Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Collection
2.3. Bacteria Preparation
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Quantitative Real-Time PCR
2.6. Transcriptomic Analysis
2.7. Flow Cytometry Analysis
2.8. Data Availability
2.9. Statistical Analysis
3. Results
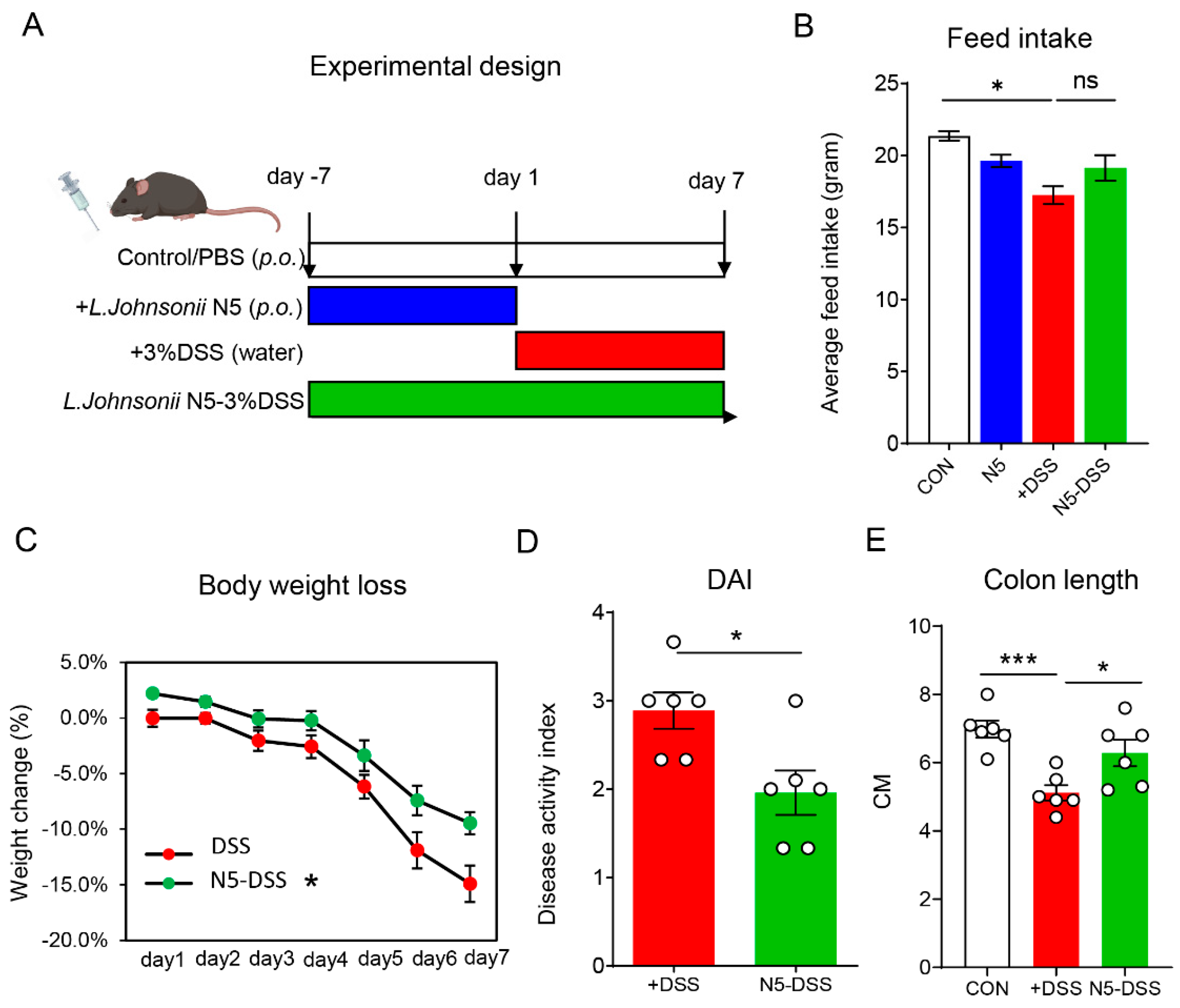
3.1. Lactobacillus johnsonii N5 Protects Against DSS-Induced Colitis in Mice
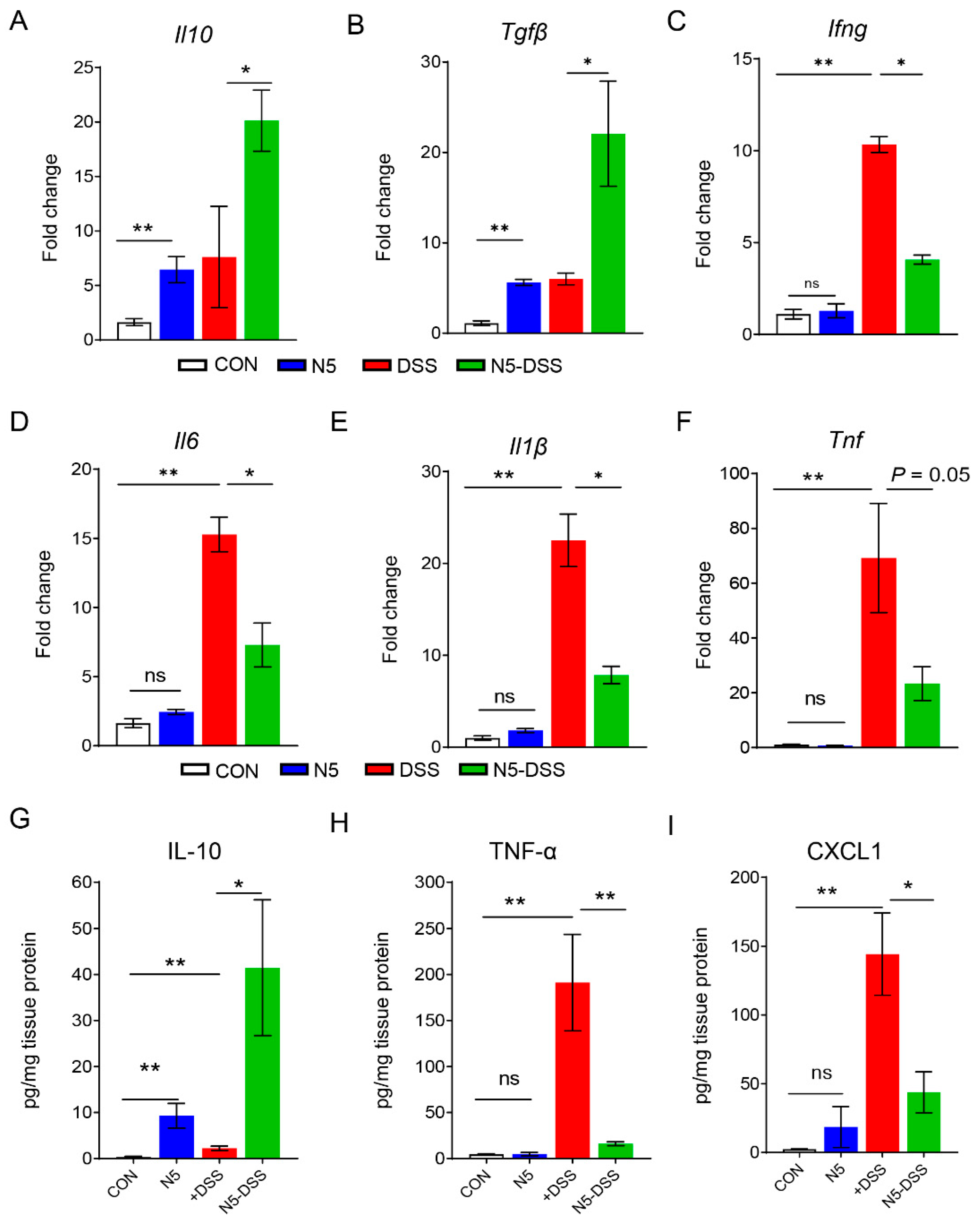
3.2. Lactobacillus johnsonii N5 Modulates Colonic Cytokine and Chemokine Levels in DSS-Induced Colitis in Mice
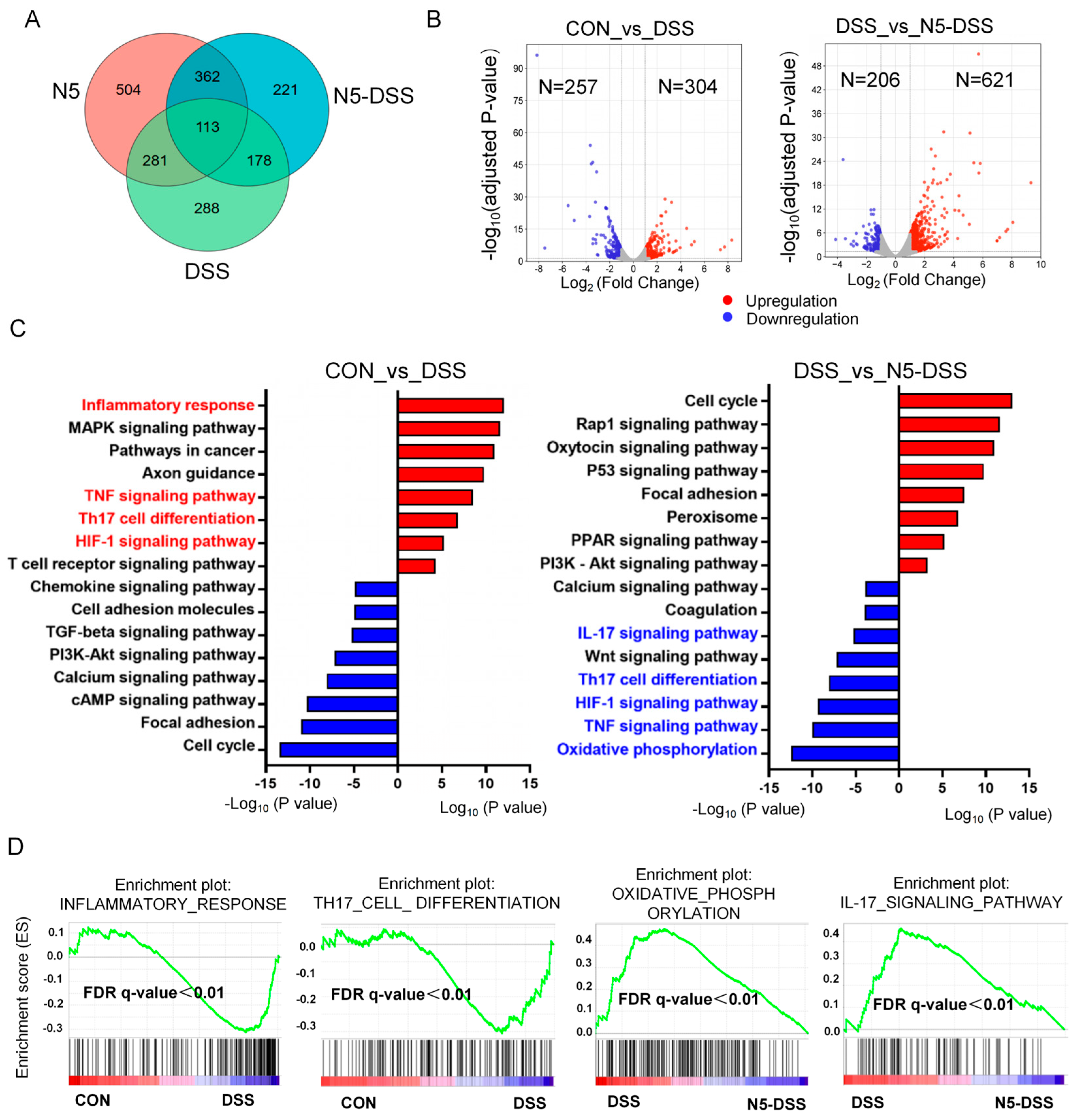
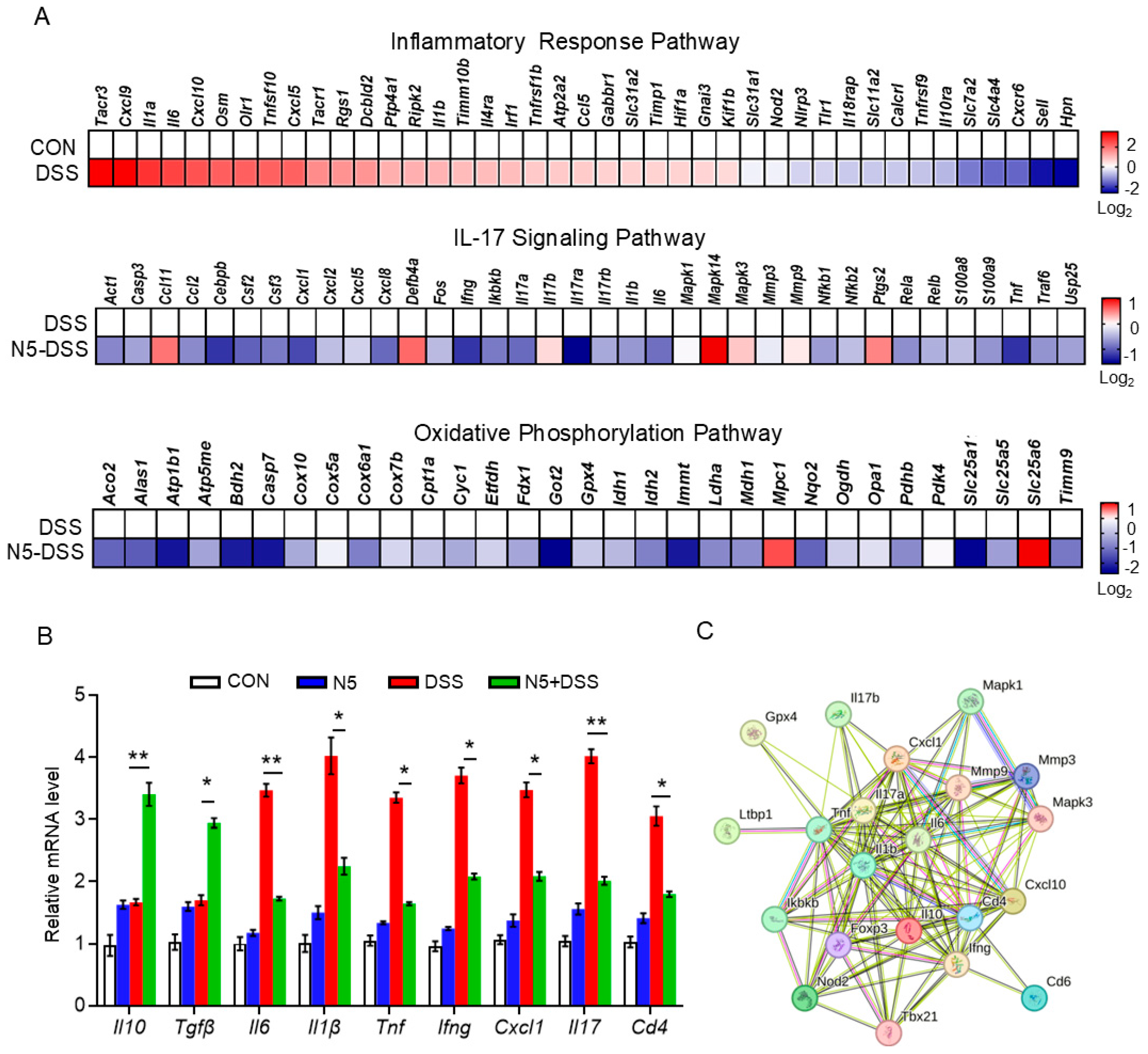
3.3. Lactobacillus johnsonii N5 Regulates the Splenic Transcriptome Against DSS-Induced Colitis in Mice
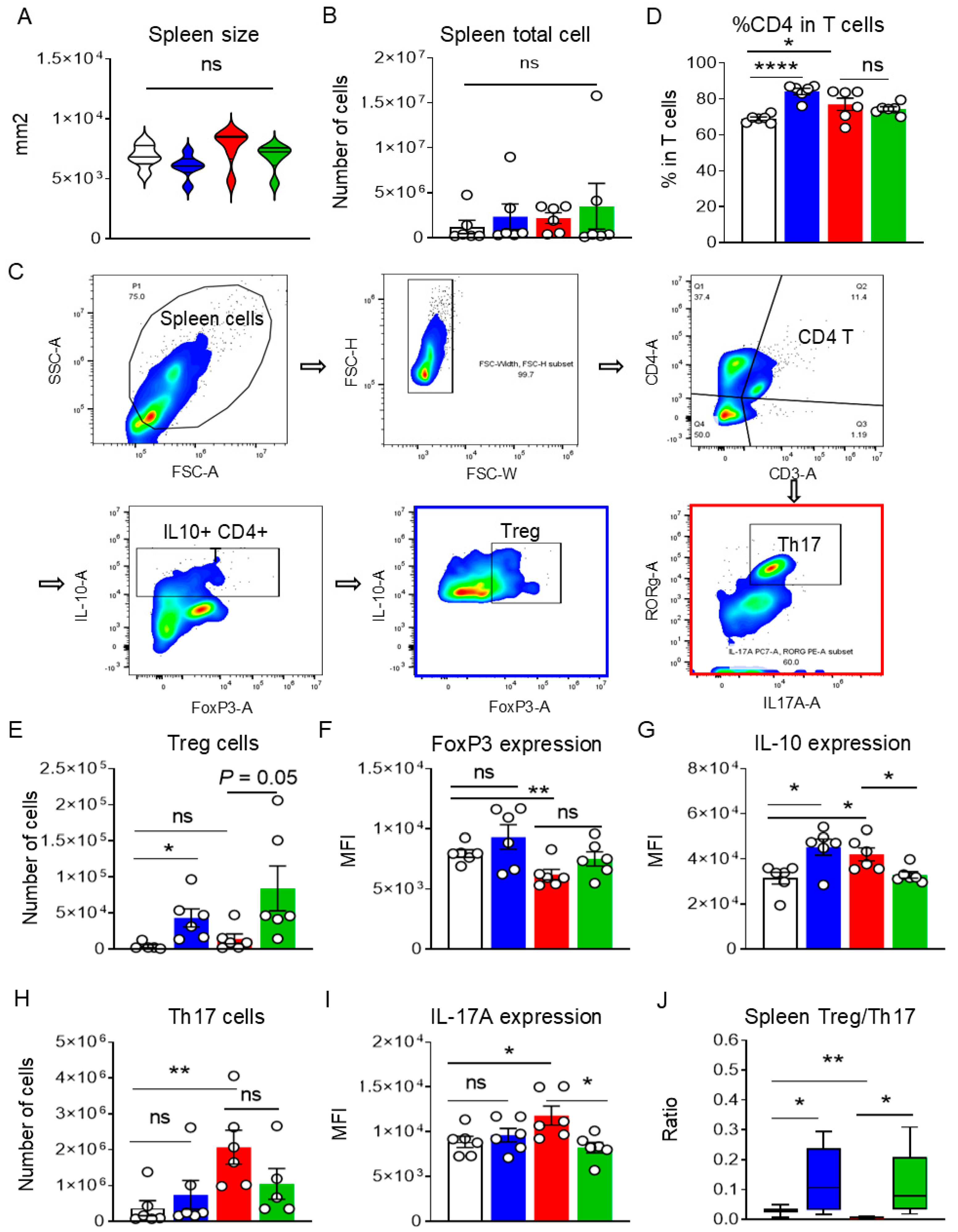
3.4. Lactobacillus johnsonii N5 Modulates the Spleen Treg/Th17 Responses Against DSS-Induced Colitis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathur, A.; McLean, M.H.; Cao, H.; Vickers, M.A. Hyposplenism and gastrointestinal diseases: Significance and mechanisms. Dig. Dis. 2022, 40, 290–298. [Google Scholar] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 10571–10584. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cai, D.; Li, Y.; Gu, H.; Qu, H.; Zong, Q.; Bao, W.; Chen, A.; Liu, H. How early-life gut microbiota alteration sets trajectories for health and inflammatory bowel disease? Front. Nutr. 2021, 8, 690073. [Google Scholar]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the microbiome—Searching the crime scene for clues. Gastroenterology 2021, 160, 524–537. [Google Scholar]
