Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine
Abstract
1. Introduction
2. Autophagy in Stem Cells
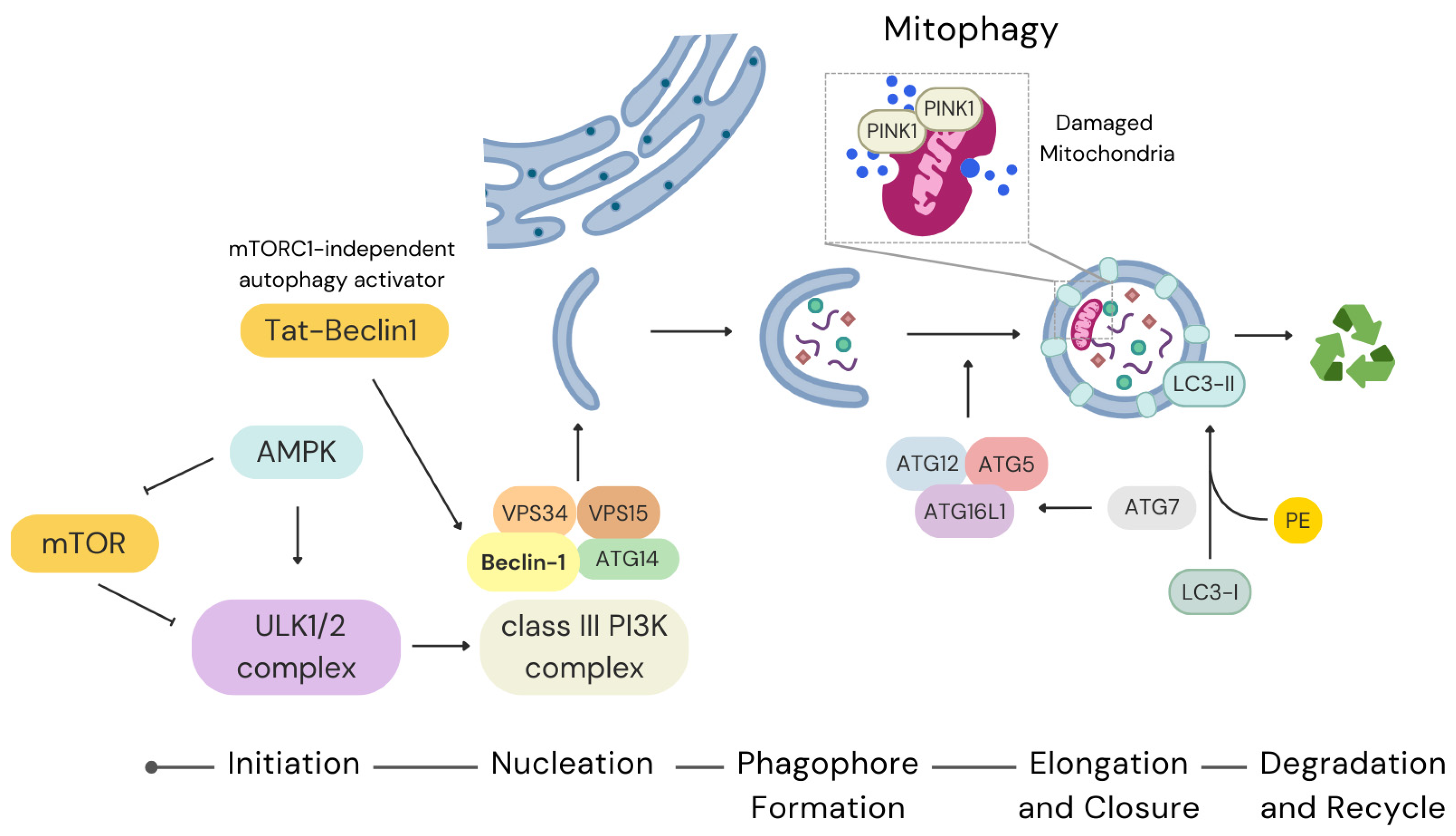
2.1. Molecular Pathways of Autophagy
2.1.1. Initiation and Regulation
2.1.2. Nucleation and Phagophore Formation
2.1.3. Elongation and Closure
2.2. Transcriptional and Post-Transcriptional Regulation
2.3. Autophagy and Stem Cell Ageing
2.4. Autophagy in Disease and Therapy
3. Oxidative Stress in Stem Cells
3.1. Sources and Regulation of ROS
3.2. Antioxidant Defence Mechanisms
3.3. Impacts of Oxidative Stress on Stem Cell Function
3.3.1. ROS and Stem Cell Differentiation
3.3.2. Excessive ROS and Stem Cell Damage
3.4. Therapeutic Implications
3.4.1. Antioxidant Therapies
3.4.2. Metabolic Reprogramming
3.4.3. Activation of Nrf2 Pathways
3.4.4. Improved Culture Conditions
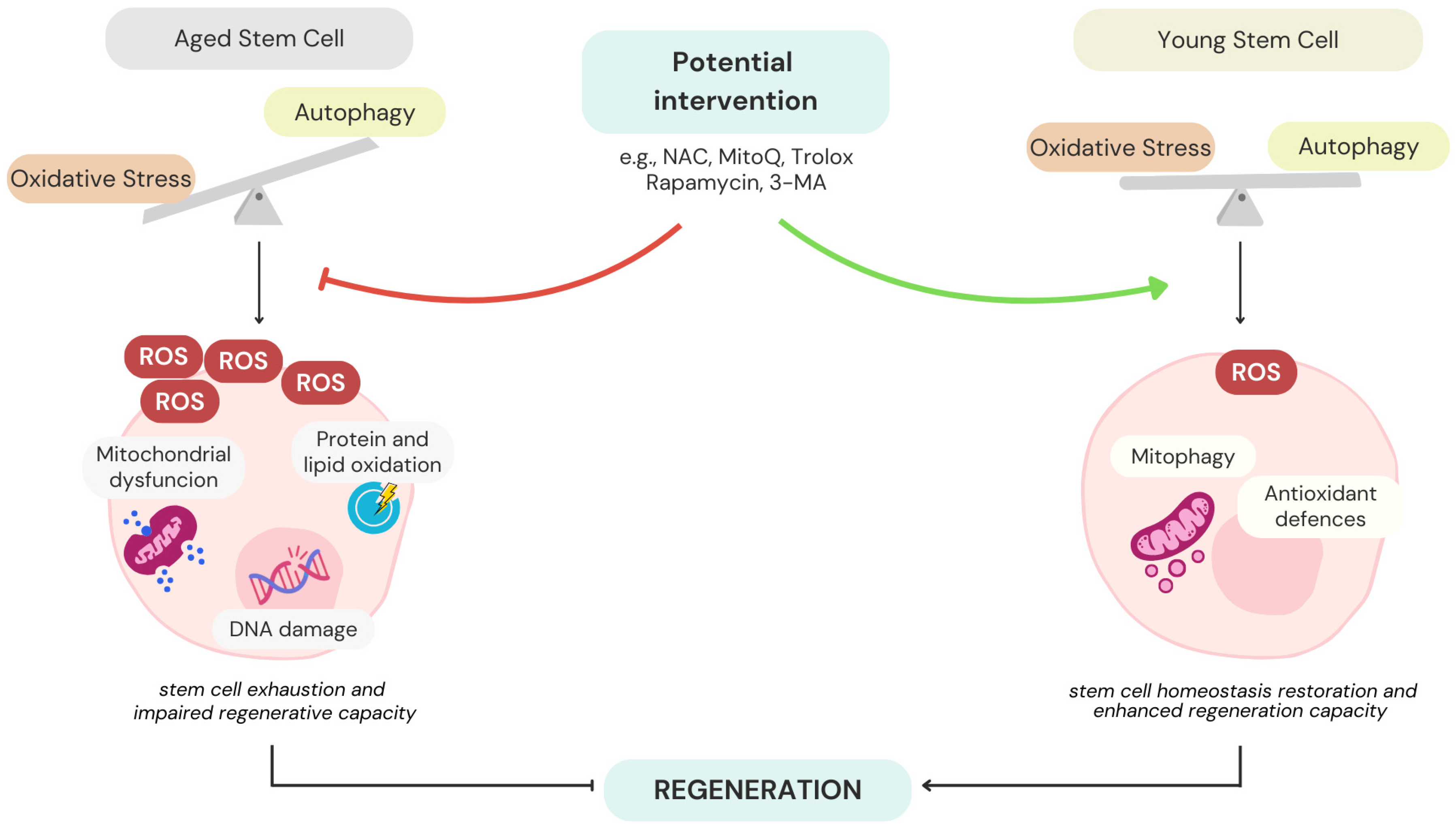
4. Crosstalk Between Autophagy and Oxidative Stress
4.1. Mitochondrial Quality Control
4.2. Transcriptional Regulation
4.3. Drugs Regulating Oxidative Stress and Autophagy Balance in Stem Cells
4.4. Impact on Stem Cell Ageing and Regeneration
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radicals |
| 3-MA | 3-methyladenine |
| AAP | ascorbic acid 2-phosphate |
| AKT | protein kinase B |
| ALA | α-lipoic acid |
| ALP | autophagy-lysosome pathway |
| Ambra1 | activating molecule in BECN1-regulated autophagy protein 1 |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AREs | antioxidant response elements |
| ASCs | adipose-derived stem cells |
| Atg | autophagy-related gene |
| ATG | autophagy-related protein |
| ATM | ataxia-telangiectasia mutated |
| ATP | adenosine triphosphate |
| Becn1 | beclin-1 |
| BMP4 | bone morphogenetic protein 4 |
| BNIP3 | BCL2 Interacting Protein 3 |
| BNIP3L | BCL2 Interacting Protein 3 like |
| CAT | catalase |
| CDK | cyclin-dependent Kinase |
| CDKIs | cyclin-dependent kinase inhibitors |
| c-MYC | cellular myelocytomatosis oncogene |
| CO2 | carbon dioxide |
| CREB | cAMP response element-binding protein |
| DCF | dichlorodihydrofluorescein |
| DNA | deoxyribonucleic acid |
| DNMT1 | DNA (cytosine-5)-methyltransferase |
| DPSCs | dental pulp stem cells |
| EPCs | endothelial precursor cells |
| EpiLCs | epiblast-like cells |
| ERK | extracellular signal-regulated kinase |
| ERRγ | estrogen-related receptor gamma |
| ESC | embryonic stem cell |
| FOXO3 | forkhead box O3 |
| FPN1 | ferroportin 1 |
| FUNDC1 | FUN14 domain containing 1 |
| GABARAPL1 | GABA type A receptor-associated protein like 1 |
| GATA4 | GATA Binding Protein 4 |
| GFP | green fluorescent protein |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSK3β | glycogen synthase kinase-3 beta |
| H2O2 | hydrogen peroxide |
| H3K4me3 | tri-methylation of lysine 4 of the H3 histone protein |
| hDFSC | human dental follicle stem cells |
| hDPSC | human dental pulp stem cells |
| hESCs | human embryonic stem cells |
| HGF | hepatocyte growth factor |
| HIF1A | hypoxia inducible factor 1 subunit alpha |
| HK2 | hexokinase |
| hMSCs | human mesenchimal stem cells |
| HO-1 | heme oxygenase-1 |
| HSCs | hematopoietic stem cells |
| HSP70 | heat shock protein 70 |
| HSPCs | hematopoietic stem and progenitor cells |
| hUCMSCs | human umbilical cord mesenchymal stem cells |
| IGF-1 | insulin-like growth factor-1 |
| iPSCs | induced pluripotent stem cells |
| JNK-1/2 | c-Jun N-terminal kinases-1/2 |
| Keap1 | kelch-like ECH-associated protein 1 |
| LC3 | microtubule-associated protein 1A/1B-light chain 3 |
| LDHA | lactate dehydrogenase |
| lncRNAs | long non-coding RNAs |
| LPS | lipopolysaccharide |
| LT-HSCs | long-term hematopoietic stem cells |
| MAP1LC3B | microtubule-associated proteins 1A/1B light chain 3B |
| MAPK | mitogen-activated protein kinase |
| mESCs | mouse embryonic stem cells |
| miRNAs | microRNAs |
| MitoQ | mitoquinone mesylate |
| MSCs | mesenchymal stem cells |
| MT1 | melatonin receptor type 1A |
| mTOR | mechanistic target of rapamycin |
| mTORC1 | mechanistic target of rapamycin complex 1 |
| MuSCs | muscle stem cells |
| NAC | N-acetylcysteine |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NANOG | homeobox protein NANOG |
| NBR1 | neighbour of BRCA1 gene 1 |
| NDP52 | pulp nuclear dot protein 52 |
| NES | neuroepithelial stem |
| NF-κB | nuclear factor kappa B |
| NIX | nip3-like protein X |
| N-MYC | neuroblastoma-derived v-myc avian myelocytomatosis viral-related oncogene |
| NOS | nitric oxide synthase |
| Notch | notch receptor |
| NOXs | NADPH oxidase enzymes |
| NPCs | neural progenitor cells |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NSCs | neural stem cells |
| NSPC | neural stem and progenitor cells |
| O2 | oxygen |
| O2•− | superoxide anions |
| OCT4 | octamer-binding transcription factor 4 |
| OGD | oxygen-glucose deprivation |
| O-GlcNAc | O-linked N-acetylglucosamine |
| Ogt | O-GlcNAc transferase gene |
| OGT | O-GlcNAc transferase |
| OxPhos | oxidative phosphorylation |
| PARK2 | parkin-2 gene |
| PE | phosphatidylethanolamine |
| PEG-CAT | polyethylene glycol-CAT |
| PEG-SOD | polyethylene glycol-SOD |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator 1-alpha |
| piRNAs | piwi-interacting RNAs |
| PI3K | phosphatidylinositol 3-kinase |
| PIP3 | phosphatidylinositol-3-phosphate |
| PINK1 | PTEN-induced putative kinase 1 |
| PKM1 | pyruvate kinase M1 |
| PKM2 | pyruvate kinase M2 |
| PRDX1/6 | peroxiredoxins 1 and 6 |
| PS1 | presenilin 1 |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| SCF | stem cell factor |
| SDF-1 | stromal cell-derived factor-1 |
| SIRT | sirtuin |
| SOD | superoxide dismutase |
| SOX2 | sex-determining region Y-box 2 |
| SQSTM1 | sequestosome 1 |
| Tat-Beclin1 | mTORC1-independent autophagy activator |
| TCA-cycle | tricarboxylic acid cycle |
| TFEB | transcription factor EB |
| TOR | target of rapamycin |
| Tsc1 | tuberous sclerosis gene |
| ULK1/2 | unc-51-like kinase ½ |
