The Western Diet and Atopic Dermatitis: The Potential Role of Nutrients, Contaminants, and Additives in Dysbiosis and Epithelial Barrier Dysfunction
Abstract
1. Introduction
2. The Exposome and Human Microbiota
Role of Diet in Microbiota Modulation
3. The Exposome and AD
Features of the Epithelial Barrier in AD
- (i)
- Filaggrin Deficiency: Filaggrin is a crucial protein involved in skin barrier formation. Loss-of-function mutations in the filaggrin gene are frequent in AD patients and can lead to a compromised barrier, increasing susceptibility to irritants and allergens. Additionally, these mutations are recognized as risk factors for bacterial and viral skin infections [21].
- (ii)
- Altered Lipid Composition: The lipid matrix in the stratum corneum is essential for barrier function. In AD, there is often a reduction in the abundance of ceramides and other lipids, leading to increased transepidermal water loss (TEWL) and skin dryness [22,23]. This dysfunction contributes to a cycle of inflammation and pruritus, significantly impacting the patient’s quality of life.
- (iii)
| GENETICS | Loss-of-function mutations in the filaggrin gene [21] | Compromised skin barrier. Increased susceptibility to irritants and allergens. Viral and bacterial infection. |
| SKIN FEATURES | Lower abundances of ceramides [22,23] | Increased TEWL. Skin dryness. Pruritus. |
| IMMUNE SYSTEM | Th1/Th2 imbalance [24,25] | Compromised skin barrier. Inflammation. |
| MICROBIOTA | Skin and gut dysbiosis [26,27] | Bacterial colonization. Inflammation. |
| ENVIRONMENT | Pollutants Temperature Humidity | Compromised skin barrier. Skin dryness. Irritation. |
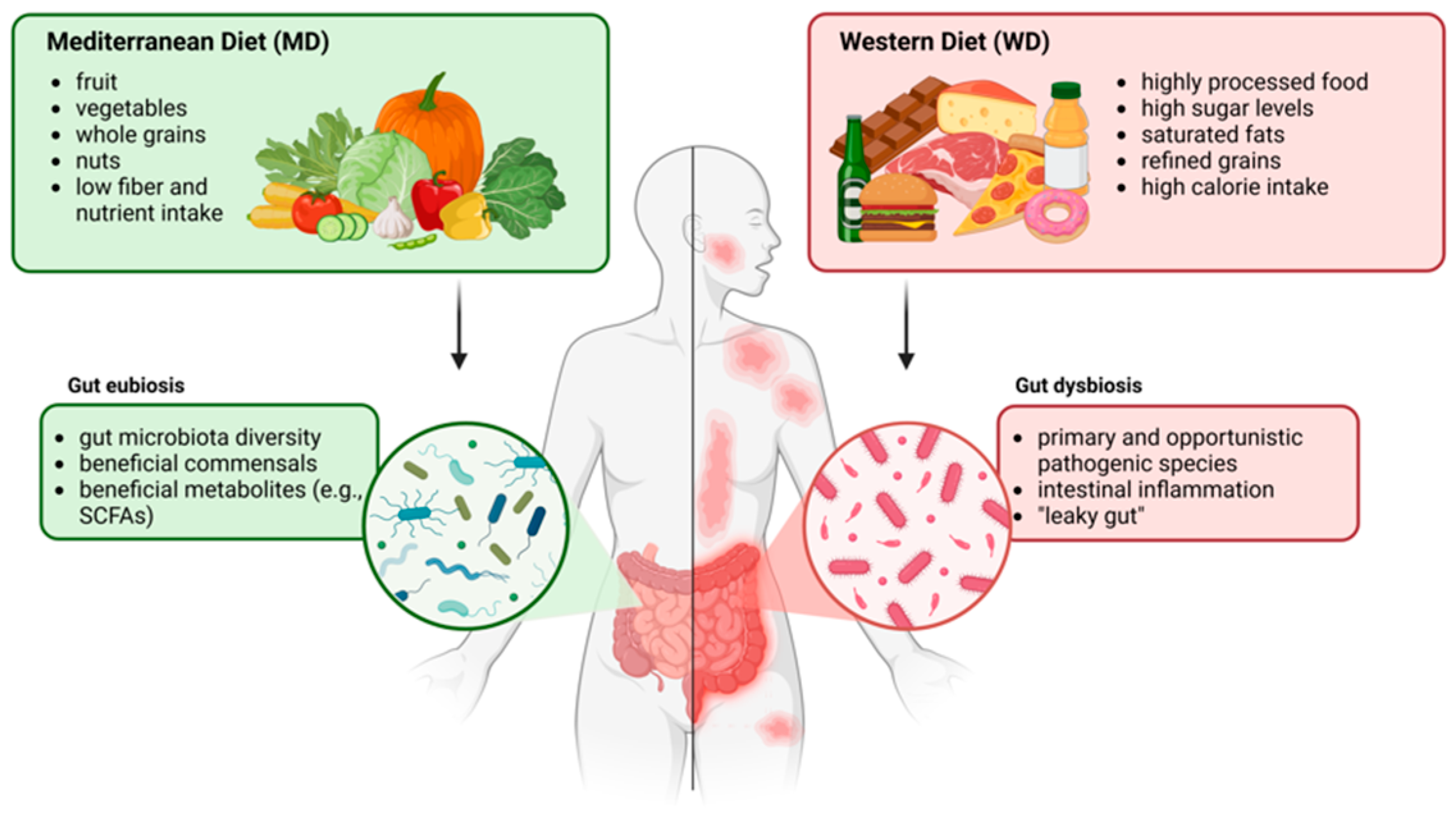
4. Role of the WD in AD
4.1. Features of a WD
4.2. Role of the Gut–Skin Axis and Microbiota
4.3. Pro-Inflammatory vs. Anti-Inflammatory Foods
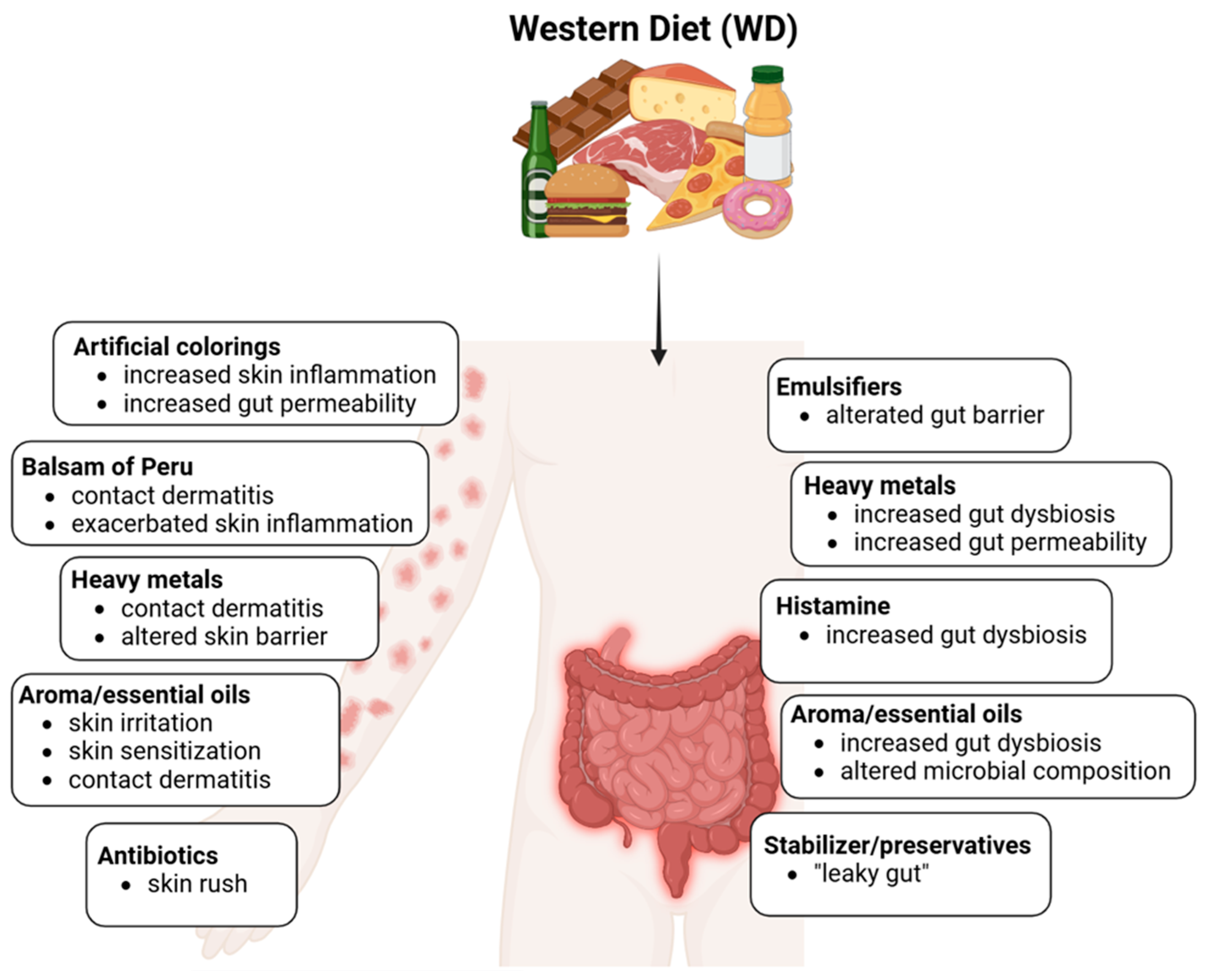
4.4. Impact of Processed Foods and Additives
4.4.1. Histamine
4.4.2. Emulsifiers
4.4.3. Stabilizers and Preservatives
4.4.4. Colorings
4.4.5. Aromatic and Essential Oils
4.4.6. Heavy Metals
4.4.7. Balsam of Peru
| INGREDIENT | COMPOSITION (%) | INGREDIENT | COMPOSITION (%) |
|---|---|---|---|
| Amyrin | α-Farnesene and β-farnesene | ||
| Aristolene | Farnesol | Traces | |
| Benzaldehyde | Ferulic acid | 0.1–0.4% | |
| Benzoic acid | 1.5–11% | Formic acid | |
| Benzyl alcohol | 1–2% | Geranyl acetone | |
| Benzyl benzoate | up to 30% | Guaiacol | |
| Benzyl cinnamate | up to 40% | Heptadecanoic acid (margaric acid) | |
| Benzyl p-coumarate (benzyl-trans-4-hydroxycinnamate) | Hexacosanoic acid (cerotic acid) | ||
| Benzyl ferulate | 1-Hexacosanol | ||
| Benzyl formate | Hexadecanoic acid (palmitic acid) | ||
| Benzyl isoferulate (cis and trans) | 0.2% | Hydroconiferyl benzoate | |
| Benzyl vanillate (benzyl 4-hydroxy-3-methoxybenzoate) | Hydroconiferyl cinnamate | ||
| cis-α-Bisabolene, β-bisabolene and cis-γ-bisabolene and trans-γ-bisabolene | Hydroxycinnamic acid | ||
| β-Caryophyllene | Isoeugenol | 0.85% in fraction BP3 | |
| 1,8-Cineole | Isoferulic acid (traces) | ||
| cis-Cinnamic acid and trans-cinnamic acid | 3–30% | Lactic acid (2-hydroxypropanoic acid) | |
| Cinnamyl alcohol | 0.4% | Limonene | |
| Cinnamyl cinnamate | 0.5% | Methoxyeugenol | |