- Ahl, D.; Liu, H.; Schreiber, O.; Roos, S.; Phillipson, M.; Holm, L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016, 217, 300–310. [Google Scholar]
- Winter, S.E.; Bäumler, A.J. Gut dysbiosis: Ecological causes and causative effects on human disease. Proc. Natl. Acad. Sci. USA 2023, 120, e2316579120. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, S.; Ogamune, K.J.; Ahmed, A.A.; Kim, I.H.; Zhang, Y.; Cai, D. Fungi in the gut microbiota: Interactions, homeostasis, and host physiology. Microorganisms 2025, 13, 70. [Google Scholar] [CrossRef]
- Wu, R.; Xiong, R.; Li, Y.; Chen, J.; Yan, R. Gut microbiome, metabolome, host immunity associated with inflammatory bowel disease and intervention of fecal microbiota transplantation. J. Autoimmun. 2023, 141, 103062. [Google Scholar]
- Sartor, R.B. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: Antibiotics, probiotics, and prebiotics. Gastroenterology 2004, 126, 1620–1633. [Google Scholar]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 6570–6575. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20; quiz 21–22. [Google Scholar] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [PubMed]
- Zheng, D.; Wang, Z.; Sui, L.; Xu, Y.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; Tang, L.; et al. Lactobacillus johnsonii activates porcine monocyte derived dendritic cells maturation to modulate Th cellular immune response. Cytokine 2021, 144, 155581. [Google Scholar]
