The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation
Abstract
1. Introduction
2. Methodology
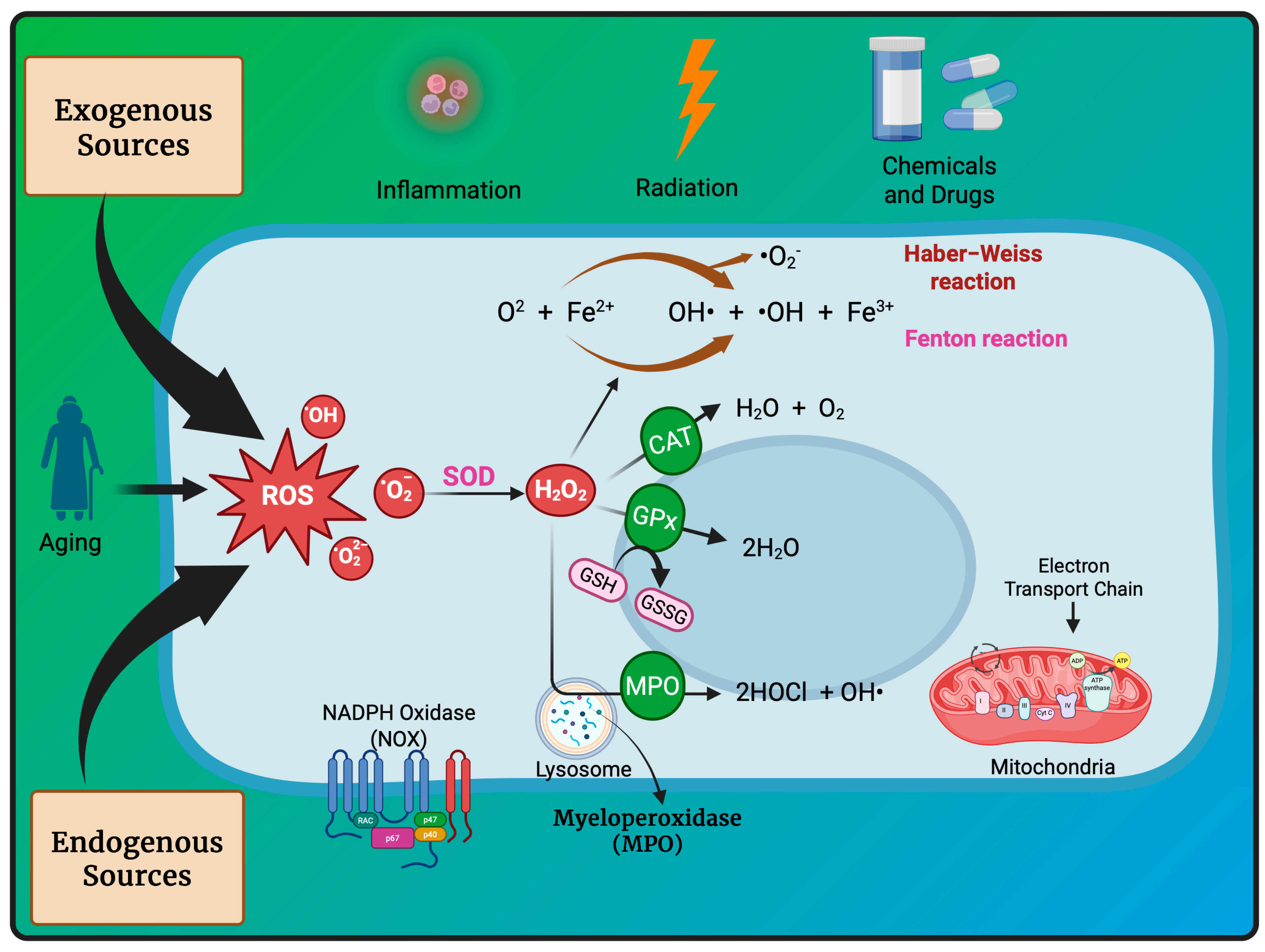
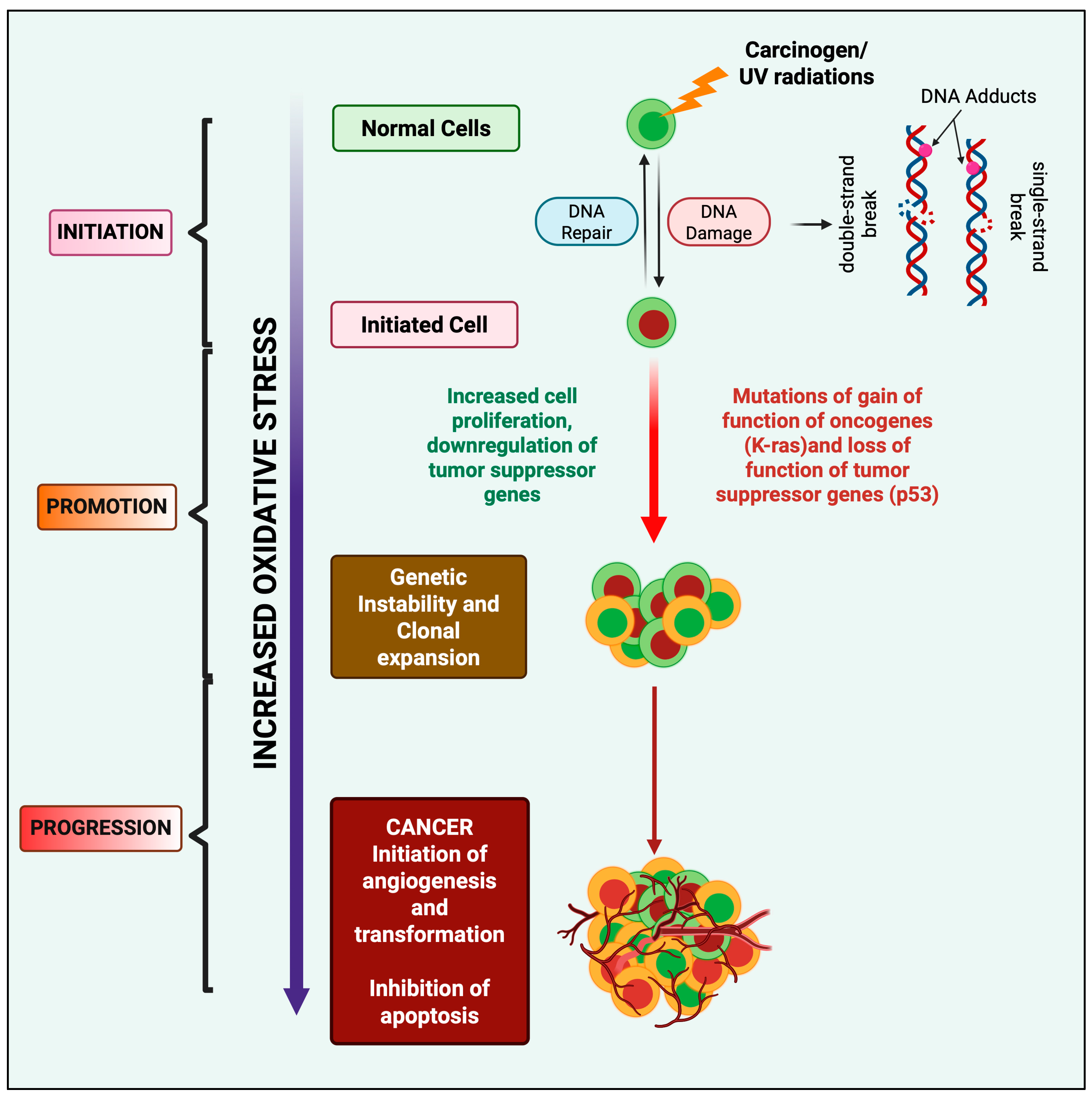
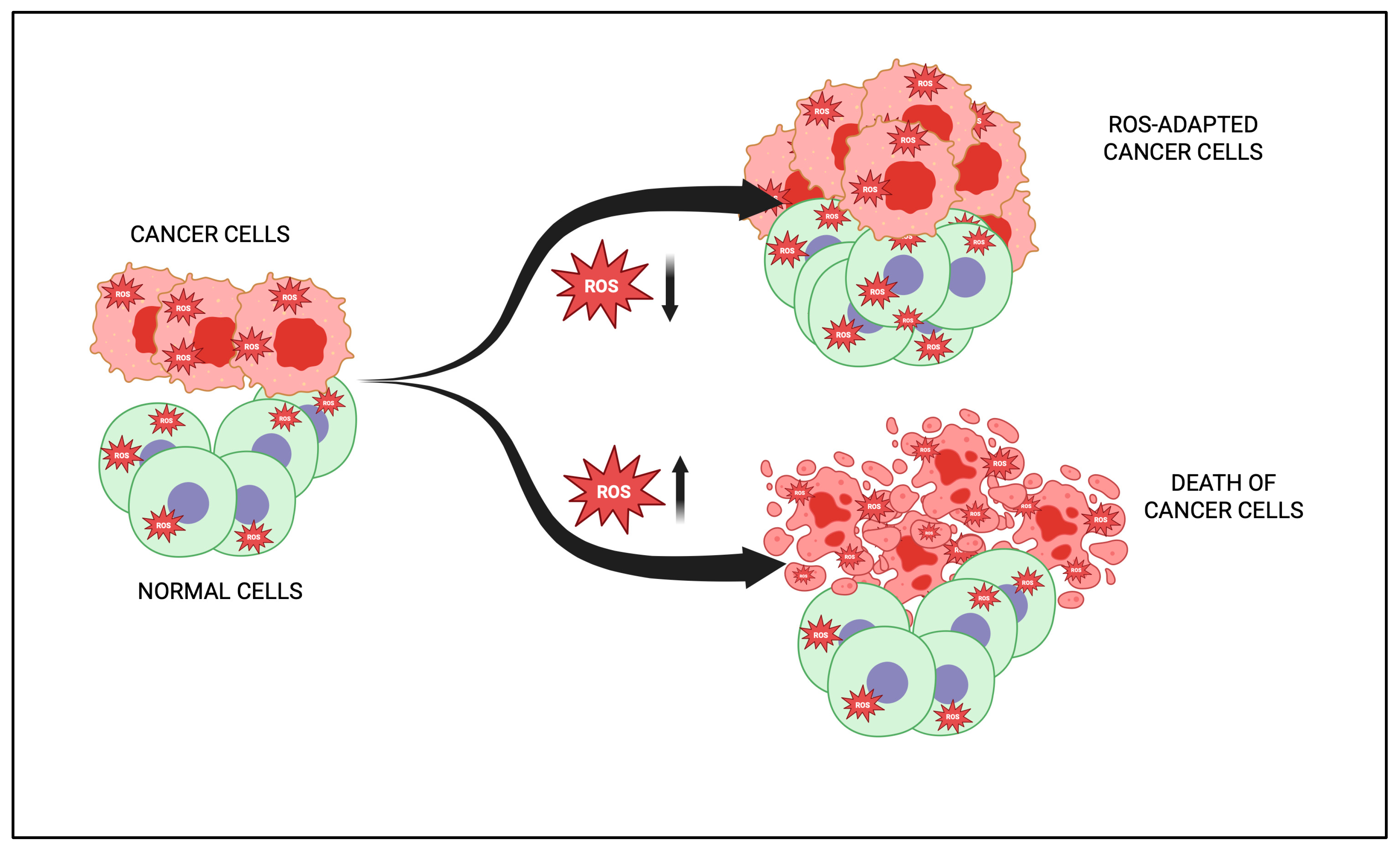
3. Redox Homeostasis and Oxidative Stress-Induced Oncogenesis
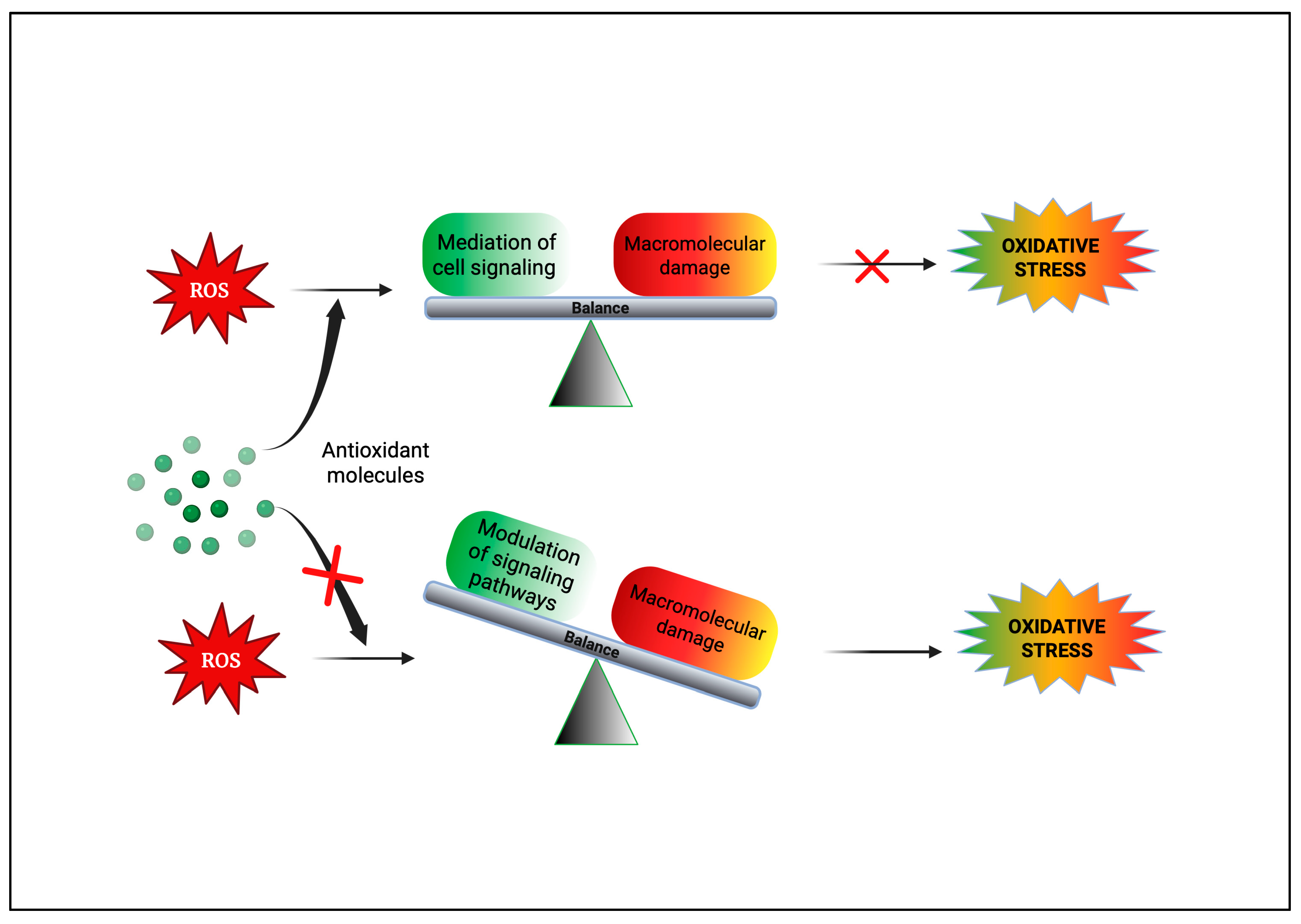
4. Dual Role of Phytochemicals in Redox Modulation
5. Bioavailability and Pharmacokinetics of Phytochemicals
6. Pharmacological Effects of Phytochemicals
6.1. Anti-Inflammatory Effects
6.2. Antioxidant Effect
7. Anticancer Action of Phytochemicals
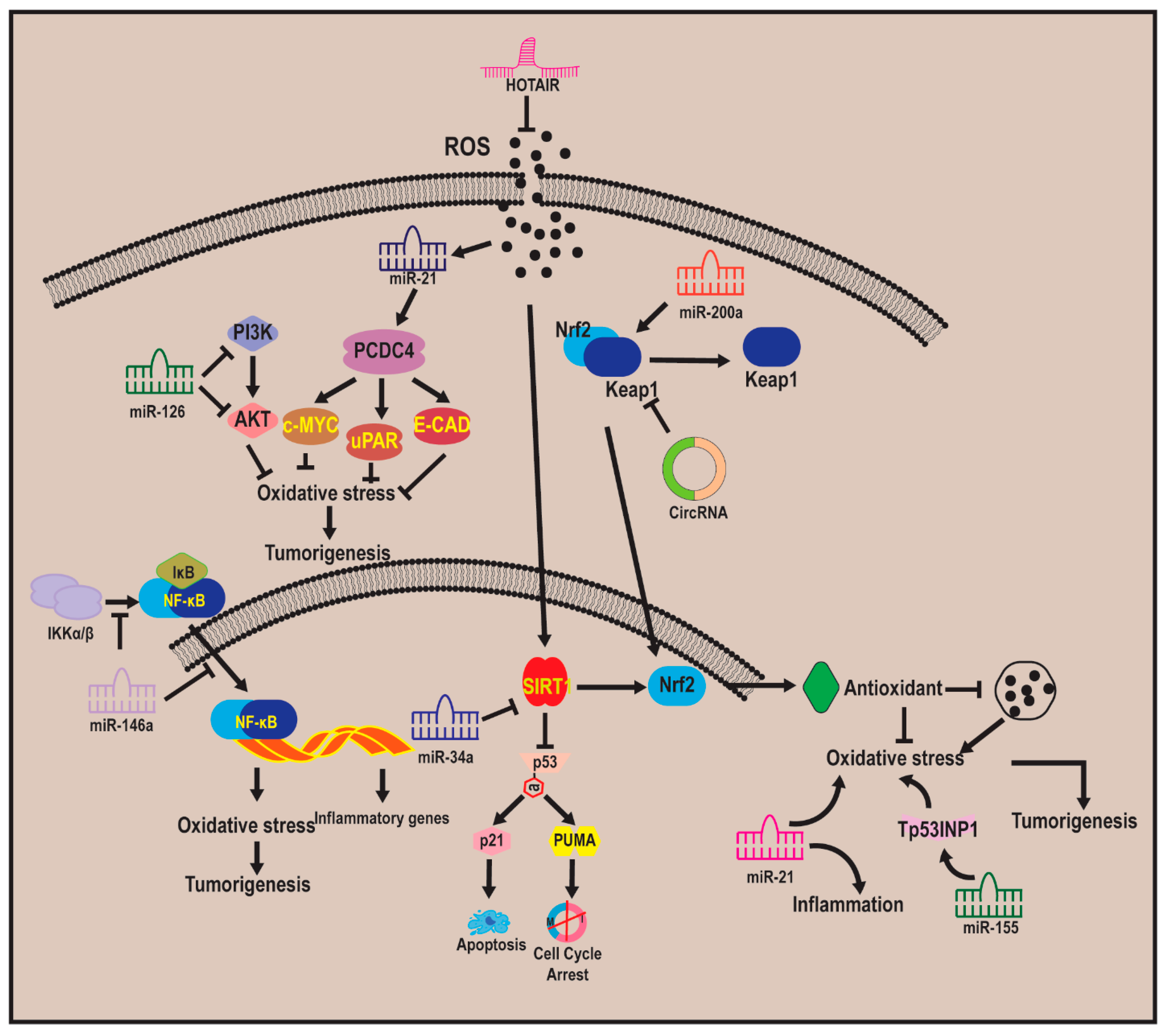
7.1. Non-Coding RNA–Oxidative Stress–Carcinogenesis Relationship
7.2. MicroRNA
7.3. Long Non-Coding RNAs
7.4. Circular RNAs
7.5. Phytochmeicals That Target ncRNA–Oxidative Stress–Carcinogenesis Relationships
7.6. Clinical Significance (In Vitro and In Vivo Studies) of Phytochemicals
8. Conclusions and Future Direction
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Oxidative stress |
| ATP | Adenosine triphosphate |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| PI3K | Phosphatidylinositol 3-kinase |
| mTOR | Mechanistic target of rapamycin |
| NF-κB | Nuclear factor κB |
| MAPK | Mitogen-activated protein kinase |
| ERK1/2 | Extracellular signal-regulated kinase1/2 |
| JNK | c-Jun N-terminal kinase |
| STAT3 | Signal transducer and activator of transcription-3 |
| EGCG | EpigalloCatechin-3-gallate |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.; Wild, C.P. (Eds.) World Cancer Report. Etiology of Cancer; IARC: Lyon, France, 2014; Volume 3, pp. 82–169. [Google Scholar]
- Weir, H.K.; Thompson, T.D.; Stewart, S.L.; White, M.C. Cancer Incidence Projections in the United States Between 2015 and 2050. Prev. Chronic Dis. 2021, 18, E59. [Google Scholar] [CrossRef]
- Pellerin, M.; Trabucco, B.; Capai, L.; Laval, M.; Maestrini, O.; Jori, F.; Falchi, A.; Doceul, V.; Charrier, F.; Casabianca, F.; et al. Low prevalence of hepatitis E virus in the liver of Corsican pigs slaughtered after 12 months despite high antibody seroprevalence. Transbound. Emerg. Dis. 2022, 69, e2706–e2718. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Fatma, H.; Siddique, H.R. Research and Patents Status of Selected Phytochemicals Against Cancer: How Close and How Far? Recent Pat. Anticancer Drug Discov. 2023, 18, 428–447. [Google Scholar] [CrossRef]
- Fabrizio, F.P.; Sparaneo, A.; Muscarella, L.A. NRF2 regulation by noncoding RNAs in cancers: The present knowledge and the way forward. Cancers 2020, 12, 3621. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Bartosz, G.; Lawrynowicz, M.; Soszyński, M. The Role of Polyphenols, β-Carotene, and Lycopene in the Antioxidative Action of the Extracts of Dried, Edible Mushrooms. J. Nutr. Metab. 2010, 2010, 173274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Balasas, T.; Callaghan, J.; Coombes, R.C.; Evans, J.; Hall, J.A.; Kinrade, S.; Jones, D.; Jones, P.S.; Jones, R.; et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 2019, 16, 185–204. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Mohammad, T.; Hasan, G.M.; Yadav, D.K.; Hassan, M.I. B Cell Lymphoma 2: A Potential Therapeutic Target for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 10442. [Google Scholar] [CrossRef]
- Alam, M.; Hasan, G.M.; Eldin, S.M.; Adnan, M.; Riaz, M.B.; Islam, A.; Khan, I.; Hassan, M.I. Investigating regulated signaling pathways in therapeutic targeting of non-small cell lung carcinoma. Biomed. Pharmacother. 2023, 161, 114452. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lei, X.; Yang, X. The crosstalk between non-coding RNAs and oxidative stress in cancer progression. Genes Dis. 2024, 12, 101286. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719, Erratum in Nat. Rev. Mol. Cell Biol. 2024, 25, 758. https://doi.org/10.1038/s41580-024-00754-8. [Google Scholar] [CrossRef]
- Fatma, H.; Jameel, M.; Siddique, H.R. An Update on Phytochemicals in Redox Homeostasis: “Virtuous or Evil” in Cancer Chemoprevention? Chemistry 2023, 5, 201–222. [Google Scholar] [CrossRef]
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bera, S.; Verma, A.; Bhatt, A.N.; Dwarakanath, B.S. Metabolic Oxidative Stress in Initiation, Progression, and Therapy of Cancer. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychoudhury, S., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2010, 3, 23–34. [Google Scholar] [CrossRef]
- Shah, M.A.; Rogoff, H.A. Implications of reactive oxygen species on cancer formation and its treatment. Semin. Oncol. 2021, 48, 238–245. [Google Scholar] [CrossRef]
- Marques, M.P.M.; de Carvalho, A.L.M.B.; Martins, C.B.; Silva, J.D.; Sarter, M.; García Sakai, V.; Stewart, J.R.; de Carvalho, L.A.E.B. Cellular dynamics as a marker of normal-to-cancer transition in human cells. Sci. Rep. 2023, 13, 21079. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef]
- Letafati, A.; Taghiabadi, Z.; Zafarian, N.; Tajdini, R.; Mondeali, M.; Aboofazeli, A.; Chichiarelli, S.; Saso, L.; Jazayeri, S.M. Emerging paradigms: Unmasking the role of oxidative stress in HPV-induced carcinogenesis. Infect. Agent Cancer 2024, 19, 30. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Byers, L.A.; Kurie, J.M. Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 2014, 12, 3–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peiris-Pagès, M.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Metastasis and Oxidative Stress: Are Antioxidants a Metabolic Driver of Progression? Cell Metab. 2015, 22, 956–958. [Google Scholar] [CrossRef]
- Shin, D.H.; Dier, U.; Melendez, J.A.; Hempel, N. Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 2593–2602. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.L.; Tang, M.S. The major lipid peroxidation product, trans-4-hydroxy-2- nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002, 23, 1781–1789. [Google Scholar] [CrossRef]
- Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Van Loenhout, J.; Peeters, M.; Bogaerts, A.; Smits, E.; Deben, C. Oxidative Stress-Inducing Anticancer Therapies: Taking a Closer Look at Their Immunomodulating Effects. Antioxidants 2020, 9, 1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013, 3, 120144. [Google Scholar] [CrossRef]
- Howes, M.J.; Simmonds, M.S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 558–566. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef]
- Pawase, P.A.; Goswami, C.; Shams, R.; Pandey, V.K.; Tripathi, A.; Rustagi, S.; Darshan, G. A conceptual review on classification, extraction, bioactive potential and role of phytochemicals in human health. Future Foods 2024, 2024, 100313. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications-A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Rodríguez-Negrete, E.V.; Morales-González, Á.; Madrigal-Santillán, E.O.; Sánchez-Reyes, K.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Valadez-Vega, C.; Chamorro-Cevallos, G.; Garcia-Melo, L.F.; Morales-González, J.A. Phytochemicals and Their Usefulness in the Maintenance of Health. Plants 2024, 13, 523. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-S.; Huang, G.-J.; Lu, Y.-H.; Chang, L.-W. Anti-inflammatory effects of an aqueous extract of Welsh onion green leaves in mice. Food Chem. 2019, 138, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Darawsha, A.; Trachtenberg, A.; Levy, J.; Sharoni, Y. The protective effect of carotenoids, polyphenols, and estradiol on dermal fibroblasts under oxidative stress. Antioxidants 2021, 10, 2023. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Rizwan, D.; Masoodi, F.A. Brassica-Derived Isothiocyanates as Anticancer Therapeutic Agents and Their Nanodelivery. Phytother. Res. 2024, 38, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Ugwu, O.P.C. Beyond Nutrients: Exploring the Potential of Phytochemicals for Human Health. IAA J. Appl. Sci. 2023, 10, 1–7. [Google Scholar] [CrossRef]