| VEGF | vascular endothelial growth factor |
| VPS15 | phosphoinositide-3-kinase regulatory subunit 4 |
| VPS34 | phosphatidylinositol-3-kinase class III |
| WJ-MSCs | Wharton jelly multipotent stem cells |
| XO | xanthine oxidase |
References
- Cheung, T.H.; Rando, T.A. Molecular Regulation of Stem Cell Quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Baraniak, P.R.; McDevitt, T.C. Stem Cell Paracrine Actions and Tissue Regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef]
- Cho, I.J.; Lui, P.P.W.; Obajdin, J.; Riccio, F.; Stroukov, W.; Willis, T.L.; Spagnoli, F.; Watt, F.M. Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence. Stem Cell Rep. 2019, 12, 1190–1200. [Google Scholar] [CrossRef]
- Mi, L.; Hu, J.; Li, N.; Gao, J.; Huo, R.; Peng, X.; Zhang, N.; Liu, Y.; Zhao, H.; Liu, R.; et al. The Mechanism of Stem Cell Aging. Stem Cell Rev. Rep. 2022, 18, 1281–1293. [Google Scholar] [CrossRef]
- de Morree, A.; Rando, T.A. Regulation of Adult Stem Cell Quiescence and Its Functions in the Maintenance of Tissue Integrity. Nat. Rev. Mol. Cell Biol. 2023, 24, 334–354. [Google Scholar] [CrossRef]
- Brunet, A.; Goodell, M.A.; Rando, T.A. Ageing and Rejuvenation of Tissue Stem Cells and Their Niches. Nat. Rev. Mol. Cell Biol. 2023, 24, 45–62. [Google Scholar] [CrossRef]
- Lampert, M.A.; Gustafsson, Å.B. Mitochondria and Autophagy in Adult Stem Cells: Proliferate or Differentiate. J. Muscle Res. Cell Motil. 2020, 41, 355–362. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Shatrova, A.N.; Deryabin, P.I.; Griukova, A.A.; Abushik, P.A.; Antonov, S.M.; Nikolsky, N.N.; Burova, E.B. Calcium Alterations Signal Either to Senescence or to Autophagy Induction in Stem Cells upon Oxidative Stress. Aging 2016, 8, 3400–3418. [Google Scholar] [CrossRef]
- Ma, Y.; Qi, M.; An, Y.; Zhang, L.; Yang, R.; Doro, D.H.; Liu, W.; Jin, Y. Autophagy Controls Mesenchymal Stem Cell Properties and Senescence during Bone Aging. Aging Cell 2018, 17, e12709. [Google Scholar] [CrossRef]
- Murmu, N.; Shravage, B.V. Autophagy Slows the Aging of Germline Stem Cells in Drosophila through Modulation of E-Cadherin. bioRxiv 2022, 2022.03.31.486570. [Google Scholar] [CrossRef]
- Houri, K.; Mori, T.; Onodera, Y.; Tsujimoto, T.; Takehara, T.; Nakao, S.; Teramura, T.; Fukuda, K. MiR-142 Induces Accumulation of Reactive Oxygen Species (ROS) by Inhibiting Pexophagy in Aged Bone Marrow Mesenchymal Stem Cells. Sci. Rep. 2020, 10, 3735. [Google Scholar] [CrossRef]
- Mortensen, M.; Soilleux, E.J.; Djordjevic, G.; Tripp, R.; Lutteropp, M.; Sadighi-Akha, E.; Stranks, A.J.; Glanville, J.; Knight, S.; Jacobsen, S.E.W.; et al. The Autophagy Protein Atg7 Is Essential for Hematopoietic Stem Cell Maintenance. J. Exp. Med. 2011, 208, 455–467. [Google Scholar] [CrossRef]
- Ho, T.T.; Warr, M.R.; Adelman, E.R.; Lansinger, O.M.; Flach, J.; Verovskaya, E.V.; Figueroa, M.E.; Passegué, E. Autophagy Maintains the Metabolism and Function of Young and Old Stem Cells. Nature 2017, 543, 205–210. [Google Scholar] [CrossRef]
- Borsa, M.; Obba, S.; Richter, F.C.; Zhang, H.; Riffelmacher, T.; Carrelha, J.; Alsaleh, G.; Jacobsen, S.E.W.; Simon, A.K. Autophagy Preserves Hematopoietic Stem Cells by Restraining MTORC1-Mediated Cellular Anabolism. Autophagy 2024, 20, 45–57. [Google Scholar] [CrossRef]
- Chu, Y.; Yuan, X.; Tao, Y.; Yang, B.; Luo, J. Autophagy in Muscle Regeneration: Mechanisms, Targets, and Therapeutic Perspective. Int. J. Mol. Sci. 2024, 25, 11901. [Google Scholar] [CrossRef]
- Dellorusso, P.V.; Proven, M.A.; Calero-Nieto, F.J.; Wang, X.; Mitchell, C.A.; Hartmann, F.; Amouzgar, M.; Favaro, P.; DeVilbiss, A.; Swann, J.W.; et al. Autophagy Counters Inflammation-Driven Glycolytic Impairment in Aging Hematopoietic Stem Cells. Cell Stem Cell 2024, 31, 1020–1037.e9. [Google Scholar] [CrossRef]
- Fang, Y.; An, N.; Zhu, L.; Gu, Y.; Qian, J.; Jiang, G.; Zhao, R.; Wei, W.; Xu, L.; Zhang, G.; et al. Autophagy-Sirt3 Axis Decelerates Hematopoietic Aging. Aging Cell 2020, 19, e13232. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Q.; Kao, Y.R.; Diaz, A.; Tasset, I.; Kaushik, S.; Thiruthuvanathan, V.; Zintiridou, A.; Nieves, E.; Dzieciatkowska, M.; et al. Chaperone-Mediated Autophagy Sustains Haematopoietic Stem-Cell Function. Nature 2021, 591, 117–123. [Google Scholar] [CrossRef]
- Ghanta, S.; Tsoyi, K.; Liu, X.; Nakahira, K.; Ith, B.; Coronata, A.A.; Fredenburgh, L.E.; Englert, J.A.; Piantadosi, C.A.; Choi, A.M.K.; et al. Mesenchymal Stromal Cells Deficient in Autophagy Proteins Are Susceptible to Oxidative Injury and Mitochondrial Dysfunction. Am. J. Respir. Cell Mol. Biol. 2017, 56, 300–309. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Wu, D.; Li, L. Modulating Autophagy in Mesenchymal Stem Cells Effectively Protects against Hypoxia- or Ischemia-Induced Injury. Stem Cell Res. Ther. 2019, 10, 120. [Google Scholar] [CrossRef]
- Song, C.; Song, C.; Tong, F. Autophagy Induction Is a Survival Response against Oxidative Stress in Bone Marrow-Derived Mesenchymal Stromal Cells. Cytotherapy 2014, 16, 1361–1370. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem Cells and the Impact of ROS Signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Prakash, R.; Fauzia, E.; Siddiqui, A.J.; Yadav, S.K.; Kumari, N.; Shams, M.T.; Naeem, A.; Praharaj, P.P.; Khan, M.A.; Bhutia, S.K.; et al. Oxidative Stress-Induced Autophagy Compromises Stem Cell Viability. Stem Cells 2022, 40, 468–478. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Wong, N.-K.; Xiao, J.; So, K.-F. Oxidative Stress in Stem Cell Aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef]
- Zhao, K.; Chan, I.T.C.; Tse, E.H.Y.; Xie, Z.; Cheung, T.H.; Zeng, Y.A. Autophagy in Adult Stem Cell Homeostasis, Aging, and Disease Therapy. Cell Regen. 2025, 14, 14. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, Z.; Zhang, Y.; Sun, J.; Xu, S.; Di, X.; Ding, X.; Ding, G. Regulation of Mesenchymal Stem Cell Differentiation by Autophagy. Open Med. 2024, 19, 20240968. [Google Scholar] [CrossRef]
