Dietary Probiotic Bacillus subtilis AAHM-BS2360 and Its Postbiotic Metabolites Enhance Growth, Immunity, and Resistance to Edwardsiellosis in Pangasianodon hypophthalmus
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Pangasius Husbandry
2.3. Bacterial Cultivation and Preparation of Experimental Diets
2.4. Growth Performance
2.5. Sample Collection
2.6. Parameter of Serum Biochemical
2.7. Humoral Innate Immune Responses Assays
2.7.1. Bactericidal Activity
2.7.2. Lysozyme Activity
2.8. Determination of Oxidative Stress and Antioxidant Status of Target Organs
2.8.1. Measurement of Catalase (CAT) Activity
2.8.2. Measurement of Glutathione Peroxidase (GPx) Activity
2.8.3. Measurement of Glutathione Reductase (GR) Enzyme Activity
2.8.4. Measurement of Reduced Glutathione (GSH) Content
2.8.5. Measurement of Glutathione-S-Transferase (GST) Activity
2.8.6. Measurement of Malondialdehyde (MDA) Content
2.8.7. Measurement of Superoxide Dismutase (SOD) Activity
2.9. cDNA Preparation and Gene Expression Analysis Using Quantitative Reverse Transcription PCR (qRT-PCR)
2.10. Disease Resistance and Survival Rate (SR) After E. tarda Challenges
2.11. Statistical Data Analysis
3. Results
3.1. Serum Biochemical Analysis
3.2. Nonspecific Humoral Immune Responses
3.2.1. Bactericidal Activity
3.2.2. Lysozyme Activity
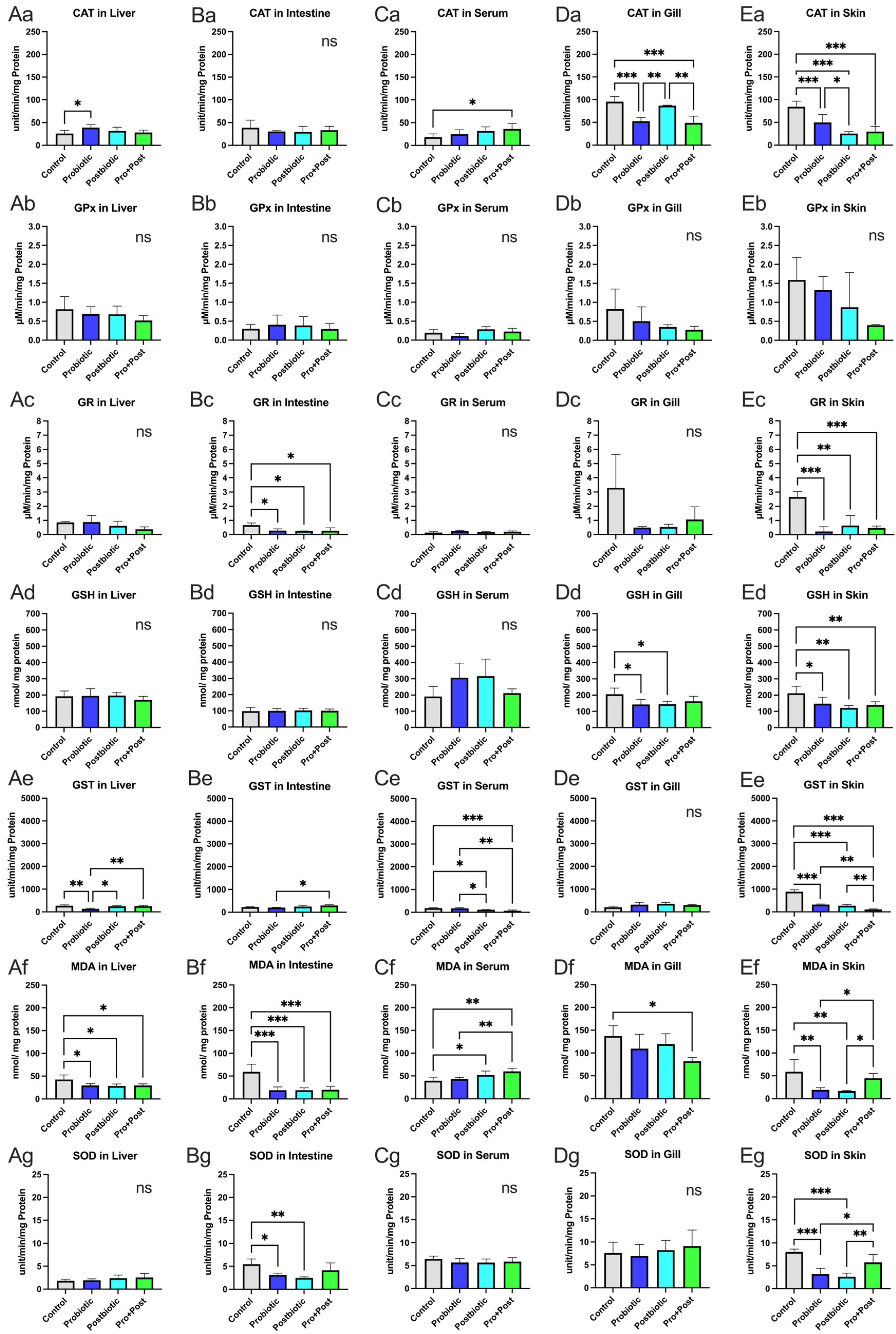
3.3. Responses of Oxidative Stress and Antioxidant Activity
3.3.1. Liver
3.3.2. Intestine
3.3.3. Serum
3.3.4. Gills
3.3.5. Skin
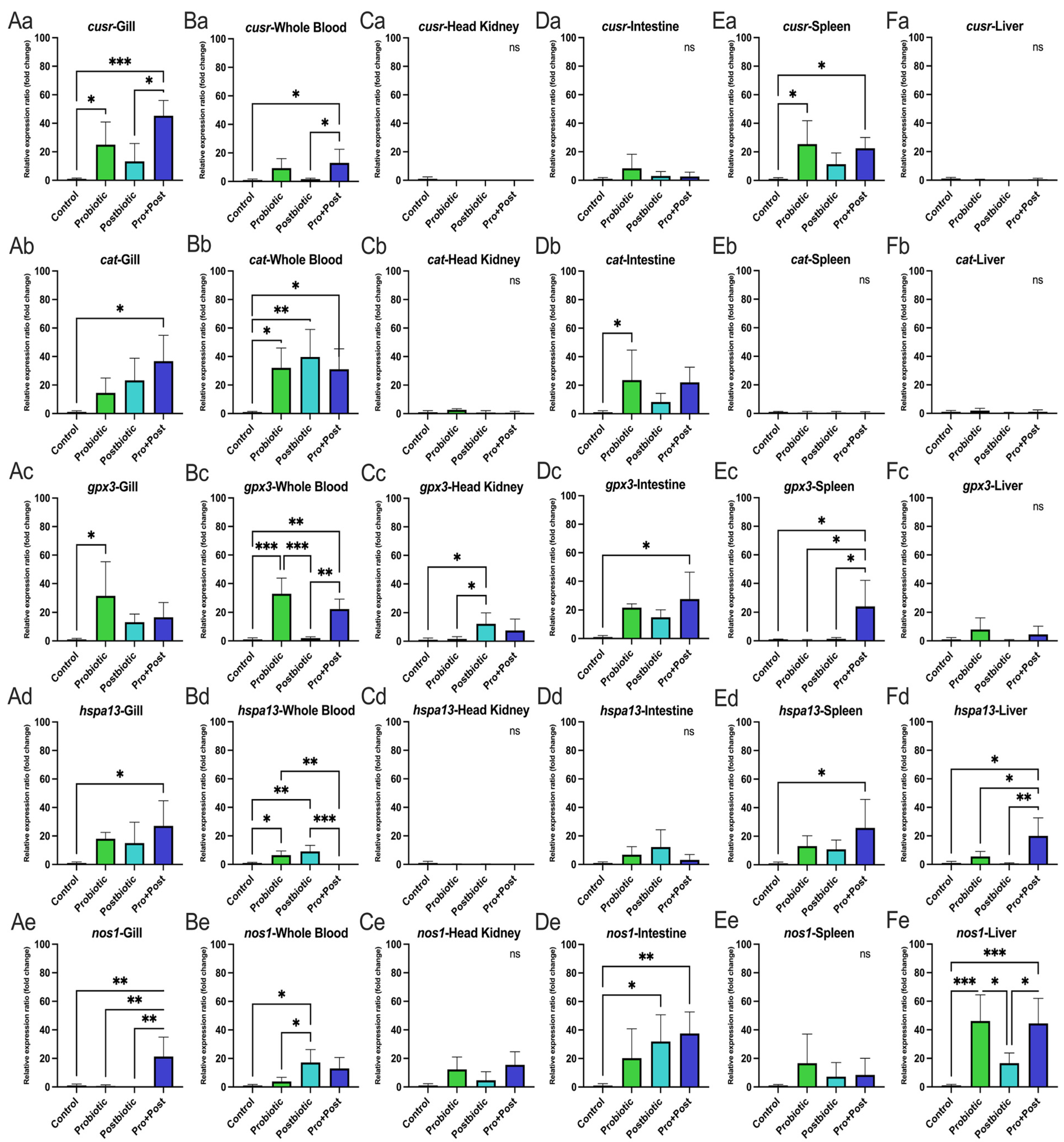
3.4. Oxidative Stress and Antioxidant-Related Gene Expression
3.4.1. Following a 30-Day Supplementation with Probiotic, Postbiotic, and Their Combination Derived from B. subtilis AAHM-BS2360
3.4.2. Post-Challenge with E. tarda
3.5. Non-Specific Immune-Related Gene Expression
3.5.1. Following a 30-Day Supplementation with Probiotic, Postbiotic, and Their Combination Derived from B. subtilis AAHM-BS2360
3.5.2. Post-Challenge with E. tarda
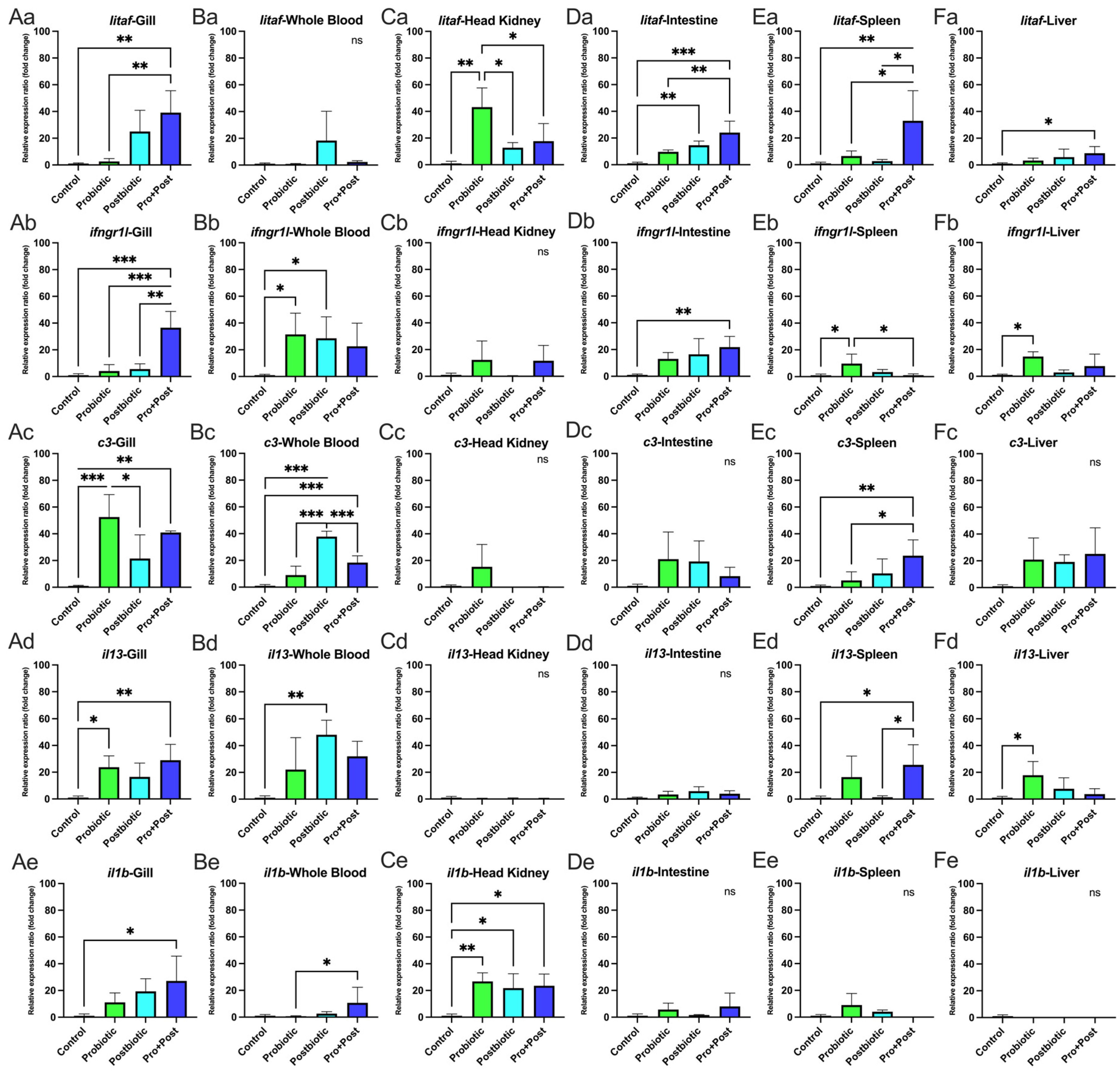
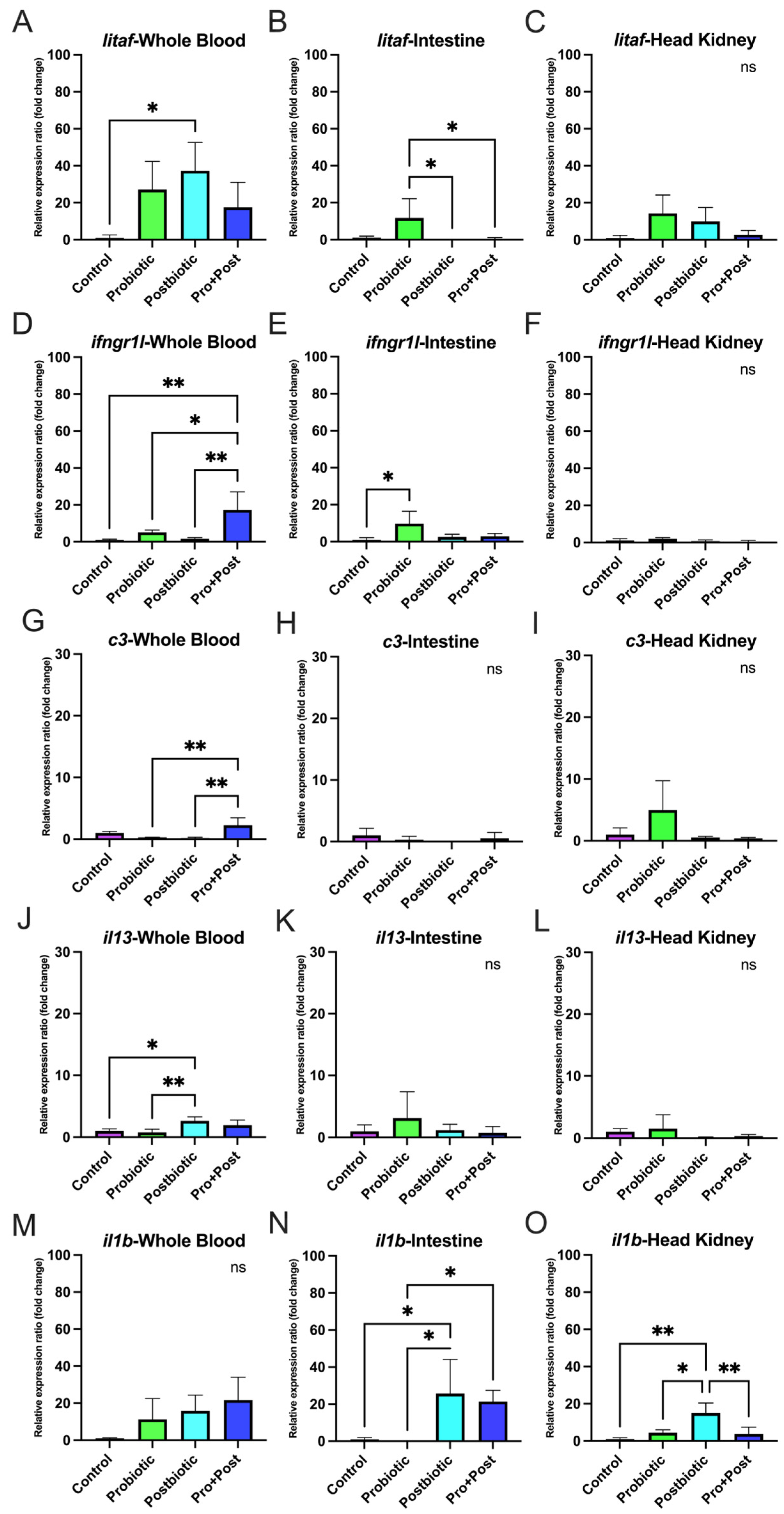
3.6. Proinflammatory-Related Gene Expression
3.6.1. Following a 30-Day Supplementation with Probiotic, Postbiotic, and Their Combination Derived from B. subtilis AAHM-BS2360
3.6.2. Post-Challenge with E. tarda
3.7. Growth-Related Gene Expression
3.8. Growth Performance Analysis
3.9. Histopathology
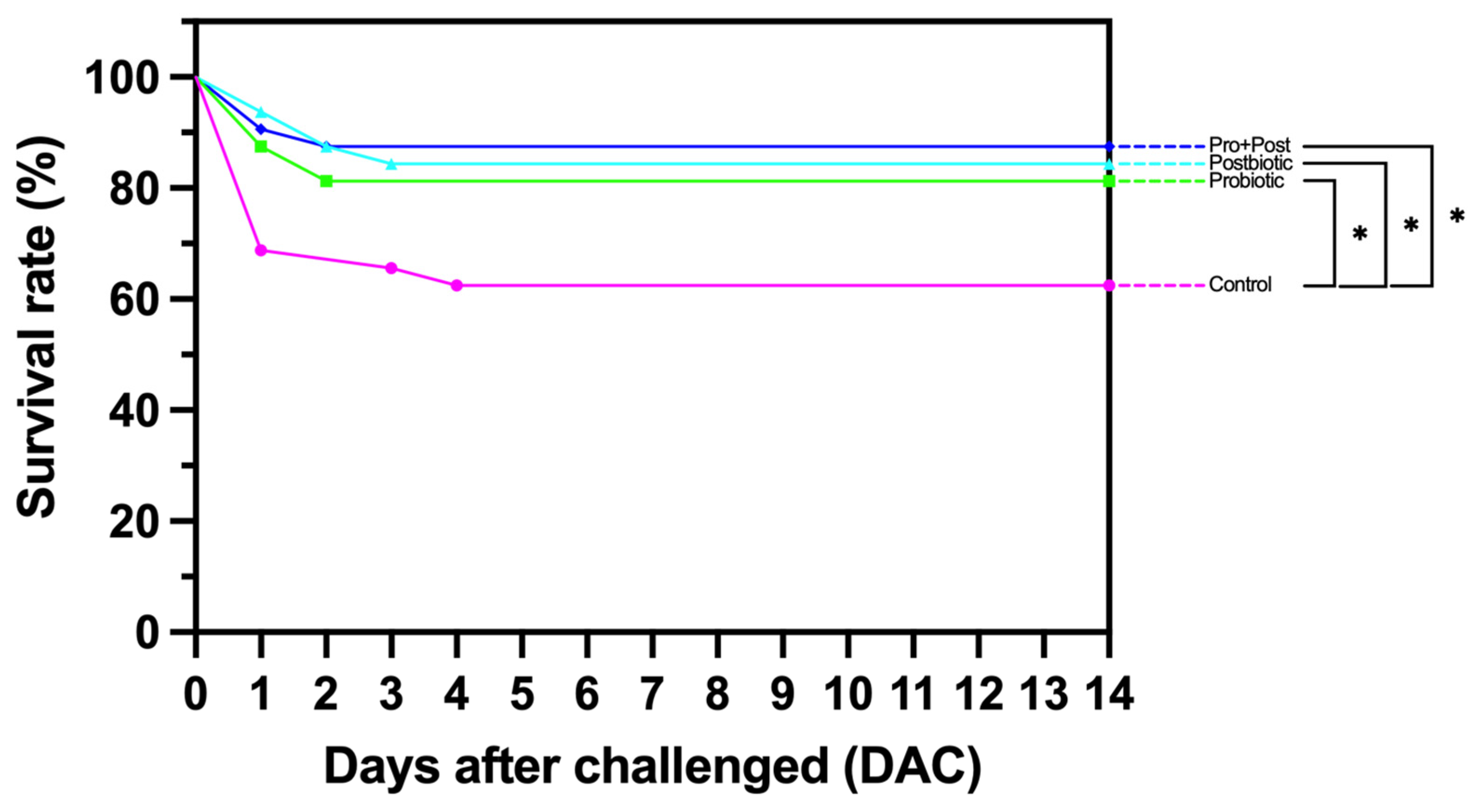
3.10. Survival Rate Analysis Following Post-Challenge Against E. tarda
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FishStatJ. FishStat: Global Aquaculture Production 1950–2022, 4.03.04; FAO: Rome, Italy, 2024. [Google Scholar]
- FAO. International markets for fisheries and aquaculture products—First issue 2024, with January September 2023 statistics. In GLOBEFISH Highlights; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef]
- Hasan, M.R.; Shipton, T.A. Aquafeed value chain analysis of striped catfish in Vietnam. Aquaculture 2021, 541, 736798. [Google Scholar] [CrossRef]
