Antioxidant Effects of SGLT2 Inhibitors on Cardiovascular–Kidney–Metabolic (CKM) Syndrome
Abstract
1. Cardiovascular–Kidney–Metabolic (CKM) Syndrome
1.1. Unmet Needs in the Care of AKI and Its CVD Complications
1.2. CKD and CKM Syndrome Definitions
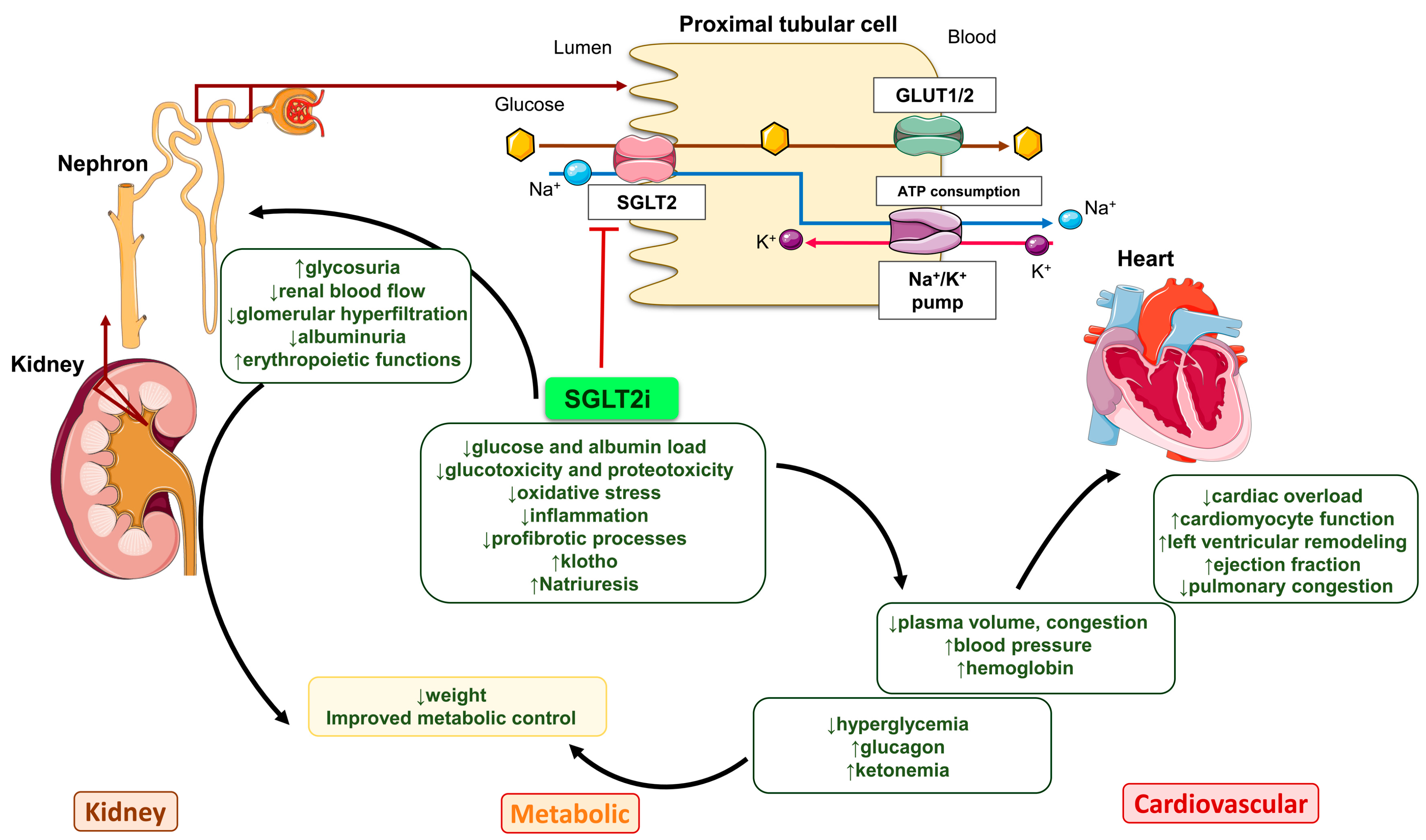
2. Mechanism of Action of SGLT2i
3. Antioxidant Effects of SGLT2i in Preclinical Models
3.1. CKD and CKM Syndrome
3.2. AKI
4. Antioxidant Effects of SGLT2i in Clinical Studies
4.1. CKM Syndrome
4.2. CKD Without T2DM
4.3. AKI and HF
5. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Collaborators, G.F. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant. 2019, 34, 1803–1805. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Coresh, J.; Matsushita, K.; Ballew, S.H.; Sang, Y.; Surapaneni, A.; Alencar de Pinho, N.; Anderson, A.; Appel, L.J.; Ärnlöv, J.; et al. Estimated Glomerular Filtration Rate, Albuminuria, and Adverse Outcomes: An Individual-Participant Data Meta-Analysis. JAMA 2023, 330, 1266–1277. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Seremetis, S.V.; Aledort, L.M.; Bergman, G.E.; Bona, R.; Bray, G.; Brettler, D.; Eyster, M.E.; Kessler, C.; Lau, T.S.; Lusher, J. Three-year randomised study of high-purity or intermediate-purity factor VIII concentrates in symptom-free HIV-seropositive haemophiliacs: Effects on immune status. Lancet 1993, 342, 700–703. [Google Scholar] [CrossRef]
- Abasheva, D.; Ortiz, A.; Fernandez-Fernandez, B. GLP-1 receptor agonists in patients with chronic kidney disease and either overweight or obesity. Clin. Kidney J. 2024, 17, 19–35. [Google Scholar] [CrossRef]
- Fernández-Fernandez, B.; Sarafidis, P.; Soler, M.J.; Ortiz, A. EMPA-KIDNEY: Expanding the range of kidney protection by SGLT2 inhibitors. Clin. Kidney J. 2023, 16, 1187–1198. [Google Scholar] [CrossRef]
- Mark, P.B.; Sarafidis, P.; Ekart, R.; Ferro, C.J.; Balafa, O.; Fernandez-Fernandez, B.; Herrington, W.G.; Rossignol, P.; Del Vecchio, L.; Valdivielso, J.M.; et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: A comprehensive review by EURECA-m and ERBP working groups of ERA. Nephrol. Dial. Transplant. 2023, 38, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lin, Y.L.; Hsu, B.G. Endothelial dysfunction in chronic kidney disease: Mechanisms, biomarkers, diagnostics, and therapeutic strategies. Tzu Chi Med. J. 2025, 37, 125–134. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; Aceves-Ripoll, J.; González-Lafuente, L.; Corbacho-Alonso, N.; Baldan-Martín, M.; Madruga, F.; Alvarez-Llamas, G.; Barderas, M.G.; Ruilope, L.M.; et al. Analysis of Global Oxidative Status Using Multimarker Scores Reveals a Specific Association Between Renal Dysfunction and Diuretic Therapy in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1198–1205. [Google Scholar] [CrossRef]
- RodrÍguez-SÁnchez, E.; Navarro-GarcÍa, J.A.; Aceves-Ripoll, J.; GonzÁlez-Lafuente, L.; Corbacho-Alonso, N.; Martinez, P.; Calvo-Bonacho, E.; Alvarez-Llamas, G.; Barderas, M.G.; Ruilope, L.M.; et al. Lifetime cardiovascular risk is associated with a multimarker score of systemic oxidative status in young adults independently of traditional risk factors. Transl. Res. 2019, 212, 54–66. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 143. [Google Scholar] [CrossRef]
- Farrokh-Eslamlou, N.; Momtaz, S.; Niknejad, A.; Hosseini, Y.; Mahdaviani, P.; Ghasemnejad-Berenji, M.; Abdolghaffari, A.H. Empagliflozin protective effects against cisplatin-induced acute nephrotoxicity by interfering with oxidative stress and inflammation in Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 7061–7070. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Shahrin, L.; Claure-Del Granado, R.; Luyckx, V.A.; Conroy, A.L. Infections and Acute Kidney Injury: A Global Perspective. Semin. Nephrol. 2023, 43, 151466. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cleary, C.; Sanz, A.B.; Avello, A.; Sanchez-Niño, M.D.; Ortiz, A. NephroCheck at 10: Addressing unmet needs in AKI diagnosis and risk stratification. Clin. Kidney J. 2023, 16, 1359–1366. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhao, J.C.; Xi, X.M.; Zheng, Y.; Li, W.X. Attributable mortality of acute kidney injury among critically ill patients with sepsis: A multicenter, retrospective cohort study. BMC Nephrol. 2024, 25, 125. [Google Scholar] [CrossRef]
