How Does HDL Participate in Atherogenesis? Antioxidant Activity Versus Role in Reverse Cholesterol Transport
Abstract
1. Introduction
2. The Oxidant Theory of Atherosclerosis
3. How HDL Protects Against Atherosclerosis
3.1. Participation in Reverse Cholesterol Transport
3.2. Impeding the Structural Modification of LDL by Oxidation and Glycation
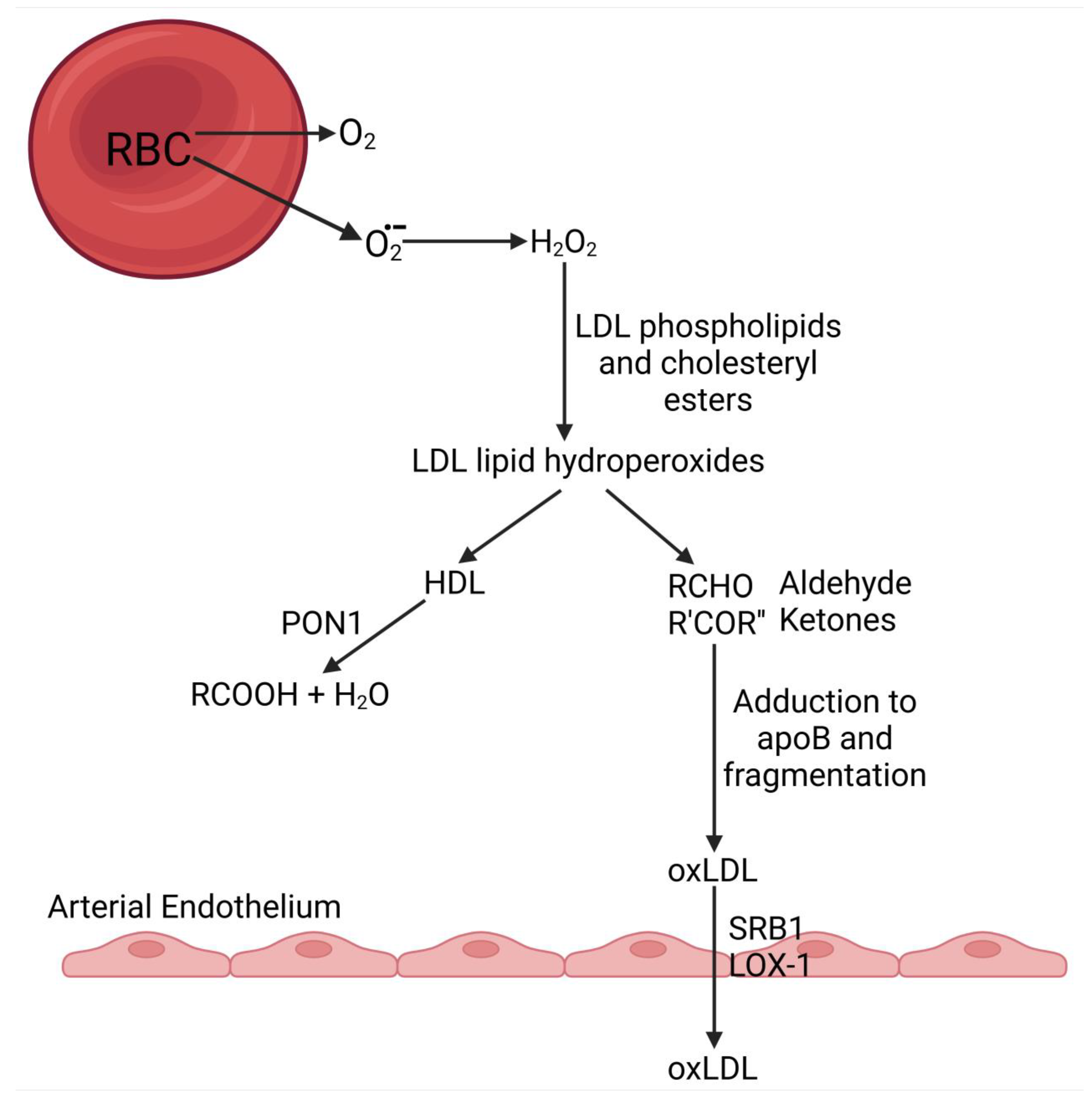
3.2.1. Source of Reactive Oxygen Species (ROS) in the Circulation