- Nguyen, T.A.T.; Jolly, C.M. Global value chain and food safety and quality standards of Vietnam pangasius exports. Aquac. Rep. 2020, 16, 100256. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, J.; Lin, M.; Weng, Q.; Huang, L.; Yan, Q. Identification of antibiotic-resistance markers of Edwardsiella tarda using aptamers. Food Biosci. 2024, 59, 104028. [Google Scholar] [CrossRef]
- Pakingking, R.V.; Nguyen, V.V. Chapter 26—Edwardsiellosis. In Aquaculture Pathophysiology; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S.-M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 345–356. [Google Scholar] [CrossRef]
- Joseph, T.C.; Dinesh, R.; Shaheer, P.; Murugadas, V.; Rekha, M.; Lalitha, K.V. Outbreak of Edwardsiella tarda in Cultured Pangasius hypophthalmus (Sauvage, 1878). Fish. Technol. 2019, 56, 151–157. [Google Scholar]
- Shetty, M.; Maiti, B.; Venugopal, M.N.; Karunasagar, I.; Karunasagar, I. First isolation and characterization of Edwardsiella tarda from diseased striped catfish, Pangasianodon hypophthalmus (Sauvage). J. Fish. Dis. 2014, 37, 265–271. [Google Scholar] [CrossRef]
- Lassen, S.B.; Ahsan, M.E.; Islam, S.R.; Zhou, X.Y.; Razzak, M.A.; Su, J.Q.; Brandt, K.K. Prevalence of antibiotic resistance genes in Pangasianodon hypophthalmus and Oreochromis niloticus aquaculture production systems in Bangladesh. Sci. Total Environ. 2022, 813, 151915. [Google Scholar] [CrossRef] [PubMed]
- Wiratama, N.; Uchuwittayakul, A.; Susanto, Y.; Utari, H.B.; Muna, N.; Satriagasa, M.C. Mapping spatial analysis of fish disease incidence and antibiotic resistance trends in selected provinces of Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2024, 1392, 012004. [Google Scholar] [CrossRef]
- Sam-on, M.F.S.; Mustafa, S.; Hashim, A.M.; Yusof, M.T.; Zulkifly, S.; Roslan, M.A.H. Determination of prebiotic utilisation capability of potential probiotic Bacillus velezensis FS26 through in silico and in vitro approaches. Food Biosci. 2023, 53, 102566. [Google Scholar] [CrossRef]
- Saengrung, J.; Bunnoy, A.; Du, X.; Huang, L.; An, R.; Liang, X.; Srisapoome, P. Effects of ribonucleotide supplementation in modulating the growth of probiotic Bacillus subtilis and the synergistic benefits for improving the health performance of Asian seabass (Lates calcarifer). Fish. Shellfish. Immunol. 2023, 140, 108983. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Kayansamruaj, P.; Srisapoome, P. Acinetobacter Strain KUO11TH, a Unique Organism Related to Acinetobacter pittii and Isolated from the Skin Mucus of Healthy Bighead Catfish and Its Efficacy Against Several Fish Pathogens. Microorganisms 2019, 7, 549. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y.; Zhang, H.; Dong, X. Host gut-derived Bacillus probiotics supplementation improves growth performance, serum and liver immunity, gut health, and resistive capacity against Vibrio harveyi infection in hybrid grouper (female Epinephelus fuscoguttatus × male Epinephelus lanceolatus). Anim. Nutr. 2023, 14, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Da, M.; Sun, J.; Ma, C.; Li, D.; Dong, L.; Wang, L.S.; Chen, F. Postbiotics: Enhancing human health with a novel concept. eFood 2024, 5, e180. [Google Scholar] [CrossRef]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Probiotic Effects of a Novel Strain, Acinetobacter KU011TH, on the Growth Performance, Immune Responses, and Resistance against Aeromonas hydrophila of Bighead Catfish (Clarias macrocephalus Gunther, 1864). Microorganisms 2019, 7, 613. [Google Scholar] [CrossRef]
- Meachasompop, P.; Bunnoy, A.; Keaswejjareansuk, W.; Dechbumroong, P.; Namdee, K.; Srisapoome, P. Development of Immersion and Oral Bivalent Nanovaccines for Streptococcosis and Columnaris Disease Prevention in Fry and Fingerling Asian Seabass (Lates calcarifer) Nursery Farms. Vaccines 2023, 12, 17. [Google Scholar] [CrossRef]
- Parry, R.M.; Chandan, R.C.; Shahani, K.M. A Rapid and Sensitive Assay of Muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Sukhsangchan, R.; Phaksopa, J.; Uchuwittayakul, A.; Chou, C.C.; Srisapoome, P. Effects of Zinc Oxide Nanoparticles (ZnO NPs) on Growth, Immune Responses and Histopathological Alterations in Asian Seabass (Lates calcarifer, Bloch 1790) under Low-Salinity Conditions. Animals 2024, 14, 2737. [Google Scholar] [CrossRef]
- Maehly, A.C. The Assay of Catalases and Peroxidases. In Methods of Biochemical Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1954; pp. 357–424. [Google Scholar] [CrossRef]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: Possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984, 33, 1801–1807. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A.; Alkreathy, H.M. Protective effects of Carissa opaca fruits against CCl4-induced oxidative kidney lipid peroxidation and trauma in rat. Food Nutr. Res. 2017, 59, 28438. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-Induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3,4-Bromobenzene Oxide as the Hepatotoxic Metabolite. Pharmacology 2008, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Paankhao, N.; Sangsawang, A.; Kantha, P.; Paankhao, S.; Promsee, K.; Soontara, C.; Kongsriprapan, S.; Srisapoome, P.; Kumwan, B.; Meachasompop, P.; et al. Antioxidant and antibacterial efficiency of the ethanolic leaf extract of Kratom (Mitragyna speciosa (Korth.) Havil) and its effects on growth, health, and disease resistance against Edwardsiella tarda infection in Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 2024, 152, 109771. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Noguchi, T.; Okabe, T.; Kajiyama, M. Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res. 1982, 42, 4233–4235. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cox, T.F.; Czanner, G. A practical divergence measure for survival distributions that can be estimated from Kaplan-Meier curves. Stat. Med. 2016, 35, 2406–2421. [Google Scholar] [CrossRef]