- Vickery, S.; Price, C.P.; John, R.I.; Abbas, N.A.; Webb, M.C.; Kempson, M.E.; Lamb, E.J. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am. J. Kidney Dis. 2005, 46, 610–620. [Google Scholar] [CrossRef]
- Gallacher, P.J.; Yeung, D.; Bell, S.; Shah, A.S.V.; Mills, N.L.; Dhaun, N. Kidney replacement therapy: Trends in incidence, treatment, and outcomes of myocardial infarction and stroke in a nationwide Scottish study. Eur. Heart J. 2024, 45, 1339–1351. [Google Scholar] [CrossRef]
- Concepcion Izquierdo, M.; Sanz, A.B.; Mezzano, S.; Blanco, J.; Carrasco, S.; Dolores Sanchez-Nino, M.; Benito-Martin, A.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. TWEAK (tumor necrosis factor-like weak inducer of apoptosis) activates CXCL16 expression during renal tubulointerstitial inflammation. Kidney Int. 2012, 81, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Massy, Z.A.; Drueke, T.B. Combination of Cardiovascular, Kidney, and Metabolic Diseases in a Syndrome Named Cardiovascular-Kidney-Metabolic, With New Risk Prediction Equations. Kidney Int. Rep. 2024, 9, 2608–2618. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Wanner, C.; Heerspink, H.J.L.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; Hantel, S.; Woerle, H.J.; Broedl, U.C.; von Eynatten, M.; et al. Empagliflozin and Kidney Function Decline in Patients with Type 2 Diabetes: A Slope Analysis from the EMPA-REG OUTCOME Trial. J. Am. Soc. Nephrol. 2018, 29, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- Bosch, C.; Carriazo, S.; Soler, M.J.; Ortiz, A.; Fernandez-Fernandez, B. Tirzepatide and prevention of chronic kidney disease. Clin. Kidney J. 2023, 16, 797–808. [Google Scholar] [CrossRef]
- El Khayari, A.; Hakam, S.M.; Malka, G.; Rochette, L.; El Fatimy, R. New insights into the cardio-renal benefits of SGLT2 inhibitors and the coordinated role of miR-30 family. Genes Dis. 2024, 11, 101174. [Google Scholar] [CrossRef]
- Sun, Z.; Cui, W.; Chen, L. Structures reveal how SGLT inhibitors work. Trends Pharmacol. Sci. 2024, 45, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Hediger, M.A.; Rhoads, D.B. Molecular physiology of sodium-glucose cotransporters. Physiol. Rev. 1994, 74, 993–1026. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, S.; Huang, Z.; Wang, T.; Tang, H. Network Meta-Analysis of Novel Glucose-Lowering Drugs on Risk of Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 16, 70–78. [Google Scholar] [CrossRef]
- Mora-Fernández, C.; Sánchez-Niño, M.D.; Donate-Correa, J.; Martín-Núñez, E.; Pérez-Delgado, N.; Valiño-Rivas, L.; Fernández-Fernández, B.; Ortiz, A.; Navarro-González, J.F. Sodium-glucose co-transporter-2 inhibitors increase Klotho in patients with diabetic kidney disease: A clinical and experimental study. Biomed. Pharmacother. 2022, 154, 113677. [Google Scholar] [CrossRef]
- Lindberg, K.; Amin, R.; Moe, O.W.; Hu, M.C.; Erben, R.G.; Östman Wernerson, A.; Lanske, B.; Olauson, H.; Larsson, T.E. The kidney is the principal organ mediating klotho effects. J. Am. Soc. Nephrol. 2014, 25, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Demiray, A.; Afsar, B.; Covic, A.; Tapoi, L.; Ureche, C.; Ortiz, A. Role of Klotho in the Development of Essential Hypertension. Hypertension 2021, 77, 740–750. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Cho, H.J.; Adams-Huet, B.; Paek, J.; Hill, K.; Shelton, J.; Amaral, A.P.; Faul, C.; Taniguchi, M.; et al. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J. Am. Soc. Nephrol. 2015, 26, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of aging in mice by the hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Vallon, V. State-of-the-Art-Review: Mechanisms of Action of SGLT2 Inhibitors and Clinical Implications. Am. J. Hypertens. 2024, 37, 841–852. [Google Scholar] [CrossRef]
