Zearalenone Exposure Damages Skeletal Muscle Through Oxidative Stress and Is Alleviated by Glutathione, Nicotinamide Mononucleotide, and Melatonin
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animals and Study Approval
2.3. Cell Counting Kit 8 (CCK-8) Assay
2.4. Total RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
2.5. Immunofluorescence Staining
2.6. Mito-Tracker Staining
2.7. Reactive Oxygen Species (ROS) Evaluation by 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA) Staining
2.8. Hematoxylin-Eosin (H&E) Staining
2.9. Transmission Electron Microscopy (TEM)
2.10. 16S rDNA Sequencing
2.11. Statistics Analysis
3. Results
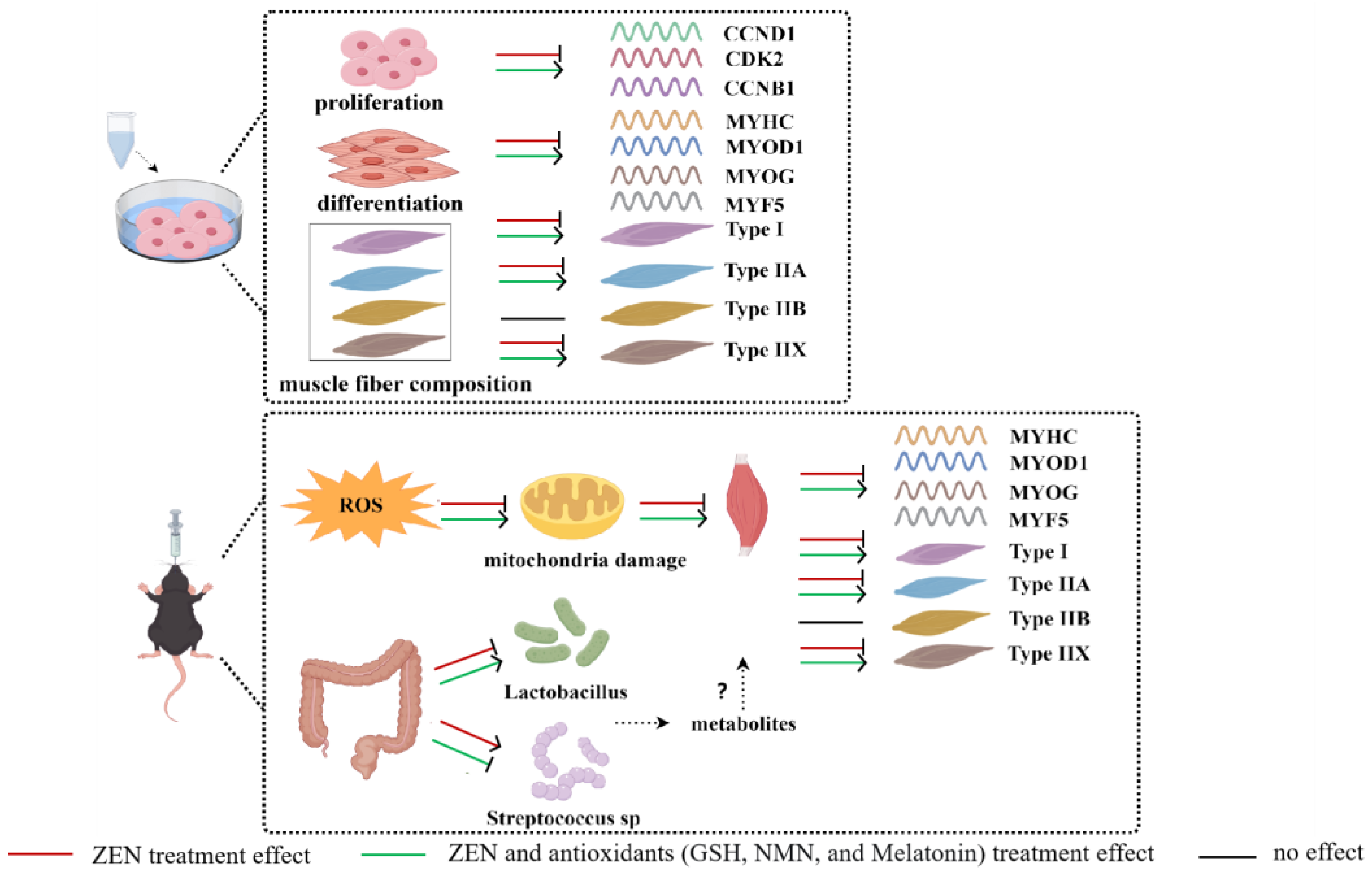
3.1. ZEN Inhibits the Proliferation of Myoblast and Antioxidant Treatments Alleviate This Inhibitory Effect
3.2. ZEN Suppresses the Differentiation of Myoblast and Antioxidant Treatment Rescues This Suppression Effect
3.3. ZEN Induces Slow-to-Fast Myofiber Shift and Antioxidant Treatment Rescues This Phenotype
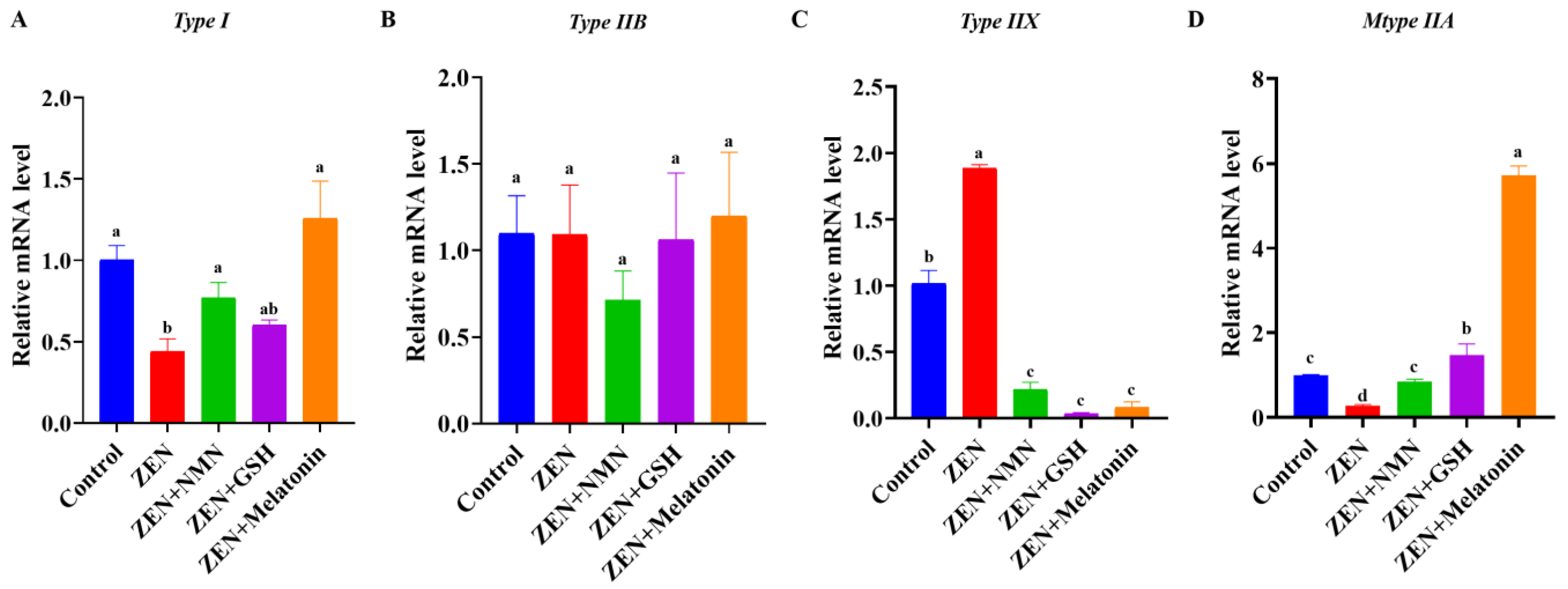
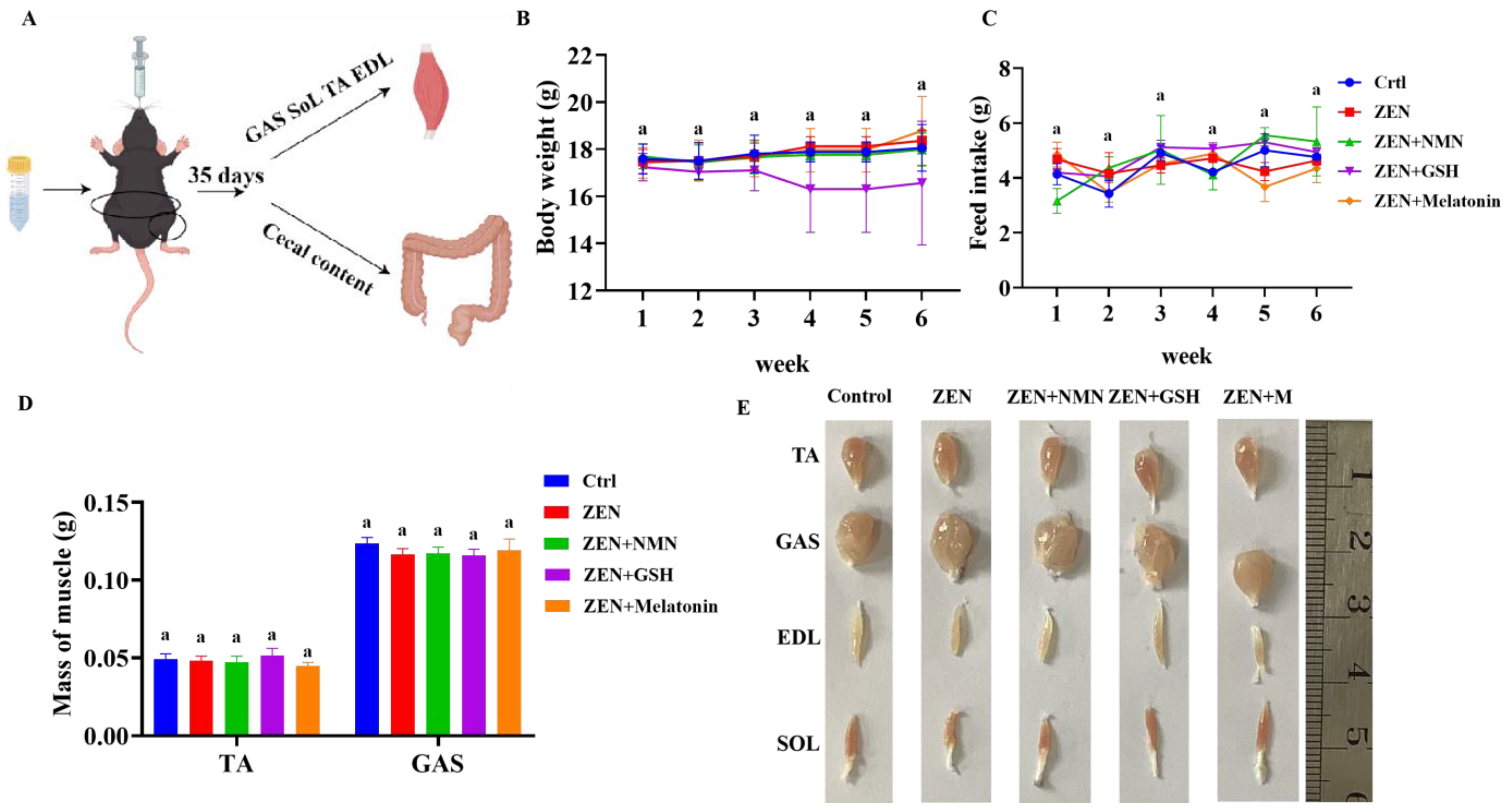
3.4. ZEN Exposure Decreases Skeletal Muscle Myogenic-Related Gene Levels and Changes Myofiber Composition In Vivo
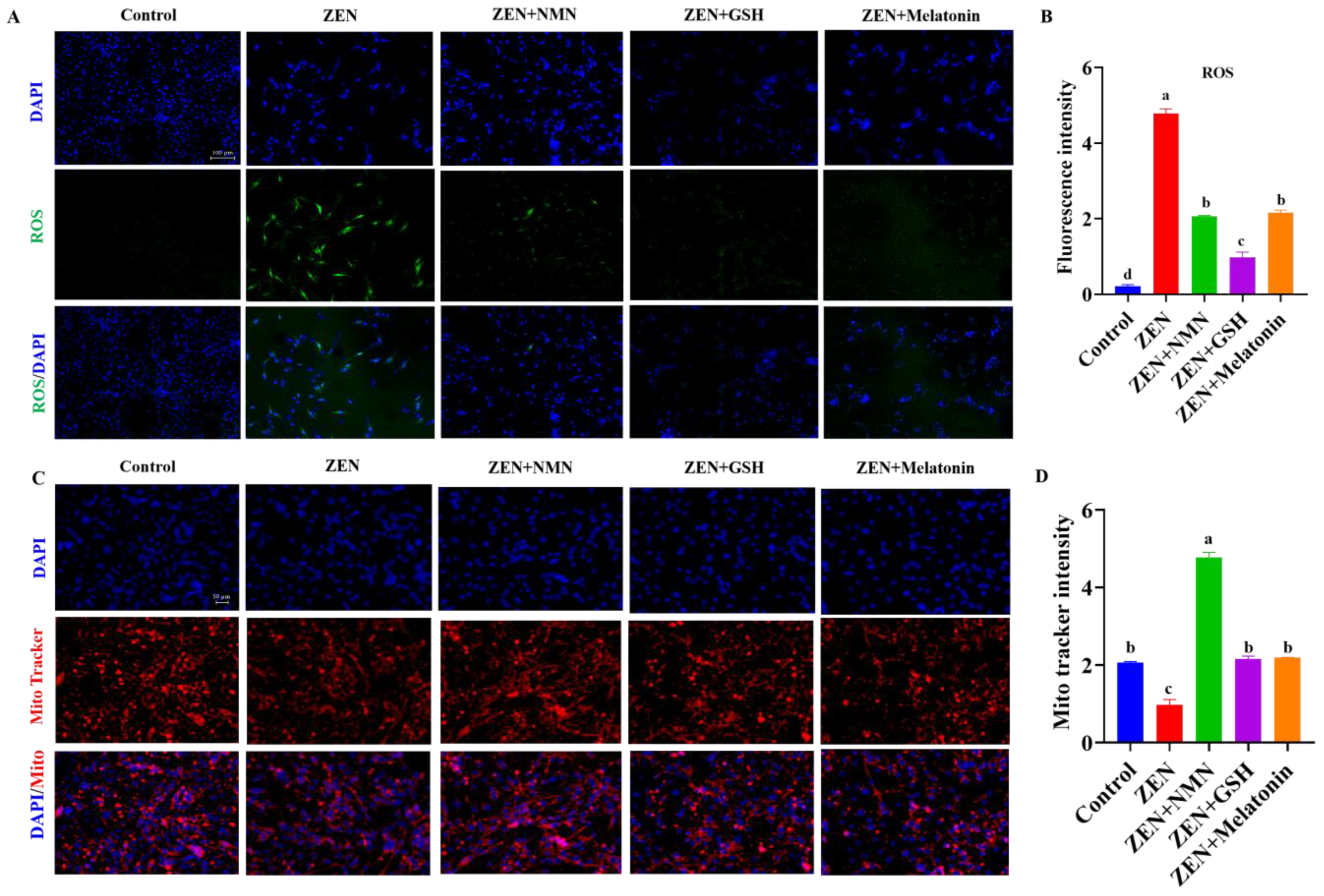
3.5. ZEN Induces Slow-to-Fast Myofiber Shift Through Oxidative Stress and Mitochondrial Dysfunction
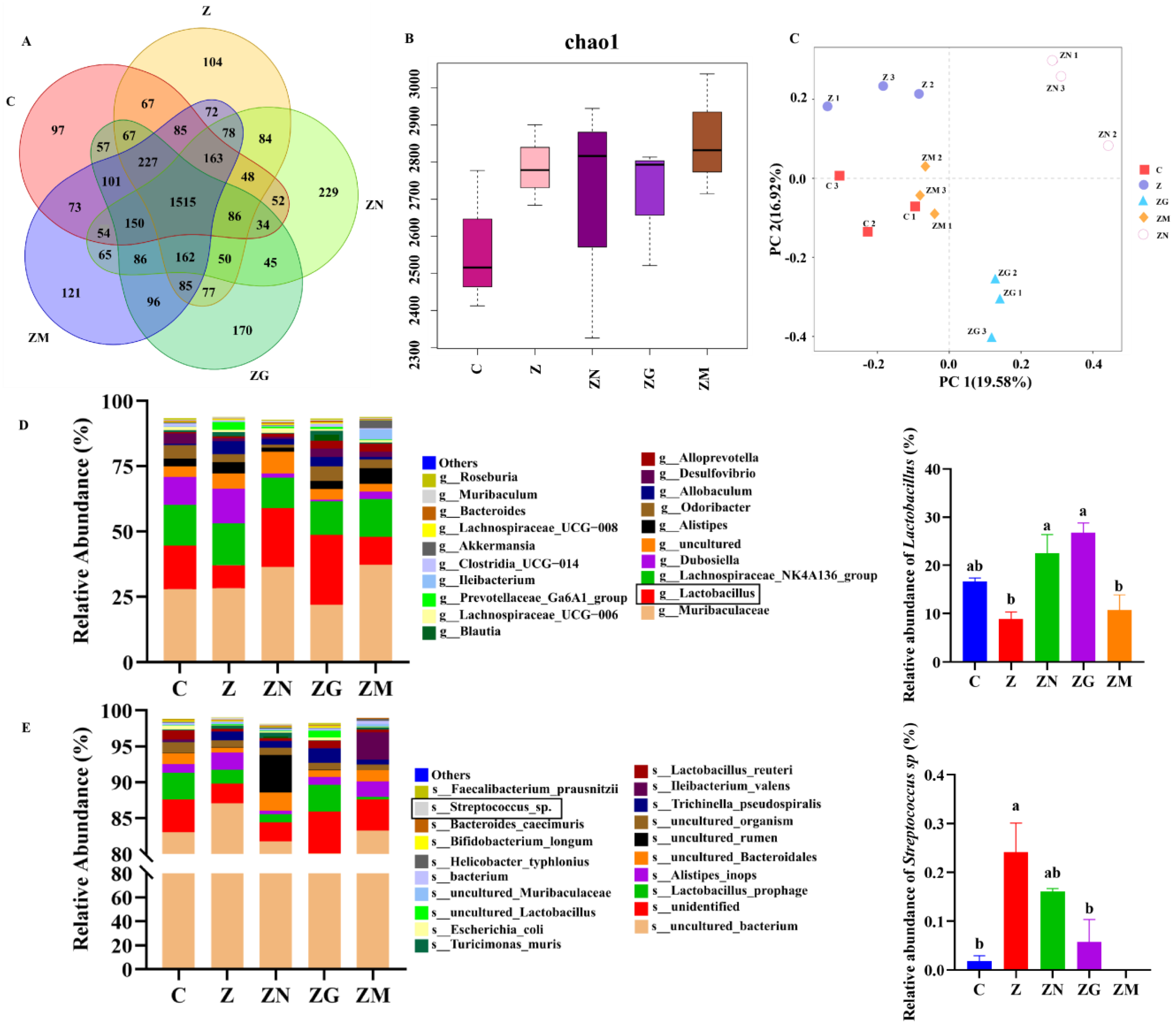
3.6. ZEN Combined with Antioxidant Treatment Indirectly Affects Slow-to-Fast Myofiber Shift by Altering the Proportion of the Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCND1 | Cyclin d1 |
| CCNB1 | Cyclin b1 |
| CDK2 | Cyclin-dependent kinase 2 |
| MYHC | Myosin heavy chain |
| MYOD1 | Myogenic differentiation 1 |
| MYOG | Myogenin |
| MYF5 | Myogenic factor 5 |
| TA | Tibialis anterior |
| GAS | Gastrocnemius muscle |
| EDL | Extensor digitorum longus |
| SOL | Soleus |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ROS | Reactive oxygen species |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| TEM | Transmission electron microscopy |
References