- Editorial. The next giant step for microbes. Nat. Biotechnol. 2023, 41, 1. [Google Scholar]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar]
- Heczko, P.B.; Giemza, M.; Ponikiewska, W.; Strus, M. Importance of Lactobacilli for human health. Microorganisms 2024, 12, 2382. [Google Scholar] [CrossRef]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar]
- Lin, C.; Zheng, Y.; Lu, J.; Zhang, H.; Wang, G.; Chen, W. Differential reinforcement of intestinal barrier function by various Lactobacillus reuteri strains in mice with DSS-induced acute colitis. Life Sci. 2023, 314, 121309. [Google Scholar]
- Liu, H.Y.; Gu, F.; Zhu, C.; Yuan, L.; Zhu, C.; Zhu, M.; Yao, J.; Hu, P.; Zhang, Y.; Dicksved, J.; et al. Epithelial heat shock proteins mediate the protective effects of Limosilactobacillus reuteri in dextran sulfate sodium-induced colitis. Front. Immunol. 2022, 13, 865982. [Google Scholar]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG--host interactions. Microb. Cell Factories 2014, 13 (Suppl. 11), S7. [Google Scholar]
- Yuan, L.; Zhu, C.; Gu, F.; Zhu, M.; Yao, J.; Zhu, C.; Li, S.; Wang, K.; Hu, P.; Zhang, Y.; et al. Lactobacillus johnsonii N5 from heat stress-resistant pigs improves gut mucosal immunity and barrier in dextran sodium sulfate-induced colitis. Anim. Nutr. 2023, 15, 210–224. [Google Scholar] [PubMed]
- Liu, H.Y.; Giraud, A.; Seignez, C.; Ahl, D.; Guo, F.; Sedin, J.; Walden, T.; Oh, J.; van Pijkeren, J.P.; Holm, L.; et al. Distinct B cell subsets in Peyer’s patches convey probiotic effects by Limosilactobacillus reuteri. Microbiome 2021, 9, 198. [Google Scholar]