3.2.2. Oxidative Modification of LDL in the Circulation
3.2.3. Oxidative Modification of LDL in the Tissues
3.2.4. Lipoprotein (a)
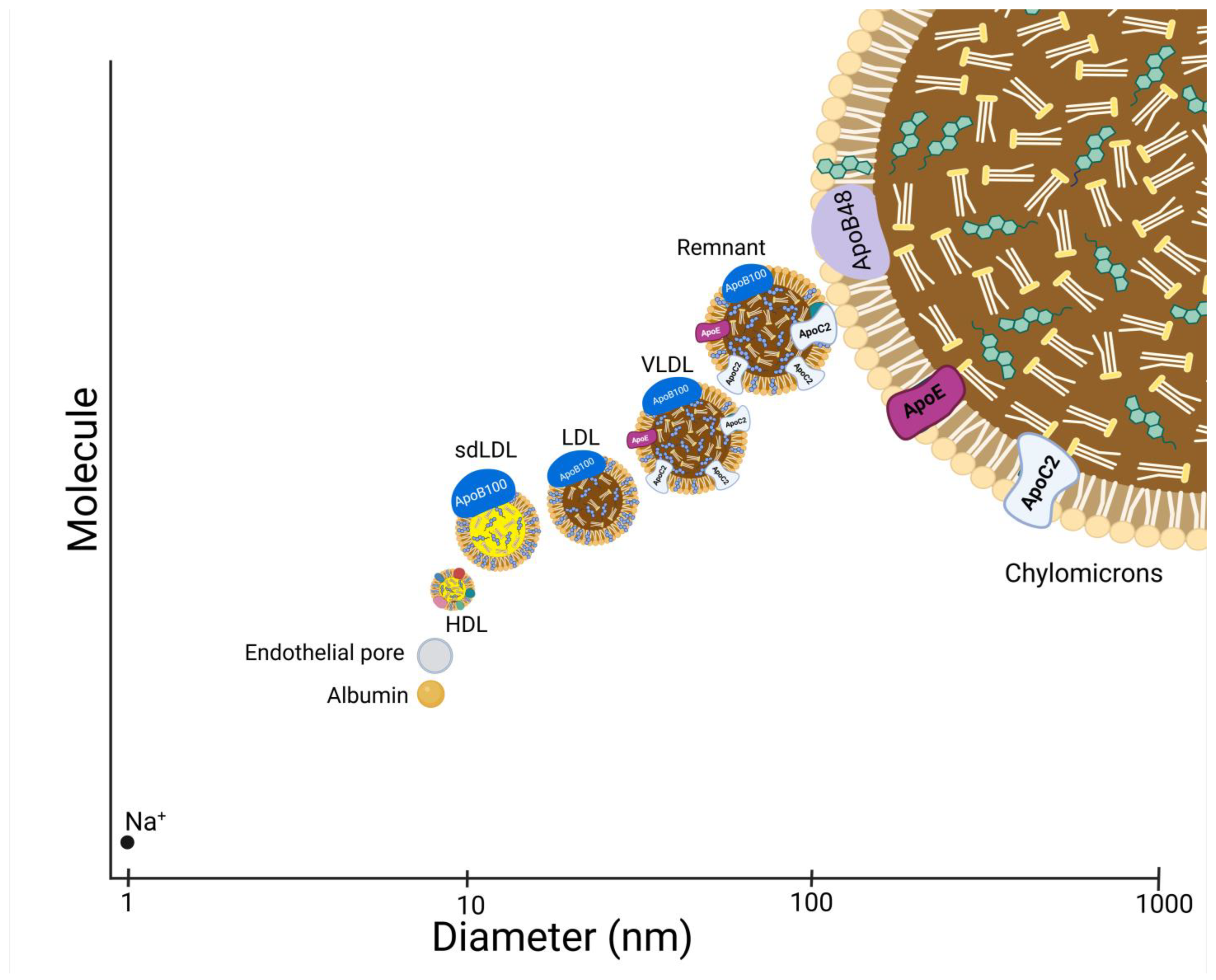
3.2.5. Small, Dense LDL
3.2.6. LDL Glycation
3.3. Mechanism by Which the Antioxidative Action of HDL Protects Against Atherogenesis
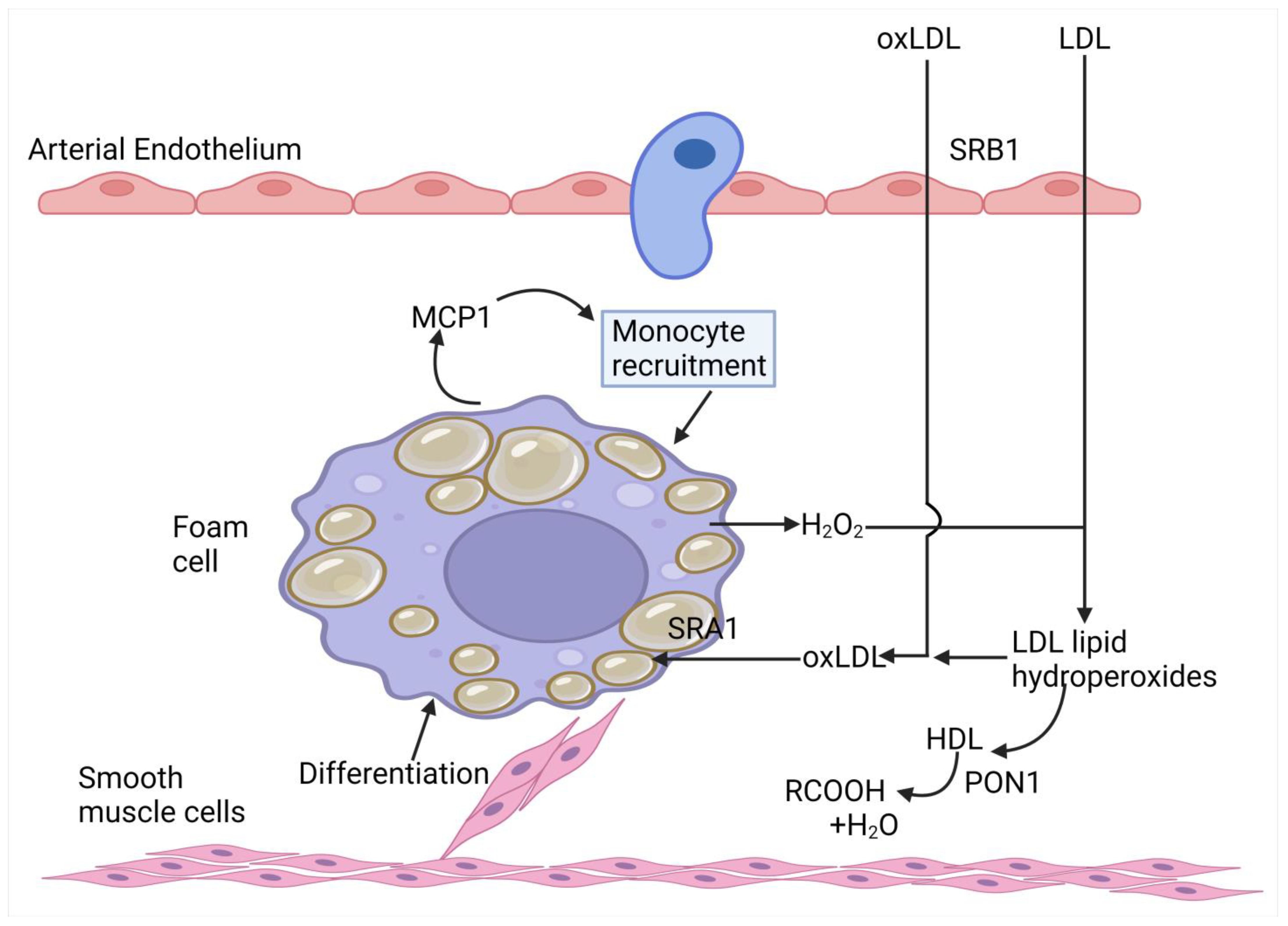
3.3.1. Impeding the Passage of Atherogenic Lipoproteins Across the Arterial Endothelium
3.3.2. Impeding Foam Cell Formation in the Arterial Wall
3.4. Antioxidant Components of HDL
4. PON1 and ASCVD Causality
- Serum PON1 activity varies greatly throughout the animal kingdom. Birds lack serum paraoxonase activity, whereas humans have substantial amounts, and rabbits, for example, even more. Human HDL protects LDL against oxidative modification, avian HDL fails to do so [155].