| Coniferyl alcohol | 0.2% | Methyl benzoate | |
| cis-Coniferyl benzoate and trans-coniferyl benzoate | up to 1.5% in fresh MP | Methyl cinnamate | |
| Coniferyl cinnamate | Methyl vanillyl ketone | ||
| α-Copaene | α-Muurolene | ||
| α-Curcumene | Naphthalene | ||
| Cycloisosativene | Nerolidol | 2–7% | |
| p-β-Cymene and trans-β-cymene | allo-β-Ocimene, cis-β-ocimene and trans-β-ocimene | ||
| Docosanoic acid | 1-Octacosanol | ||
| Dodecanoic acid | Patchoulene | ||
| Eicosanoic acid (arachidic acid) | α-Phellandrene and β-phellandrene | ||
| Ethylbenzene | 1-Phenylethanol (α-methylbenzyl alcohol) | ||
| Ethyl benzoate | 3-Phenylpropanol | ||
| Ethyl cinnamate | α-Pinene and β-pinene | ||
| Ethylhexanoic acid (tentatively identified) | β-Sesquiphellandrene | ||
| Eugenol | 0.2% in fraction BP3 | Stearic acid (octadecanoic acid) | |
| Styrene | Tetradecanoic acid (myristic acid) | ||
| α-Terpinene and γ-terpinene | 1-Undecanol | ||
| 4-Terpineol (terpinen-4-ol) | Vanillic acid (4-hydroxy-3-methoxybenzoic acid) | ||
| α-Terpineol | Vanillin | 0.2–1.3% | |
| 1-Tetracosanol (lignoceryl alcohol) | p-Vinylguaiacol |
4.4.8. Antibiotics
4.5. Nutritional Deficiencies and Skin Barrier Function
4.6. Antioxidants and Skin Protection
5. Maternal Diet and AD
6. Pitfalls and Future Possibilities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.L.; Miller, G.W. The exposome and health: Where chemistry meets biology. Science 2020, 367, 392–396. [Google Scholar] [PubMed]
- Lichtenstein, P.; Holm, N.V.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [PubMed]
- Hindorff, L.A.; Sethupathy, P.; Junkins, H.A.; Ramos, E.M.; Mehta, J.P.; Collins, F.S.; Manolio, T.A. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc. Natl. Acad. Sci. USA 2009, 106, 9362–9367. [Google Scholar]
- Willett, W.C. Balancing Life-Style and Genomics Research for Disease Prevention. Science 2002, 296, 695–698. [Google Scholar]
- Rappaport, S.M.; Smith, M.T. Environment and Disease Risks. Science 2010, 330, 460–461. [Google Scholar] [CrossRef]
- Merra, G.; Gualtieri, P.; La Placa, G.; Frank, G.; Della Morte, D.; De Lorenzo, A.; Di Renzo, L. The Relationship between Exposome and Microbiome. Microorganisms 2024, 12, 1386. [Google Scholar] [CrossRef]
- Mahmud, M.d.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Wang, R.; An, Y.; Gao, W.; Bai, L.; Li, Y.; Zhao, S.; Fan, J.; Liu, E. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis. 2018, 17, 159. [Google Scholar]
- Hills, R.; Pontefract, B.; Mishcon, H.; Black, C.; Sutton, S.; Theberge, C. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on Growth and Biofilm Formation in Laboratory Media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef]
- McKenzie, C.; Silverberg, J.I. The prevalence and persistence of atopic dermatitis in urban United States children. Ann. Allergy Asthma Immunol. 2019, 123, 173–178.e1. [Google Scholar] [CrossRef]
- Celebi Sozener, Z.; Özbey Yücel, Ü.; Altiner, S.; Ozdel Oztürk, B.; Cerci, P.; Türk, M.; Gorgülü Akin, B.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The External Exposome and Allergies: From the Perspective of the Epithelial Barrier Hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar] [CrossRef]