- Guan, J.L.; Simon, A.K.; Prescott, M.; Menendez, J.A.; Liu, F.; Wang, F.; Wang, C.; Wolvetang, E.; Vazquez-Martin, A.; Zhang, J. Autophagy in Stem Cells. Autophagy 2013, 9, 830–849. [Google Scholar] [CrossRef]
- Holczer, M.; Hajdú, B.; Lőrincz, T.; Szarka, A.; Bánhegyi, G.; Kapuy, O. Fine-Tuning of AMPK-ULK1-MTORC1 Regulatory Triangle Is Crucial for Autophagy Oscillation. Sci. Rep. 2020, 10, 17803. [Google Scholar] [CrossRef]
- Wu, F.; Chen, Z.; Liu, J.; Hou, Y. The Akt–MTOR Network at the Interface of Hematopoietic Stem Cell Homeostasis. Exp. Hematol. 2021, 103, 15–23. [Google Scholar] [CrossRef]
- Magri, L.; Galli, R. MTOR Signaling in Neural Stem Cells: From Basic Biology to Disease. Cell Mol. Life Sci. 2013, 70, 2887–2898. [Google Scholar] [CrossRef]
- Xiang, X.; Zhao, J.; Xu, G.; Li, Y.; Zhang, W. MTOR and the Differentiation of Mesenchymal Stem Cells. Acta Biochim. Biophys. Sin. 2011, 43, 501–510. [Google Scholar] [CrossRef]
- Alhasan, B.A.; Gordeev, S.A.; Knyazeva, A.R.; Aleksandrova, K.V.; Margulis, B.A.; Guzhova, I.V.; Suvorova, I.I. The MTOR Pathway in Pluripotent Stem Cells: Lessons for Understanding Cancer Cell Dormancy. Membranes 2021, 11, 858. [Google Scholar] [CrossRef]
- Fan, C.; Zhao, C.; Zhang, F.; Kesarwani, M.; Tu, Z.; Cai, X.; Davis, A.K.; Xu, L.; Hochstetler, C.L.; Chen, X.; et al. Adaptive Responses to MTOR Gene Targeting in Hematopoietic Stem Cells Reveal a Proliferative Mechanism Evasive to MTOR Inhibition. Proc. Natl. Acad. Sci. USA 2021, 118, e2020102118. [Google Scholar] [CrossRef]
- Chang, N.C. Autophagy and Stem Cells: Self-Eating for Self-Renewal. Front. Cell Dev. Biol. 2020, 8, 138. [Google Scholar] [CrossRef]
- Wang, C.; Haas, M.; Yeo, S.K.; Sebti, S.; Fernández, Á.F.; Zou, Z.; Levine, B.; Guan, J.L. Enhanced Autophagy in Becn1F121A/F121A Knockin Mice Counteracts Aging-Related Neural Stem Cell Exhaustion and Dysfunction. Autophagy 2022, 18, 409–422. [Google Scholar] [CrossRef]
- He, Y.; Lu, H.; Zhao, Y. Development of an Autophagy Activator from Class III PI3K Complexes, Tat-BECN1 Peptide: Mechanisms and Applications. Front. Cell Dev. Biol. 2022, 10, 851166. [Google Scholar] [CrossRef]
- Mizushima, N.; Ohsumi, Y.; Yoshimori, T. Autophagosome Formation in Mammalian Cells. Cell Struct. Funct. 2002, 27, 421–429. [Google Scholar] [CrossRef]
- Cassidy, L.D.; Narita, M. Autophagy at the Intersection of Aging, Senescence, and Cancer. Mol. Oncol. 2022, 16, 3259–3275. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Chen, Y.; Luo, S.; Chen, Y.; Chen, H.; Lan, W.; Lu, X.; Cao, Z.; Ye, Z.; et al. Autophagy Regulates the Maturation of Hematopoietic Precursors in the Embryo. Nat. Commun. 2024, 15, 2255. [Google Scholar] [CrossRef]
- Nitta, A.; Hori, K.; Tanida, I.; Igarashi, A.; Deyama, Y.; Ueno, T.; Kominami, E.; Sugai, M.; Aoki, K. Blocking LC3 Lipidation and ATG12 Conjugation Reactions by ATG7 Mutant Protein Containing C572S. Biochem. Biophys. Res. Commun. 2019, 508, 521–526. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Cao, L.; Lu, S.; Zhang, S.; Yang, R.; Wang, Y.; Zhang, N.; Yu, Y.; Wang, X.; et al. Insights on E1-like Enzyme ATG7: Functional Regulation and Relationships with Aging-Related Diseases. Commun. Biol. 2024, 7, 382. [Google Scholar] [CrossRef]
- Zhou, J.; He, H.; Zhang, J.J.; Liu, X.; Yao, W.; Li, C.; Xu, T.; Yin, S.Y.; Wu, D.Y.; Dou, C.L.; et al. ATG7-Mediated Autophagy Facilitates Embryonic Stem Cell Exit from Naive Pluripotency and Marks Commitment to Differentiation. Autophagy 2022, 18, 2946–2968. [Google Scholar] [CrossRef]
- Shen, Y.; Li, T.; Sun, C.; Cheng, X.; Chen, Z.; Wang, G.; Yang, X. Atg7 Autophagy-Independent Role on Governing Neural Stem Cell Fate Could Be Potentially Applied for Regenerative Medicine. Cell Death Differ. 2024, 31, 1375–1388. [Google Scholar] [CrossRef]
- Joshi, A.; Kundu, M. Mitophagy in Hematopoietic Stem Cells: The Case for Exploration. Autophagy 2013, 9, 1737–1749. [Google Scholar] [CrossRef]
- Narendra, D.P.; Youle, R.J. The Role of PINK1-Parkin in Mitochondrial Quality Control. Nat. Cell Biol. 2024, 26, 1639–1651. [Google Scholar] [CrossRef]
- Liu, K.; Li, X.; Li, Z.; Cao, J.; Li, X.; Xu, Y.; Liu, L.; Zhao, T. Evaluating Mitophagy in Embryonic Stem Cells by Using Fluorescence-Based Imaging. Front. Cell Dev. Biol. 2022, 10, 910464. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, J.; Gu, L.; Dan, X.; Zhang, C.; Yang, Y. New Insights into Mitophagy and Stem Cells. Stem Cell Res. Ther. 2021, 12, 452. [Google Scholar] [CrossRef]
- Lampert, M.A.; Orogo, A.M.; Najor, R.H.; Hammerling, B.C.; Leon, L.J.; Wang, B.J.; Kim, T.; Sussman, M.A.; Gustafsson, Å.B. BNIP3L/NIX and FUNDC1-Mediated Mitophagy Is Required for Mitochondrial Network Remodeling during Cardiac Progenitor Cell Differentiation. Autophagy 2019, 15, 1182–1198. [Google Scholar] [CrossRef]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Audesse, A.J.; Dhakal, S.; Hassell, L.A.; Gardell, Z.; Nemtsova, Y.; Webb, A.E. FOXO3 Directly Regulates an Autophagy Network to Functionally Regulate Proteostasis in Adult Neural Stem Cells. PLoS Genet. 2019, 15, e1008097. [Google Scholar] [CrossRef]
- Gómez-Puerto, M.C.; Verhagen, L.P.; Braat, A.K.; Lam, E.W.F.; Coffer, P.J.; Lorenowicz, M.J. Activation of Autophagy by FOXO3 Regulates Redox Homeostasis during Osteogenic Differentiation. Autophagy 2016, 12, 1804–1816. [Google Scholar] [CrossRef]
- Warr, M.R.; Binnewies, M.; Flach, J.; Reynaud, D.; Garg, T.; Malhotra, R.; Debnath, J.; Passegué, E. FOXO3A Directs a Protective Autophagy Program in Haematopoietic Stem Cells. Nature 2013, 494, 323–327. [Google Scholar] [CrossRef]
- Tan, A.; Prasad, R.; Jho, E.H. TFEB Regulates Pluripotency Transcriptional Network in Mouse Embryonic Stem Cells Independent of Autophagy-Lysosomal Biogenesis. Cell Death Dis. 2021, 12, 343. [Google Scholar] [CrossRef]