- Li, Z.; Ma, T.; Liu, Y.; Liu, W.; Zhao, X.; Zhang, G.; Wang, J.; Zhang, Y. Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes. Antioxidants 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kong, L.; Zhang, X.; Yu, M.; Zhu, K.; Zhao, A.; Shi, D.; Sun, Y.; Wang, J.; Shen, W.; et al. Maternal Zearalenone Exposure Affects Gut Microbiota and Follicular Development in Suckled Offspring. J. Agric. Food Chem. 2022, 70, 15570–15582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, L.; Huang, S.; Liu, Q.; Ao, X.; Lei, Y.; Ji, C.; Ma, Q. Zearalenone toxicosis on reproduction as estrogen receptor selective modulator and alleviation of zearalenone biodegradative agent in pregnant sows. J. Anim. Sci. Biotechnol. 2022, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Huang, Y.; Gu, F.; Liu, W.; Guo, Y.; Chen, H.; Wang, K.; Wu, Z.; Li, J. Zearalenone induces liver injury in mice through ferroptosis pathway. Sci. Total. Environ. 2024, 952, 175875. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Jiang, L.; Gao, X.; Liu, W.; Zhu, X.; Huang, W.; Zhao, H.; Wei, Z.; Wang, K.; et al. Baicalin protects against zearalenone-induced chicks liver and kidney injury by inhibiting expression of oxidative stress, inflammatory cytokines and caspase signaling pathway. Int. Immunopharmacol. 2021, 100, 108097. [Google Scholar] [CrossRef]
- Shirakawa, T.; Miyawaki, A.; Kawamoto, T.; Kokabu, S. Natural Compounds Attenuate Denervation-Induced Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2021, 22, 8310. [Google Scholar] [CrossRef]
- Campbell, W.G.; Gordon, S.E.; Carlson, C.J.; Pattison, J.S.; Hamilton, M.T.; Booth, F.W. Differential global gene expression in red and white skeletal muscle. Am. J. Physiol. Cell Physiol. 2001, 280, C763–C768. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef]