- Thim-Uam, A.; Makjaroen, J.; Issara-Amphorn, J.; Saisorn, W.; Wannigama, D.; Chancharoenthana, W.; Leelahavanichkul, A. Enhanced bacteremia in dextran sulfate-induced colitis in splenectomy mice correlates with gut dysbiosis and LPS tolerance. Int. J. Mol. Sci. 2022, 23, 1676. [Google Scholar] [CrossRef]
- Campos Canesso, M.C.; de Castro, T.B.R.; Nakandakari-Higa, S.; Lockhart, A.; Luehr, J.; Bortolatto, J.; Parsa, R.; Esterházy, D.; Lyu, M.; Liu, T.; et al. Identification of antigen-presenting cell-T cell interactions driving immune responses to food. Science 2025, 387, eado5088. [Google Scholar]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar]
- Harbour, S.N.; Maynard, C.L.; Zindl, C.L.; Schoeb, T.R.; Weaver, C.T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl. Acad. Sci. USA 2015, 112, 7061–7066. [Google Scholar]
- Buchele, V.; Konein, P.; Vogler, T.; Kunert, T.; Enderle, K.; Khan, H.; Büttner-Herold, M.; Lehmann, C.H.K.; Amon, L.; Wirtz, S.; et al. Th17 cell-mediated colitis is positively regulated by interferon regulatory factor 4 in a T Cell-extrinsic manner. Front. Immunol. 2021, 11, 590893. [Google Scholar]
- Ohara, D.; Takeuchi, Y.; Hirota, K. Type 17 immunity: Novel insights into intestinal homeostasis and autoimmune pathogenesis driven by gut-primed T cells. Cell Mol. Immunol. 2024, 21, 1183–1200. [Google Scholar]