- García-Prat, L.; Kaufmann, K.B.; Schneiter, F.; Voisin, V.; Murison, A.; Chen, J.; Chan-Seng-Yue, M.; Gan, O.I.; McLeod, J.L.; Smith, S.A.; et al. TFEB-Mediated Endolysosomal Activity Controls Human Hematopoietic Stem Cell Fate. Cell Stem Cell 2021, 28, 1838–1850.e10. [Google Scholar] [CrossRef]
- Balzano, F.; Campesi, I.; Cruciani, S.; Garroni, G.; Bellu, E.; Giudici, S.D.; Angius, A.; Oggiano, A.; Rallo, V.; Capobianco, G.; et al. Epigenetics, Stem Cells, and Autophagy: Exploring a Path Involving MiRNA. Int. J. Mol. Sci. 2019, 20, 5091. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Shen, Q.; Feng, L.; Jin, H. Long Non-Coding RNAs Involved in Autophagy Regulation. Cell Death Dis. 2017, 8, e3073. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Duan, C.; Tian, W.; Gao, L. LncRNA NEAT1-206 Regulates Autophagy of Human Umbilical Cord Mesenchymal Stem Cells through the WNT5A/Ca2+ Signaling Pathway under Senescence Stress. Noncoding RNA Res. 2025, 11, 234–248. [Google Scholar] [CrossRef]
- Vitale, E.; Perveen, S.; Rossin, D.; Lo Iacono, M.; Rastaldo, R.; Giachino, C. Role of Chaperone-Mediated Autophagy in Ageing Biology and Rejuvenation of Stem Cells. Front. Cell Dev. Biol. 2022, 10, 912470. [Google Scholar] [CrossRef]
- Lim, S.H.Y.; Hansen, M.; Kumsta, C. Molecular Mechanisms of Autophagy Decline during Aging. Cells 2024, 13, 1364. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy Maintains Stemness by Preventing Senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Tang, A.H.; Rando, T.A. Induction of Autophagy Supports the Bioenergetic Demands of Quiescent Muscle Stem Cell Activation. EMBO J. 2014, 33, 2782–2797. [Google Scholar] [CrossRef]
- Rastaldo, R.; Vitale, E.; Giachino, C. Dual Role of Autophagy in Regulation of Mesenchymal Stem Cell Senescence. Front. Cell Dev. Biol. 2020, 8, 276. [Google Scholar] [CrossRef]
- Ma, W.; Lu, Y.; Jin, X.; Lin, N.; Zhang, L.; Song, Y. Targeting Selective Autophagy and beyond: From Underlying Mechanisms to Potential Therapies. J. Adv. Res. 2024, 65, 297–327. [Google Scholar] [CrossRef]
- Chong, C.M.; Ke, M.; Tan, Y.; Huang, Z.; Zhang, K.; Ai, N.; Ge, W.; Qin, D.; Lu, J.H.; Su, H. Presenilin 1 Deficiency Suppresses Autophagy in Human Neural Stem Cells through Reducing γ-Secretase-Independent ERK/CREB Signaling. Cell Death Dis. 2018, 9, 879. [Google Scholar] [CrossRef]
- Balnis, J.; Jackson, E.L.; Drake, L.A.; Singer, D.V.; Bossardi Ramos, R.; Singer, H.A.; Jaitovich, A. Rapamycin Improves Satellite Cells’ Autophagy and Muscle Regeneration during Hypercapnia. JCI Insight 2025, 10, e182842. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Y.-L.; Wang, H.-J.; Wu, J.-H.; Tan, Y.-Z. Rapamycin-Preactivated Autophagy Enhances Survival and Differentiation of Mesenchymal Stem Cells After Transplantation into Infarcted Myocardium. Stem Cell Rev. Rep. 2020, 16, 344–356. [Google Scholar] [CrossRef]
- Ceccariglia, S.; Cargnoni, A.; Silini, A.R.; Parolini, O. Autophagy: A Potential Key Contributor to the Therapeutic Action of Mesenchymal Stem Cells. Autophagy 2020, 16, 28–37. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive Oxygen Species in Cell Signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Boldogh, I.R.; Pon, L.A. Mitochondria on the Move. Trends Cell Biol. 2007, 17, 502–510. [Google Scholar] [CrossRef]
- Urao, N.; Ushio-Fukai, M. Redox Regulation of Stem/Progenitor Cells and Bone Marrow Niche. Free Radic. Biol. Med. 2013, 54, 26–39. [Google Scholar] [CrossRef]
- Tahara, E.B.; Navarete, F.D.T.; Kowaltowski, A.J. Tissue-, Substrate-, and Site-Specific Characteristics of Mitochondrial Reactive Oxygen Species Generation. Free Radic. Biol. Med. 2009, 46, 1283–1297. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Maraldi, T.; Angeloni, C.; Prata, C.; Hrelia, S. NADPH Oxidases: Redox Regulators of Stem Cell Fate and Function. Antioxidants 2021, 10, 973. [Google Scholar] [CrossRef]
- Ushio-Fukai, M. Compartmentalization of Redox Signaling through NaDPH Oxidase-Derived ROS. Antioxid. Redox Signal. 2009, 11, 1289–1299. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The Emerging Antioxidant Paradigm of Mesenchymal Stem Cell Therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Kasper, G.; Mao, L.; Geissler, S.; Draycheva, A.; Trippens, J.; Kühnisch, J.; Tschirschmann, M.; Kaspar, K.; Perka, C.; Duda, G.N.; et al. Insights into Mesenchymal Stem Cell Aging: Involvement of Antioxidant Defense and Actin Cytoskeleton. Stem Cells 2009, 27, 1288–1297. [Google Scholar] [CrossRef]
- Jang, Y.Y.; Sharkis, S.J. A Low Level of Reactive Oxygen Species Selects for Primitive Hematopoietic Stem Cells That May Reside in the Low-Oxygenic Niche. Blood 2007, 110, 3056–3063. [Google Scholar] [CrossRef]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, as Well as Their Bone Marrow Microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef]
- Wilson, A.; Laurenti, E.; Oser, G.; van der Wath, R.C.; Blanco-Bose, W.; Jaworski, M.; Offner, S.; Dunant, C.F.; Eshkind, L.; Bockamp, E.; et al. Hematopoietic Stem Cells Reversibly Switch from Dormancy to Self-Renewal during Homeostasis and Repair. Cell 2008, 135, 1118–1129. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Matsuoka, S.; Takubo, K.; Hamaguchi, I.; Nomiyama, K.; Hosokawa, K.; Sakurada, K.; Nakagata, N.; et al. Regulation of Oxidative Stress by ATM Is Required for Self-Renewal of Haematopoietic Stem Cells. Nature 2004, 431, 997–1002. [Google Scholar] [CrossRef]
- Jing, Q.; Zhou, C.; Zhang, J.; Zhang, P.; Wu, Y.; Zhou, J.; Tong, X.; Li, Y.; Du, J.; Wang, Y. Role of Reactive Oxygen Species in Myelodysplastic Syndromes. Cell Mol. Biol. Lett. 2024, 29, 53. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human Mesenchymal Stem Cells Efficiently Manage Oxidative Stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Garrison, B.R.; McDaniel, D.P.; Zhai, M.; Liao, P.J.; Nurmemet, D.; Kiang, J.G. Adaptive Redox Response of Mesenchymal Stromal Cells to Stimulation with Lipopolysaccharide Inflammagen: Mechanisms of Remodeling of Tissue Barriers in Sepsis. Oxid. Med. Cell Longev. 2013, 2013, 139–148. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Ouyang, T.; Chen, H.; Lin, J.; Liu, J.; Xiao, Y.; Yu, J.; Huang, Y. SIRT1 Reverses Senescence via Enhancing Autophagy and Attenuates Oxidative Stress-Induced Apoptosis through Promoting P53 Degradation. Int. J. Biol. Macromol. 2018, 117, 225–234. [Google Scholar] [CrossRef]