- Gajecka, M.; Slawuta, P.; Nicpon, J.; Kolacz, R.; Kielbowicz, Z.; Zielonka, L.; Dabrowski, M.; Szweda, W.; Gajecki, M.; Nicpon, J. Zearalenone and its metabolites in the tissues of female wild boars exposed per os to mycotoxins. Toxicon 2016, 114, 1–12. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, L.; Xu, J.; Wang, Y.; Wang, Y. Effects of moldy corn on the performance, antioxidant capacity, immune function, metabolism and residues of mycotoxins in eggs, muscle, and edible viscera of laying hens. Poult. Sci. 2023, 102, 102502. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Longobardi, C.; Ferrara, G.; Piscopo, N.; Riccio, L.; Russo, V.; Meucci, V.; De Marchi, L.; Esposito, L.; Florio, S.; et al. Oxidative Status and Histological Evaluation of Wild Boars’ Tissues Positive for Zearalenone Contamination in the Campania Region, Southern Italy. Antioxidants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Meng, Q.; Li, J.; Liu, M.; Zhang, Y.; Bi, C.; Shan, A. Modified halloysite nanotubes reduce the toxic effects of zearalenone in gestating sows on growth and muscle development of their offsprings. J. Anim. Sci. Biotechnol. 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, C.; Sharaf El Dein, O.; El Golli, E.; Abid-Essefi, S.; Brenner, C.; Lemaire, C.; Bacha, H. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 2008, 254, 19–28. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Huang, G.; He, Y.; Hong, L.; Zhou, M.; Zuo, X.; Zhao, Z. Restoration of NAD(+) homeostasis protects C2C12 myoblasts and mouse levator ani muscle from mechanical stress-induced damage. Anim. Cells Syst. 2022, 26, 192–202. [Google Scholar] [CrossRef]
- Morel, G.; Bonnet, P.; Cossec, B.; Morel, S.; Cour, C.; Lambert, A.M.; Roure, M.B.; Brondeau, M.T. The role of glutathione and cysteine conjugates in the nephrotoxicity of o-xylene in rats. Arch. Toxicol. 1998, 72, 553–558. [Google Scholar] [CrossRef]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized Glutathione as a Key Antioxidant for the Treatment of Oxidative Stress in Neurodegenerative Conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A review of its potential functions and effects on neurological diseases. Rev. Neurol. 2020, 176, 148–165. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.H.; Shi, W.; Li, P.; Guo, Y.; Guo, J.; He, J.B.; Zhang, Y. Protective effect of proanthocyanidin on mice Sertoli cell apoptosis induced by zearalenone via the Nrf2/ARE signalling pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 26724–26733. [Google Scholar] [CrossRef]