- Hernandez, M.; Sullivan, R.D.; McCune, M.E.; Reed, G.L.; Gladysheva, I.P. Sodium-Glucose Cotransporter-2 Inhibitors Improve Heart Failure with Reduced Ejection Fraction Outcomes by Reducing Edema and Congestion. Diagnostics 2022, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.D.; McCune, M.E.; Hernandez, M.; Reed, G.L.; Gladysheva, I.P. Suppression of Cardiogenic Edema with Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure with Reduced Ejection Fraction: Mechanisms and Insights from Pre-Clinical Studies. Biomedicines 2022, 10, 2016. [Google Scholar] [CrossRef]
- Biegus, J.; Fudim, M.; Salah, H.M.; Heerspink, H.J.L.; Voors, A.A.; Ponikowski, P. Sodium-glucose cotransporter-2 inhibitors in heart failure: Potential decongestive mechanisms and current clinical studies. Eur. J. Heart Fail. 2023, 25, 1526–1536. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chang, D.Y.; Zhao, M.H.; Chen, M. Dapagliflozin Alleviates Diabetic Kidney Disease. Antioxid. Redox Signal. 2024, 40, 492–509. [Google Scholar] [CrossRef]
- Chi, P.J.; Lee, C.J.; Hsieh, Y.J.; Lu, C.W.; Hsu, B.G. Dapagliflozin Ameliorates Lipopolysaccharide Related Acute Kidney Injury in Mice with Streptozotocin-induced Diabetes Mellitus. Int. J. Med. Sci. 2022, 19, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Hirashima, Y.; Nakano, T.; Torisu, K.; Aihara, S.; Wakisaka, M.; Kitazono, T. SGLT2 inhibition mitigates transition from acute kidney injury to chronic kidney disease by suppressing ferroptosis. Sci. Rep. 2024, 14, 20386. [Google Scholar] [CrossRef]
- Maki, T.; Maeno, S.; Maeda, Y.; Yamato, M.; Sonoda, N.; Ogawa, Y.; Wakisaka, M.; Inoguchi, T. Amelioration of diabetic nephropathy by SGLT2 inhibitors independent of its glucose-lowering effect: A possible role of SGLT2 in mesangial cells. Sci. Rep. 2019, 9, 4703. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Bilić Ćurčić, I.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors-Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef]
- Patel, T.A.; Zheng, H.; Patel, K.P. Sodium-Glucose Cotransporter 2 Inhibitors as Potential Antioxidant Therapeutic Agents in Cardiovascular and Renal Diseases. Antioxidants 2025, 14, 336. [Google Scholar] [CrossRef]
- Arab, H.H.; Al-Shorbagy, M.Y.; Saad, M.A. Activation of autophagy and suppression of apoptosis by dapagliflozin attenuates experimental inflammatory bowel disease in rats: Targeting AMPK/mTOR, HMGB1/RAGE and Nrf2/HO-1 pathways. Chem. Biol. Interact. 2021, 335, 109368. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Suda, M.; Yoshida, Y.; Furihata, T.; Joki, Y.; Hsiao, C.L.; Jiaqi, L.; Fujiki, S.; Abe, M.; et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat. Aging 2024, 4, 926–938. [Google Scholar] [CrossRef]
- Tan, Y.; Ouyang, Y.; Ma, Z.; Huang, J.; Tan, C.; Qiu, J.; Wu, F. Mitochondrial Quality Control Systems in Septic AKI: Molecular Mechanisms and Therapeutic Implications. Int. J. Med. Sci. 2025, 22, 1852–1864. [Google Scholar] [CrossRef]
- Koizumi, T.; Watanabe, M.; Yokota, T.; Tsuda, M.; Handa, H.; Koya, J.; Nishino, K.; Tatsuta, D.; Natsui, H.; Kadosaka, T.; et al. Empagliflozin suppresses mitochondrial reactive oxygen species generation and mitigates the inducibility of atrial fibrillation in diabetic rats. Front. Cardiovasc. Med. 2023, 10, 1005408. [Google Scholar] [CrossRef]