- Kumar, S.; Raman, R.P.; Prasad, K.P.; Srivastava, P.P.; Kumar, S.; Rajendran, K.V. Effects on haematological and serum biochemical parameters of Pangasianodon hypophthalmus to an experimental infection of Thaparocleidus sp. (Monogenea: Dactylogyridae). Exp. Parasitol. 2018, 188, 1–7. [Google Scholar] [CrossRef]
- Hasan, N.A.; Haque, M.M.; Bashar, A.; Hasan, M.T.; Faruk, M.A.R.; Ahmed, G.U. Effects of dietary Papaveraceae extract on growth, feeding response, nutritional quality and serum biochemical indices of striped catfish (Pangasianodon hypophthalmus). Aquac. Rep. 2021, 21, 100793. [Google Scholar] [CrossRef]
- Uchuwittayakul, A.; Thompson, K.D.; Thangsunan, P.; Phaksopa, J.; Buncharoen, W.; Saenphet, K.; Kumwan, B.; Meachasompop, P.; Saenphet, S.; Wiratama, N.; et al. Evaluation of a hydrogel platform for encapsulated multivalent Vibrio antigen delivery to enhance immune responses and disease protection against vibriosis in Asian seabass (Lates calcarifer). Fish. Shellfish. Immunol. 2025, 160, 110230. [Google Scholar] [CrossRef]
- Hoque, F.; Abraham, T.J.; Dash, G.; Boda, S.; Nagesh, T.S.; Ghosh, T.K.; Joardar, S.N.; Sundaray, J.K. Effect of Dietary Yeast Extract on the Innate Immunity, Serum Biochemistry and Disease Resistance of Pangasius pangasius Against Edwardsiella tarda Infection. Proc. Zool. Soc. 2024, 77, 47–57. [Google Scholar] [CrossRef]
- Abdelkhalek, N.K.; Ghazy, E.W.; Abdel-Daim, M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: Impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Escamilla, I.; López, L.M.; Gisbert, E.; Sanchez, S.; Rodarte-Venegas, D.; Álvarez, C.A.; Galaviz, M.A. Soybean protein concentrate as a protein source for totoaba (Totoaba macdonaldi) juveniles: Effect on intermediary metabolism and liver histological organization. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 262, 111062. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, Y.; Cao, Q.; Shan, H.; Zong, J.; Feng, L.; Jiang, W.; Wu, P.; Zhao, J.; Liu, H.; et al. Hepatic Oxidative Stress and Cell Death Influenced by Dietary Lipid Levels in a Fresh Teleost. Antioxidants 2024, 13, 808. [Google Scholar] [CrossRef]
- Lu, K.-L.; Xu, W.-N.; Liu, W.-B.; Wang, L.-N.; Zhang, C.-N.; Li, X.-F. Association of Mitochondrial Dysfunction with Oxidative Stress and Immune Suppression in Blunt Snout Bream Megalobrama amblycephala Fed a High-Fat Diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef]
- Xu, R.; Wang, T.; Ding, F.F.; Zhou, N.N.; Qiao, F.; Chen, L.Q.; Du, Z.Y.; Zhang, M.L. Lactobacillus plantarum Ameliorates High-Carbohydrate Diet-Induced Hepatic Lipid Accumulation and Oxidative Stress by Upregulating Uridine Synthesis. Antioxidants 2022, 11, 1238. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Q.; Wu, D.; Hu, Y.; Chen, S.; Ding, T.; Ye, X.; Liu, D.; Chen, J. What is new in lysozyme research and its application in food industry? A review. Food Chem 2019, 274, 698–709. [Google Scholar] [CrossRef]