- Forster, S.C.; Clare, S.; Beresford-Jones, B.S.; Harcourt, K.; Notley, G.; Stares, M.D.; Kumar, N.; Soderholm, A.T.; Adoum, A.; Wong, H.; et al. Identification of gut microbial species linked with disease variability in a widely used mouse model of colitis. Nat. Microbiol. 2022, 7, 590–599. [Google Scholar] [PubMed]
- Wang, J.; Hou, Y.; Mu, L.; Yang, M.; Ai, X. Gut microbiota contributes to the intestinal and extraintestinal immune homeostasis by balancing Th17/Treg cells. Int. Immunopharmacol. 2024, 143 Pt 143, 113570. [Google Scholar] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 12769–12778. [Google Scholar]
- Wang, K.; Wang, Y.; Gu, L.; Yu, J.; Liu, Q.; Zhang, R.; Liang, G.; Chen, H.; Gu, F.; Liu, H.; et al. Characterization of probiotic properties and whole-genome analysis of Lactobacillus johnsonii N5 and N7 isolated from swine. Microorganisms 2024, 12, 672. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar]
- Muzaki, A.R.; Tetlak, P.; Sheng, J.; Loh, S.C.; Setiagani, Y.A.; Poidinger, M.; Zolezzi, F.; Karjalainen, K.; Ruedl, C. Intestinal CD103(+)CD11b(-) dendritic cells restrain colitis via IFN-γ-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. 2016, 9, 336–351. [Google Scholar]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar]
- Kedmi, R.; Najar, T.A.; Mesa, K.R.; Grayson, A.; Kroehling, L.; Hao, Y.; Hao, S.; Pokrovskii, M.; Xu, M.; Talbot, J.; et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nat. Biotechnol. 2022, 610, 737–743. [Google Scholar]