- Uehara-Watanabe, N.; Okuno-Ozeki, N.; Nakamura, I.; Nakata, T.; Nakai, K.; Yagi-Tomita, A.; Ida, T.; Yamashita, N.; Kamezaki, M.; Kirita, Y.; et al. Proximal tubular epithelia-specific transcriptomics of diabetic mice treated with dapagliflozin. Heliyon 2022, 8, e10615. [Google Scholar] [CrossRef]
- Martin-Sanchez, D.; Fontecha-Barriuso, M.; Martinez-Moreno, J.M.; Ramos, A.M.; Sanchez-Niño, M.D.; Guerrero-Hue, M.; Moreno, J.A.; Ortiz, A.; Sanz, A.B. Ferroptosis and kidney disease. Nefrologia 2020, 40, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Sanchez-Niño, M.D.; Ramos, A.M.; Ortiz, A. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023, 19, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhou, C.; Yan, Q.; Li, Z.; Chen, D.; Feng, B.; Song, J. Dapagliflozin improves diabetic kidney disease by inhibiting ferroptosis through β-hydroxybutyrate production. Ren. Fail. 2025, 47, 2438857. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Wu, Y.; Miao, L.; Wang, Z.; Wang, Y.; Xu, S.; Lou, Y. Dapagliflozin alleviates high-fat-induced obesity cardiomyopathy by inhibiting ferroptosis. ESC Heart Fail. 2025, 12, 1358–1373. [Google Scholar] [CrossRef]
- Shao, M.; Chen, D.; Wang, Q.; Guo, F.; Wei, F.; Zhang, W.; Gan, T.; Luo, Y.; Fan, X.; Du, P.; et al. Canagliflozin regulates metabolic reprogramming in diabetic kidney disease by inducing fasting-like and aestivation-like metabolic patterns. Diabetologia 2024, 67, 738–754. [Google Scholar] [CrossRef]
- Wang, Y.J.; Paneni, F.; Stein, S.; Matter, C.M. Modulating Sirtuin Biology and Nicotinamide Adenine Diphosphate Metabolism in Cardiovascular Disease-From Bench to Bedside. Front. Physiol. 2021, 12, 755060. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, W.C.; Chiu, C.C.; Chi, N.Y.; Liu, Y.H.; Huang, T.C.; Wu, W.T.; Lin, T.H.; Lai, W.T.; Sheu, S.H.; et al. The Beneficial Effect of the SGLT2 Inhibitor Dapagliflozin in Alleviating Acute Myocardial Infarction-Induced Cardiomyocyte Injury by Increasing the Sirtuin Family SIRT1/SIRT3 and Cascade Signaling. Int. J. Mol. Sci. 2024, 25, 8541. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Li, N.; Yang, X.; Zhou, L.; Li, H.; Wang, T.; Xie, M.; Liu, H. Dapagliflozin delays renal fibrosis in diabetic kidney disease by inhibiting YAP/TAZ activation. Life Sci. 2023, 322, 121671. [Google Scholar] [CrossRef]
- Dia, B.; Alkhansa, S.; Njeim, R.; Al Moussawi, S.; Farhat, T.; Haddad, A.; Riachi, M.E.; Nawfal, R.; Azar, W.S.; Eid, A.A. SGLT2 Inhibitor-Dapagliflozin Attenuates Diabetes-Induced Renal Injury by Regulating Inflammation through a CYP4A/20-HETE Signaling Mechanism. Pharmaceutics 2023, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Vaios, V.; Roumeliotis, S.; Leivaditis, K.; Eleftheriadis, T.; Liakopoulos, V. Evidence for Cardiorenal Protection with SGLT-2 Inhibitors and GLP-1 Receptor Agonists in Patients with Diabetic Kidney Disease. J. Pers. Med. 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Jacobs-Cacha, C.; Llorens-Cebria, C.; Ortiz, A.; Martinez-Diaz, I.; Martos, N.; Dominguez-Báez, P.; Van den Bosch, M.M.; Bermejo, S.; Pieper, M.P.; et al. Enhanced Cardiorenal Protective Effects of Combining SGLT2 Inhibition, Endothelin Receptor Antagonism and RAS Blockade in Type 2 Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 12823. [Google Scholar] [CrossRef] [PubMed]
- Martos-Guillami, N.; Vergara, A.; Llorens-Cebrià, C.; Motto, A.E.; Martínez-Díaz, I.; Gonçalves, F.; Garcias-Ramis, M.M.; Allo-Urzainqui, E.; Narváez, A.; Bermejo, S.; et al. SGLT2i and GLP1-RA exert additive cardiorenal protection with a RAS blocker in uninephrectomized db/db mice. Front. Pharmacol. 2024, 15, 1415879. [Google Scholar] [CrossRef]