- Ashraf, M.V.; Khan, S.; Misri, S.; Gaira, K.S.; Rawat, S.; Rawat, B.; Khan, M.H.; Shah, A.A.; Asgher, M.; Ahmad, S. High-Altitude Medicinal Plants as Promising Source of Phytochemical Antioxidants to Combat Lifestyle-Associated Oxidative Stress-Induced Disorders. Pharmaceuticals 2024, 17, 975. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Khan, M.A.; Malik, Z.; Massey, S.; Parveen, R.; Mustafa, S.; Shamsi, A.; Husain, S.A. Phytocompounds Targeting Epigenetic Modulations: An Assessment in Cancer. Front. Pharmacol. 2024, 14, 1273993. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.; Kumar, M.; Guarve, K.; Dhanawat, M.; Sharma, V. Therapeutic Potential of Genistein and Its Derivatives as a Target for Anticancer Agents. ChemistrySelect 2023, 8, e202204924. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.; Yao, Y.; Fang, L.; Yu, M.; Wang, Z. Curcumin in the Treatment of Inflammation and Oxidative Stress Responses in Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Front. Neurol. 2024, 15, 1380353. [Google Scholar] [CrossRef]
- Thacker, H.; Ram, V. Medicinal Properties of Phytochemicals: A Review. J. Pharmacogn. Phytochem. 2024, 13, 78–82. [Google Scholar] [CrossRef]
- Pons, D.G. Roles of Phytochemicals in Cancer Prevention and Therapeutics. Int. J. Mol. Sci. 2024, 25, 5450. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Qu, L.; Li, H.; Ren, X.; Yan, N.; Fu, X. Functional Properties of Dietary Quercetin in Cardiovascular Health and Disease. Food Front. 2024, 5, 1951–1967. [Google Scholar] [CrossRef]
- Rasmi, Y.; da Silva, A.P.G.; Rezaei, S.; Rafique, S.; Ahmed, M.Z. Biochemical, Molecular, Pharmacokinetic, and Toxicological Aspects of Dietary Polyphenols. In Dietary Polyphenols in Human Diseases; CRC Press: Boca Raton, FL, USA, 2022; pp. 27–52. [Google Scholar] [CrossRef]
- Batool, S.; Asim, L.; Qureshi, F.R.; Masood, A.; Mushtaq, M.; Saleem, R.S.Z. Molecular Targets of Plant-Based Alkaloids and Polyphenolics in Liver and Breast Cancer- An Insight Into Anticancer Drug Development. Anti-Cancer Agents Med. Chem. 2025, 25, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Varadharajaperumal, P.; Muthuswamy, S.; Pothagar, D.; Ganesan, M.; Santhanam, A. Adenia Hondala-Derived Biopolymer Nanoparticles Cause G2/M Cell Cycle Arrest in Breast Cancer Cells. Uttar Pradesh J. Zool. 2024, 45, 550–560. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.E.; Radu, A.; Dumitru, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A Review on the Biological Activity and Applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.P.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A.; et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food. 2008, 11, 390–394. [Google Scholar] [CrossRef]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Palozza, P.; Parrone, N.; Catalano, A.; Simone, R. Tomato Lycopene and Inflammatory Cascade: Basic Interactions and Clinical Implications. Curr. Med. Chem. 2010, 17, 2547–2563. [Google Scholar] [CrossRef]
- Ono, M.; Takeshima, M.; Nakano, S. Mechanism of the Anticancer Effect of Lycopene (Tetraterpenoids). Enzymes.echanism of the anticancer effect of lycopene (tetraterpenoids). Enzymes 2015, 37, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Aly, S.M.; Ali, H.; Babiker, A.Y.; Srikar, S.; Khan, A.A. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, antioxidant and anti-tumour activity. Int. J. Clin. Exp. Med. 2014, 7, 483–491. [Google Scholar] [PubMed]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Yadav, D.K. Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 2021, 22, 12162. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef]
- Gustin, D.M.; Rodvold, K.A.; Sosman, J.A.; Diwadkar-Navsariwala, V.; Stacewicz-Sapuntzakis, M.; Viana, M.; Crowell, J.A.; Murray, J.; Tiller, P.; Bowen, P.E. Single-dose pharmacokinetic study of lycopene delivered in a well-defined food-based lycopene delivery system (tomato paste-oil mixture) in healthy adult male subjects. Cancer Epidemiol. Biomark. Prev. 2004, 13, 850–860. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef]
- Fielding, J.M.; Rowley, K.G.; Cooper, P.; O’Dea, K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac. J. Clin. Nutr. 2005, 14, 131–136. [Google Scholar]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Mikkelsen, D.; Gidley, M.J. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 2013, 4, 906–916. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; González-Laredo, R.F.; Rocha-Guzmán, N.E. Absorption and distribution of Lupeol in CD-1 mice evaluated by UPLC-APCI+ -MS/MS. Biomed. Chromatogr. 2019, 33, e4432. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2021, 11, 594852. [Google Scholar] [CrossRef]
- Bučević Popović, V.; Karahmet Farhat, E.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Zhao, W.X.; Wang, L.; Yang, J.L.; Li, L.Z.; Xu, W.M.; Li, T. Caffeic acid phenethyl ester attenuates pro-inflammatory and fibrogenic phenotypes of LPS Stimulated hepatic stellate cells through the inhibition of NF-kB signaling. Int. J. Mol. Med. 2014, 33, 687–694. [Google Scholar] [CrossRef]
- Ohishi, T.; Goto, S.; Monira, P.; Isemura, M.; Nakamura, Y. Anti-inflammatory action of green tea. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2016, 15, 74–90. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Singh, D.D.; Han, I.; Choi, E.H.; Yadav, D.K. Recent Advances in Pathophysiology, Drug Development and Future Perspectives of SARS-CoV-2. Front. Cell Dev. Biol. 2020, 8, 580202. [Google Scholar] [CrossRef] [PubMed]
- Javed, C.; Noreen, R.; Niazi, S.G.; Kiyani, M.M.; Ul Ain, Q. Anti-gouty arthritis and anti-inflammatory effects of Curcumin nanoparticles in monosodium urate crystals induced Balb/C mice. Inflammopharmacology 2024, 32, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Oz, A.T.; Kafkas, E. Phytochemicals in fruits and vegetables. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumaran, V.; Arulmathi, K.; Srividhya, R.; Kalaiselvi, P. Repletion of antioxidant status by EGCG and retardation of oxidative damage induced macromolecular anomalies in aged rats. Exp. Gerontol. 2008, 43, 176–183. [Google Scholar] [CrossRef]
- Raza, H.; John, A. Green tea polyphenol epigallocatechin-3-gallate differentially modulates oxidative stress in PC12 cell compartments. Toxicol. Appl. Pharmacol. 2005, 207, 212–220. [Google Scholar] [CrossRef]
- Levites, Y.; Amit, T.; Mandel, S.; Y Oudim, M.B. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003, 17, 952–954. [Google Scholar] [CrossRef]
- Huang, J.; Wu, L.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J. Pharmacol. Sci. 2008, 107, 370–379. [Google Scholar] [CrossRef]
- Du, Y.; Villeneuve, N.F.; Wang, X.J.; Sun, Z.; Chen, W.; Li, J.; Lou, H.; Wong, P.K.; Zhang, D.D. Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response. Environ. Health Perspect. 2008, 116, 1154–1161. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Anita, R. In vitro and in silico evaluation of antioxidant activity of a sesquiterpene lactone, costunolide, isolated from costus specious rhizome on MCF-7 and MDA-MB-231 human breast cancer cell lines. World J. Pharm. Pharm. Sci. 2014, 3, 1334–1347. [Google Scholar]
- Parray, H.A.; Lone, J.; Park, J.P.; Choi, J.W.; Yun, J.W. Magnolol promotes thermogenesis and attenuates oxidative stress in 3T3-L1 adipocytes. Nutrition 2018, 50, 82–90. [Google Scholar] [CrossRef]
- Chilampalli, C.; Zhang, X.; Kaushik, R.S.; Young, A.; Zeman, D.; Hildreth, M.B.; Fahmy, H.; Dwivedi, C. Chemopreventive effects of combination of honokiol and magnolol with α-santalol on skin cancer developments. Drug Discov. Ther. 2013, 7, 109–115. [Google Scholar] [PubMed]