- Fu, W.; Dai, C.; Ma, Z.; Li, Q.; Lan, D.; Sun, C.; Wu, X.; Li, J.; Wang, S. Enhanced glutathione production protects against zearalenone-induced oxidative stress and ferroptosis in female reproductive system. Food Chem. Toxicol. 2024, 185, 114462. [Google Scholar] [CrossRef] [PubMed]
- Jani, N.; Ziogas, J.; Angus, J.A.; Wright, C.E. Exogenous glutathione is essential in the testing of antioxidant capacity using radical-induced haemolysis. J. Pharmacol. Toxicol. Methods 2012, 65, 142–146. [Google Scholar] [CrossRef]
- Chen, B.; You, W.; Shan, T. Myomaker, and Myomixer-Myomerger-Minion modulate the efficiency of skeletal muscle development with melatonin supplementation through Wnt/beta-catenin pathway. Exp. Cell Res. 2019, 385, 111705. [Google Scholar] [CrossRef]
- Dai, C.; Hou, M.; Yang, X.; Wang, Z.; Sun, C.; Wu, X.; Wang, S. Increased NAD(+) levels protect female mouse reproductive system against zearalenone-impaired glycolysis, lipid metabolism, antioxidant capacity and inflammation. Reprod. Toxicol. 2024, 124, 108530. [Google Scholar] [CrossRef]
- Han, B.H.; Lee, H.K.; Jang, S.H.; Tai, A.L.; Jang, Y.J.; Yoon, J.J.; Kim, H.Y.; Lee, H.S.; Lee, Y.J.; Kang, D.G. Effect of Geumgwe-Sinkihwan on Renal Dysfunction in Ischemia/Reperfusion-Induced Acute Renal Failure Mice. Nutrients 2021, 13, 3859. [Google Scholar] [CrossRef]
- Khan, S.; Adhikari, J.S.; Rizvi, M.A.; Chaudhury, N.K. Radioprotective potential of melatonin against (6)(0)Co gamma-ray-induced testicular injury in male C57BL/6 mice. J. Biomed. Sci. 2015, 22, 61. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Arneson-Wissink, P.C.; Doles, J.D. Quantification of Muscle Stem Cell Differentiation Using Live-Cell Imaging and Eccentricity Measures. Methods. Mol. Biol. 2022, 2429, 455–471. [Google Scholar]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef]