- Mujalli, A.; Farrash, W.F.; Obaid, A.A.; Khan, A.A.; Almaimani, R.A.; Idris, S.; Elzubier, M.E.; Khidir, E.B.A.; Aslam, A.; Minshawi, F.; et al. Improved Glycaemic Control and Nephroprotective Effects of Empagliflozin and Paricalcitol Co-Therapy in Mice with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 17380. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; El Salem, A.; Awad, M.M. Empagliflozin, SGLT. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1347–1360. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Soulié, M.; Stephan, Y.; Durand, M.; Lima-Posada, I.; Palacios-Ramírez, R.; Nicol, L.; Lopez-Andres, N.; Mulder, P.; Jaisser, F. Benefit of combination therapy with dapagliflozin and eplerenone on cardiac function and fibrosis in rats with non-diabetic chronic kidney disease. Sci. Rep. 2024, 14, 23955. [Google Scholar] [CrossRef]
- Nakatsukasa, T.; Ishizu, T.; Ouchi, M.; Murakoshi, N.; Sato, K.; Yamamoto, M.; Kawanishi, K.; Seo, Y.; Ieda, M. Sodium Glucose Co-Transporter 2 Inhibitors Improve Renal Congestion and Left Ventricular Fibrosis in Rats With Hypertensive Heart Failure. Circ. J. 2022, 86, 2029–2039. [Google Scholar] [CrossRef]
- Bailey, C.R. Implants in dental prosthetics. Part 1. Br. Dent. Surg. Assist. 1989, 48, 2–4. [Google Scholar]
- Venot, M.; Weis, L.; Clec’h, C.; Darmon, M.; Allaouchiche, B.; Goldgran-Tolédano, D.; Garrouste-Orgeas, M.; Adrie, C.; Timsit, J.F.; Azoulay, E. Acute Kidney Injury in Severe Sepsis and Septic Shock in Patients with and without Diabetes Mellitus: A Multicenter Study. PLoS ONE 2015, 10, e0127411. [Google Scholar] [CrossRef]
- Li, H.; Xia, Y.; Zha, H.; Zhang, Y.; Shi, L.; Wang, J.; Huang, H.; Yue, R.; Hu, B.; Zhu, J.; et al. Dapagliflozin attenuates AKI to CKD transition in diabetes by activating SIRT3/PGC1-α signaling and alleviating aberrant metabolic reprogramming. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167433. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, X.; Yan, G.; Zhang, Y.; Yao, Y.; Qiao, Y.; Wang, D.; Chen, G.; Zhang, W.; Tang, C.; et al. Dapagliflozin Attenuates Contrast-induced Acute Kidney Injury by Regulating the HIF-1α/HE4/NF-κB Pathway. J. Cardiovasc. Pharmacol. 2022, 79, 904–913. [Google Scholar] [CrossRef]
- Oe, Y.; Kim, Y.C.; Sidorenko, V.S.; Zhang, H.; Kanoo, S.; Lopez, N.; Goodluck, H.A.; Crespo-Masip, M.; Vallon, V. SGLT2 inhibitor dapagliflozin protects the kidney in a murine model of Balkan nephropathy. Am. J. Physiol. Renal Physiol. 2024, 326, F227–F240. [Google Scholar] [CrossRef]
- Kimura, Y.; Kuno, A.; Tanno, M.; Sato, T.; Ohno, K.; Shibata, S.; Nakata, K.; Sugawara, H.; Abe, K.; Igaki, Y.; et al. Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J. Diabetes Investig. 2019, 10, 933–946. [Google Scholar] [CrossRef]
- Kuno, A.; Kimura, Y.; Mizuno, M.; Oshima, H.; Sato, T.; Moniwa, N.; Tanaka, M.; Yano, T.; Tanno, M.; Miki, T.; et al. Empagliflozin attenuates acute kidney injury after myocardial infarction in diabetic rats. Sci. Rep. 2020, 10, 7238. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Mitsuo, H.; Nishijima, T.; Uchiyama, H.; Nita, T.; Matsunaga, S.; Fujimoto, N.; Ushijima, T.; Ando, Y.; Kan-O, M.; et al. Acute Dapagliflozin Administration Ameliorates Cardiac Surgery-Associated Acute Kidney Injury in a Rabbit Model. Circ. J. 2024, 88, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S239–S251. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S207–S238. [Google Scholar] [CrossRef]
- Kadowaki, T.; Maegawa, H.; Watada, H.; Yabe, D.; Node, K.; Murohara, T.; Wada, J. Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan. Diabetes Obes. Metab. 2022, 24, 2283–2296. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Lim, H.S.; MacFadyen, R.J.; Lip, G.Y. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch. Intern. Med. 2004, 164, 1737–1748. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Whaley-Connell, A.; Sowers, J.R. Oxidative stress in the cardiorenal metabolic syndrome. Curr. Hypertens. Rep. 2012, 14, 360–365. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Georgianos, P.I.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Is Associated with eGFR Decline in Proteinuric Diabetic Kidney Disease: A Cohort Study. Oxidative Med. Cell. Longev. 2021, 2021, 2968869. [Google Scholar] [CrossRef]