- Khan, A.; Adalsteinsson, J.; Whitaker-Worth, D.L. Atopic dermatitis and nutrition. Clin. Dermatol. 2022, 40, 135–144. [Google Scholar] [CrossRef]
- Graham, F.; Eigenmann, P.A. Atopic dermatitis and its relation to food allergy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 305–310. [Google Scholar] [CrossRef]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond. Ann. Allergy Asthma Immunol. 2024, 132, 187–195. [Google Scholar] [CrossRef]
- Manti, S.; Amorini, M.; Cuppari, C.; Salpietro, A.; Porcino, F.; Leonardi, S.; Giudice, M.; Marseglia, G.; Caimmi, D.P.; Salpietro, C. Filaggrin mutations and Molluscum contagiosum skin infection in patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 2017, 119, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; Van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [PubMed]
- Boer, D.E.C.; Van Smeden, J.; Al-Khakany, H.; Melnik, E.; Van Dijk, R.; Absalah, S.; Van Dijk, R.; Absalah, S.; Vreeken, R.; Haenen, C.C.P.; et al. Skin of atopic dermatitis patients shows disturbed β-glucocerebrosidase and acid sphingomyelinase activity that relates to changes in stratum corneum lipid composition. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2020, 1865, 158673. [Google Scholar]
- De Benedetto, A.; Agnihothri, R.; McGirt, L.Y.; Bankova, L.G.; Beck, L.A. Atopic Dermatitis: A Disease Caused by Innate Immune Defects? J. Investig. Dermatol. 2009, 129, 14–30. [Google Scholar]
- Cesare, A.D.; Meglio, P.D.; Nestle, F.O. A Role for Th17 Cells in the Immunopathogenesis of Atopic Dermatitis? J. Investig. Dermatol. 2008, 128, 2569–2571. [Google Scholar] [CrossRef]
- Bjerre, R.D.; Holm, J.B.; Palleja, A.; Sølberg, J.; Skov, L.; Johansen, J.D. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiol. 2021, 21, 256. [Google Scholar]
- Lai, A.; Owens, K.; Patel, S.; Nicholas, M. The Impact of Air Pollution on Atopic Dermatitis. Curr. Allergy Asthma Rep. 2023, 23, 435–442. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar]
- Lee, W.; Chaudhary, F.; KAgrawal, D. Environmental Influences on Atopic Eczema. J. Environ. Sci. Public Health 2024, 8, 101–115. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.-C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar]
- Jaworowska, A.; Blackham, T.; Davies, I.G.; Stevenson, L. Nutritional challenges and health implications of takeaway and fast food. Nutr. Rev. 2013, 71, 310–318. [Google Scholar] [PubMed]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef] [PubMed]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.T.K.; Costa, S.A.; Ashe, M.; Zwicker, L.; Cawley, J.H.; Brownell, K.D. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Monir, R.L.; Schoch, J.J. Clinical Relevance of the Microbiome in Pediatric Skin Disease: A Review. Dermatol. Clin. 2022, 40, 117–126. [Google Scholar]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354. [Google Scholar]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Hrestak, D.; Matijašić, M.; Čipčić Paljetak, H.; Ledić Drvar, D.; Ljubojević Hadžavdić, S.; Perić, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Weinberg, J.M.; Yamauchi, P.S.; Patil, A.; Grabbe, S.; Goldust, M. Psoriasis: Embarking a dynamic shift in the skin microbiota. J. Cosmet. Dermatol. 2022, 21, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Buhaș, M.C.; Gavrilaș, L.I.; Candrea, R.; Cătinean, A.; Mocan, A.; Miere, D.; Tătaru, A. Gut Microbiota in Psoriasis. Nutrients 2022, 14, 2970. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, X.J.; Zhu, Y.; Yang, M.; Gu, F. Association between rosacea and helicobacter pylori infection: A meta-analysis. PLoS ONE 2024, 19, e0301703. [Google Scholar] [CrossRef]