- Misra, S.; Raghuwanshi, S.; Gupta, P.; Saxena, R.K. Examine growth inhibition pattern and lactic acid production in Streptococcus mutans using different concentrations of xylitol produced from Candida tropicalis by fermentation. Anaerobe 2012, 18, 273–279. [Google Scholar] [CrossRef]
- Friman, G.; Ilback, N.G.; Beisel, W.R. Effects of Streptococcus pneumoniae, Salmonella typhimurium and Francisella tularensis infections on oxidative, glycolytic and lysosomal enzyme activity in red and white skeletal muscle in the rat. Scand. J. Infect. Dis. 1984, 16, 111–119. [Google Scholar] [CrossRef]
- Hansen, A.K.; Clausen, T.; Nielsen, O.B. Effects of lactic acid and catecholamines on contractility in fast-twitch muscles exposed to hyperkalemia. Am. J. Physiol. Cell Physiol. 2005, 289, C104–C112. [Google Scholar] [CrossRef]
- Zhang, G.L.; Song, J.L.; Ji, C.L.; Feng, Y.L.; Yu, J.; Nyachoti, C.M.; Yang, G.S. Zearalenone Exposure Enhanced the Expression of Tumorigenesis Genes in Donkey Granulosa Cells via the PTEN/PI3K/AKT Signaling Pathway. Front. Genet. 2018, 9, 293. [Google Scholar] [CrossRef]
- Zhang, G.L.; Song, J.L.; Zhou, Y.; Zhang, R.Q.; Cheng, S.F.; Sun, X.F.; Qin, G.Q.; Shen, W.; Li, L. Differentiation of sow and mouse ovarian granulosa cells exposed to zearalenone in vitro using RNA-seq gene expression. Toxicol. Appl. Pharmacol. 2018, 350, 78–90. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Cheng, Z.; Zhang, Z.; Xu, Y.; Zhang, H.; Xu, T.; Chen, J.; Yin, D.; Yan, W.; et al. Caspase-8 dependent apoptosis contributes to dyskinesia caused by muscle defects and neurotoxicity in zebrafish exposed to zearalenone. Food Chem. Toxicol. 2024, 186, 114516. [Google Scholar] [CrossRef]
- Tatay, E.; Font, G.; Ruiz, M.J. Cytotoxic effects of zearalenone and its metabolites and antioxidant cell defense in CHO-K1 cells. Food Chem. Toxicol. 2016, 96, 43–49. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Zhao, A.H.; Wang, J.J.; Tian, Y.; Yan, Z.H.; Dri, M.; Shen, W.; De Felici, M.; Li, L. Oxidative stress as a plausible mechanism for zearalenone to induce genome toxicity. Gene 2022, 829, 146511. [Google Scholar] [CrossRef]