- Chen, L.C.; Liu, Y.C.; Liang, Y.C.; Ho, Y.S.; Lee, W.S. Magnolol inhibits human glioblastoma cell proliferation through upregulation of p21/Cip1. J. Agric. Food Chem. 2009, 57, 7331–7337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.H.; Ren, H.Y.; Shen, J.X.; Zhang, X.Y.; Ye, H.M.; Shen, D.Y. Magnolol suppresses the proliferation and invasion of cholangiocarcinoma cells via inhibiting the NF-κB signaling pathway. Biomed. Pharmacother. 2017, 94, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ding, Y.; Wang, X.; Lu, S.; Wang, C.; He, C.; Wang, L.; Piao, M.; Chi, G.; Luo, Y.; et al. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 2018, 428, 21–33. [Google Scholar] [CrossRef]
- Huang, H.; Li, P.; Ye, X.; Zhang, F.; Lin, Q.; Wu, K.; Chen, W. Isoalantolactone increases the sensitivity of prostate cancer cells to cisplatin treatment by inducing oxidative stress. Front. Cell Dev. Biol. 2021, 9, 632779. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Duan, D.; Yao, J.; Gao, K.; Fang, J. Inhibition of thioredoxin reductase by alantolactone prompts oxidative stress-mediated apoptosis of HeLa cells. Biochem. Pharmacol. 2016, 102, 34–44. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, D.H.; Lee, K.W.; Yoon, D.Y.; Surh, Y.J. Jaceosidin induces apoptosis in ras-transformed human breast epithelial cells through generation of reactive oxygen species. Ann. N. Y. Acad. Sci. 2007, 1095, 483–495. [Google Scholar] [CrossRef]
- Khan, M.; Yu, B.; Rasul, A.; Al Shawi, A.; Yi, F.; Yang, H.; Ma, T. Jaceosidin induces apoptosis in U87 glioblastoma cells through G2/M phase arrest. J. Evid. Based Complement. Altern. Med. 2012, 2012, 703034. [Google Scholar] [CrossRef]
- Kobayakawa, J.; Sato-Nishimori, F.; Moriyasu, M.; Matsukawa, Y. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifoliaLinne fil.). Cancer Lett. 2004, 208, 59–64. [Google Scholar] [CrossRef]
- Xie, J.; Bai, J.; Sheng, X.; Cao, J.; Xie, W. Proliferation inhibition of human cervical cancer HeLa cells by Casticin in vitro. Chin. -Ger. J. Clin. Oncol. 2011, 10, 47–50. [Google Scholar] [CrossRef]
- Shen, J.K.; Du, H.P.; Yang, M.; Wang, Y.G.; Jin, J. Casticin induces leukemic cell death through apoptosis and mitotic catastrophe. Ann. Hematol. 2009, 88, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Cai, B.; Cui, C.B.; Zhang, D.Y.; Yang, B.F. Vitexicarpin, a flavonoid from Vitex trifolia L., induces apoptosis in K562 cells via mitochondria-controlled apoptotic pathway. Acta Pharm. Sin. 2005, 40, 27–31. [Google Scholar]
- Qu, L.; Liu, F.X.; Cao, X.C.; Xiao, Q.; Yang, X.; Ren, K.Q. Activation of the apoptosis signal-regulating kinase 1/c-Jun N-terminal kinase pathway is involved in the casticin-induced apoptosis of colon cancer cells. Exp. Ther. Med. 2014, 8, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Chou, J.; Yeh, M.Y.; Chou, H.M.; Chou, H.C.; Lu, H.F.; Shang, H.S.; Chueh, F.S.; Chu, Y.L.; Hsueh, S.C.; et al. Casticin induces DNA damage and inhibits DNA repair-associated protein expression in B16F10 mouse melanoma cancer cells. Oncol. Rep. 2016, 36, 2094–2100. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.P.; Jiang, R.; Chen, Y.; Lv, X.T.; Guo, X.X.; Tian, K.; Yuan, D.Z.; Lv, Y.W.; Ran, J.H.; et al. Evodiamine inhibits migration and invasion by Sirt1-mediated post-translational modulations in colorectal cancer. Anti-Cancer Drugs 2019, 30, 611–617. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin regulates cancer progression: Focus on ncRNAs and molecular signaling pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef]
- D’anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A.; et al. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, J.; Yao, J.; Liu, Y.; Fang, J. Targeting thioredoxin reductase by parthenolide contributes to inducing apoptosis of HeLa cells. J. Biol. Chem. 2016, 291, 10021–10031. [Google Scholar] [CrossRef]
- Liu, J.; Ding, D.; Liu, F.; Chen, Y. Rhein inhibits the progression of chemoresistant lung cancer cell lines via the Stat3/snail/MMP2/MMP9 pathway. BioMed Res. Intern. 2022, 2022, 7184871. [Google Scholar] [CrossRef]
- Ren, B.; Guo, W.; Tang, Y.; Zhang, J.; Xiao, N.; Zhang, L.; Li, W. Rhein inhibits the migration of ovarian cancer cells through down-regulation of matrix metalloproteinases. Biol. Pharm. Bullet. 2019, 42, 568–572. [Google Scholar] [CrossRef]
- Lin, S.; Fujii, M.; Hou, D.X. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys. 2003, 418, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Inter. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.A.; Elsaed, W.M.; Eladl, M.A.; El-Sherbiny, M.; Ebrahim, H.A.; Asseri, S.M.; Eltahir, Y.A.M.; Elsherbiny, N.; Eldesoqui, M. Curcumin modulates oxidative stress, fibrosis, and apoptosis in drug-resistant cancer cell lines. Life 2022, 12, 1427. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Hassanian, S.M.; Rahmani, F.; Aghaee-Bakhtiari, S.H.; Avan, A.; Khazaei, M. PhytosomalCurcumin elicits anti-tumor properties through suppression of angiogenesis, cell proliferation and induction of oxidative stress in colorectal cancer. Curr. Pharm. Des. 2018, 24, 4626–4638. [Google Scholar] [CrossRef]
- Weisburg, J.H.; Weissman, D.B.; Sedaghat, T.; Babich, H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin. Pharmacol. Toxicol. 2004, 95, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, Y.; Zhou, H.F. Esculetin inhibits proliferation, invasion, and migration of laryngeal cancer in vitro and in vivo by inhibiting Janus kinas (JAK)-signal transducer and activator of transcription-3 (STAT3) activation. Med. Sci. Monit. 2019, 25, 7853–7863. [Google Scholar] [CrossRef]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer 2016, 15, 64. [Google Scholar] [CrossRef]
- Nadal-Serrano, M.; Pons, D.G.; Sastre-Serra, J.; del Mar Blanquer-Rosselló, M.; Roca, P.; Oliver, J. Genistein modulates oxidative stress in breast cancer cell lines according to ERα/ERβ ratio: Effects on mitochondrial functionality, sirtuins, uncoupling protein 2 and antioxidant enzymes. Int. J. Biochem. Cell Biol. 2013, 45, 2045–2051. [Google Scholar] [CrossRef]
- Alorda-Clara, M.; Torrens-Mas, M.; Morla-Barcelo, P.M.; Roca, P.; Sastre-Serra, J.; Pons, D.G.; Oliver, J. High concentrations of genistein decrease cell viability depending on oxidative stress and inflammation in colon cancer cell lines. Int. J. Mol. Sci. 2022, 23, 7526. [Google Scholar] [CrossRef]
- Jeong, S.A.; Yang, C.; Song, J.; Song, G.; Jeong, W.; Lim, W. Hesperidin suppresses the proliferation of prostate cancer cells by inducing oxidative stress and disrupting Ca2+ homeostasis. Antioxidants 2022, 11, 1633. [Google Scholar] [CrossRef]
- Banjerdpongchai, R.; Wudtiwai, B.; Khaw-On, P.; Rachakhom, W.; Duangnil, N.; Kongtawelert, P. Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol. 2016, 37, 227–237. [Google Scholar] [CrossRef]
- Mostafavi-Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin C in Nrf2-dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017, 13, 1965–1973. [Google Scholar] [CrossRef]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive effects of rosmarinic acid rich fraction from Perilla on oxidative stress, inflammation and metastasis ability in A549 cells exposed to PM via c-Jun, P-65-Nf-Κb and AktSignaling pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Gunes-Bayir, A.; Guler, E.M.; Bilgin, M.G.; Ergun, I.S.; Kocyigit, A.; Dadak, A. Anti-inflammatory and antioxidant effects of carvacrol on N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) induced gastric carcinogenesis in Wistar rats. Nutrients 2022, 14, 2848. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Naiki, T.; Naiki-Ito, A.; Suzuki, S.; Kato, H.; Nozaki, S.; Nagai, T.; Etani, T.; Nagayasu, Y.; Ando, R.; et al. Luteolin suppresses bladder cancer growth via regulation of the mechanistic target of rapamycin pathway. Cancer Sci. 2020, 111, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induces ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef]