- Zinöcker, M.; Lindseth, I. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Herieka, M.; Faraj, T.A.; Erridge, C. Reduced dietary intake of pro-inflammatory Toll-like receptor stimulants favourably modifies markers of cardiometabolic risk in healthy men. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 194–200. [Google Scholar] [CrossRef]
- Erridge, C. The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br. J. Nutr. 2011, 105, 15–23. [Google Scholar] [CrossRef]
- Park, S.; Bae, J.H. Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination Survey 2012–2013). Nutr. Res. 2016, 36, 125–133. [Google Scholar] [CrossRef]
- Unno, T.; Choi, J.H.; Hur, H.G.; Sadowsky, M.J.; Ahn, Y.T.; Huh, C.S.; Kim, G.-B.; Cha, C.-J. Changes in human gut microbiota influenced by probiotic fermented milk ingestion. J. Dairy Sci. 2015, 98, 3568–3576. [Google Scholar] [CrossRef]
- Hu, C.; Zeng, D.; Huang, Y.; Deng, Q.; Liu, S.; Zhou, W.; Zhou, W. Sodium Butyrate Ameliorates Atopic Dermatitis-Induced Inflammation by Inhibiting HDAC3-Mediated STAT1 and NF-κB Pathway. Inflammation 2024, 47, 989–1001. [Google Scholar]
- Won, T.J.; Kim, B.; Lim, Y.T.; Song, D.S.; Park, S.Y.; Park, E.S.; Lee, D.I.; Hwang, K.W. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC/Nga mice: Lactobacilli from Kimchi inhibit atopic dermatitis. J. Appl. Microbiol. 2011, 110, 1195–1202. [Google Scholar] [PubMed]
- Alves, E.; Gregório, J.; Baby, A.R.; Rijo, P.; Rodrigues, L.M.; Rosado, C. Homemade Kefir Consumption Improves Skin Condition—A Study Conducted in Healthy and Atopic Volunteers. Foods 2021, 10, 2794. [Google Scholar] [CrossRef] [PubMed]
- Gardner, K.G.; Gebretsadik, T.; Hartman, T.J.; Rosa, M.J.; Tylavsky, F.A.; Adgent, M.A.; Moore, P.E.; Kocak, M.; Bush, N.R.; Davis, R.L.; et al. Prenatal Omega-3 and Omega-6 Polyunsaturated Fatty Acids and Childhood Atopic Dermatitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 937–944. [Google Scholar]
- Li, Y.; Su, J.; Luo, D.; Duan, Y.; Huang, Z.; He, M.; Tao, J.; Xiao, S.; Xiao, Y.; Chen, X.; et al. Processed Food and Atopic Dermatitis: A Pooled Analysis of Three Cross-Sectional Studies in Chinese Adults. Front. Nutr. 2021, 8, 754663. [Google Scholar]
- Matthias, J.; Maul, J.; Noster, R.; Meinl, H.; Chao, Y.Y.; Gerstenberg, H.; Jeschke, F.; Gasparoni, G.; Welle, A.; Walter, J.; et al. Sodium chloride is an ionic checkpoint for human TH 2 cells and shapes the atopic skin microenvironment. Sci. Transl. Med. 2019, 11, eaau0683. [Google Scholar] [CrossRef]
- Sacotte, R.; Silverberg, J.I. Epidemiology of adult atopic dermatitis. Clin. Dermatol. 2018, 36, 595–605. [Google Scholar]
- Proietti, I.; Tolino, E.; Bernardini, N.; Trovato, F.; Vizzaccaro, A.V.; Skroza, N.; Potenza, C. The Role of the Western Diet on Atopic Dermatitis: Our Experience and Review of the Current Literature. Nutrients 2023, 15, 3896. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Olika Keyata, E. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 2022, 9901018. [Google Scholar]