5. Objections to PON1 Being Essential for the Antioxidative, Antiglycative Role of HDL
- There are those that focus on the role of HDL in RCT in which so much research has been invested. They tend to dismiss the effect of HDL in decreasing LDL oxidation as due to the hydrolytic activity of PAFAH present on HDL [14,162,163,164]. Undoubtedly, HDL has PAFAH activity, and proteomic studies show PAFAH itself to be present in HDL. However, the majority of the PAFAH activity of HDL (not so LDL) is due to PON1. Furthermore, the effect of both whole HDL and partially purified PON1 in protecting LDL against oxidative modification is unaffected by inhibitors of PAFAH [39].
- PON1 when highly purified loses its capacity to protect LDL against oxidation, therefore the effect of less highly purified PON1 is due to contamination by another enzyme truly responsible [165]. PON1 has hydrophobic domains and requires a lipid environment for its activity towards lipophilic substrates. It is difficult to maintain this lipid environment whilst achieving the final stage of purification, whether of PON1 from serum or of rPON1 from the culture media of genetically engineered cells [166,167,168,169,170].
- There are numerous commercially available rPON1 preparations. Most, if not all, do not protect LDL against oxidation. Methods used to isolate the rPON1 have been optimized to maximize the yield with the aim of organophosphate hydrolysis. Initial screening is usually by phenyl acetate hydrolysis and then by testing potency as potential antidotes to organophosphate toxicity. The rPON1 thus produced may be lacking its hydrophobic environment depending on its method of isolation and may have structural modifications and tagging to enhance ease of isolation and potency as an organophosphate detoxicating enzyme. The antioxidant, antiatherogenic properties of PON1 were, however, reported to persist in rPON1 [171] prepared by one published method [172].
- HDL can still protect LDL against oxidation in the absence of Ca2+ when paraoxon hydrolysis has thus been abolished [173]. However, soon after this report, it was revealed that Ca2+ is much less critical for phospholipid peroxide hydrolysis than for its aryl esterase and paraoxonase hydrolytic activities [174].
- Mendelian randomization epidemiology based on the 192 polymorphism shows that the PON1 isoform is more active in promoting paraoxon hydrolysis. However, although this polymorphism is associated with ASCVD, in one report the association was not so strong as to rule out publication bias [175]. As has been earlier discussed, the impact of the R isoform on serum PON1 activity is not as strong as the authors of this report supposed. More recent meta-analyses have largely discounted publication bias as the explanation.