- Van Tits, L.; De Graaf, J.; Hak-Lemmers, H.; Bredie, S.; Demacker, P.; Holvoet, P.; Stalenhoef, A. Increased levels of low-density lipoprotein oxidation in patients with familial hypercholesterolemia and in end-stage renal disease patients on hemodialysis. Lab. Investig. 2003, 83, 13–21. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, E.; Navarro-García, J.A.; González-Lafuente, L.; Aceves-Ripoll, J.; Vázquez-Sánchez, S.; Poveda, J.; Mercado-García, E.; Corbacho-Alonso, N.; Calvo-Bonacho, E.; Fernández-Velasco, M.; et al. Oxidized Low-Density Lipoprotein Associates with Ventricular Stress in Young Adults and Triggers Intracellular Ca. Antioxidants 2020, 9, 1213. [Google Scholar] [CrossRef]
- Matsuda, M.; Tamura, R.; Kanno, K.; Segawa, T.; Kinoshita, H.; Nishimoto, O.; Nishiyama, H.; Kawamoto, T. Impact of dyslipidemic components of metabolic syndrome, adiponectin levels, and anti-diabetes medications on malondialdehyde-modified low-density lipoprotein levels in statin-treated diabetes patients with coronary artery disease. Diabetol. Metab. Syndr. 2013, 5, 77. [Google Scholar] [CrossRef][Green Version]
- Zoccali, C.; Bode-Böger, S.; Mallamaci, F.; Benedetto, F.; Tripepi, G.; Malatino, L.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Hinokio, Y.; Suzuki, S.; Hirai, M.; Suzuki, C.; Suzuki, M.; Toyota, T. Urinary excretion of 8-oxo-7, 8-dihydro-2’-deoxyguanosine as a predictor of the development of diabetic nephropathy. Diabetologia 2002, 45, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Watanabe, M.; Qureshi, A.R.; Mukai, H.; Machowska, A.; Heimbürger, O.; Barany, P.; Lindholm, B.; Stenvinkel, P. Serum 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage, is associated with mortality independent of inflammation in chronic kidney disease. Eur. J. Intern. Med. 2019, 68, 60–65. [Google Scholar] [CrossRef]
- Dilworth, L.; Stennett, D.; Facey, A.; Omoruyi, F.; Mohansingh, S.; Omoruyi, F.O. Diabetes and the associated complications: The role of antioxidants in diabetes therapy and care. Biomed. Pharmacother. 2024, 181, 117641. [Google Scholar] [CrossRef]
- Rahayu, I.; Arfian, N.; Kustanti, C.Y.; Wahyuningsih, M.S.H. The effectiveness of antioxidant agents in delaying progression of diabetic nephropathy: A systematic review of randomized controlled trials. Bioimpacts 2025, 15, 30129. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef]
- Iannantuoni, F.; de Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef]
- Sezai, A.; Sekino, H.; Unosawa, S.; Taoka, M.; Osaka, S.; Tanaka, M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc. Diabetol. 2019, 18, 76. [Google Scholar] [CrossRef]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef]
- Mulder, S.; Hammarstedt, A.; Nagaraj, S.B.; Nair, V.; Ju, W.; Hedberg, J.; Greasley, P.J.; Eriksson, J.W.; Oscarsson, J.; Heerspink, H.J.L. A metabolomics-based molecular pathway analysis of how the sodium-glucose co-transporter-2 inhibitor dapagliflozin may slow kidney function decline in patients with diabetes. Diabetes Obes. Metab. 2020, 22, 1157–1166. [Google Scholar] [CrossRef]
- Goto, Y.; Otsuka, Y.; Ashida, K.; Nagayama, A.; Hasuzawa, N.; Iwata, S.; Hara, K.; Tsuruta, M.; Wada, N.; Motomura, S.; et al. Improvement of skeletal muscle insulin sensitivity by 1 week of SGLT2 inhibitor use. Endocr. Connect. 2020, 9, 599–606. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Imre, E.; Gunhan, H.G.; Erel, P.; Ustay, O. SGLT2 inhibitors improve plasma atherogenic biomarkers in patients with type 2 diabetes: A real-world retrospective observational study. Minerva Endocrinol. 2023, 48, 295–304. [Google Scholar] [CrossRef]