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo De Edelenyi, F.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and nitrates from food additives and natural sources and cancer risk: Results from the NutriNet-Santé cohort. Int. J. Epidemiol. 2022, 51, 1106–1119. [Google Scholar]
- Turesky, R.J. Mechanistic Evidence for Red Meat and Processed Meat Intake and Cancer Risk: A Follow-up on the International Agency for Research on Cancer Evaluation of 2015. Chimia 2018, 72, 718. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Mostafa, M.H. N-Nitroso compounds induce changes in carcinogen-metabolizing enzymes. Cancer Lett. 1996, 106, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Gonza, I.; Goya-Jorge, E.; Douny, C.; Boutaleb, S.; Taminiau, B.; Daube, G.; Scippo, M.-L.; Delcenserie, V. Food additives impair gut microbiota from healthy individuals and IBD patients in a colonic in vitro fermentation model. Food Res. Int. 2024, 182, 114157. [Google Scholar] [CrossRef] [PubMed]
- Willmott, T.; Serrage, H.J.; Cottrell, E.C.; Humphreys, G.J.; Myers, J.; Campbell, P.M.; McBain, A.J.; Elkins, C.A. Investigating the association between nitrate dosing and nitrite generation by the human oral microbiota in continuous culture. Appl. Environ. Microbiol. 2024, 90, e02035-23. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- San Mauro Martin, I.; Brachero, S.; Garicano Vilar, E. Histamine intolerance and dietary management: A complete review. Allergol. Immunopathol. 2016, 44, 475–483. [Google Scholar] [CrossRef]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Schaper-Gerhardt, K.; Rossbach, K.; Nikolouli, E.; Werfel, T.; Gutzmer, R.; Mommert, S. The role of the histamine H4 receptor in atopic dermatitis and psoriasis. Br. J. Pharmacol. 2020, 177, 490–502. [Google Scholar] [CrossRef]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar]
- Shin, N.; Covington, M.; Bian, D.; Zhuo, J.; Bowman, K.; Li, Y.; Soloviev, M.; Qian, D.Q.; Feldman, P.; Leffet, L.; et al. INCB38579, a novel and potent histamine H4 receptor small molecule antagonist with anti-inflammatory pain and anti-pruritic functions. Eur. J. Pharmacol. 2012, 675, 47–56. [Google Scholar] [CrossRef]
- Chung, B.Y.; Cho, S.I.; Ahn, I.S.; Lee, H.B.; Kim, H.O.; Park, C.W.; Lee, C.H. Treatment of Atopic Dermatitis with a Low-histamine Diet. Ann. Dermatol. 2011, 23 (Suppl. S1), S91. [Google Scholar] [PubMed]
- Son, J.H.; Chung, B.Y.; Kim, H.O.; Park, C.W. A Histamine-Free Diet Is Helpful for Treatment of Adult Patients with Chronic Spontaneous Urticaria. Ann. Dermatol. 2018, 30, 164. [Google Scholar] [PubMed]
- Sánchez-Pérez, S.; Comas-Basté, O.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef] [PubMed]
- Van Bever, H.P.; Docx, M.; Stevens, W.J. Food and food additives in severe atopic dermatitis. Allergy 1989, 44, 588–594. [Google Scholar]
- Food and Agriculture Organization; World Health Organization. CODEX Alimentarius: International Food Standards. Class Names and the International Numbering System for Food Additives. Available online: https://www.fao.org/fao-who-codexalimentarius/%20GSFA-online%20/Functional-Classes/en/ (accessed on 21 March 2025).