- Xie, J.; Xu, Y.; Huang, X.; Chen, Y.; Fu, J.; Xi, M.; Wang, L. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour Biol. 2015, 36, 1279–1288. [Google Scholar] [CrossRef]
- Hou, D.; Xu, G.; Zhang, C.; Li, B.; Qin, J.; Hao, X.; Liu, Q.; Zhang, X.; Liu, J.; Wei, J.; et al. Berberine induces oxidative DNA damage and impairs homologous recombination repair in ovarian cancer cells to confer increased sensitivity to PARP inhibition. Cell Death Dis. 2017, 8, e3070. [Google Scholar] [CrossRef]
- Shakeel, I.; Haider, S.; Khan, S.; Ahmed, S.; Hussain, A.; Alajmi, M.F.; Chakrabarty, A.; Afzal, M.; Imtaiyaz Hassan, M. Thymoquinone, artemisinin, and thymol attenuate proliferation of lung cancer cells as Sphingosine kinase 1 inhibitors. Biomed. Pharmacother. = Biomed. Pharmacother. 2024, 177, 117123. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, J.M.; Du, C.J.; Hu, S.M.; Chen, J.X.; Zhang, S.G.; Zhang, N.; Gao, F.; Li, S.J.; Mao, X.W.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Goyal, A.K.; Ghosh, G.; Si, S.C.; Rath, G. Development and characterization of pellets for targeted delivery of 5-fluorouracil and phytic acid for treatment of colon cancer in Wistar rat. Heliyon 2020, 6, e03125. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell Physiol. 2017, 232, 3786–3797. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, Y.; Yang, Y.; Huang, H.; Feng, Y.; Wang, Y.; Zhan, L.; Wei, B. Chrysin induces autophagy through the inactivation of the ROS mediated Akt/mTOR signaling pathway in endometrial cancer. Int. J. Mol. Med. 2021, 48, 172. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef]
- Liu, T.; Chi, H.; Chen, J.; Chen, C.; Huang, Y.; Xi, H.; Xue, J.; Si, Y. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene 2017, 631, 29–38. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Surangkul, D.; Srikummool, M.; Richert, L.; Pekthong, D.; Parhira, S.; Somran, J.; Srisawang, P. Epigallocatechin gallate triggers apoptosis by suppressing de novo lipogenesis in colorectal carcinoma cells. FEBS Open Bio 2022, 12, 937–958. [Google Scholar] [CrossRef]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of Curcumin and Curcuminoids. J. Mol. Str. 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary supplementation of Curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: Possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 2003, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by Curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
- Palsamy, P.; Sivakumar, S.; Subramanian, S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem. Biol. Interact. 2010, 186, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, L.; Chen, S.; Zheng, B.; Chen, H.; Zeng, T.; Sun, H.; Zhong, S.; Wu, W.; Lin, X.; et al. The Curcumin analogue WZ35 affects glycolysis inhibition of gastric cancer cells through ROS-YAP-JNK pathway. Food Chem. Toxicol. 2020, 137, 111131. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and its Analogues in Oral Squamous Cell Carcinoma: State-of-the-art and Therapeutic Potential. Anti-Cancer Agents Med. Chem. 2025, 25, 313–329. [Google Scholar] [CrossRef]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. C. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar] [CrossRef]
- Pintea, A.; Rugină, D.; Pop, R.; Bunea, A.; Socaciu, C.; Diehl, H.A. Antioxidant effect of trans-Resveratrol in cultured human retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2011, 27, 315–321. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yi, K.; Zhou, X.; Li, X.; Zhong, C.; Cao, H.; Xie, C.; Zhu, J. Destruction of the cellular antioxidant pool contributes to Resveratrol-induced senescence and apoptosis in lung cancer. Phytother. Res. 2023, 37, 2995–3008. [Google Scholar] [CrossRef] [PubMed]
- Srivani, G.; Behera, S.K.; Dariya, B.; Aliya, S.; Alam, A.; Nagaraju, G.P. Resveratrol binds and inhibits transcription factor HIF-1α in pancreatic cancer. Exp. Cell Res. 2020, 394, 112126. [Google Scholar] [CrossRef] [PubMed]
- Poortalebi, H.; ZareDini, M.; Foroughi-Nematollahi, S.; Farkhondeh, T.; Samarghandian, S.; Pourhanifeh, M.H. Therapeutic Effect of Resveratrol and its Novel Formulations on Lung Cancer: Focus on Biological Aspects and Underlying Pathways. Curr. Med. Chem. 2024, 31, 4340–4361. [Google Scholar] [CrossRef]
- Farhan, M. Cytotoxic Activity of the Red Grape Polyphenol Resveratrol against Human Prostate Cancer Cells: A Molecular Mechanism Mediated by Mobilization of Nuclear Copper and Generation of Reactive Oxygen Species. Life 2024, 14, 611. [Google Scholar] [CrossRef]
- Valcic, S.; Muders, A.; Jacobsen, N.E.; Liebler, D.C.; Timmermann, B.N. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem. Res. Toxicol. 1999, 12, 382–386. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022, 80, 256–275. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Arumugam, G.; Alagar Yadav, S. Synergistic inhibitory actions of Resveratrol, epigallocatechin-3-gallate, and diallyl trisulfide against skin cancer cell line A431 through mitochondrial caspase dependent pathway: A combinational drug approach. Med. Oncol. 2024, 41, 64. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef]
- Shi, J.; Ji, X.; Shan, S.; Zhao, M.; Bi, C.; Li, Z. The interaction between Apigenin and PKM2 restrains progression of colorectal cancer. J. Nutr. Biochem. 2023, 121, 109430. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Shafiee, S.; Burcher, J.T.; Lagoa, R.; Farzaei, M.H.; Bishayee, A. Anticancer Potential of Apigenin and Isovitexin with Focus on Oncogenic Metabolism in Cancer Stem Cells. Metabolites 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Naponelli, V.; Rocchetti, M.T.; Mangieri, D. Apigenin: Molecular Mechanisms and Therapeutic Potential against Cancer Spreading. Int. J. Mol. Sci. 2024, 25, 5569. [Google Scholar] [CrossRef]
- Bhatt, M.; Patel, M.; Adnan, M.; Reddy, M.N. Anti-Metastatic Effects of Lupeol via the Inhibition of MAPK/ERK Pathway in Lung Cancer. Anticancer Agents Med. Chem. 2021, 21, 201–206. [Google Scholar] [CrossRef]
- Eldohaji, L.M.; Fayed, B.; Hamoda, A.M.; Ershaid, M.; Abdin, S.; Alhamidi, T.B.; Mohammad, M.G.; Omar, H.A.; Soliman, S.S.M. Potential targeting of Hep3B liver cancer cells by Lupeol isolated from Avicennia marina. Arch. Pharm. 2021, 354, e2100120. [Google Scholar] [CrossRef]
- Min, T.R.; Park, H.J.; Ha, K.T.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Suppression of EGFR/STAT3 activity by Lupeol contributes to the induction of the apoptosis of human non-small cell lung cancer cells. Int. J. Oncol. 2019, 55, 320–330. [Google Scholar] [CrossRef]
- Fatma, H.; Jameel, M.; Siddiqui, A.J.; Kuddus, M.; Buali, N.S.; Bahrini, I.; Siddique, H.R. Chemotherapeutic potential of Lupeol against cancer in pre-clinical model: A systematic review and meta-analysis. Phytomedicine 2024, 132, 155777, Advance online publication. [Google Scholar] [CrossRef]
- Torres-Sanchez, A.; Torres, G.; Estrade, S.; Perez, D.; Garcia, S.; Millian, M.; Velazquez, E.; Molina, V.; Delgado, Y. Unraveling the Influence of Six Lupane-, Oleanane-, and Ursane- type Pentacyclic Triterpenes’ Structure-Activity Relationship on Non-Small Lung Adenocarcinoma Cells. Pharmaceuticals 2024, 17, 694. [Google Scholar] [CrossRef]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M. The role of antioxidants in disease prevention. Medicine 2006, 34, 533–535. [Google Scholar] [CrossRef]
- Kocyigit, A.; Koyuncu, I.; Dikilitas, M.; Bahadori, F.; Turkkan, B. Cytotoxic, genotoxic and apoptotic effects of naringenin-oxime relative to naringenin on normal and cancer cell lines. Asian Pac. J. Trop. Biomed. 2016, 6, 872–880. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, S.; Sandhya, C.; Sarah Koshy, A.; Vijayalakshmi, N.R. Antioxidant activity of flavonoids from Solanum melongena. Phytother. Res. 1999, 13, 393–396. [Google Scholar] [CrossRef]