- Pan, H.; Guan, D.; Liu, X.; Li, J.; Wang, L.; Wu, J.; Zhou, J.; Zhang, W.; Ren, R.; Zhang, W.; et al. SIRT6 Safeguards Human Mesenchymal Stem Cells from Oxidative Stress by Coactivating NRF2. Cell Res. 2016, 26, 190–205. [Google Scholar] [CrossRef]
- Kobayashi, C.I.; Suda, T. Regulation of Reactive Oxygen Species in Stem Cells and Cancer Stem Cells. J. Cell Physiol. 2012, 227, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.; Taneja, R.; Ghaffari, S. Oxidative Stress Regulation of Stem and Progenitor Cells. Antioxid. Redox Signal. 2009, 11, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative Neural Stem Cells Have High Endogenous ROS Levels That Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Juntilla, M.M.; Patil, V.D.; Calamito, M.; Joshi, R.P.; Birnbaum, M.J.; Koretzky, G.A. AKT1 and AKT2 Maintain Hematopoietic Stem Cell Function by Regulating Reactive Oxygen Species. Blood 2010, 115, 4030–4038. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS Dependent DNA Damage, Mitochondria and P38 MAPK Underlies Senescence of Human Adult Stem Cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef]
- Burova, E.; Borodkina, A.; Shatrova, A.; Nikolsky, N. Sublethal Oxidative Stress Induces the Premature Senescence of Human Mesenchymal Stem Cells Derived from Endometrium. Oxid. Med. Cell Longev. 2013, 2013, 474931. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.A.; Cai, L.; Keller, B.B.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, S.Y.; Park, S.G.; Song, S.U.; Xia, Y.; Sung, J.H. Primary Involvement of NADPH Oxidase 4 in Hypoxia-Induced Generation of Reactive Oxygen Species in Adipose-Derived Stem Cells. Stem Cells Dev. 2012, 21, 2212–2221. [Google Scholar] [CrossRef]
- Li, S.Y.; Deng, Y.B.; Feng, J.Q.; Ye, W.B. Oxidative Preconditioning Promotes Bone Marrow Mesenchymal Stem Cells Migration and Prevents Apoptosis. Cell Biol. Int. 2009, 33, 411–418. [Google Scholar] [CrossRef]
- Busletta, C.; Novo, E.; Valfrè Di Bonzo, L.; Povero, D.; Paternostro, C.; Ievolella, M.; Mareschi, K.; Ferrero, I.; Cannito, S.; Compagnone, A.; et al. Dissection of the Biphasic Nature of Hypoxia-Induced Motogenic Action in Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem Cells 2011, 29, 952–963. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. P38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.R.; Ku, S.Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive Oxygen Species Enhance Differentiation of Human Embryonic Stem Cells into Mesendodermal Lineage. Exp. Mol. Med. 2010, 42, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Moliner, A.; Enfors, P.; Ibáñez, C.F.; Andäng, M. Mouse Embryonic Stem Cell-Derived Spheres with Distinct Neurogenic Potentials. Stem Cells Dev. 2008, 17, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Tsatmali, M.; Walcott, E.C.; Makarenkova, H.; Crossin, K.L. Reactive Oxygen Species Modulate the Differentiation of Neurons in Clonal Cortical Cultures. Mol. Cell Neurosci. 2006, 33, 345–357. [Google Scholar] [CrossRef]
- Sart, S.; Song, L.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid. Med. Cell Longev. 2015, 2015, 105135. [Google Scholar] [CrossRef]
- Spitkovsky, D.; Sasse, P.; Kolossov, E.; Böttinger, C.; Fleischmann, B.K.; Hescheler, J.; Wiesner, R.J. Activity of Complex III of the Mitochondrial Electron Transport Chain Is Essential for Early Heart Muscle Cell Differentiation. FASEB J. 2004, 18, 1300–1302. [Google Scholar] [CrossRef]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Perez-Terzic, C.; Behfar, A.; Terzic, A. Mitochondrial Oxidative Metabolism Is Required for the Cardiac Differentiation of Stem Cells. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4 (Suppl. S1), S60–S67. [Google Scholar] [CrossRef]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Terzic, A. Developmental Restructuring of the Creatine Kinase System Integrates Mitochondrial Energetics with Stem Cell Cardiogenesis. Ann. N. Y. Acad. Sci. 2008, 1147, 254–263. [Google Scholar] [CrossRef]
- Zhou, D.; Shao, L.; Spitz, D.R. Reactive Oxygen Species in Normal and Tumor Stem Cells. Adv. Cancer Res. 2014, 122, 1–67. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Graziano, V.; Galavotti, S.; Henriquez, N.V.; Betts, J.; Saxena, J.; Deli, A.; Karlsson, A.; Martins, L.M.; Capasso, M.; et al. Inhibition of Oxidative Metabolism Leads to P53 Genetic Inactivation and Transformation in Neural Stem Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1059–1064. [Google Scholar] [CrossRef]
- Marin Navarro, A.; Pronk, R.J.; van der Geest, A.T.; Oliynyk, G.; Nordgren, A.; Arsenian-Henriksson, M.; Falk, A.; Wilhelm, M. P53 Controls Genomic Stability and Temporal Differentiation of Human Neural Stem Cells and Affects Neural Organization in Human Brain Organoids. Cell Death Dis. 2020, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, Q.; Jiang, Z.H. Epigenetic Alterations under Oxidative Stress in Stem Cells. Oxid. Med. Cell Longev. 2022, 2022, 6439097. [Google Scholar] [CrossRef] [PubMed]
- Berniakovich, I.; Laricchia-Robbio, L.; Belmonte, J.C.I. N-Acetylcysteine Protects Induced Pluripotent Stem Cells from In Vitro Stress: Impact on Differentiation Outcome. Int. J. Dev. Biol. 2012, 56, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-Acetylcysteine Regulates Dental Follicle Stem Cell Osteogenesis and Alveolar Bone Repair via ROS Scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef]