- PON1 is the most active as a lactonase [149]. Although PON1 may have evolved as a lactonase, this does not mean that it is not involved in preventing a disease, such as atheroma, the clinical manifestations of which have only become apparent as a major epidemic in the last century [176]. If we allow that the protective effect of PON1 against ASCVD is not by protecting LDL against oxidative modification, then we must entertain that the discovery of its antiatherogenic role was serendipitous. Certainly, the possibility that its lactonase activity might be important, for example, against homocysteine thiolactone [177], should not be discounted, and further research should be directed in that area. However, the current evidence for an antioxidative role is compelling.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsimikas, S.; Witztum, J.L. Oxidized phospholipids in cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef]
- Maggio, E.; Bocchini, V.P.; Carnevale, R.; Pignatelli, P.; Violi, F.; Loffredo, L. Vitamin E supplementation (alone or with other antioxidants) and stroke: A meta-analysis. Nutr. Rev. 2024, 82, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef]
- Arrol, S.; Mackness, M.I.; Durrington, P.N. Vitamin E supplementation increases the resistance of both LDL and HDL to oxidation and increases cholesteryl ester transfer activity. Atherosclerosis 2000, 150, 129–134. [Google Scholar] [CrossRef]
- Myant, N.B. The Biology of Cholesterol and Related Steroids; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. How LDL receptors influence cholesterol and atherosclerosis. Sci. Am. 1984, 251, 58–66. [Google Scholar] [CrossRef]
- Miller, G.J.; Miller, N.E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1975, 1, 16–19. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, X.; Zhou, Q.; Zhang, Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: A systematic review and meta-analysis. Lipids Health Dis. 2017, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Remaley, A.T.; Cuchel, M. Is Low-Density Lipoprotein Cholesterol the Key to Interpret the Role of Lecithin: Cholesterol Acyltransferase in Atherosclerosis? Circulation 2018, 138, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2023, 44, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P. HDL in risk prediction and its direct and indirect involvement in atherogenesis. Atheroscler. Suppl. 2002, 3, 3–12. [Google Scholar] [CrossRef]
- Cedó, L.; Metso, J.; Santos, D.; García-León, A.; Plana, N.; Sabate-Soler, S.; Rotllan, N.; Rivas-Urbina, A.; Méndez-Lara, K.A.; Tondo, M.; et al. LDL Receptor Regulates the Reverse Transport of Macrophage-Derived Unesterified Cholesterol via Concerted Action of the HDL-LDL Axis: Insight From Mouse Models. Circ. Res. 2020, 127, 778–792. [Google Scholar] [CrossRef]
- Assmann, G.; von Eckardstein, A.; Funke, H. High density lipoproteins, reverse transport of cholesterol, and coronary artery disease. Insights from mutations. Circulation 1993, 87, III28–III34. [Google Scholar]