- Salem, I.B.; Boussabbeh, M.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S.; Bacha, H. Zearalenone-induced changes in biochemical parameters, oxidative stress and apoptosis in cardiac tissue: Protective role of crocin. Hum. Exp. Toxicol. 2016, 35, 623–634. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Ma, L.; Chen, J.; Xu, J.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112737. [Google Scholar] [CrossRef]
- Ben Salem, I.; Boussabbeh, M.; Pires Da Silva, J.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. SIRT1 protects cardiac cells against apoptosis induced by zearalenone or its metabolites alpha- and beta-zearalenol through an autophagy-dependent pathway. Toxicol. Appl. Pharmacol. 2017, 314, 82–90. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, K.H.; Sun, M.H.; Lan, M.; Wan, X.; Zhang, Y.; Sun, S.C. Protective Effects of Melatonin Against Zearalenone Toxicity on Porcine Embryos in vitro. Front. Pharmacol. 2019, 10, 327. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Rudolph, T.E.; Roach, C.M.; Baumgard, L.H.; Ross, J.W.; Keating, A.F.; Selsby, J.T. The impact of Zearalenone on heat-stressed skeletal muscle in pigs. J. Anim. Sci. 2022, 100, skac215. [Google Scholar] [CrossRef]
- Huang, S.; Sun, H.; Lin, D.; Huang, X.; Chen, R.; Li, M.; Huang, J.; Guo, F. Camellia oil exhibits anti-fatigue property by modulating antioxidant capacity, muscle fiber, and gut microbial composition in mice. J. Food. Sci. 2024, 89, 2465–2481. [Google Scholar] [CrossRef]
- Zollner, P.; Jodlbauer, J.; Kleinova, M.; Kahlbacher, H.; Kuhn, T.; Hochsteiner, W.; Lindner, W. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. J. Agric. Food Chem. 2002, 50, 2494–2501. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Ferrara, G.; Montagnaro, S.; Meucci, V.; Intorre, L.; Bacci, S.; Esposito, L.; Piscopo, N.; Rubino, A.; et al. Zearalenone (ZEN) and Its Metabolite Levels in Tissues of Wild Boar (Sus scrofa) from Southern Italy: A Pilot Study. Toxins 2023, 15, 56. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue. Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Hor, Y.Y.; Ooi, C.H.; Lew, L.C.; Jaafar, M.H.; Lau, A.S.; Lee, B.K.; Azlan, A.; Choi, S.B.; Azzam, G.; Liong, M.T. The molecular mechanisms of probiotic strains in improving ageing bone and muscle of d-galactose-induced ageing rats. J. Appl. Microbiol. 2021, 130, 1307–1322. [Google Scholar] [CrossRef]
- Kupp, L.I.; Rosen, S.; Beck, F.M. Effect of anti-oxidants on growth and lactic acid production by Streptococcus mutans. J. Dent. Res. 1985, 64, 1016–1018. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, Y. Lactic acid is absorbed from the small intestine of sheep. J. Exp. Zool. A Comp. Exp. Biol. 2003, 295, 29–36. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Margier, M.; Kuehnemann, C.; Hulo, N.; Morales, J.; Ashok Kumaar, P.V.; Cros, C.; Cannelle, H.; Charmetant, J.; Verdin, E.; Canault, M.; et al. Nicotinamide Mononucleotide Administration Prevents Doxorubicin-Induced Cardiotoxicity and Loss in Physical Activity in Mice. Cells 2022, 12, 108. [Google Scholar] [CrossRef]
- Benjamin, D.I.; Brett, J.O.; Both, P.; Benjamin, J.S.; Ishak, H.L.; Kang, J.; Kim, S.; Chung, M.; Arjona, M.; Nutter, C.W.; et al. Multiomics reveals glutathione metabolism as a driver of bimodality during stem cell aging. Cell. Metab. 2023, 35, 472–486.e476. [Google Scholar] [CrossRef]
- Sokolovic, D.T.; Lilic, L.; Milenkovic, V.; Stefanovic, R.; Ilic, T.P.; Mekic, B.; Ilic, I.; Stojanovic, N.M.; Ilic, I.R. Effects of melatonin on oxidative stress parameters and pathohistological changes in rat skeletal muscle tissue following carbon tetrachloride application. Saudi. Pharm. J. 2018, 26, 1044–1050. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Length (bp) | Gene Accession Number |
|---|---|---|---|---|
| MYHC | GCGAATCGAGGCTCAGAACAA | GTAGTTCCGCCTTCGGTCTTG | 138 | XM_017314318.3 |