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar]
- Chassaing, B.; Van De Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar]
- Oscarsson, E.; Lindberg, T.; Zeller, K.S.; Lindstedt, M.; Agardh, D.; Håkansson, Å.; Östbring, K. Changes in Intestinal Permeability Ex Vivo and Immune Cell Activation by Three Commonly Used Emulsifiers. Molecules 2020, 25, 5943. [Google Scholar] [CrossRef]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome 2021, 9, 66. [Google Scholar]
- Singh, S.; Olayinka, O.T.; Fr, J.; Nisar, M.R.; Kotha, R.; Saad-Omer, S.I.; Nath, T.S. Food Additives’ Impact on Gut Microbiota and Metabolic Syndrome: A Systematic Review. Cureus 2024, 16, e66822. [Google Scholar] [CrossRef]
- Worm; Ehlers; Sterry; Zuberbier. Clinical relevance of food additives in adult patients with atopic dermatitis. Clin. Exp. Allergy 2000, 30, 407–414. [Google Scholar] [CrossRef]
- Zaknun, D.; Schroecksnadel, S.; Kurz, K.; Fuchs, D. Potential Role of Antioxidant Food Supplements, Preservatives and Colorants in the Pathogenesis of Allergy and Asthma. Int. Arch. Allergy Immunol. 2012, 157, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Elder, R.; Vancuren, S.J.; Botschner, A.J.; Josephy, P.D.; Allen-Vercoe, E. Metabolism of azo food dyes by bacterial members of the human gut microbiome. Anaerobe 2023, 83, 102783. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Sharmeen, J.; Mahomoodally, F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Sato, H.; Kato, K.; Koreishi, M.; Nakamura, Y.; Tsujino, Y.; Satoh, A. Aromatic oil from lavender as an atopic dermatitis suppressant. PLoS ONE 2024, 19, e0296408. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, S.D.; Kim, M.; Mony, T.J.; Lee, E.S.; Kim, K.M.; Choi, S.H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sapkota, A.; Bae, Y.J.; Choi, S.H.; Bae, H.J.; Kim, H.J.; Cho, Y.E.; Choi, Y.Y.; An, J.Y.; Cho, S.Y.; et al. The Anti-Atopic Dermatitis Effects of Mentha arvensis Essential Oil Are Involved in the Inhibition of the NLRP3 Inflammasome in DNCB-Challenged Atopic Dermatitis BALB/c Mice. Int. J. Mol. Sci. 2023, 24, 7720. [Google Scholar] [CrossRef]
- Posadzki, P.; Alotaibi, A.; Ernst, E. Adverse effects of aromatherapy: A systematic review of case reports and case series. Int. J. Risk Saf. Med. 2012, 24, 147–161. [Google Scholar] [CrossRef]
- Lalko, J.; Api, A.M. Investigation of the dermal sensitization potential of various essential oils in the local lymph node assay. Food Chem. Toxicol. 2006, 44, 739–746. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Teffera, M.; Veith, A.C.; Ronnekleiv-Kelly, S.; Bradfield, C.A.; Nikodemova, M.; Tussing-Humphreys, L.; Malecki, K. Diverse mechanisms by which chemical pollutant exposure alters gut microbiota metabolism and inflammation. Environ. Int. 2024, 190, 108805. [Google Scholar] [PubMed]
- Porru, S.; Esplugues, A.; Llop, S.; Delgado-Saborit, J.M. The effects of heavy metal exposure on brain and gut microbiota: A systematic review of animal studies. Environ. Pollut. 2024, 348, 123732. [Google Scholar] [PubMed]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar]
- Callewaert, C.; Ravard Helffer, K.; Lebaron, P. Skin Microbiome and its Interplay with the Environment. Am. J. Clin. Dermatol. 2020, 21, 4–11. [Google Scholar] [CrossRef]
- Bist, P.; Choudhary, S. Impact of Heavy Metal Toxicity on the Gut Microbiota and Its Relationship with Metabolites and Future Probiotics Strategy: A Review. Biol. Trace Elem. Res. 2022, 200, 5328–5350. [Google Scholar]
- Yao, J.; Du, Z.; Yang, F.; Duan, R.; Feng, T. The relationship between heavy metals and metabolic syndrome using machine learning. Front. Public Health 2024, 12, 1378041. [Google Scholar]
- Chi, L.; Bian, X.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The Effects of an Environmentally Relevant Level of Arsenic on the Gut Microbiome and Its Functional Metagenome. Toxicol. Sci. 2017, 160, 193–204. [Google Scholar] [CrossRef]
- Brabec, J.L.; Wright, J.; Ly, T.; Wong, H.T.; McClimans, C.J.; Tokarev, V.; Lamandella, R.; Sherchand, S.; Shrestha, D.; Uprety, S.; et al. Arsenic disturbs the gut microbiome of individuals in a disadvantaged community in Nepal. Heliyon 2020, 6, e03313. [Google Scholar]
- Li, X.; Brejnrod, A.D.; Ernst, M.; Rykær, M.; Herschend, J.; Olsen, N.M.C.; Dorrestein, P.C.; Rensing, C.; Sørensen, S. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ. Int. 2019, 126, 454–467. [Google Scholar]