- Etyemez Buyukdeveci, M.; Cengizler, I.; Balcazar, J.L.; Demirkale, I. Effects of two host-associated probiotics Bacillus mojavensis B191 and Bacillus subtilis MRS11 on growth performance, intestinal morphology, expression of immune-related genes and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus iniae. Dev. Comp. Immunol. 2023, 138, 104553. [Google Scholar] [CrossRef]
- Say, P.; Nimikul, S.; Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Long-Term Application of a Synbiotic Chitosan and Acinetobacter KU011TH Mixture on the Growth Performance, Health Status, and Disease Resistance of Hybrid Catfish (Clarias gariepinus × C. macrocephalus) during Winter. Microorganisms 2023, 11, 1807. [Google Scholar] [CrossRef]
- Say, P.; Nimitkul, S.; Bunnoy, A.; Na-Nakorn, U.; Srisapoome, P. Effects of the combination of chitosan and Acinetobacter KU011TH on the growth and health performances and disease resistance of juvenile hybrid catfish (Clarias gariepinus × C. macrocephalus). Fish Shellfish Immunol. 2023, 142, 109177. [Google Scholar] [CrossRef]
- Mohammadi, G.; Hafezieh, M.; Karimi, A.A.; Azra, M.N.; Van Doan, H.; Tapingkae, W.; Abdelrahman, H.A.; Dawood, M.A.O. The synergistic effects of plant polysaccharide and Pediococcus acidilactici as a synbiotic additive on growth, antioxidant status, immune response, and resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 120, 304–313. [Google Scholar] [CrossRef]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef]
- Chen, W.; Gao, F.; Chu, F.; Zhang, J.; Gao, G.F.; Xia, C. Crystal structure of a bony fish beta2-microglobulin: Insights into the evolutionary origin of immunoglobulin superfamily constant molecules. J. Biol. Chem. 2010, 285, 22505–22512. [Google Scholar] [CrossRef]
- Song, C.; Sun, C.; Liu, B.; Xu, P. Oxidative Stress in Aquatic Organisms. Antioxidants 2023, 12, 1223. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Fu, X.; Zhang, Y.; Zhang, H.; Ye, Y.; Deng, J.; Tan, B. Effects of Malondialdehyde on Growth Performance, Gastrointestinal Health, and Muscle Quality of Striped Catfish (Pangasianodon hypophthalmus). Antioxidants 2024, 13, 1524. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Huang, H.; Fan, J.; Zhou, H.; Deng, J.; Tan, B. High Dietary Histamine Induces Digestive Tract Oxidative Damage in Juvenile Striped Catfish (Pangasianodon hypophthalmus). Antioxidants 2022, 11, 2276. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Jafarzadeh, F.; Roomiani, L.; Dezfoulnejad, M.C.; Baboli, M.J.; Sary, A.A. Harnessing paraprobiotics and postbiotics for enhanced immune function in Asian seabass (Lates calcarifer): Insights into pattern recognition receptor signaling. Fish. Shellfish. Immunol. 2024, 151, 109725. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; El-Son, M.A.M. Immune protective, stress indicators, antioxidant, histopathological status, and heat shock protein gene expression impacts of dietary Bacillus spp. against heat shock in Nile tilapia, Oreochromis niloticus. BMC Vet. Res. 2024, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.-T.; Lu, H.; Xiong, J.; Zhang, L.; Sun, W.-W.; Shan, X.-F. The application and potential of postbiotics as sustainable feed additives in aquaculture. Aquaculture 2024, 592, 741237. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dwivedi, M.K. The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 1–11. [Google Scholar] [CrossRef]
- Qi, X.; Luo, F.; Zhang, Y.; Wang, G.; Ling, F. Exploring the protective role of Bacillus velezensis BV1704-Y in zebrafish health and disease resistance against Aeromonas hydrophila infection. Fish. Shellfish. Immunol. 2024, 152, 109789. [Google Scholar] [CrossRef]
- Yun, L.; Kang, M.; Shen, Y.; Feng, J.; Yang, G.; Zhang, J.; Meng, X.; Chang, X. Dietary Bacillus velezensis R-71003 and sodium gluconate improve antioxidant capacity, immune response and resistance against Aeromonas hydrophila in common carp. Fish. Shellfish. Immunol. 2023, 139, 108921. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, X.; Liu, Y.; Liu, J.; Liang, X.; Wang, H.; Li, G.; Xue, M. Growth, Antioxidant Capacity, and Liver Health in Largemouth Bass (Micropterus salmoides) Fed Multi-Strain Yeast-Based Paraprobiotic: A Lab-to-Pilot Scale Evaluation. Antioxidants 2024, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Jenik, K.; Oberhoffner, S.; DeWitte-Orr, S.J. Response to pathogens—Innate immunity. In Encyclopedia of Fish Physiology, 2nd ed.; Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Oxford, UK, 2024; pp. 334–345. [Google Scholar] [CrossRef]
- Semple, S.L.; Barreda, D.R. Adaptive immunity in teleostean fishes. In Encyclopedia of Fish Physiology, 2nd ed.; Alderman, S.L., Gillis, T.E., Eds.; Academic Press: Oxford, UK, 2024; pp. 346–354. [Google Scholar] [CrossRef]
- Hoque, F.; Abraham, T.J.; Joardar, S.N.; Paria, P.; Behera, B.K.; Das, B.K. Effects of dietary supplementation of Pseudomonas aeruginosa FARP72 on the immunomodulation and resistance to Edwardsiella tarda in Pangasius pangasius. Fish. Shellfish. Immunol. Rep. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- García Mansilla, M.J.; Rodríguez Sojo, M.J.; Lista, A.R.; Ayala Mosqueda, C.V.; Ruiz Malagón, A.J.; Ho Plagaro, A.; Gálvez, J.; Rodríguez Nogales, A.; Rodríguez Sánchez, M.J. Microbial-Derived Antioxidants in Intestinal Inflammation: A Systematic Review of Their Therapeutic Potential. Antioxidants 2025, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.H.; Nguyen, H.T.; Le, B.T.N.; Tran, P.H.; Van Nguyen, S.; Kim, O.T.P. Characterization of single nucleotide polymorphism in IGF1 and IGF1R genes associated with growth traits in striped catfish (Pangasianodon hypophthalmus Sauvage, 1878). Aquaculture 2021, 538, 736542. [Google Scholar] [CrossRef]