- Asgari, R.; Mehran, Y.Z.; Weber, H.M.; Weber, M.; Golestanha, S.A.; Hosseini Kazerouni, S.M.; Panahi, F.; Mohammadi, P.; Mansouri, K. Management of Oxidative Stress for Cell Therapy through Combinational Approaches of Stem Cells, Antioxidants, and Photobiomodulation. Eur. J. Pharm. Sci. 2024, 196, 106715. [Google Scholar] [CrossRef]
- Li, C.J.; Sun, L.Y.; Pang, C.Y. Synergistic Protection of N-Acetylcysteine and Ascorbic Acid 2-Phosphate on Human Mesenchymal Stem Cells against Mitoptosis, Necroptosis and Apoptosis. Sci. Rep. 2015, 5, 9819. [Google Scholar] [CrossRef]
- Rodriguez, S.; Wang, L.; Mumaw, C.; Srour, E.F.; Lo Celso, C.; Nakayama, K.I.; Carlesso, N. The SKP2 E3 Ligase Regulates Basal Homeostasis and Stress-Induced Regeneration of HSCs. Blood 2011, 117, 6509–6519. [Google Scholar] [CrossRef]
- Zeng, W.; Xiao, J.; Zheng, G.; Xing, F.; Tipoe, G.L.; Wang, X.; He, C.; Chen, Z.Y.; Liu, Y. Antioxidant Treatment Enhances Human Mesenchymal Stem Cell Anti-Stress Ability and Therapeutic Efficacy in an Acute Liver Failure Model. Sci. Rep. 2015, 5, 11100. [Google Scholar] [CrossRef]
- Cai, J.; Yu, X.; Zhang, B.; Zhang, H.; Fang, Y.; Liu, S.; Liu, T.; Ding, X. Atorvastatin Improves Survival of Implanted Stem Cells in a Rat Model of Renal Ischemia-Reperfusion Injury. Am. J. Nephrol. 2014, 39, 466–475. [Google Scholar] [CrossRef]
- Song, L.; Yang, Y.J.; Dong, Q.T.; Qian, H.Y.; Gao, R.L.; Qiao, S.B.; Shen, R.; He, Z.X.; Lu, M.J.; Zhao, S.H.; et al. Atorvastatin Enhance Efficacy of Mesenchymal Stem Cells Treatment for Swine Myocardial Infarction via Activation of Nitric Oxide Synthase. PLoS ONE 2013, 8, e65702. [Google Scholar] [CrossRef]
- Shyh-Chang, N.; Ng, H.H. The Metabolic Programming of Stem Cells. Genes. Dev. 2017, 31, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Suda, T. Metabolic Requirements for the Maintenance of Self-Renewing Stem Cells. Nat. Rev. Mol. Cell Biol. 2014, 15, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Boyer, L.; Jin, M.; Mertens, J.; Kim, Y.; Ma, L.; Ma, L.; Hamm, M.; Gage, F.H.; Hunter, T. Metabolic Reprogramming during Neuronal Differentiation from Aerobic Glycolysis to Neuronal Oxidative Phosphorylation. Elife 2016, 5, e13374. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Ríos, P.; Chartier, A.; Enjolras, C.; Cremaschi, J.; Garret, C.; Boughlita, A.; Ramat, A.; Simonelig, M. PiRNAs Are Regulators of Metabolic Reprogramming in Stem Cells. Nat. Commun. 2024, 15, 8405. [Google Scholar] [CrossRef]
- Dodson, M.; Anandhan, A.; Zhang, D.D.; Madhavan, L. An NRF2 Perspective on Stem Cells and Ageing. Front. Aging 2021, 2, 690686. [Google Scholar] [CrossRef]
- Kim, S.U.; Park, Y.H.; Kim, J.M.; Sun, H.N.; Song, I.S.; Huang, S.M.; Lee, S.H.; Chae, J.I.; Hong, S.; Sik Choi, S.; et al. Dominant Role of Peroxiredoxin/JNK Axis in Stemness Regulation during Neurogenesis from Embryonic Stem Cells. Stem Cells 2014, 32, 998–1011. [Google Scholar] [CrossRef]
- Park, K.R.; Yun, H.M.; Yeo, I.J.; Cho, S.; Hong, J.T.; Jeong, Y.S. Peroxiredoxin 6 Inhibits Osteogenic Differentiation and Bone Formation Through Human Dental Pulp Stem Cells and Induces Delayed Bone Development. Antioxid. Redox Signal. 2019, 30, 1969–1982. [Google Scholar] [CrossRef]
- Scadden, D.T. The Stem-Cell Niche as an Entity of Action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef]
- Di Mattia, M.; Mauro, A.; Citeroni, M.R.; Dufrusine, B.; Peserico, A.; Russo, V.; Berardinelli, P.; Dainese, E.; Cimini, A.; Barboni, B. Insight into Hypoxia Stemness Control. Cells 2021, 10, 2161. [Google Scholar] [CrossRef]
- Li, X.; Jiang, O.; Wang, S. Molecular Mechanisms of Cellular Metabolic Homeostasis in Stem Cells. Int. J. Oral. Sci. 2023, 15, 52. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Q.; Zhang, Y.; Yu, M.; Jing, W.; Tian, W. Physioxia: A More Effective Approach for Culturing Human Adipose-Derived Stem Cells for Cell Transplantation. Stem Cell Res. Ther. 2018, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Yako, H.; Niimi, N.; Kato, A.; Takaku, S.; Tatsumi, Y.; Nishito, Y.; Kato, K.; Sango, K. Role of Pyruvate in Maintaining Cell Viability and Energy Production under High-Glucose Conditions. Sci. Rep. 2021, 11, 18910. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xu, F.; Ren, Z.; Zhang, Y.; Meng, Y.; Yang, Y.; Lingadahalli, S.; Cheung, E.; Li, G.; Liu, W.; et al. Elevated Exogenous Pyruvate Potentiates Mesodermal Differentiation through Metabolic Modulation and AMPK/MTOR Pathway in Human Embryonic Stem Cells. Stem Cell Rep. 2019, 13, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lan, X.; Li, Y.; Yan, C.; Lv, J.; Zhang, T.; Jiang, W. Fatty Acid Synthesis and Oxidation Regulate Human Endoderm Differentiation by Mediating SMAD3 Nuclear Localization via Acetylation. Dev. Cell 2023, 58, 1670–1687.e4. [Google Scholar] [CrossRef]
- Cao, Z.; Xie, Y.; Yu, L.; Li, Y. Hepatocyte Growth Factor (HGF) and Stem Cell Factor (SCF) Maintained the Stemness of Human Bone Marrow Mesenchymal Stem Cells (HBMSCs) during Long-Term Expansion by Preserving Mitochondrial Function via the PI3K/AKT, ERK1/2, and STAT3 Signaling Pathways. Stem Cell Res. Ther. 2020, 11, 329. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, R.; Li, C.; Chen, X.; Xiao, H.; Hou, J.; Hu, L.; Huang, C.; Wang, Y. Ginsenoside Rg1 Prevents Bone Marrow Mesenchymal Stem Cell Senescence via NRF2 and PI3K/Akt Signaling. Free Radic. Biol. Med. 2021, 174, 182–194. [Google Scholar] [CrossRef]
- Ning, B.; Hang, S.; Zhang, W.; Mao, C.; Li, D. An Update on the Bridging Factors Connecting Autophagy and Nrf2 Antioxidant Pathway. Front. Cell Dev. Biol. 2023, 11, 1232241. [Google Scholar] [CrossRef]
- Chang, K.C.; Liu, P.F.; Chang, C.H.; Lin, Y.C.; Chen, Y.J.; Shu, C.W. The Interplay of Autophagy and Oxidative Stress in the Pathogenesis and Therapy of Retinal Degenerative Diseases. Cell Biosci. 2022, 12, 1. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.F.; Youle, R.J. Parkin Is Recruited Selectively to Impaired Mitochondria and Promotes Their Autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Cairns, G.; Thumiah-Mootoo, M.; Burelle, Y.; Khacho, M. Mitophagy: A New Player in Stem Cell Biology. Biology 2020, 9, 481. [Google Scholar] [CrossRef]