- Durrington, P.N. Hyperlipidaemia: Diagnosis and Management, 3rd ed.; Hodder Arnold: London, UK, 2007. [Google Scholar]
- Tall, A.R.; Wang, N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J. Clin. Investig. 2000, 106, 1205–1207. [Google Scholar] [CrossRef]
- Koseki, M.; Yamashita, S.; Ogura, M.; Ishigaki, Y.; Ono, K.; Tsukamoto, K.; Hori, M.; Matsuki, K.; Yokoyama, S.; Harada-Shiba, M. Current Diagnosis and Management of Tangier Disease. J. Atheroscler. Thromb. 2021, 28, 802–810. [Google Scholar] [CrossRef]
- Rousset, X.; Vaisman, B.; Amar, M.; Sethi, A.A.; Remaley, A.T. Lecithin: Cholesterol acyltransferase--from biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 163–171. [Google Scholar] [CrossRef]
- Zanoni, P.; von Eckardstein, A. Inborn errors of apolipoprotein A-I metabolism: Implications for disease, research and development. Curr. Opin. Lipidol. 2020, 31, 62–70. [Google Scholar] [CrossRef]
- Hancock-Cerutti, W.; Lhomme, M.; Dauteuille, C.; Lecocq, S.; Chapman, M.J.; Rader, D.J.; Kontush, A.; Cuchel, M. Paradoxical coronary artery disease in humans with hyperalphalipoproteinemia is associated with distinct differences in the high-density lipoprotein phosphosphingolipidome. J. Clin. Lipidol. 2017, 11, 1192–1200.e3. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.I.; Yamashita, S.; Nakajima, N.; Arai, T.; Maruyama, T.; Yoshida, Y.; Ishigami, M.; Sakai, N.; Kameda-Takemura, K.; Matsuzawa, Y. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1053–1059. [Google Scholar] [CrossRef]
- Yamashita, S.; Matsuzawa, Y. Re-evaluation of cholesteryl ester transfer protein function in atherosclerosis based upon genetics and pharmacological manipulation. Curr. Opin. Lipidol. 2016, 27, 459–472. [Google Scholar] [CrossRef]
- Cimen, M.Y.B. Free radical metabolism in human erythrocytes. Clin. Chim. Acta 2008, 390, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.J.; Houtchens, R.A.; Maxwell, J.C.; Caughey, W.S. Mechanism of autooxidation for hemoglobins and myoglobins. Promotion of superoxide production by protons and anions. J. Biol. Chem. 1982, 257, 4966–4977. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry-Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte metabolism. Acta Physiol. 2024, 240, e14081. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.N.; Kozhevnikova, K.A.; Kuzmin, A.A.; Kuznetsov, A.S.; Belova, E.V. On the ability of high density lipoproteins to remove phospholipid peroxidation products from erythrocyte membranes. Biochemistry 2001, 66, 300–304. [Google Scholar] [CrossRef]
- Arrol, S.; Mackness, M.; Durrington, P. High-density-lipoprotein-associated enzymes and the prevention of lower density lipoprotein oxidation. Eur. J. Lab. Med. 1996, 4, 33–38. [Google Scholar]
- el-Saadani, M.; Esterbauer, H.; el-Sayed, M.; Goher, M.; Nassar, A.Y.; Jürgens, G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar]
- Mackness, M.I.; Abbott, C.; Arrol, S.; Durrington, P.N. The role of high-density lipoprotein and lipid-soluble antioxidant vitamins in inhibiting low-density lipoprotein oxidation. Biochem. J. 1993, 294 Pt 3, 829–834. [Google Scholar] [CrossRef] [PubMed]