- Meidong, R.; Khotchanalekha, K.; Doolgindachbaporn, S.; Nagasawa, T.; Nakao, M.; Sakai, K.; Tongpim, S. Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish. Shellfish. Immunol. 2018, 73, 1–10. [Google Scholar] [CrossRef]
- Han, C.; Shi, H.; Cui, C.; Wang, J.; Li, L.; Bei, W.; Cai, Y.; Wang, S. Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper. Antioxidants 2024, 13, 317. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Z.; Zhao, F.; Liu, H.; Yu, L.; Zha, J.; Wang, G. Probiotic potential of Bacillus velezensis JW: Antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish. Shellfish. Immunol. 2018, 78, 322–330. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Chaklader, M.R.; Shukry, M.; Ahmed, H.A.; Khallaf, M.A. A multispecies probiotic modulates growth, digestive enzymes, immunity, hepatic antioxidant activity, and disease resistance of Pangasianodon hypophthalmus fingerlings. Aquaculture 2023, 563, 738948. [Google Scholar] [CrossRef]









| Gene Group | Genes | Nucleotide Sequences (5′ → 3′) | Tm (°C) | Efficiency (%) | Accession Number |
|---|---|---|---|---|---|
| Housekeeping gene | Actin, beta 1 (actb1) | F-5′-GAGCGCAAGTACTCTGTATGGA-3′ | 60 | 98.5 | XM_026928832.3 |
| R-5′-CTGTGGTGGTTACAGTCCTGTT-3′ | |||||
| 18S rRNA (rna18s) | F-5′-TGACTCAACACGGGAAACCTC-3′ | 60 | 102.4 | XR_004577708 | |
| R-5′-CAGACAAATCGCTCCACCAAC-3′ | |||||
| Stress oxidative and Antioxidant-related gene | Copper-only SOD repeat protein (cusr) | F-5′-GTCCATCTTACCCGGTGCCC-3′ | 60 | 99 | XM_034299545.1 |
| R-5′- CGAGAGAAGACCCGGAACGC-3′ | |||||
| Catalase (cat) | F-5′-AGCAGGCGGAGAAGTACCCA-3′ | 60 | 104.7 | XM_026919141.2 | |
| R-5′-GCTGCTCCACCTCAGCGAAA-3′ | |||||
| Glutathione peroxidase 3 (gpx3) | F-5′-GTCACTGCAGGATGCAACAC-3′ | 60 | 106.3 | XM_026947312.2 | |
| R-5′-TTGGAATTCCGCTCATTGAT-3′ | |||||
| Heat shock protein 70 family, member 13 (hspa13) | F-5′-CTCCTCCTAAACCCCGAGTC-3′ | 60 | 100.4 | XM_026934573.2 | |
| R-5′-CCACCAGCACGTTAAACACA-3′ | |||||
| Nitric oxide synthase 1 (neuronal) (nos1) | F-5′-ACACCACGGAGTGTGTTCGT-3′ | 60 | 99.6 | XM_026931613.2 | |
| R-5′-GGATGCATGGGACGTTGCTG-3′ | |||||
| Immune-related gene | Lysozyme g-like 1 (lygl1) | F-5′- TTTTTGGAGACGTCACAAAGATCG-3′ | 60 | 101.3 | XM_026940542.3 |
| R-5′- TGGTGATGATGTTCTTGTACTGCT-3′ | |||||
| Transforming growth factor, beta (tgfb) | F-5′-GAACACTGTCCTCCTTGTCCTC-3′ | 60 | 93.4 | XM_026909968.3 | |
| R-5′-TCGTATTTGGTGGTGAGGATGG-3′ | |||||
| Tumor necrosis factor (tnf) | F-5′-AGTGCAAAGTCAAAAAGCGAGG-3′ | 60 | 100.2 | XM_026921878.3 | |
| R-5′-TGATACTTGGAGCCAGAGCATC-3′ | |||||
| Beta-2-microglobulin, like (b2ml) | F-5′-AGAGAACACACTGATCTGCCAC-3′ | 60 | 94.6 | XM_026925487.3 | |
| R-5′-GCGACGCTCTTAGTCAGATGAA-3′ | |||||
| Proinflammatory-related gene | Lipopolysaccharide-induced TNF factor (litaf) | F-5′-TGCATTATTGGCTGTATGTATGGC-3′ | 60 | 95.1 | XM_026930110.3 |
| R-5′-GTGGATATGTGCTCAGTTCCTGTT-3′ | |||||
| Interferon gamma receptor 1-like (ifngr1l) | F-5′-TACATAACAGTGGAAGCTCAACCA-3′ | 60 | 90.2 | XM_026939395.3 | |