- Kondoh, H.; Lleonart, M.E.; Nakashima, Y.; Yokode, M.; Tanaka, M.; Bernard, D.; Gil, J.; Beach, D. A High Glycolytic Flux Supports the Proliferative Potential of Murine Embryonic Stem Cells. Antioxid. Redox Signal. 2007, 9, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhao, Q.; Liu, P.; Cao, J.; Gong, J.; Wang, C.; Wang, W.; Li, X.; Sun, H.; Zhang, C.; et al. ATG3-Dependent Autophagy Mediates Mitochondrial Homeostasis in Pluripotency Acquirement and Maintenance. Autophagy 2016, 12, 2000–2008. [Google Scholar] [CrossRef]
- Vazquez-Martin, A.; Van den Haute, C.; Cufí, S.; Corominas-Faja, B.; Cuyàs, E.; Lopez-Bonet, E.; Rodriguez-Gallego, E.; Fernández-Arroyo, S.; Joven, J.; Baekelandt, V.; et al. Mitophagy-Driven Mitochondrial Rejuvenation Regulates Stem Cell Fate. Aging 2016, 8, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Yang, L.; Long, Q.; Chen, K.; Tang, H.; Wu, Y.; Liu, Z.; Zhou, Y.; Qi, J.; Zheng, L.; et al. BNIP3L-Dependent Mitophagy Accounts for Mitochondrial Clearance during 3 Factors-Induced Somatic Cell Reprogramming. Autophagy 2017, 13, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Cairns, G.; Thumiah-Mootoo, M.; Abbasi, M.R.; Gourlay, M.; Racine, J.; Larionov, N.; Prola, A.; Khacho, M.; Burelle, Y. PINK1 Deficiency Alters Muscle Stem Cell Fate Decision and Muscle Regenerative Capacity. Stem Cell Rep. 2024, 19, 673–688. [Google Scholar] [CrossRef]
- Murakami, K.; Kurotaki, D.; Kawase, W.; Soma, S.; Fukuchi, Y.; Kunimoto, H.; Yoshimi, R.; Koide, S.; Oshima, M.; Hishiki, T.; et al. OGT Regulates Hematopoietic Stem Cell Maintenance via PINK1-Dependent Mitophagy. Cell Rep. 2021, 34, 108579. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, L.; He, J.; Wu, M.; Jia, L.; Guo, J. Role of FOXO3a Transcription Factor in the Regulation of Liver Oxidative Injury. Antioxidants 2022, 11, 2478. [Google Scholar] [CrossRef]
- Zhou, J.; Liao, W.; Yang, J.; Ma, K.; Li, X.; Wang, Y.; Wang, D.; Wang, L.; Zhang, Y.; Yin, Y.; et al. FOXO3 Induces FOXO1-Dependent Autophagy by Activating the AKT1 Signaling Pathway. Autophagy 2012, 8, 1712–1723. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/P62 Activates NFE2L2/NRF2 via ULK1-Mediated Autophagic KEAP1 Degradation and Protects Mouse Liver from Lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Li, J.; Rimmelé, P.; Liang, R.; Sobol, R.W.; Ghaffari, S. FOXO3 Transcription Factor Is Essential for Protecting Hematopoietic Stem and Progenitor Cells from Oxidative DNA Damage. J. Biol. Chem. 2017, 292, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Tothova, Z.; Kollipara, R.; Huntly, B.J.; Lee, B.H.; Castrillon, D.H.; Cullen, D.E.; McDowell, E.P.; Lazo-Kallanian, S.; Williams, I.R.; Sears, C.; et al. FoxOs Are Critical Mediators of Hematopoietic Stem Cell Resistance to Physiologic Oxidative Stress. Cell 2007, 128, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Luciano, J.P.; Zhang, X.; Vercherat, C.; Taneja, R.; Ghaffari, S. Foxo3 Modulation of ATM and Oxidative Stress Mediates Distinct Functions in the Regulation of Hematopoietic Stem and Progenitor Cell Fate. Blood 2007, 110, 1272. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Halabian, R.; Gharehbaghian, A.; Amirizadeh, N.; Jahanian-Najafabadi, A.; Roushandeh, A.M.; Roudkenar, M.H. Nrf-2 Overexpression in Mesenchymal Stem Cells Reduces Oxidative Stress-Induced Apoptosis and Cytotoxicity. Cell Stress. Chaperones 2012, 17, 553–565. [Google Scholar] [CrossRef]
- Liu, P.; Cao, B.; Zhou, Y.; Zhang, H.; Wang, C. Human Umbilical Cord-Derived Mesenchymal Stem Cells Alleviate Oxidative Stress-Induced Islet Impairment via the Nrf2/HO-1 Axis. J. Mol. Cell Biol. 2023, 15, mjad035. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Jiang, L.L.; Liu, L. Effect of Metformin on Stem Cells: Molecular Mechanism and Clinical Prospect. World J. Stem Cells 2020, 12, 1455–1473. [Google Scholar] [CrossRef]
- Cekuc, M.S.; Ergul, Y.S.; Pius, A.K.; Meagan, M.; Shinohara, I.; Murayama, M.; Susuki, Y.; Ma, C.; Morita, M.; Chow, S.K.H.; et al. Metformin Modulates Cell Oxidative Stress to Mitigate Corticosteroid-Induced Suppression of Osteogenesis in a 3D Model. J. Inflamm. Res. 2024, 17, 10383–10396. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.J.; Wang, H.; Dong, Q.T.; Wang, T.J.; Qian, H.Y.; Xu, H. Autophagy Activation: A Novel Mechanism of Atorvastatin to Protect Mesenchymal Stem Cells from Hypoxia and Serum Deprivation via AMP-Activated Protein Kinase/Mammalian Target of Rapamycin Pathway. Stem Cells Dev. 2012, 21, 1321–1332. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Pirro, M.; Bianconi, V.; Jamialahmadi, T.; Sahebkar, A. Effects of Statins on the Biological Features of Mesenchymal Stem Cells and Therapeutic Implications. Heart Fail. Rev. 2021, 26, 1259–1272. [Google Scholar] [CrossRef]
- Li, X.-H.; Qian, S.-D.; Chen, D.; Li, Z.-Z.; Chen, K.-Y.; Pan, Y.-P.; Lv, X.-H.; Jia, R.-Q.; Yu, X.-F. A New Mechanism in Steroid-Induced Osteonecrosis of the Femoral Head and the Protective Role of Simvastatin. Exp. Cell Res. 2025, 446, 114471. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Xu, X.M.; Zhang, S.X. Low-Dose Dexamethasone Promotes Osteoblast Viability by Activating Autophagy via the SGK1/FOXO3a Signaling Pathway. Cell Biol. Int. 2023, 47, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xu, H.; Chen, J.; Cai, W.; Zhou, J.; Peng, H. Human Umbilical Cord Mesenchymal Stem Cells Prevent Steroid-Induced Avascular Necrosis of the Femoral Head by Modulating Cellular Autophagy. Biomedicines 2024, 12, 2817. [Google Scholar] [CrossRef] [PubMed]
- Eldien, H.S.; Mostafa, N.A.; Sayed, D.; Mansor, S.G.; Elewa, F.M.; Meligy, F.Y. Resveratrol Antiaging Impact on Bone Marrow-Derived Mesenchymal Stem Cells in A Duration-Dependent Manner. Egypt. J. Histol. 2023, 46, 335–354. [Google Scholar] [CrossRef]