- Gao, B.; Chi, L.; Mahbub, R.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol. 2017, 30, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Chavatte, L.; Juan, M.; Mounicou, S.; Leblanc Noblesse, E.; Pays, K.; Nizard, C.; Bulteau, A.L. Elemental and molecular imaging of human full thickness skin after exposure to heavy metals. Metallomics 2020, 12, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Perfetto, B.; Lamberti, M.; Giuliano, M.T.; Canozo, N.; Cammarota, M.; Baroni, A. Analysis of the signal transduction pathway of nickel-induced matrix metalloproteinase-2 expression in the human keratinocytes in vitro: Preliminary findings. J. Cutan. Pathol. 2007, 34, 441–447. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Menné, T. Metal Allergy—A Review on Exposures, Penetration, Genetics, Prevalence, and Clinical Implications. Chem. Res. Toxicol. 2010, 23, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Deng, D.; Roffel, S.; Gibbs, S. Differential influence of Streptococcus mitis on host response to metals in reconstructed human skin and oral mucosa. Contact Dermat. 2020, 83, 347–360. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, Y.; Niu, Z.; Li, X. Effects of cadmium and diethylhexyl phthalate on skin microbiota of Rana chinensis tadpoles. Environ. Sci. Pollut. Res. 2023, 30, 64285–64299. [Google Scholar] [CrossRef]
- Ho, J.C.; Wen, H.J.; Sun, C.W.; Tsai, S.F.; Su, P.H.; Chang, C.L.; Sun, H.L.; Wang, S.L.; Lee, C.H. Prenatal exposure to nickel and atopic dermatitis at age 3 years: A birth cohort study with cytokine profiles. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 2414–2422. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, K.S.; Ha, E.H.; Park, H.; Ha, M.; Hong, Y.C.; Lee, S.J.; Lee, K.Y.; Jeong, J.; Kim, Y. Association between Prenatal Exposure to Cadmium and Atopic Dermatitis in Infancy. J. Korean Med. Sci. 2013, 28, 516. [Google Scholar] [CrossRef]
- Pesce, G.; Sesé, L.; Calciano, L.; Travert, B.; Dessimond, B.; Maesano, C.N.; Ferrante, G.; Huel, G.; Prud’homme, J.; Guinot, M.; et al. Foetal exposure to heavy metals and risk of atopic diseases in early childhood. Pediatr. Allergy Immunol. 2021, 32, 242–250. [Google Scholar] [CrossRef]
- Hausen, B.M. Contact allergy to balsam of Peru. II. Patch test results in 102 patients with selected balsam of Peru constituents. Am. J. Contact Dermat. Off. J. Am. Contact Dermat. Soc. 2001, 12, 93–102. [Google Scholar]
- Herro, E.M.; Jacob, S.E. Systemic Contact Dermatitis—Kids and Ketchup. Pediatr. Dermatol. 2013, 30, e32–e33. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; de Jong, H. Monographs in Contact Allergy; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- De Groot, A.C. Myroxylon pereirae resin (balsam of Peru)—A critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermat. 2019, 80, 335–353. [Google Scholar]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [PubMed]
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar]
- Fujii, M.; Nakashima, H.; Tomozawa, J.; Shimazaki, Y.; Ohyanagi, C.; Kawaguchi, N.; Ohya, S.; Kohno, S.; Nabe, T. Deficiency of n-6 polyunsaturated fatty acids is mainly responsible for atopic dermatitis-like pruritic skin inflammation in special diet-fed hairless mice. Exp. Dermatol. 2013, 22, 272–277. [Google Scholar]
- Trikamjee, T.; Comberiati, P.; D’Auria, E.; Peroni, D.; Zuccotti, G.V. Nutritional Factors in the Prevention of Atopic Dermatitis in Children. Front. Pediatr. 2021, 8, 577413. [Google Scholar]
- Foster, R.H.; Hardy, G.; Alany, R.G. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718. [Google Scholar]
- Daniluk, U.; Filimoniuk, A.; Kowalczuk-Krystoń, M.; Alifier, M.; Karpińska, J.; Kaczmarski, M.G.; Lebensztejn, D.M. Association of antioxidants and vitamin D level with inflammation in children with atopic dermatitis. Int. J. Dermatol. 2019, 58, 1056–1061. [Google Scholar]
- Wang, S.S.; Hon, K.L.; Kong, A.P.; Pong, H.N.; Wong, G.W.; Leung, T.F. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr. Allergy Immunol. 2014, 25, 30–35. [Google Scholar]
- Xiang, J.; Wang, H.; Li, T. Comorbidity of Vitamin A and Vitamin D Deficiency Exacerbates the Severity of Atopic Dermatitis in Children. Dermatology 2019, 235, 196–204. [Google Scholar] [PubMed]
- Roche, F.C.; Harris-Tryon, T.A. Illuminating the Role of Vitamin A in Skin Innate Immunity and the Skin Microbiome: A Narrative Review. Nutrients 2021, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Droitcourt, C.; Arellano, J. Atopic dermatitis and vitamin D supplementation: The end of the story? J. Eur. Acad. Dermatol. Venereol. 2024, 38, 1677–1678. [Google Scholar] [PubMed]
- Kim, S.; Lee, S.I.; Kang, S.S. Nutritional Intervention for a Korean Adolescent with Atopic Dermatitis: A Case Report. Clin. Nutr. Res. 2021, 10, 364. [Google Scholar] [CrossRef]
- Januszewski, J.; Forma, A.; Zembala, J.; Flieger, M.; Tyczyńska, M.; Dring, J.C.; Dudek, I.; Świątek, K.; Baj, J. Nutritional Supplements for Skin Health—A Review of What Should Be Chosen and Why. Medicina 2023, 60, 68. [Google Scholar] [CrossRef]
- Plevnik Kapun, A.; Salobir, J.; Levart, A.; Tavčar Kalcher, G.; Nemec Svete, A.; Kotnik, T. Vitamin E supplementation in canine atopic dermatitis: Improvement of clinical signs and effects on oxidative stress markers. Vet. Rec. 2014, 175, 560. [Google Scholar]
- Teo, C.W.L.; Tay, S.H.Y.; Tey, H.L.; Ung, Y.W.; Yap, W.N. Vitamin E in Atopic Dermatitis: From Preclinical to Clinical Studies. Dermatology 2021, 237, 553–564. [Google Scholar]
- Wang, S.; Dan, W.; Wang, Z.; Sun, Y.; Zhang, G. Causal relationships between dietary antioxidant vitamin intake and atopic dermatitis: A two-sample Mendelian randomization study. Skin Res. Technol. 2024, 30, e13883. [Google Scholar]
