Mean ± SE | |
|---|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | ||||
| 0 | 100 | 100 | - | 1.00 | 100 A |
| 24 | 125.71 a | 135.38 b | 8.68 | 0.034 | 130.54 C ± 2.59 |
| 48 | 127.08 a | 136.09 b | 10.59 | 0.042 | 131.59 C ± 3.65 |
| 72 | 114.94 a | 123.49 b | 5.38 | 0.039 | 119.2 B ± 2.12 |
| 96 | 107.41 a | 116.17 b | 2.95 | 0.012 | 111.79 B ± 2.13 |
| Treatment mean ± SE | 115.03 a ± 1.93 | 122.23 b ± 3.26 | 10.56 | 0.024 | |
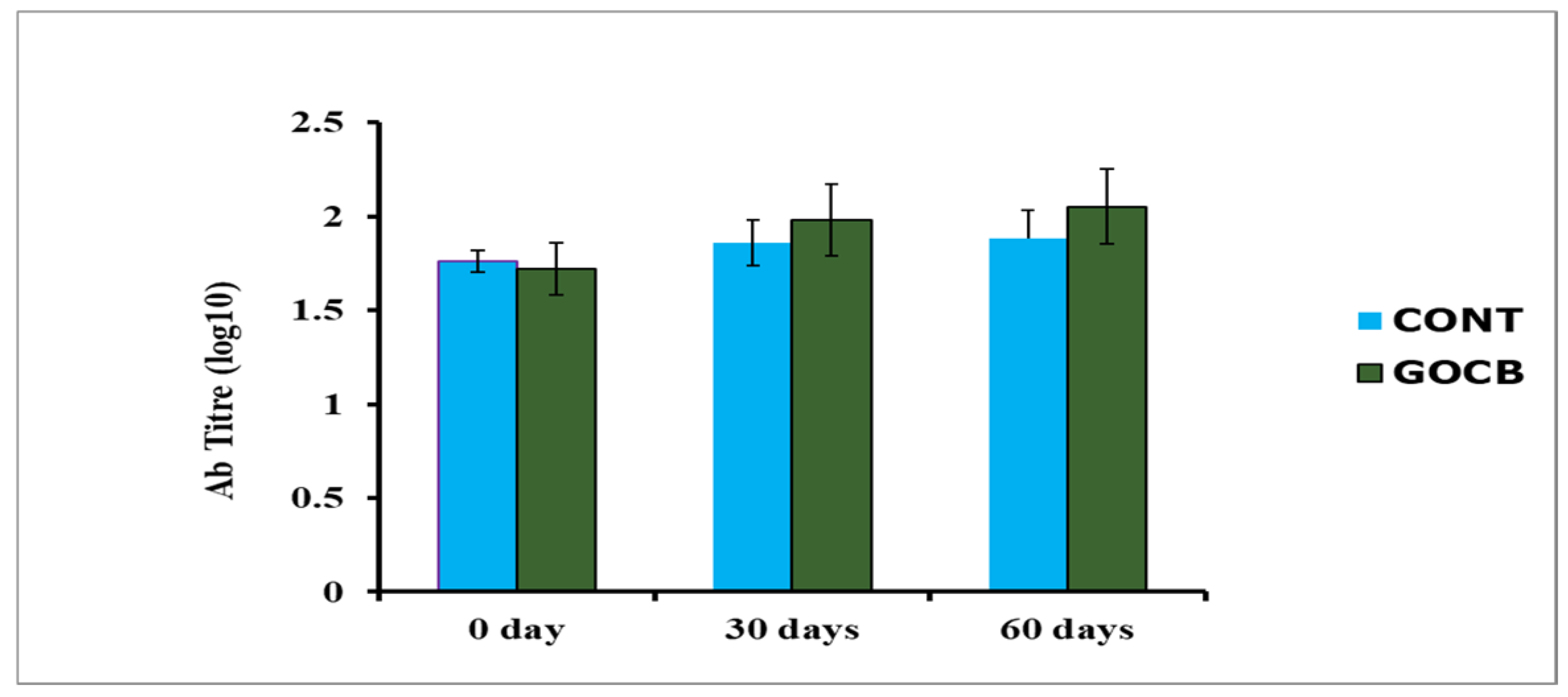
| Days Post-Immunisation | Antibody Titre (log10) | SEM | p Value | |
|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | |||
| 0 | 1.76 | 1.72 | 0.05 | 0.322 |
| 30 | 1.86 a | 1.98 b | 0.06 | 0.019 |
| 60 | 1.88 a | 2.05 b | 0.07 | 0.048 |
| Attributes | Treatments | SEM | p Value | |
|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | |||
| Dry Matter | 56.72 a | 60.20 b | 0.874 | 0.036 |
| Organic Matter | 60.28 a | 69.28 b | 1.68 | 0.001 |
| Crude Protein | 61.58 a | 67.66 b | 1.21 | 0.002 |
| Ether Extract | 69.82 | 72.75 | 2.73 | 0.621 |
| Neutral Detergent Fibre | 47.56 | 50.70 | 1.34 | 0.263 |
| Acid Detergent Fibre | 43.27 | 48.40 | 1.79 | 0.162 |
| Attribute | Treatments | SEM | p Value | |
|---|---|---|---|---|
| CONT (n = 8) | GOCB (n = 8) | |||
| Methane conc. | 431.29 b | 285.19 a | 27.16 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, A.; Thakur, S.; Singh, R.K.; Sheoran, S.; Andonissamy, J.; Kumar, S. Developing a Feeding Module with a Blend of Garlic Oil and Cinnamon Bark for Enhancing Antioxidant Status and Immunity of Murrah Buffalo (Bubalus bubalis) with an Improvement in Feed Efficiency and Reduced Methane Emissions. Antioxidants 2025, 14, 702. https://doi.org/10.3390/antiox14060702
Dey A, Thakur S, Singh RK, Sheoran S, Andonissamy J, Kumar S. Developing a Feeding Module with a Blend of Garlic Oil and Cinnamon Bark for Enhancing Antioxidant Status and Immunity of Murrah Buffalo (Bubalus bubalis) with an Improvement in Feed Efficiency and Reduced Methane Emissions. Antioxidants. 2025; 14(6):702. https://doi.org/10.3390/antiox14060702
Chicago/Turabian StyleDey, Avijit, Shubham Thakur, Ram Kumar Singh, Sandeep Sheoran, Jerome Andonissamy, and Sanjay Kumar. 2025. "Developing a Feeding Module with a Blend of Garlic Oil and Cinnamon Bark for Enhancing Antioxidant Status and Immunity of Murrah Buffalo (Bubalus bubalis) with an Improvement in Feed Efficiency and Reduced Methane Emissions" Antioxidants 14, no. 6: 702. https://doi.org/10.3390/antiox14060702
APA StyleDey, A., Thakur, S., Singh, R. K., Sheoran, S., Andonissamy, J., & Kumar, S. (2025). Developing a Feeding Module with a Blend of Garlic Oil and Cinnamon Bark for Enhancing Antioxidant Status and Immunity of Murrah Buffalo (Bubalus bubalis) with an Improvement in Feed Efficiency and Reduced Methane Emissions. Antioxidants, 14(6), 702. https://doi.org/10.3390/antiox14060702