- Hu, C.; Li, L. The Application of Resveratrol to Mesenchymal Stromal Cell-Based Regenerative Medicine. Stem Cell Res. Ther. 2019, 10, 307. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Petukhov, A.V.; Aksenov, N.D.; Pospelov, V.A. Resveratrol Enhances Pluripotency of Mouse Embryonic Stem Cells by Activating AMPK/Ulk1 Pathway. Cell Death Discov. 2019, 5, 61. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, S.D.; Liu, H.J.; Yuan, Q.H.; Liu, S.M.; Zhang, Y.M.; Ling, E.A.; Hao, A.J. Melatonin Promotes Proliferation and Differentiation of Neural Stem Cells Subjected to Hypoxia In Vitro. J. Pineal Res. 2011, 51, 104–112. [Google Scholar] [CrossRef]
- Song, J.; Kang, S.M.; Lee, K.M.; Lee, J.E. The Protective Effect of Melatonin on Neural Stem Cell against LPS-Induced Inflammation. Biomed. Res. Int. 2015, 2015, 854359. [Google Scholar] [CrossRef]
- Gong, X.; Ivanov, V.N.; Hei, T.K. 2,3,5,6-Tetramethylpyrazine (TMP) down-Regulated Arsenic-Induced Heme Oxygenase-1 and ARS2 Expression by Inhibiting Nrf2, NF-ΚB, AP-1 and MAPK Pathways in Human Proximal Tubular Cells. Arch. Toxicol. 2016, 90, 2187–2200. [Google Scholar] [CrossRef]
- Ma, W.; He, F.; Ding, F.; Zhang, L.; Huang, Q.; Bi, C.; Wang, X.; Hua, B.; Yang, F.; Yuan, Y.; et al. Pre-Treatment with Melatonin Enhances Therapeutic Efficacy of Cardiac Progenitor Cells for Myocardial Infarction. Cell. Physiol. Biochem. 2018, 47, 1287–1298. [Google Scholar] [CrossRef]
- Yoo, Y.M.; Han, T.Y.; Kim, H.S. Melatonin Suppresses Autophagy Induced by Clinostat in Preosteoblast MC3T3-E1 Cells. Int. J. Mol. Sci. 2016, 17, 526. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.W.; Wang, Z.; Zhang, Y.M.; Du, Z.X.; Zhang, X.L.; Liu, Q.; Guo, Y.J.; Li, X.G.; Hao, A.J. Protective Effect of Melatonin on Bone Marrow Mesenchymal Stem Cells against Hydrogen Peroxide-Induced Apoptosis In Vitro. J. Cell Biochem. 2013, 114, 2346–2355. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, W.; Li, Z.; Ren, J.; Li, X.; Li, S.; Liu, Q.; Song, F.; Hao, A.; Wang, F. Melatonin Protects Neural Stem Cells Against Tri-Ortho-Cresyl Phosphate-Induced Autophagy. Front. Mol. Neurosci. 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jacobi, A.; Vater, C.; Zou, L.; Zou, X.; Stiehler, M. Icariin Promotes Angiogenic Differentiation and Prevents Oxidative Stress-Induced Autophagy in Endothelial Progenitor Cells. Stem Cells 2015, 33, 1863–1877. [Google Scholar] [CrossRef]
- Yao, X.; Jing, X.; Guo, J.; Sun, K.; Deng, Y.; Zhang, Y.; Guo, F.; Ye, Y. Icariin Protects Bone Marrow Mesenchymal Stem Cells against Iron Overload Induced Dysfunction through Mitochondrial Fusion and Fission, PI3K/Akt/MTOR and MAPK Pathways. Front. Pharmacol. 2019, 10, 163. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Zhang, H.; Huang, H.; Zhang, Z.; Tang, D.; Jiao, F. Icariin Protects Rabbit BMSCs against OGD-Induced Apoptosis by Inhibiting ERs-Mediated Autophagy via MAPK Signaling Pathway. Life Sci. 2020, 253, 117730. [Google Scholar] [CrossRef]
- Li, W.; Huang, Y.; Fan, L.; Yangzom, D.; Zhang, K.; Shen, L.; Cao, S.; Gu, C.; Yu, S. Curcumin Liposomes Alleviate Senescence of Bone Marrow Mesenchymal Stem Cells by Activating Mitophagy. Sci. Rep. 2024, 14, 31291. [Google Scholar] [CrossRef]
- Ruan, Y.; Luo, H.; Tang, J.; Ji, M.; Yu, D.; Yu, Q.; Cao, Z.; Mai, Y.; Zhang, B.; Chen, Y.; et al. Curcumin Inhibits Oxidative Stress and Autophagy in C17.2 Neural Stem Cell through ERK1/2 Signaling Pathways. Aging Med. 2024, 7, 559–570. [Google Scholar] [CrossRef]
- Jiang, E.; Chen, X.; Bi, Y.; Pan, C.; Li, X.; Lan, X. Curcumin Inhibits Oxidative Stress and Apoptosis Induced by H2O2 in Bovine Adipose-Derived Stem Cells (BADSCs). Animals 2024, 14, 3421. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Liu, Y.; Zheng, P. MTOR Regulation and Therapeutic Rejuvenation of Aging Hematopoietic Stem Cells. Sci. Signal. 2009, 2, ra75. [Google Scholar] [CrossRef]
- Rimmelé, P.; Lofek-Czubek, S.; Ghaffari, S. Resveratrol Increases the Bone Marrow Hematopoietic Stem and Progenitor Cell Capacity. Am. J. Hematol. 2014, 89, E235–E238. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Asada, S.; Goyama, S.; Kitamura, T. Mechanisms Involved in Hematopoietic Stem Cell Aging. Cell Mol. Life Sci. 2022, 79, 473. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of Antioxidant Supplements on the Survival and Differentiation of Stem Cells. Oxid. Med. Cell Longev. 2017, 2017, 5032102. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; Moawed, F.S.M.; Abdel-Hamid, G.R. Icariin Promote Stem Cells Regeneration and Repair Acinar Cells in L-Arginine/Radiation-Inducing Chronic Pancreatitis in Rats. Dose-Response 2020, 18, 1559325820970810. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Zhang, X.; Chen, P.; Hang, R.; Xiao, Y.; Wang, J.; Liu, C. Osteoporosis Remission via an Anti-Inflammaging Effect by Icariin Activated Autophagy. Biomaterials 2023, 297, 122125. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossin, D.; Perrelli, M.-G.; Lo Iacono, M.; Rastaldo, R.; Giachino, C. Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine. Antioxidants 2025, 14, 691. https://doi.org/10.3390/antiox14060691
Rossin D, Perrelli M-G, Lo Iacono M, Rastaldo R, Giachino C. Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine. Antioxidants. 2025; 14(6):691. https://doi.org/10.3390/antiox14060691
Chicago/Turabian StyleRossin, Daniela, Maria-Giulia Perrelli, Marco Lo Iacono, Raffaella Rastaldo, and Claudia Giachino. 2025. "Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine" Antioxidants 14, no. 6: 691. https://doi.org/10.3390/antiox14060691
APA StyleRossin, D., Perrelli, M.-G., Lo Iacono, M., Rastaldo, R., & Giachino, C. (2025). Dynamic Interplay Between Autophagy and Oxidative Stress in Stem Cells: Implications for Regenerative Medicine. Antioxidants, 14(6), 691. https://doi.org/10.3390/antiox14060691