| MYOG | GAGACATCCCCCTATTTCTACCA | GCTCAGTCCGCTCATAGCC | 106 | NM_031189.2 |
| MYF5 | AAGGCTCCTGTATCCCCTCAC | AAGGCTCCTGTATCCCCTCAC | 213 | NM_175686.3 |
| RPL7 | TGGTTTAGGAGAGTAAGGTTGCT | TGGTTTAGGAGAGTAAGGTTGCT | 351 | XM_006529040.3 |
| MYOD1 | CCACTCCGGGACATAGACTTG | AAAAGCGCAGGTCTGGTGAG | 109 | NM_010866.2 |
| CCND1 | TAGGCCCTCAGCCTCACTC | CCACCCCTGGGATTGGTTTA | 338 | XM_006529043.2 |
| CCNB1 | CTTGCAGTGAGTGACGTAGAC | CCAGTTGTCGGAGATAAGCATAG | 94 | NM_172301.3 |
| CDK2 | CAAAGCCAAGCACGTAGAGAC | TGCACCACATATTGACTGTCC | 141 | NM_053180.2 |
| MYH7 | GAATGGCAAGACGGTGACTGTG | GAAGCGTAGCGCTCCTTGAG | 233 | gi|1698894 |
| MYH2 | ATCAACCAGCAGCTGGACACCA | TCCAGCACGAACATGTGGTGGT | 249 | gi|5360745 |
| MYH4 | ACAGACTAAAGTGAAAGCCTACAA | CACATTTTGTGATTTCTCCTGTCAC | 257 | gi|5360749 |
| MYH1 | CCAATGAAACCAAGACTCCTGG | TGCTATCGATGAACTGTCCCTC | 234 | gi|5360747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Fu, W.; Zhang, J.; Lin, Y.; Xiong, X.; Li, J.; Xiong, Y. Zearalenone Exposure Damages Skeletal Muscle Through Oxidative Stress and Is Alleviated by Glutathione, Nicotinamide Mononucleotide, and Melatonin. Antioxidants 2025, 14, 528. https://doi.org/10.3390/antiox14050528
Li D, Fu W, Zhang J, Lin Y, Xiong X, Li J, Xiong Y. Zearalenone Exposure Damages Skeletal Muscle Through Oxidative Stress and Is Alleviated by Glutathione, Nicotinamide Mononucleotide, and Melatonin. Antioxidants. 2025; 14(5):528. https://doi.org/10.3390/antiox14050528
Chicago/Turabian StyleLi, Dandan, Wei Fu, Jiyue Zhang, Yaqiu Lin, Xianrong Xiong, Jian Li, and Yan Xiong. 2025. "Zearalenone Exposure Damages Skeletal Muscle Through Oxidative Stress and Is Alleviated by Glutathione, Nicotinamide Mononucleotide, and Melatonin" Antioxidants 14, no. 5: 528. https://doi.org/10.3390/antiox14050528
APA StyleLi, D., Fu, W., Zhang, J., Lin, Y., Xiong, X., Li, J., & Xiong, Y. (2025). Zearalenone Exposure Damages Skeletal Muscle Through Oxidative Stress and Is Alleviated by Glutathione, Nicotinamide Mononucleotide, and Melatonin. Antioxidants, 14(5), 528. https://doi.org/10.3390/antiox14050528