- Ferrannini, E.; Muscelli, E.; Frascerra, S.; Baldi, S.; Mari, A.; Heise, T.; Broedl, U.C.; Woerle, H.J. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Investig. 2014, 124, 499–508. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, A.; Fernández-Fernández, B.; Ortiz, A.; Gil, M.; Rodríguez-Martín, S.; Ruiz-Hurtado, G.; Fernández-Antón, E.; Ruilope, L.M.; de Abajo, F.J. Glucose-Lowering Drugs and Primary Prevention of Chronic Kidney Disease in Type 2 Diabetes Patients: A Real-World Primary Care Study. Pharmaceuticals 2024, 17, 1299. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.G.; Staplin, N.; Agrawal, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Zhu, D.; et al. Long-Term Effects of Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2025, 392, 777–787. [Google Scholar] [CrossRef]
- Amioka, M.; Sanada, R.; Matsumura, H.; Kinoshita, H.; Sairaku, A.; Morishima, N.; Nakano, Y. Impact of SGLT2 inhibitors on old age patients with heart failure and chronic kidney disease. Int. J. Cardiol. 2023, 370, 294–299. [Google Scholar] [CrossRef]
- Boeckhaus, J.; Gross, O. Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Hereditary Podocytopathies, Alport Syndrome, and FSGS: A Case Series to Better Plan a Large-Scale Study. Cells 2021, 10, 1815. [Google Scholar] [CrossRef]
- Packer, M. Hyperuricemia and Gout Reduction by SGLT2 Inhibitors in Diabetes and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2024, 83, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Kobayashi, M.; Wada, M.; Tokuchi, M.; Misegawa, S.; Saito, R.; Nomi, H.; Haga, R.; Nagatoya, K.; Yamauchi, A. Effect of Dapagliflozin on Serum Uric Acid Levels in Patients with Advanced Chronic Kidney Disease. Intern. Med. 2024, 63, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Anker, S.D.; Butler, J.; Zannad, F.; Filippatos, G.; Coats, A.J.S.; Ferreira, J.P.; Henrichmoeller, I.; Brueckmann, M.; Schueler, E.; et al. Uric Acid and SGLT2 Inhibition With Empagliflozin in Heart Failure With Preserved Ejection Fraction: The EMPEROR-Preserved Trial. JACC Heart Fail. 2024, 12, 2057–2070. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Kramer, A.; Rychlík, I.; Nangaku, M.; Yanagita, M.; Jager, K.J.; Caskey, F.J.; Stel, V.S.; Kashihara, N.; Kuragano, T.; et al. Maintaining kidney health in aging societies: A JSN and ERA call to action. Nephrol. Dial. Transplant. 2025, gfaf068. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Farkouh, M.; Altman, D.G.; Hopewell, S.; Emdin, C.A.; Hunn, B.H. AKI and Long-Term Risk for Cardiovascular Events and Mortality. J. Am. Soc. Nephrol. 2017, 28, 377–387. [Google Scholar] [CrossRef]
- Chawla, L.S.; Amdur, R.L.; Shaw, A.D.; Faselis, C.; Palant, C.E.; Kimmel, P.L. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin. J. Am. Soc. Nephrol. 2014, 9, 448–456. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Wu, V.C.; Wu, C.H.; Huang, T.M.; Wang, C.Y.; Lai, C.F.; Shiao, C.C.; Chang, C.H.; Lin, S.L.; Chen, Y.Y.; Chen, Y.M.; et al. Long-term risk of coronary events after AKI. J. Am. Soc. Nephrol. 2014, 25, 595–605. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Parikh, C.R.; Himmelfarb, J.; Chinchilli, V.M.; Liu, K.D.; Coca, S.G.; Garg, A.X.; Hsu, C.Y.; Siew, E.D.; Wurfel, M.M.; et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021, 99, 456–465. [Google Scholar] [CrossRef]
- Lee, S.A.; Cozzi, M.; Bush, E.L.; Rabb, H. Distant Organ Dysfunction in Acute Kidney Injury: A Review. Am. J. Kidney Dis. 2018, 72, 846–856. [Google Scholar] [CrossRef]