- Ma, S.; Ming, Y.; Wu, J.; Cui, G. Cellular metabolism regulates the differentiation and function of T-cell subsets. Cell Mol. Immunol. 2024, 21, 419–435. [Google Scholar]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Zhu, X.M.; Shi, Y.Z.; Cheng, M.; Wang, D.F.; Fan, J.F. Serum IL-6, IL-23 profile and Treg/Th17 peripheral cell populations in pediatric patients with inflammatory bowel disease. Pharmazie 2017, 72, 283–287. [Google Scholar]
- Wang, L.; Ray, A.; Jiang, X.; Wang, J.Y.; Basu, S.; Liu, X.; Qian, T.; He, R.; Dittel, B.N.; Chu, Y. T regulatory cells and B cells cooperate to form a regulatory loop that maintains gut homeostasis and suppresses dextran sulfate sodium-induced colitis. Mucosal Immunol. 2015, 8, 1297–1312. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S.; et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 2017, 357, 6806–6810. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Olson, S.; Kalina, W.V.; Ruthel, G.; Demmin, G.L.; Warfield, K.L.; Bavari, S.; Klaenhammer, T.R. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immun. 2023, 24, 19–29. [Google Scholar] [CrossRef]
- Shelton, C.D.; Sing, E.; Mo, J.; Shealy, N.G.; Yoo, W.; Thomas, J.; Fitz, G.N.; Castro, P.R.; Hickman, T.T.; Torres, T.P.; et al. An early-life microbiota metabolite protects against obesity by regulating intestinal lipid metabolism. Cell Host Microbe 2023, 31, 1604–1619.e10. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Park, J.; Kim, M.; Kang, S.G.; Jannasch, A.H.; Cooper, B.; Patterson, J.; Kim, C.H. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015, 8, 80–93. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Huws, S.A.; Xu, G.; Li, J.; Ren, J.; Xu, J.; Guan, L.L.; Yao, J.; Wu, S. Ileal microbial microbiome and its secondary bile acids modulate susceptibility to nonalcoholic steatohepatitis in dairy goats. Microbiome 2024, 12, 247. [Google Scholar] [CrossRef]
- Jia, L.; Wu, R.; Han, N.; Fu, J.; Luo, Z.; Guo, L.; Su, Y.; Du, J.; Liu, Y. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin. Transl. Immunol. 2020, 9, e1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Physiological characteristics of Lactobacillus casei strains and their alleviation effects against inflammatory bowel disease. J. Microbiol. Biotechnol. 2021, 31, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Tang, C.; Baba, S.; Hamada, T.; Shimazu, T.; Iwakura, Y. Ovalbumin-Induced airway inflammation is ameliorated in dectin-1-deficient mice, in which pulmonary regulatory T cells are expanded through modification of intestinal commensal bacteria. J. Immunol. 2021, 206, 1991–2000. [Google Scholar] [CrossRef] [PubMed]

| Name | Primer Sequences (5′-3′) | Accession Number |
|---|---|---|
| Il10 | F: CGGGAAGACAATAACTGCACC R: CGGTTAGCAGTATGTTGTCCA | NM_010548.2 |
| Il6 | F: AGCCCACCGGGAACGA R: GGACCGAAGGCGCTTGT | NM_031168.2 |
| Il1β | F: CCACAGACCTTCCAGGAGAATG R: GTGCAGTTCAGTGATCGTACAGG | NM_008361.4 |
| Il17 | F: TCAACCCGATTGTCCACCAT R: GAGTTTAGTCCGAAATGAGGCTG | NM_010552.3 |
| Tnfα | F: ATGAGCACTGAAAGCATGATCC R: GAGGGCTGATTAGAGAGAGGTC | NM_013693.3 |