- Das, P.; Babaei, P.; Nielsen, J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genom. 2019, 20, 208. [Google Scholar]
- Magnúsdóttir, S.; Ravcheev, D.; De Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Steinert, R.E.; Lee, Y.K.; Sybesma, W. Vitamins for the Gut Microbiome. Trends Mol. Med. 2020, 26, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Chesini Ms, D.; Caminati, M.M. Vitamin B12 and Atopic Dermatitis: Any Therapeutic Relevance For Oral Supplementation? J. Diet. Suppl. 2022, 19, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.A.; Esterhuizen, T.M.; Khumalo, N.P.; Stein, D.J. Investigating hair zinc concentrations in children with and without atopic dermatitis. S. Afr. Med. J. Suid-Afr. Tydskr. Vir. Geneeskd. 2020, 110, 409–415. [Google Scholar] [CrossRef]
- Eriksen, B.B.; Kåre, D.L. Open trial of supplements of omega 3 and 6 fatty acids, vitamins and minerals in atopic dermatitis. J. Dermatol. Treat. 2006, 17, 82–85. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, S.; Jeong, M.; Ko, J.; Ro, Y. Hair Zinc Levels and the Efficacy of Oral Zinc Supplementation in Patients with Atopic Dermatitis. Acta Derm. Venereol. 2014, 94, 558–562. [Google Scholar] [CrossRef]
- Poljšak, B.; Fink, R. The Protective Role of Antioxidants in the Defence against ROS/RNS-Mediated Environmental Pollution. Oxid. Med. Cell. Longev. 2014, 2014, 671539. [Google Scholar] [CrossRef]
- Takahashi, N.; Kake, T.; Hasegawa, S.; Imai, M. Effects of Post-administration of β-Carotene on Diet-induced Atopic Dermatitis in Hairless Mice. J. Oleo Sci. 2019, 68, 793–802. [Google Scholar] [CrossRef]
- Hiragun, M.; Hiragun, T.; Oseto, I.; Uchida, K.; Yanase, Y.; Tanaka AOkame, T.; Ishikawa, S.; Mihara, S.; Hide, M. Oral administration of β-carotene or lycopene prevents atopic dermatitis-like dermatitis in HR-1 mice. J. Dermatol. 2016, 43, 1188–1192. [Google Scholar] [CrossRef]
- Rosenlund, H.; Kull, I.; Pershagen, G.; Wolk, A.; Wickman, M.; Bergström, A. Fruit and vegetable consumption in relation to allergy: Disease-related modification of consumption? J. Allergy Clin. Immunol. 2011, 127, 1219–1225. [Google Scholar] [CrossRef]
- Fsadni, C.; Fsadni, P.; Montefort, S.; Fava, S. Food consumption and the risk of childhood allergy. Asia Pac. Allergy 2018, 8, e35. [Google Scholar] [CrossRef] [PubMed]
- Kouda, K.; Tanaka, T.; Kouda, M.; Takeuchi, H.; Takeuchi, A.; Nakamura, H.; Takigawa, M. Low-Energy Diet in Atopic Dermatitis Patients. Clinical Findings and DNA Damage. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 225–228. [Google Scholar]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant Intake in Pregnancy in Relation to Wheeze and Eczema in the First Two Years of Life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [PubMed]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Król, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Świrta, E.; Kałużny, P.; Hanke, W.; Bal-Gierańczyk, K.; et al. Maternal diet during pregnancy and risk of allergic diseases in children up to 7–9 years old from Polish Mother and Child Cohort study. Environ. Res. 2022, 208, 112682. [Google Scholar]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Georghegan, N.; Ruparella, A.; Devani, P.; Trivella, M.; et al. Diet during pregnancy and infancy and risk of allergic or autoimmune disease: A systematic review and meta-analysis. PLoS Med. 2018, 15, e1002507. [Google Scholar] [CrossRef]
- Dong, Y.; Lau, H.X.; Suaini, N.H.A.; Kee, M.Z.L.; Ooi, D.S.Q.; Shek, L.P.; Lee, B.W.; Godfrey, K.M.; Tham, E.H.; Ong, M.E.H.; et al. A machine-learning exploration of the exposome from preconception in early childhood atopic eczema, rhinitis and wheeze development. Environ. Res. 2024, 250, 118523. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boggio, C.M.T.; Veronese, F.; Armari, M.; Zavattaro, E.; Esposto, E.; Savoia, P.; Azzimonti, B. The Western Diet and Atopic Dermatitis: The Potential Role of Nutrients, Contaminants, and Additives in Dysbiosis and Epithelial Barrier Dysfunction. Antioxidants 2025, 14, 386. https://doi.org/10.3390/antiox14040386
Boggio CMT, Veronese F, Armari M, Zavattaro E, Esposto E, Savoia P, Azzimonti B. The Western Diet and Atopic Dermatitis: The Potential Role of Nutrients, Contaminants, and Additives in Dysbiosis and Epithelial Barrier Dysfunction. Antioxidants. 2025; 14(4):386. https://doi.org/10.3390/antiox14040386
Chicago/Turabian StyleBoggio, Chiara Maria Teresa, Federica Veronese, Marta Armari, Elisa Zavattaro, Elia Esposto, Paola Savoia, and Barbara Azzimonti. 2025. "The Western Diet and Atopic Dermatitis: The Potential Role of Nutrients, Contaminants, and Additives in Dysbiosis and Epithelial Barrier Dysfunction" Antioxidants 14, no. 4: 386. https://doi.org/10.3390/antiox14040386
APA StyleBoggio, C. M. T., Veronese, F., Armari, M., Zavattaro, E., Esposto, E., Savoia, P., & Azzimonti, B. (2025). The Western Diet and Atopic Dermatitis: The Potential Role of Nutrients, Contaminants, and Additives in Dysbiosis and Epithelial Barrier Dysfunction. Antioxidants, 14(4), 386. https://doi.org/10.3390/antiox14040386