- Van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Constantinou, C.; Papas, A.; Constantinou, A.I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 123, 739–752. [Google Scholar] [CrossRef]
- Howes, R.M. Dangers of Antioxidants in Cancer Patients: A Review. Medicine, Chemistry. 2009. Available online: https://www.semanticscholar.org/paper/Dangers-of-Antioxidants-in-Cancer-Patients%3A-A-Howes/69bb0dd751ce7ed05afc948d68ad4bce66201484 (accessed on 3 May 2024).
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Berneis, K.; Bollag, W.; Kofler, M.; Lüthy, H. The enhancement of the after effect of ionizing radiation by a cytotoxic methylhydrazine derivative. Eur. J. Cancer. 2004, 40, 1928–1933. [Google Scholar] [CrossRef]
- Azzam, E.I.; De Toledo, S.M.; Little, J.B. Stress signaling from irradiated to non-irradiated cells. Curr. Cancer Drug Targets. 2004, 4, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wochna, A.; Niemczyk, E.; Kurono, C.; Masaoka, M.; Kędzior, J.; Słomińska, E.; Lipiński, M.; Wakabayashi, T. A possible role of oxidative stress in the switch mechanism of the cell death mode from apoptosis to necrosis-studies on ρ0 cells. Mitochondrion 2007, 7, 119–124. [Google Scholar] [CrossRef]
- Denning, T.L.; Takaishi, H.; Crowe, S.E.; Boldogh, I.; Jevnikar, A.; Ernst, P.B. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic. Biol. Med. 2002, 33, 1641–1650. [Google Scholar] [CrossRef]
- Gibson, S.B. A matter of balance between life and death: Targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy. Autophagy 2010, 6, 835–837. [Google Scholar] [CrossRef]
- Kong, Q.; Beel, J.A.; Lillehei, K.O. A threshold concept for cancer therapy. Med. Hypotheses 2000, 55, 29–35. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J. Current evidence on healthy eating. Annu. Rev. Public Health 2013, 34, 77–95. [Google Scholar] [CrossRef]
- Boyle, P.; Levin, B. World Cancer Report 2008; International Agency for Research on Cancer; WHO Press: Geneva, Switzerland, 2008. [Google Scholar]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed. Pharmacother. 2016, 81, 31–37. [Google Scholar] [CrossRef]
- Demiray, M.; Sahinbas, H.; Atahan, S.; Demiray, H.; Selcuk, D.; Yildirim, I.; Atayoglu, A.T. Successful treatment of c-kit-positive metastatic Adenoid Cystic Carcinoma (ACC) with a combination of Curcumin plus imatinib: A case report. Complement. Ther. Med. 2016, 27, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Hassanian, S.M.; Mohammadzadeh, E.; ShahidSales, S.; Maftouh, M.; Fayazbakhsh, H.; Khazaei, M.; Avan, A. Therapeutic Potential of Curcumin in Treatment of Pancreatic Cancer: Current Status and Future Perspectives. J. Cell Biochem. 2017, 118, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Cheng, H.L.; Huang, L.W.; Hsieh, B.S.; Hu, Y.C.; Chih, T.T.; Shyu, H.W.; Su, S.J. Combined effects of terazosin and genistein on a metastatic, hormone-independent human prostate cancer cell line. Cancer Lett. 2009, 276, 14–20. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Haznedaroglu, I.C.; Malkan, U.Y. Ankaferdhe most at induces DNA damage, apoptosis and cytotoxic activity by generating reactive oxygen species in melanoma and normal cell lines. Int. J. Clin. Exp. Med. 2017, 10, 2116–2126. [Google Scholar]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Huang, Z.; Nice, E.; Zou, B.; Huang, C. Revisiting cancer hallmarks: Insights from the interplay between oxidative stress and non-coding RNAs. Mol. Biomed. 2020, 1, 4. [Google Scholar] [CrossRef]
- Sharma, V.; Misteli, T. Non-coding RNAs in DNA damage and repair. FEBS Lett. 2013, 587, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, C.; Huang, L.; Syrigos, K.; Li, Y.; Li, P. Non-coding RNAs regulate mitochondrial dynamics in the development of gastric cancer. Front. Mol. Biosci. 2023, 10, 1107651. [Google Scholar] [CrossRef]
- Liu, P.; Wang, M.; Tang, W.; Li, G.; Gong, N. Circ_SATB2 attenuates the anti-tumor role of celastrol in non-small-cell lung carcinoma through targeting miR-33a-5p/E2F7 axis. OncoTargets Ther. 2020, 13, 11899–11912. [Google Scholar] [CrossRef]
- Kura, B.; Pavelkova, P.; Kalocayova, B.; Pobijakova, M.; Slezak, J. MicroRNAs as Regulators of Radiation-Induced Oxidative Stress. Curr. Issues Mol. Biol. 2024, 46, 7097–7113. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. MiR-21: An environmental driver of malignant melanoma? J. Transl. Med. 2015, 13, 202. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.O.; Divya, S.P.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Wang, L.; Kim, D.; Dai, J.; Asha, P.; et al. Hexavalent chromium induces malignant transformation of human lung bronchial epithelial cells via ROS-dependent activation of miR-21-PDCD4 signaling. Oncotarget 2016, 7, 51193–51210. [Google Scholar] [CrossRef] [PubMed]
- Hiraku, Y.; Watanabe, J.; Kaneko, A.; Ichinose, T.; Murata, M. MicroRNA expression in lung tissues of asbestos-exposed mice: Upregulation of miR-21 and downregulation of tumor suppressor genes Pdcd4 and Reck. J. Occup. Health 2021, 63, e12282. [Google Scholar] [CrossRef]
- Czochor, J.R.; Sulkowski, P.; Glazer, P.M. miR-155 overexpression promotes genomic instability by reducing high-fidelity polymerase delta expression and activating error-prone DSB repair. Mol. Cancer Res. 2016, 14, 363–373. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An important role in inflammation response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Wu, Q.; Qi, B.; Duan, X.; Ming, X.; Yan, F.; He, Y.; Bu, X.; Sun, S.; Zhu, H. MicroRNA-126 enhances the biological function of endothelial progenitor cells under oxidative stress via PI3K/Akt/GSK3β and ERK1/2 signaling pathways. Bosn. J. Basic Med. Sci. 2021, 21, 71. [Google Scholar] [PubMed]
- Rasoulinejad, S.A.; Akbari, A.; Nasiri, K. Interaction of miR-146a-5p with oxidative stress and inflammation in complications of type 2 diabetes mellitus in male rats: Anti-oxidant and anti-inflammatory protection strategies in type 2 diabetic retinopathy. Iran. J. Basic Med. Sci. 2021, 24, 1078. [Google Scholar]
- Uszczynska-Ratajczak, B.; Lagarde, J.; Frankish, A.; Guigó, R.; Johnson, R. Towards a complete map of the human long non-coding RNA transcriptome. Nat. Rev. Genet. 2018, 19, 535–548. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Wang, K.C.; Chang, H.Y. Molecular Mechanisms of Long Noncoding RNAs. Mol. Cell. 2011, 43, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kacso, T.P.; Zahu, R.; Tirpe, A.; Paslari, E.V.; Nuțu, A.; Berindan-Neagoe, I. Reactive Oxygen Species and Long Non-Coding RNAs, an Unexpected Crossroad in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10133. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Cui, X.; O’Connell, D.; Yang, Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. 2022, 29, 1850–1863. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Wang, L.; Piao, H.L.; Ma, L. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim. Biophys. Sin. 2014, 46, 1–5. [Google Scholar] [CrossRef]
- Loewen, G.; Jayawickramarajah, J.; Zhuo, Y.; Shan, B. Functions of lncRNA HOTAIR in lung cancer. J. Hematol. Oncol. 2014, 7, 90. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Li, J.; Dashwood, R.H. Emerging crosstalk between long non-coding RNAs and Nrf2 signaling. Cancer Lett. 2020, 490, 154–164. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Wen, J.F.; Jiang, Y.Q.; Li, C.; Dai, X.K.; Wu, T.; Yin, W.Z. LncRNA-XIST promotes the oxidative stress-induced migration, invasion, and epithelial-to-mesenchymal transition of osteosarcoma cancer cells through miR-153-SNAI1 axis. Cell Biol. Int. 2020, 44, 1991–2001. [Google Scholar] [CrossRef]