| R-5′-AAGTTTGTGTTGTTGGACGGAAAT-3′ | |||||
| Complement C3 (c3) | F-5′-GAATAGCTCCTGACTGTCCCAC-3′ | 60 | 98.3 | XM_034300087.2 | |
| R-5′-ATGTGTAGCCCAGTCTGTTCTG-3′ | |||||
| Interleukin 13 (il13) | F-5′-GTTTATATCCGGCTTTCATGTGCA-3′ | 60 | 102.3 | XM_034311451.2 | |
| R-5′-AGAAAAACAACCACGACCTTCATC-3′ | |||||
| Interleukin 1, beta (il1b) | F-5′-CTATTCTGCTGGCCATTACTCTGA-3′ | 60 | 100.8 | XM_034312378.2 | |
| R-5′-ATGAGAGAAAGAGGTTGCTCTTCA-3′ | |||||
| Growth-related gene | Growth hormone 1 (gh1) | F-5′-CCCAGCAAGAACCTCGGCAA-3′ | 60 | 94.1 | GQ859589.1 |
| R-5′-GCGGAGCCAGAGAGTCGTTC-3′ | |||||
| Insulin-like growth factor 1 (igf1) | F-5′-GCAACGGCACACAGACACGC-3′ | 60 | 96.2 | XM_034313382.2 | |
| R-5′-CAGACGTTCCCTCACCATCCTCT-3′ |
| Growth Parameter | Treatment | |||
|---|---|---|---|---|
| Control | Probiotic | Postbiotic | Pro + Post | |
| Spesific growth rate (SGR); %/day | 0.74 ± 0.43 a | 2.81 ± 0.61 b | 2.61 ± 0.79 ab | 3.29 ± 0.98 b |
| Average daily gain (ADG); g/day | 0.37 ± 0.23 a | 1.31 ± 0.35 ab | 1.24 ± 0.40 ab | 1.42 ± 0.52 b |
| Feed conversion ratio (FCR) | 1.06 ± 0.35 a | 0.79 ± 0.04 a | 0.77 ± 0.05 a | 0.81 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiratama, N.; Meachasompop, P.; Kumwan, B.; Adisornprasert, Y.; Srisapoome, P.; Phrompanya, P.; Thangsunan, P.; Thangsunan, P.; Saenphet, K.; Saenphet, S.; et al. Dietary Probiotic Bacillus subtilis AAHM-BS2360 and Its Postbiotic Metabolites Enhance Growth, Immunity, and Resistance to Edwardsiellosis in Pangasianodon hypophthalmus. Antioxidants 2025, 14, 629. https://doi.org/10.3390/antiox14060629
Wiratama N, Meachasompop P, Kumwan B, Adisornprasert Y, Srisapoome P, Phrompanya P, Thangsunan P, Thangsunan P, Saenphet K, Saenphet S, et al. Dietary Probiotic Bacillus subtilis AAHM-BS2360 and Its Postbiotic Metabolites Enhance Growth, Immunity, and Resistance to Edwardsiellosis in Pangasianodon hypophthalmus. Antioxidants. 2025; 14(6):629. https://doi.org/10.3390/antiox14060629
Chicago/Turabian StyleWiratama, Nugroho, Pakapon Meachasompop, Benchawan Kumwan, Yosapon Adisornprasert, Prapansak Srisapoome, Phornphan Phrompanya, Patcharapong Thangsunan, Pattanapong Thangsunan, Kanokporn Saenphet, Supap Saenphet, and et al. 2025. "Dietary Probiotic Bacillus subtilis AAHM-BS2360 and Its Postbiotic Metabolites Enhance Growth, Immunity, and Resistance to Edwardsiellosis in Pangasianodon hypophthalmus" Antioxidants 14, no. 6: 629. https://doi.org/10.3390/antiox14060629
APA StyleWiratama, N., Meachasompop, P., Kumwan, B., Adisornprasert, Y., Srisapoome, P., Phrompanya, P., Thangsunan, P., Thangsunan, P., Saenphet, K., Saenphet, S., Buncharoen, W., & Uchuwittayakul, A. (2025). Dietary Probiotic Bacillus subtilis AAHM-BS2360 and Its Postbiotic Metabolites Enhance Growth, Immunity, and Resistance to Edwardsiellosis in Pangasianodon hypophthalmus. Antioxidants, 14(6), 629. https://doi.org/10.3390/antiox14060629