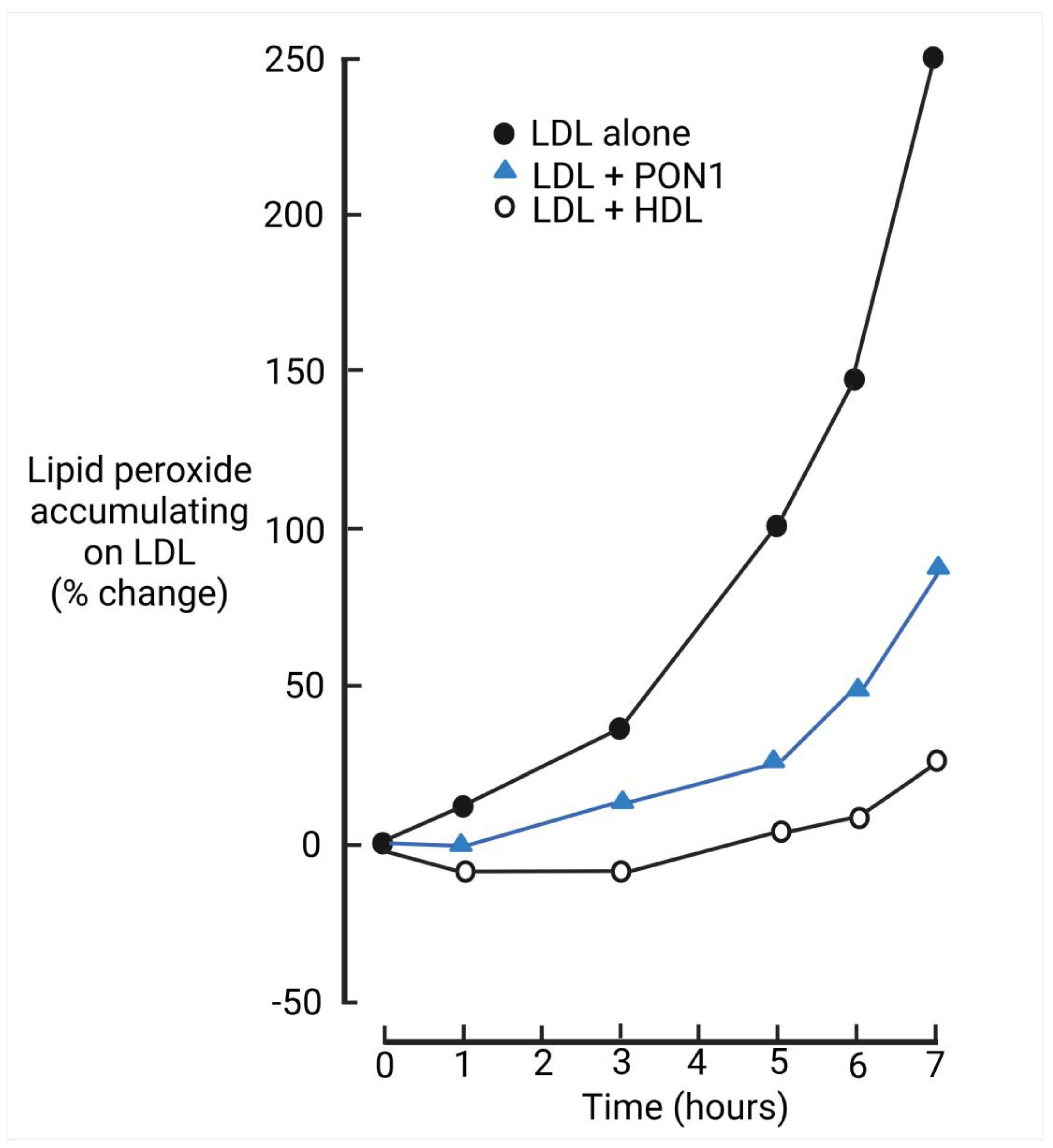
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Abbott, C.; Durrington, P.N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 1993, 104, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Berliner, J.A.; Hama, S.Y.; La Du, B.N.; Faull, K.F.; Fogelman, A.M.; Navab, M. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Investig. 1995, 96, 2882–2891. [Google Scholar] [CrossRef]
- Hine, D.; Mackness, B.; Mackness, M. Cholesteryl-ester transfer protein enhances the ability of high-density lipoprotein to inhibit low-density lipoprotein oxidation. IUBMB Life 2011, 63, 772–774. [Google Scholar] [CrossRef]
- Pantazi, D.; Tellis, C.; Tselepis, A.D. Oxidized phospholipids and lipoprotein-associated phospholipase A2 (Lp-PLA2 ) in atherosclerotic cardiovascular disease: An update. Biofactors 2022, 48, 1257–1270. [Google Scholar] [CrossRef]
- Rodrigo, L.; Mackness, B.; Durrington, P.N.; Hernandez, A.; Mackness, M.I. Hydrolysis of platelet-activating factor by human serum paraoxonase. Biochem. J. 2001, 354 Pt 1, 1–7. [Google Scholar] [CrossRef]
- Neary, R.; Bhatnagar, D.; Durrington, P.; Ishola, M.; Arrol, S.; Mackness, M. An investigation of the role of lecithin:cholesterol acyltransferase and triglyceride-rich lipoproteins in the metabolism of pre-beta high density lipoproteins. Atherosclerosis 1991, 89, 35–48. [Google Scholar] [CrossRef]
- Rye, K.A.; Barter, P.J. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 421–428. [Google Scholar] [CrossRef]
- Steinbrecher, U.P.; Parthasarathy, S.; Leake, D.S.; Witztum, J.L.; Steinberg, D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc. Natl. Acad. Sci. USA 1984, 81, 3883–3887. [Google Scholar] [CrossRef]
- Morel, D.W.; DiCorleto, P.E.; Chisolm, G.M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis 1984, 4, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Pepin, J.M.; O’Neil, J.A.; Hoff, H.F. Quantification of apo[a] and apoB in human atherosclerotic lesions. J. Lipid Res. 1991, 32, 317–327. [Google Scholar]
- Packard, C.J. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem. Soc. Trans. 2003, 31 Pt 5, 1066–1069. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Cicero, A.F.; Janez, A.; Stoian, A.P.; Sonmez, A.; Rizzo, M. Atherosclerosis Development and Progression: The Role of Atherogenic Small, Dense LDL. Medicina 2022, 58, 299. [Google Scholar] [CrossRef]
- Younis, N.; Charlton-Menys, V.; Sharma, R.; Soran, H.; Durrington, P.N. Glycation of LDL in non-diabetic people: Small dense LDL is preferentially glycated both in vivo and in vitro. Atherosclerosis 2009, 202, 162–168. [Google Scholar] [CrossRef]
- Younis, N.N.; Soran, H.; Sharma, R.; Charlton-Menys, V.; Greenstein, A.; Elseweidy, M.M.; Durrington, P.N. Small-dense LDL and LDL glycation in metabolic syndrome and in statin-treated and non-statin-treated type 2 diabetes. Diabetes Vasc. Dis. Res. 2010, 7, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Soran, H.; Pemberton, P.; Charlton-Menys, V.; Elseweidy, M.M.; Durrington, P.N. Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin. Sci. 2013, 124, 343–349. [Google Scholar] [CrossRef]