- Dong, P.; Xu, D.; Xiong, Y.; Yue, J.; Ihira, K.; Konno, Y.; Watari, H. The expression, functions and mechanisms of circular RNAs in gynecological cancers. Cancers 2020, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017, 24, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gao, H.; Zhou, W.; Cai, H.; Liao, L.; Wang, C. Circular RNA HIPK3 facilitates ferroptosis in gestational diabetes mellitus by regulating glutathione peroxidase 4 DNA methylation. J. Gene Med. 2023, 25, e3526. [Google Scholar] [CrossRef]
- Bajan, S.; Hutvagner, G. RNA-based therapeutics: From antisense oligonucleotides to miRNAs. Cells 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, K.R.; Challa, H.J.; Gaur, A.; Jain, R.; Vardhani, K.K.; Geddam, A.; Natya, K.; Aswini, K.; Palasamudram, K. Unlocking the potential of non-coding RNAs in cancer research and therapy. Transl. Oncol. 2023, 35, 101730. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Novak Kujundžić, R.; Grbeša, I.; Ivkić, M.; Katdare, M.; Gall-Trošelj, K. Curcumin downregulates H19 gene transcription in tumor cells. J. Cell Biochem. 2008, 104, 1781–1792. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, Z.; Wang, S.; Li, T.; Mastriani, E.; Li, Q.H.; Bao, H.X.; Zhou, Y.J.; Wang, X.; Liu, Y.; et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017, 18, 990–999. [Google Scholar] [CrossRef]
- Chen, T.; Yang, P.; Wang, H.; He, Z.Y. Silence of long noncoding RNA PANDAR switches low-dose curcumin-induced senescence to apoptosis in colorectal cancer cells. OncoTargets Ther. 2017, 10, 483–491. [Google Scholar] [CrossRef]
- Pei, C.-S.; Wu, H.-Y.; Fan, F.-T.; Wu, Y.; Shen, C.-S.; Pan, L.-Q. Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 4239–4243. [Google Scholar] [CrossRef]
- Al Aameri, R.F.; Sheth, S.; Alanisi, E.M.; Borse, V.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Tonic suppression of PCAT29 by the IL-6 signaling pathway in prostate cancer: Reversal by resveratrol. PLoS ONE 2017, 12, e0177198. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Tang, X.; Peterson, D.R.; Kilari, D.; Chow, C.-W.; Fujimoto, J.; Kalhor, N.; Swisher, S.G.; Stewart, D.J.; Wistuba, I.I. Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 2014, 85, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, P.; Wang, P.; Yang, C.S.; Wang, X.; Feng, Q. EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS ONE 2015, 10, e0125402. [Google Scholar] [CrossRef]
- Sabry, D.; Abdelaleem, O.O.; El Amin Ali, A.M.; Mohammed, R.A.; Abdel-Hameed, N.D.; Hassouna, A.; Khalifa, W.A. Anti-proliferative and anti-apoptotic potential effects of epigallocatechin-3-gallate and/or metformin on hepatocellular carcinoma cells: In vitro study. Mol. Biol. Rep. 2019, 46, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, C.; Wang, L.; Luo, G.; Huang, C.; Li, Y.; Liu, D.; Zeng, F.; Jiang, G.; Xiao, X. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018, 9, 238. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Tang, X.; Li, Y.; Xia, P.; Gao, X. Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 2015, 13, 24. [Google Scholar] [CrossRef]
- Awasthee, N.; Rai, V.; Verma, S.S.; Sajin Francis, K.; Nair, M.S.; Gupta, S.C. Anti-cancer activities of Bharangin against breast cancer: Evidence for the role of NF-kappaB and lncRNAs. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2738–2749. [Google Scholar] [CrossRef]
- Venkatadri, R.; Muni, T.; Iyer, A.K.V.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef]
- Gordon, M.W.; Yan, F.; Zhong, X.; Mazumder, P.B.; Xu-Monette, Z.Y.; Zou, D.; Young, K.H.; Ramos, K.S.; Li, Y. Regulation of p53-targeting microRNAs by polycyclic aromatic hydrocarbons: Implications in the etiology of multiple myeloma. Mol. Carcinog. 2015, 54, 1060–1069. [Google Scholar] [CrossRef]
- Ahmed, F.; Ijaz, B.; Ahmad, Z.; Farooq, N.; Sarwar, M.B.; Husnain, T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: Mechanism and translational significance. Phytomedicine 2020, 68, 153168. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Hargraves, K.G.; He, L.; Firestone, G.L. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol. Carcinog. 2016, 55, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Y.; Zhang, Z. Expression profile of miRNAs involved in the hepatoprotective effects of curcumin against oxidative stress in Nile tilapia. Aquat. Toxicol. 2021, 237, 105896. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, X.; Yu, F.; Zhang, L.; Zhang, Y.; Chang, W. The targeting of noncoding RNAs by quercetin in cancer prevention and therapy. Oxidative Med. Cell. Longev. 2022, 2022, 4330681. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Wang, L.; Sun, Y. Effects of berberine on circular RNA expression profiles in human gastric cancer cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 6688629. [Google Scholar] [CrossRef]
- Tian, F.; Yu, C.T.; Ye, W.D.; Wang, Q. Cinnamaldehyde induces cell apoptosis mediated by a novel circular RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1260–1266. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158. [Google Scholar] [CrossRef]
- Achi, I.T.; Sarbadhikary, P.; George, B.P.; Abrahamse, H. Multi-target potential of berberine as an antineoplastic and antimetastatic agent: A special focus on lung cancer treatment. Cells 2022, 11, 3433. [Google Scholar] [CrossRef]
- Jia, Q.; Thomas, M.F. 7-Hydroxy Chromones as Potent Antioxidants. US9078891B2, 14 July 2015. [Google Scholar]
- Seeram, N.P.; Liya, L.; Geneviève, B.; Barbeau, J. Novel Phytochemicals from Extracts of Maple Syrups and Maple Trees and Uses Thereof. WO2012021981A1, 23 February 2012. [Google Scholar]
- Khandelwal, S.; Pratibha, O.; Tripathi, V.K. A Bio-Stabilized Resveratrol Formulation. WO2012017451A, 9 February 2012. [Google Scholar]
- Cohen, I. Anticancer Methods Employing Extracts of Gleditsiasinensis Lam. US20120045532A1, 23 February 2012. [Google Scholar]
- Gupta, R.C.; Vadhanam, M.V. Methods and Compositions for Controlled Delivery of Phytochemical Agents. WO2013148682A1, 3 October 2013. [Google Scholar]
- Barbeau, J.; Beland, G.; Seeram, N.P.; Yuan, T. Maple Tree-Derived Products and Uses Thereof. US20130310332A1, 21 November 2013. [Google Scholar]
- Sang, K.; Jung, H.K.; Sang, H. Anti-Inflammatory, Anti-Oxidative or Anti-Bacterial Compositions. KR101219520B1, 8 January 2013. [Google Scholar]
- Shraibom, N. Molecular Combinations for Cancer or Other Disease Treatment. US8734859B1, 27 May 2014. [Google Scholar]
- Myung, J.; Lim, G.; Jumg, D.; Hwang, Y.; Sun, S.E. Pharmaceutical Composition Having Antioxidant Comprising Chlorophylls from Isolated Soybean. KR101450480B1, 16 October 2014. [Google Scholar]
- Gupta, R.C.; Vadhanam, M.V.; Aqil, F. Methods and Compositions for Controlled Delivery of Phytochemical Agents. US8858995B2, 14 October 2014. [Google Scholar]
- Thornthwaite, J.T. Natural Killer Cell Formulations. US20140127179A1, 8 May 2014. [Google Scholar]
- Biswal, S.; Thimmulappa, R.; Kumar, S.; Malhotra, S.V.; Kumar, V.; Jung-Hyun, K. Chalcone Derivatives as nrf2 Activators. US20140088052A1, 27 March 2014. [Google Scholar]
- Crooks, P.A.; Jordan, C.T.; Pei, S.; Nasim, S. Melampomagnolide B Derivatives as Antileukemic and Cytotoxic Agents. US20120122943A1, 11 November 2014. [Google Scholar]
- Vander Jagt, D.l.; Deck, L.M.; Abcouwer, S.F.; Orlando, R.A.; Royer, R.E.; Weber, W.M.; Bobrovnikova-Marjon, E.V.; Hunsaker, L.A. Therapeutic Curcumin Derivatives. US9187397B2, 17 November 2015. [Google Scholar]
- Gao, S. Compositions Containing Enriched Natural Crocin and/or Crocetin, and Their Therapeutic or Nutraceutical Uses. US20140141082A1, 15 December 2015. [Google Scholar]
- Nair, V. Fermented Soy Nutritional Supplements Including Mushroom Components. US20150216918A1, 25 August 2011. [Google Scholar]
- Babish, J.G.; Pacioretty, L.M.; Debenedetto, J. Compositions from Nigella Sativa. US9180155B2, 10 November 2015. [Google Scholar]
- Takamatsu. Compositions Containing Enriched Natural Crocin and/or Crocetin, and Their Therapeutic or Nutraceutical Uses. CN104623670A, 20 May 2015.