| Ifng | F: CAGCAACAGCAAGGCGAAAAAG R: TTTCCGCTTCCTGAGGCTGGA | NM_008337.4 |
| Hif1a | F: CTATGGAGGCCAGAAGAGGGTAT R: CCCACATCAGGTGGCTCATAA | NM_010431.3 |
| Cd4 | F: TCTGGAACTGCACCGTGAC R: CCGTGATAGCTGTGCTCTGA | NM_013488.3 |
| Tgfβ | F: GGCCAGATCCTGTCCAAGC R: GTGGGTTTCCACCATTAGCAC | NM_011577.2 |
| Gapdh | F: GTCTCCTCTGACTTCAACAGCG R: ACCACCCTGTTGCTGTAGCCA | NM_008084.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-Y.; Li, S.; Ogamune, K.J.; Yuan, P.; Shi, X.; Ennab, W.; Ahmed, A.A.; Kim, I.H.; Hu, P.; Cai, D. Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis. Antioxidants 2025, 14, 433. https://doi.org/10.3390/antiox14040433
Liu H-Y, Li S, Ogamune KJ, Yuan P, Shi X, Ennab W, Ahmed AA, Kim IH, Hu P, Cai D. Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis. Antioxidants. 2025; 14(4):433. https://doi.org/10.3390/antiox14040433
Chicago/Turabian StyleLiu, Hao-Yu, Shicheng Li, Kennedy Jerry Ogamune, Peng Yuan, Xinyu Shi, Wael Ennab, Abdelkareem A. Ahmed, In Ho Kim, Ping Hu, and Demin Cai. 2025. "Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis" Antioxidants 14, no. 4: 433. https://doi.org/10.3390/antiox14040433
APA StyleLiu, H.-Y., Li, S., Ogamune, K. J., Yuan, P., Shi, X., Ennab, W., Ahmed, A. A., Kim, I. H., Hu, P., & Cai, D. (2025). Probiotic Lactobacillus johnsonii Reduces Intestinal Inflammation and Rebalances Splenic Treg/Th17 Responses in Dextran Sulfate Sodium-Induced Colitis. Antioxidants, 14(4), 433. https://doi.org/10.3390/antiox14040433