- González-Lafuente, L.; Navarro-García, J.A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Poveda, J.; Vázquez-Sánchez, S.; Mercado-García, E.; Fernández-Velasco, M.; Kuro-O, M.; Liaño, F.; et al. Interplay between mineral bone disorder and cardiac damage in acute kidney injury: From Ca. Transl. Res. 2022, 243, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Berlyne, G.M.; Adler, A.J.; Ferran, N.; Bennett, S.; Holt, J. Silicon metabolism. I. Some aspects of renal silicon handling in normal man. Nephron 1986, 43, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Valiño-Rivas, L.; Cuarental, L.; Ceballos, M.I.; Pintor-Chocano, A.; Perez-Gomez, M.V.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Growth differentiation factor-15 preserves Klotho expression in acute kidney injury and kidney fibrosis. Kidney Int. 2022, 101, 1200–1215. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jang, H.R.; Rabb, H. Lymphocytes and innate immune cells in acute kidney injury and repair. Nat. Rev. Nephrol. 2024, 20, 789–805. [Google Scholar] [CrossRef]
- Eshraghi, A.; Khalesi, S.; Amini, K.; Salleh, F.H.; Sharifikia, M.; Hajmiri, M.S.; Zamanirafe, M.; Yazdi, A.; Mehrpooya, M. Empagliflozin Ameliorates the Oxidative Stress Profile in Type 2 Diabetic Patients with Heart Failure and Reduced Ejection Fraction: Results of a Randomized, Double-blind, Placebo-controlled Study. Rev. Recent Clin. Trials 2025, 20, 167–179. [Google Scholar] [CrossRef]
- Magurno, M.; Cassano, V.; Maruca, F.; Pastura, C.A.; Divino, M.; Fazio, F.; Severini, G.; Clausi, E.; Armentaro, G.; Miceli, S.; et al. Effects of SGLT2-Inhibitors on Comprehensive Geriatric Assessment, Biomarkers of Oxidative Stress, and Platelet Activation in Elderly Diabetic Patients with Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2024, 25, 8811. [Google Scholar] [CrossRef]
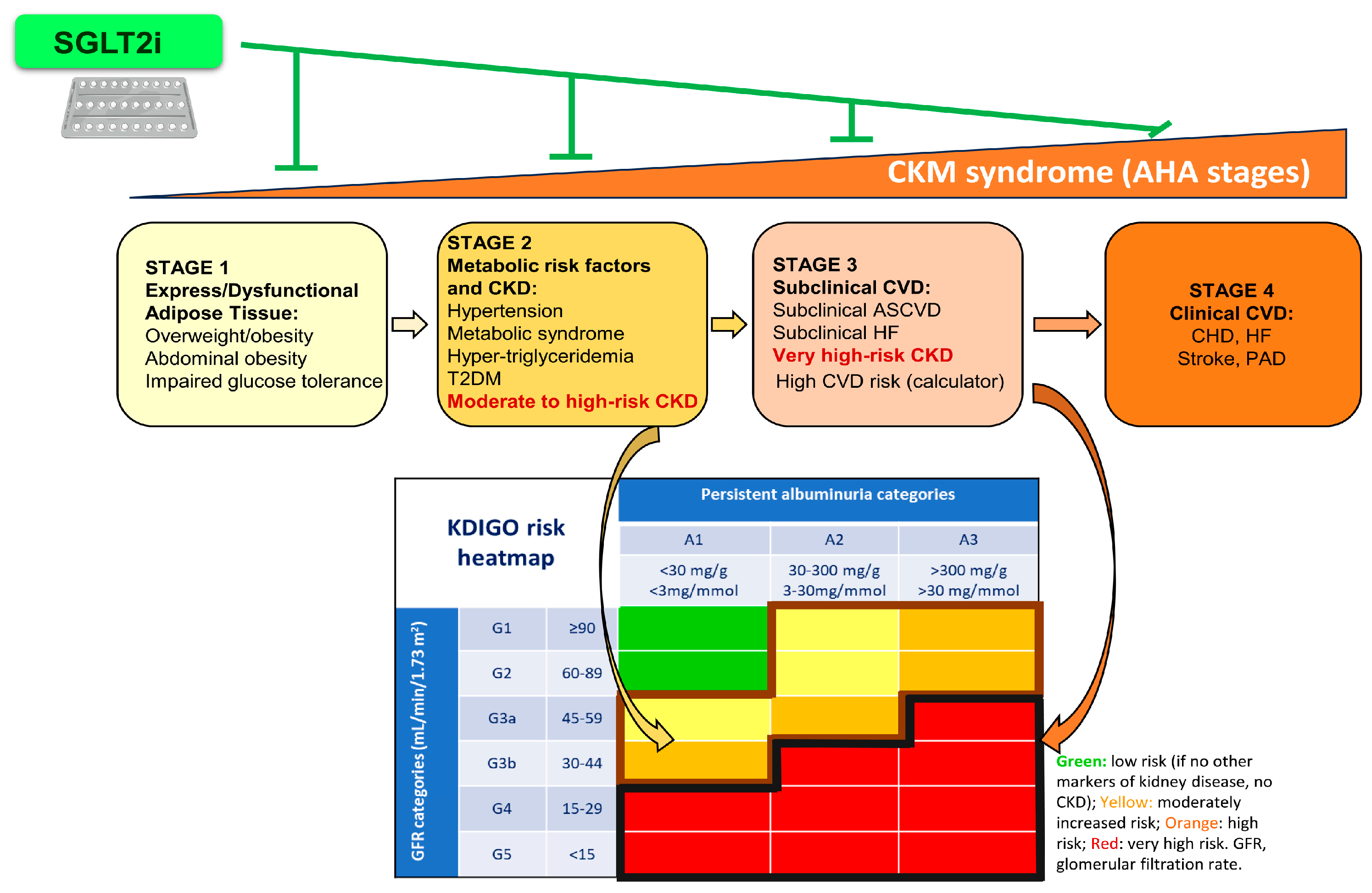
| CKM Syndrome | Definition |
|---|---|
| Stage 0: No risk factors | Includes individuals without metabolic risk factors, or no evidence of CKD or CVD.
|
| Stage 1: Excess/dysfunctional adiposity | Excess/dysfunctional adipose tissue characterized by overweight, abdominal obesity, impaired glucose tolerance, or prediabetes. |
| Stage 2: Metabolic risk factors and CKD | Metabolic risk factors (hypertriglyceridemia, hypertension, metabolic syndrome, diabetes mellitus type 2) and moderate- to high-risk of CKD. |
| Stage 3: Subclinical CVD in CKM | CKM risk factors, subclinical CVD (subclinical health failure, atherosclerosis), very high risk of CKD, and high predicted CVD risk. |
| Stage 4: Clinical CVD in CKM | CKM risk factors and clinical CVD (coronary heart disease, heart failure, stroke, peripheral artery disease, atrial fibrillation) subdivided into: (a) Without kidney failure; (b) With kidney failure. |
| CKM Component | Preclinical and Clinical Benefits of SGLT2i (* Preclinical-Only Findings) |
|---|---|
| Metabolic |
|
| Kidney |
|
| Cardiac |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Mauvecin, J.; Villar-Gómez, N.; Miño-Izquierdo, L.; Povo-Retana, A.; Ramos, A.M.; Ruiz-Hurtado, G.; Sanchez-Niño, M.D.; Ortiz, A.; Sanz, A.B. Antioxidant Effects of SGLT2 Inhibitors on Cardiovascular–Kidney–Metabolic (CKM) Syndrome. Antioxidants 2025, 14, 701. https://doi.org/10.3390/antiox14060701
Guerrero-Mauvecin J, Villar-Gómez N, Miño-Izquierdo L, Povo-Retana A, Ramos AM, Ruiz-Hurtado G, Sanchez-Niño MD, Ortiz A, Sanz AB. Antioxidant Effects of SGLT2 Inhibitors on Cardiovascular–Kidney–Metabolic (CKM) Syndrome. Antioxidants. 2025; 14(6):701. https://doi.org/10.3390/antiox14060701
Chicago/Turabian StyleGuerrero-Mauvecin, Juan, Natalia Villar-Gómez, Lucia Miño-Izquierdo, Adrián Povo-Retana, Adrian M. Ramos, Gema Ruiz-Hurtado, Maria D. Sanchez-Niño, Alberto Ortiz, and Ana B. Sanz. 2025. "Antioxidant Effects of SGLT2 Inhibitors on Cardiovascular–Kidney–Metabolic (CKM) Syndrome" Antioxidants 14, no. 6: 701. https://doi.org/10.3390/antiox14060701
APA StyleGuerrero-Mauvecin, J., Villar-Gómez, N., Miño-Izquierdo, L., Povo-Retana, A., Ramos, A. M., Ruiz-Hurtado, G., Sanchez-Niño, M. D., Ortiz, A., & Sanz, A. B. (2025). Antioxidant Effects of SGLT2 Inhibitors on Cardiovascular–Kidney–Metabolic (CKM) Syndrome. Antioxidants, 14(6), 701. https://doi.org/10.3390/antiox14060701