- Minatelli, J.A.; Hill, S.; Moerck, R.E. Krill Oil and Carotenoid Composition, Associated Method and Delivery System. US9295698B2, 29 March 2016. [Google Scholar]
- Kubow, S.; Donnelly, D.; Piccolomini, A.; Agellon, L. Compositions and Methods for Preventing and Treating Diseases and Environmentally Induced Health Disorders. US9849153B2, 26 December 2017. [Google Scholar]
- Seeram, N.P.; Heber, D. Therapeutic Uses of Urolithins. EP2068864B1, 23 August 2017. [Google Scholar]
- Bommagani, S.; Crooks, P.; Penthala, N.R.; Janganati, V.; Ponderm, J.J. Melampomagnolide B Derivatives. US9920063B2, 20 March 2018. [Google Scholar]
- Deng, S.Y.; Hongyan, Z.L. Radix Tetrastigme Compound Composition for Enhancing Antitumor and Antioxidant Activities and Preparation Method Thereof. CN110538301A, 6 December 2019. [Google Scholar]
- Zelkha, M.; Blatt, Y.; Levy, Y.; Sharoni, Y. Pharmaceutical Compositions for Oral Administration Comprising a Tomato Oleoresin. CA2832273C, 12 November 2019. [Google Scholar]
- Howes, R.M. Pro-Oxidant Cancer Chemo-Suppressors and Chemo-Protectors and Methods of Use Related Thereto. US20190275119A1, 12 September 2019. [Google Scholar]
- Koren, Z. Compositions Comprising a Cannabinoid and Punicalagin and Methods of Use Thereof. WO2019155337A1, 15 August 2019. [Google Scholar]
- Kovarik, J.E. Method for Reducing the Likelihood of Developing Bladder or Colorectal Cancer in an Individual Human Being. US11026982B2, 8 June 2021. [Google Scholar]
- Zielinski, J.; Moon, T.R.; Allen, E.P. Antioxidant Compositions for Treatment of Inflammation or Oxidative Damage. US20160331707A1, 23 August 2016. [Google Scholar]
- Viera, K. Herbal Nutraceutical Formulation to Reduce Oxidative Stress, Viral and Microbial Infections, and Inflammation. US10967025B2, 6 April 2021. [Google Scholar]
- Firger, R.; Haase, G.M. Compositions with Ketogenic Agents, Cannabinoids, Plant-Derived Substances and Micronutrients. US10912758B2, 9 February 2021. [Google Scholar]
- CHungm, H.H. Nutritional Formulation for Cancer Prevention. US20220257640A1, 18 August 2022. [Google Scholar]
- Sloey, C.J.; Ko, J.; Li, T. Aqueous Formulation of Erythropoiesis Stimulating Protein Stabilised by Antioxidants for Parenteral Administration. US11433134B2, 6 September 2022. [Google Scholar]
- Hybertson, B.M.; McCord, J.M. Compositions for Improved nrf2 Activation and Methods of Their Use. US20180250264A1, 10 November 2022. [Google Scholar]
- Singh, M.; Suman, S.; Shukla, Y. New Enlightenment of Skin Cancer Chemoprevention through Phytochemicals: In Vitro and In Vivo Studies and the Underlying Mechanisms. Biomed. Res. Int. 2014, 2014, 243452. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Velmurugan, B.; Kaur, M.; Singh, R.P.; Agarwal, R. Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res. 2010, 70, 2368–2378. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Theoretical study on physicochemical properties of Curcumin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 672007, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure–activity relationship evidence. In Nutrition, Wellbeing and Health; InTechOpen Limited: London, UK, 2012; pp. 24–48. [Google Scholar]
- Hadi, S.M.; Ullah, M.F.; Azmi, A.S.; Ahmad, A.; Shamim, U.; Zubair, H.; Khan, H.Y. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for chemoprevention of cancer. Pharm. Res. 2010, 27, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Batteux, F.; Nicco, C.; Chéreau, C.; Laurent, A.; Guillevin, L.; Weill, B.; Goldwasser, F. Accumulation of hydrogen peroxide is an early and crucial step for Paclitaxel-induced cancer cell death both in vitro and in vivo. Int. J. Cancer 2006, 119, 41–48. [Google Scholar] [CrossRef]
- López-Lázaro, M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol. Med. 2010, 16, 144–153. [Google Scholar] [CrossRef]

| Compound Name | Structure (PubChem) | Function | Target Cells | Modes of Action | Ref. |
|---|---|---|---|---|---|
| Oridonin Or Rubesecensin A | 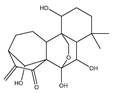 | Anti-proliferative | HeLa | Induction of oxidative stress via targeting thioredoxin reductase (TrxR). | |
| Apoptotic | HepG2 | Oxidative stress induction via mitochondrial signaling pathway. | [91] | ||
| Protection against arsenic-induced toxicity | UROtsa | Activation of NrF-2-mediated response, reduced formation of reactive oxygen species (ROS), and improved cell survival after arsenic challenge. | [92] | ||
| Costunolide | 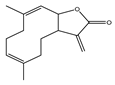 | Antioxidant effect | MCI-7 and MDA-MB-231 | Decrease TBARs level and increase in SOD, catalase, and GPx. | [93] |
| Magnolol | 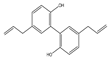 | Antioxidant | 3T3-L1 | Increase in SIRT-1 and decrease in ROS and FAS levels. | [94] |
| Oxidative stress-induced apoptosis. | [95] | ||||
| Anticancer | Skin cancer in SKH-1 mice and A431 | Go/G1 arrest and decrease in levels of CyclinD1 and Cyclin A. | [96] | ||
| U373 CCA | Inhibition of NF-κB pathway and decrease in Ki67, MMP-2, MMP7, and MMP9 levels. | [97] | |||
| Pseudolaric Acid | 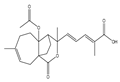 | ferroptosis | Glioma cells | Increase in ferrous ion levels and H2O2 levels, lipid peroxidation, GSH depletion, and Nox$ activation. | [98] |
| Isoalantolactone | 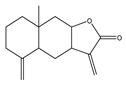 | Anticancer | DU-145 and PC-3 | Induction of oxidative stress, increase in ROS, and activation of JNK pathway. | [99] |
| Apoptosis | HeLa | Oxidative stress by TrxR inhibition and increase in ROS. | [100] | ||
| Jaceosidin | 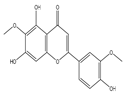 | Apoptosis | MCF10A-RAS | Increase in ROS and Bax and decrease in Bcl-2 levels. Inhibition of ERK1/2 activation. | [101] |
| U87 | G2/M arrest, upregulation of p53 and Bax, and release of cyt-c and activation of caspase 3. | [102] | |||
| Caticin Or Casticine Or Vitexicarpin | 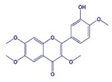 | Anticancer | HeLa | G2/M arrest and antimitotic activity. | [103] |
| K562 and A2780 | Downregulation of cyclin B and activation of p21. | [104] | |||
| HCT-15 | Mitotic arrest via PI3k/Akt pathway. | [105] | |||
| Apoptosis | HT-29, HCT-116, SW480, and CaCo-2 | Increase in caspase 3. | [106] | ||
| B16F10 | Increase in ROS and ASK1/JNK/Bim signaling cascade. | [107] | |||
| Induction of DNA damage via inhibition of DNA repair proteins and decrease in MGMT and MDC1 levels. | [108] | ||||
| Evodiamine |  | Anticancer | HT-29 and HCT-116 | Inhibition of migration of invasion via increase in SIRT-1 and decrease in MMP-9 and acetyl NR-kappaBp65. | [109] |
| Apoptosis | A375-S2 | Via PI3K/Akt/caspase and FasL/NF-κB signaling. | [110] | ||
| Parthenolide |  | Anticancer | MDA-MB23, BT-20, and MDA-MB43 | Oxidative stress, mitochondrial dysfunction, and necrosis. | [111] |
| HeLa | TrxR1- and TrxR2-mediated increase in ROS. | [112] | |||
| Rhein Or Cassic acid |  | Anticancer | A549, PC9, and PC9 | Inhibition of proliferation and migration via Stat3/Snail/MMP2/MMP9 pathway. | [113] |
| A2780 and OV2008 | Inhibition of migration through downregulation of MMP. | [114] | |||
| Apoptosis | HL-60 | ROS-independent mitochondrial death pathway. | [115] | ||
| Resveratrol |  | Anticancer | HeLa and MDA-MB-231 | Increase in ROS production and decrease in SOD activity and GSH levels. | [116] |
| Apoptosis | A375SM | Induction of ROS generation and ER stress and cell cycle arrest. Downregulation of Bcl-2 expression and upregulation Bax. | [117] | ||
| Curcumin |  | Anti-proliferative | MCF7, HCT116, and A549 | Modulation of oxidative stress, regulation of fibrosis, SIRT1 activation, and induction of cellular apoptosis. | [118] |
| Antitumor | Colorectal cancer and CT-26 cell | Suppression of angiogenesis and cell proliferation and induction of oxidative stress. | [119] | ||
| EGCG |  | Anti-proliferative | CAL27, HSC-2, and HSG1 | Pro-oxidant effect by potentiation of Fe2+-induced lipid peroxidation. | [120] |
| Esculetin |  | Anti-proliferative | Hep-2, TU-212, and M4e | Inhibition of Janus Kinas (JAK)-signal transducer and activator of transcription-3 (STAT3) activation. Cell cycle arrest at G1/S phase. | [121] |
| Apoptosis | PANC-1, MIA PaCa-2, and AsPC-1 | Loss of Nrf2-KEAP1 interaction by binding of esculetin with KEAP1 directly. | [122] | ||
| Genistein | 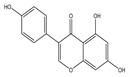 | Anticancer | MCF-7 | Modulation of oxidative stress according to ERα/ERβ ratioG2/M arrest, increased H2O2, and production of filopodia. | [123] |
| HT29 and SW620 | Expression of inflammation-related genes increased. NF-kB translocation to the nucleus was increased. | [124] | |||
| Hesperidin | 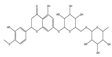 | Anti-proliferative | Prostate cancer cells, PC3, and DU145 | Generation of ROS and induction of mitochondrial membrane depolarization and endoplasmic reticulum stress. | [125] |
| Apoptosis | HepG2 | Induction of mitochondrial pathway and death receptor pathway. | [126] | ||
| Quercetin | 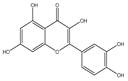 | Antiproliferation | MDA-MB-231, MDA-MB-468, and MCF cells | Nrf2-dependent oxidative stress. | [127] |
| Rosmarinic Acid | 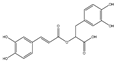 | Antioxidant, anti-inflammatory, and anti-metastasis | A549 | Modulation of c-Jun, NF-κB, and Akt signaling pathways. | [128] |
| Carvacrol | 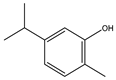 | Antioxidant effects and apoptosis | Gastric carcinoma in Wistar rats | Induction of oxidative stress. | [129] |
| Luteolin |  | Apoptosis | HT-29 | Upregulation of Bax, downregulation of Bcl-2, activation of caspase-9, and caspase-3. | [130] |
| SNU-407 | Upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53. | [130] | |||
| Bladder cancer cell line and T24 | Inhibition of cell survival and induction of G2/M cell cycle arrest, p21 upregulation, and downregulation of phospho(p)-S6, via mTOR signaling. Upregulation of TRX1 and reduction in intracellular ROS production. | [131] | |||
| Tannic acid |  | Apoptosis | Prostate cancer cell, C4-2, DU145, and PC-3 | Induction of ER stress by ROS. Inhibition of lipogenic signaling and suppression of lipid metabolic pathways. Downregulation of proteins responsible for lipogenesis. | [132] |
| Berberine | 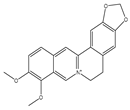 | Apoptosis | MCF-7 and MDA-MB-231 cells | Increased production of ROS with activation of the pro-apoptotic JNK signaling. | [133] |
| Ovarian cancer cells | Induction of oxidative DNA damage and impairment of homologous recombination repair combined increases sensitivity to PARP inhibition. | [134] | |||
| Thymoquinone | 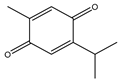 | Antitumor and apoptosis | NSCLC | Generation of ROS. | [135] |
| Thymol |  | Anticancer and apoptosis | T24 and SW780 | Generation of ROS. | [136] |
| Phytic acid | 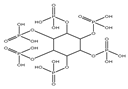 | Anticancer | Colon cancers | Increased ROS. | [137] |
| Chrysin | 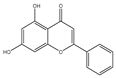 | Anticancer and apoptosis | Prostate cancers | Inactivation of the ROS-mediated Akt/mTOR pathway. | [138,139] |
| Phytochemical | Target ncRNA Type | ncRNA Example(s) | Mechanism/Effect | Cancer/Condition | References |
|---|---|---|---|---|---|
| Curcumin | lncRNA | ↓ H19 and ↓ ROR | Suppresses Wnt/β-catenin pathway. | Various cancer types | [110,233,234] |
| ↓ HOTAIR | Inhibits cancer cell migration and induces growth arrest. | Renal cell carcinoma | [236] | ||
| miRNA | ↓ miR-153b, ↑ miR-200a, and ↑ miR-29 | Enhances Nrf2 pathway and provides hepatoprotection. | Liver cells | [249] | |
| ↓ miR-21 | Induced the expression of the tumour suppressor PDCD4 and inhibited invasion and metastasis. | Colorectal cancer | [250] | ||
| circRNA | ↓ circSATB2 and ↓ circFOXM1 | Modulates invasion, migration, and apoptosis. | Diverse cancers | [141] | |
| Resveratrol | lncRNA | ↑ PCAT29 | Boosts tumor suppressor lncRNA and the inhibition of metastasis. | Prostate cancer cell | [237] |
| miRNA | ↓ miR-200c | Induces apoptosis. | Breast cancer | [244] | |
| ↓ miR-155 and ↑ miR-663 | Targets JunB and JunD, reduces ROS, and inhibits inflammation-driven carcinogenesis. | Human monocytic leukemia | [247] | ||
| EGCG (Green Tea) | lncRNA | Modulation of multiple lncRNA | Induces apoptosis and disrupts energy metabolism. | Lung and ovarian cancer | [238,239] |
| ↓ SOX2OT variation 7 | EGCG reduced Dox-induced pro-survival autophagy. Decreased the stemness and abated the drug resistance in osteosarcoma cells and deactivated the Notch3/DLL3 signaling cascade. | Osteosarcoma | [240] | ||
| ↓ AF085935 | Suppress cell proliferation. | Hepatocellular carcinoma | [240] | ||
| miRNA | ↓ miR-21 | Modulates NF-κB pathway. | Breast cancer | [246] | |
| ↑ miR-200c | Induces apoptosis. | Breast cancer | [245] | ||
| ↓ miR-200c | Suppressing Notch and Bmi1, Ezh2, and Suz12. | Colorectal cancer | [254] | ||
| Gamboic Acid (GA) | lncRNA | ↓ EZH2 | Induces apoptosis. | Bladder cancer | [241] |
| Bharangin | lncRNA | ↑ MEG-3, ↓ GAS-5, and ↓ H19 | Inhibition of NF-κB and induction of apoptosis and cycle arrest. | Breast cancer | [243] |
| Artemisinin/Artesunate | miRNA | ↑ miR-34a | Increases expression and downregulates CDK4. | Breast cancer | [248] |
| Quercetin | circRNA | ↓ PI3K/AKT/mTOR-related circRNAs | Suppresses tumorigenesis by targeting oncogenic circRNAs. | Diverse cancers | [251] |
| Berberine | lncRNA | ↑ MRAK052686 | Restores Nrf2/lncRNA levels. | NAFLD and liver dysfunction | [242] |
| ↓ HOTAIR | Induction of metastasis. | Lung cancer | [255] | ||
| miRNA | ↑ miR-34a-5p | Suppression of KRAS and c-MYC to prevent and reverse tumorigenesis. | |||
| circRNA | Diverse circRNA | Affects cell proliferation via Notch, MAPK, and NF-κB signaling pathways. | Gastric cancer | [252] | |
| Cinnamon Aldehyde | circRNA | ↑ hsa_circ_0043256 | Modulates apoptosis, autophagy, and proliferation. | Diverse cancers | [253] |
| Celastrol | circRNA | ↓ circSATB2 | Inhibiting miR-33a-5p/E2F7 axis. | Lung cancer | [205] |
| Year | Patent Number | Title | Applicants | Ref. |
|---|---|---|---|---|
| 2012 | US9078891B2 | 7-Hydroxychromones as potent antioxidants. | Unigen Inc. Tacoma, WA, USA | [256] |
| 2012 | WO2012021981A1 | Novel phytochemicals from extracts of maple syrups and maple trees and uses thereof. | Fédération Des Producteurs Acéricoles Du Québec, University Of Rhode Island, KGN, USA | [257] |
| 2012 | WO2012017451A1 | A bio-stabilized resveratrol formulation. | Sanjeev Khandelwal | [258] |
| 2012 | US20120045532A1 | Anticancer methods employing extracts of Gleditsiasinensis Lam. | Bionovo Inc., Emeryville, CA, USA | [259] |
| 2013 | WO2013148682A1 | Methods and compositions for controlled delivery of phytochemical agents. | University of Louisville Research Foundation ULRF | [260] |
| 2013 | US20130310332A1 | Maple tree-derived products and uses thereof. | Federation Des Producteurs Acericoles Du Quebec Rhode Island University, KGN, USA | [261] |
| 2013 | KR101219520B1 | Anti-inflammatory, anti-oxidative, or antibacterial compositions. | Michel Bio Co., Ltd., Dallas, TX, USA | [262] |
| 2014 | US8734859B1 | Molecular combinations for cancer or other disease treatment. | Sirbal Ltd., Limassol, Cyprus | [263] |
| 2014 | KR101450480B1 | Pharmaceutical composition having antioxidants comprising chlorophylls from isolated soybean. | Kangwon National University Industry-University Cooperation Foundation | [264] |
| 2014 | US8858995B2 | Methods and compositions for controlled delivery of phytochemical agents. | University of Louisville Research Foundation ULRF | [265] |
| 2014 | US20140127179A1 | Natural killer cell formulations. | Scientific Formulations LLC, Allentown, PA, USA | [266] |
| 2014 | US20140088052A1 | Chalcone derivatives as nrf2 activators. | United States, Health and Human Services C/O Office of Technology Transfer National Institutes Of Health, Secretary of, Department of Johns Hopkins University | [267] |
| 2014 | US20120122943A1 | Melampomagnolide B derivatives as antileukemic and cytotoxic agents. | University of Kentucky Research Foundation University of Rochester | [268] |
| 2015 | US9187397B2 | Therapeutic curcumin derivatives. | STC UNM | [269] |
| 2015 | US20140141082A1 | Compositions containing enriched natural crocin and/or crocetin and their therapeutic or nutraceutical uses. | Song Gao | [270] |
| 2015 | US20150216918A1 | Fermented soy nutritional supplements including mushroom components. | Vijaya Nair | [271] |
| 2015 | US9180155B2 | Compositions from Nigella Sativa. | Bio Nexus Ltd., Netanya, Israel | [272] |
| 2015 | CN104623670A | Compositions containing enriched natural crocin and/or crocetin and their therapeutic or nutraceutical uses. | Takamatsu | [273] |
| 2016 | US9295698B2 | Krill oil and carotenoid composition and its associated method and delivery system. | US Nutraceuticals LLC, Eustis, FL, USA | [274] |
| 2017 | US9849153B2 | Compositions and methods for preventing and treating diseases and environmentally induced health disorders. | Royal Institution for the Advancement of Learning | [275] |
| 2017 | EP2068864B1 | Therapeutic uses of urolithins. | University of California | [276] |
| 2018 | US9920063B2 | Melampomagnolide B derivatives. | BioVentures LLC University of Colorado | [277] |
| 2019 | CN110538301A | Radix tetrastigme compound composition for enhancing antitumor and antioxidant activities and preparation method thereof. | Nanchang University | [278] |
| 2019 | CA2832273C | Pharmaceutical compositions for oral administration, comprising a tomato oleoresin. | Lycored Ltd., Beer Sheva, Israel | [279] |
| 2019 | US20190275119A1 | Pro-oxidant cancer chemo-suppressors and chemo-protectors and methods of use related thereto. | Randolph M Howes | [280] |
| 2019 | WO2019155337A1 | Compositions comprising a cannabinoid and punicalagin and methods of use thereof. | Scicann Therapeutics Inc., Dr. Mark Friedman Ltd., Toronto, ON, Canada | [281] |
| 2021 | US11026982B2 | Method for reducing the likelihood of developing bladder or colorectal cancer in an individual human being. | Seed Health Inc., Venice, CA, USA | [282] |
| 2021 | US20160331707A1 | Antioxidant compositions for treatment of inflammation or oxidative damage. | Perio Sciences LLC, Rockford, IL, USA | [283] |
| 2021 | US10967025B2 | Herbal nutraceutical formulation to reduce oxidative stress, viral and microbial infections, and inflammation. | Moringo Organics Inc., Vancouver, BC, Canada | [284] |
| 2021 | US10912758B2 | Compositions with ketogenic agents, cannabinoids, plant-derived substances, and micronutrients. | Robert Firger, Gerald M. Haase | [285] |
| 2022 | US20220257640A1 | Nutritional formulation for cancer prevention. | Individual | [286] |
| 2022 | US11433134B2 | Aqueous formulation of erythropoiesis-stimulating protein stabilized by antioxidants for parenteral administration. | Amgen Inc., Oaks, PA, USA | [287] |
| 2022 | US20180250264A1 | Compositions for improved nrf2 activation and methods of their use. | Pathways Bioscience LLC, Aurora, CO, USA | [288] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhri, K.U.; Sharma, D.; Fatma, H.; Yasin, D.; Alam, M.; Sami, N.; Ahmad, F.J.; Shamsi, A.; Rizvi, M.A. The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation. Antioxidants 2025, 14, 620. https://doi.org/10.3390/antiox14060620
Fakhri KU, Sharma D, Fatma H, Yasin D, Alam M, Sami N, Ahmad FJ, Shamsi A, Rizvi MA. The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation. Antioxidants. 2025; 14(6):620. https://doi.org/10.3390/antiox14060620
Chicago/Turabian StyleFakhri, Khalid Umar, Deepti Sharma, Homa Fatma, Durdana Yasin, Manzar Alam, Neha Sami, Farhan Jalees Ahmad, Anas Shamsi, and Moshahid Alam Rizvi. 2025. "The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation" Antioxidants 14, no. 6: 620. https://doi.org/10.3390/antiox14060620
APA StyleFakhri, K. U., Sharma, D., Fatma, H., Yasin, D., Alam, M., Sami, N., Ahmad, F. J., Shamsi, A., & Rizvi, M. A. (2025). The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation. Antioxidants, 14(6), 620. https://doi.org/10.3390/antiox14060620