- Charlton-Menys, V.; Durrington, P. Apolipoproteins AI and B as therapeutic targets. J. Intern. Med. 2006, 259, 462–472. [Google Scholar] [CrossRef]
- Soran, H.; Ho, J.H.; Adam, S.; Durrington, P.N. Non-HDL cholesterol should not generally replace LDL cholesterol in the management of hyperlipidaemia. Curr. Opin. Lipidol. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Tames, F.J.; Mackness, M.I.; Arrol, S.; Laing, I.; Durrington, P.N. Non-enzymatic glycation of apolipoprotein B in the sera of diabetic and non-diabetic subjects. Atherosclerosis 1992, 93, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Sharma, R.; Soran, H.; Charlton-Menys, V.; Elseweidy, M.; Durrington, P.N. Glycation as an atherogenic modification of LDL. Curr. Opin. Lipidol. 2008, 19, 378–384. [Google Scholar] [CrossRef]
- Younis, N.N.; Soran, H.; Charlton-Menys, V.; Sharma, R.; Hama, S.; Pemberton, P.; Elseweidy, M.M.; Durrington, P.N. High-density lipoprotein impedes glycation of low-density lipoprotein. Diab Vasc. Dis. Res. 2013, 10, 152–160. [Google Scholar] [CrossRef]
- Yim, M.B.; Yim, H.S.; Lee, C.; Kang, S.O.; Chock, P.B. Protein glycation: Creation of catalytic sites for free radical generation. Ann. N. Y. Acad. Sci. 2001, 928, 48–53. [Google Scholar] [PubMed]
- Guimarães, E.L.M.; Empsen, C.; Geerts, A.; van Grunsven, L.A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 2010, 52, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lankin, V.Z.; Konovalova, G.G.; Tikhaze, A.K.; Nedosugova, L.V. The influence of glucose on the free radical peroxidation of low density lipoproteins in vitro and in vivo. Biomed. Khim. 2012, 58, 339–352. [Google Scholar] [CrossRef][Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Lindsay, R.M.; Smith, W.; Lee, W.K.; Dominiczak, M.H.; Baird, J.D. The effect of delta-gluconolactone, an oxidised analogue of glucose, on the nonenzymatic glycation of human and rat haemoglobin. Clin. Chim. Acta 1997, 263, 239–247. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, L.; Liu, S.; Li, Y.; Xia, S.; Chen, D.; Wang, M.; Wu, S.; Dai, Q.; Vu, H.; et al. γ-6-Phosphogluconolactone, a Byproduct of the Oxidative Pentose Phosphate Pathway, Contributes to AMPK Activation through Inhibition of PP2A. Mol. Cell 2019, 76, 857–871.e9. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Langborg, A.; Minhas, H.S. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 1999, 344 Pt 1, 109–116. [Google Scholar]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by methylglyoxal increases arterial atherogenicity: A possible contributor to increased risk of cardiovascular disease in diabetes. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015, 458, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Dicarbonyl-Dependent Modification of LDL as a Key Factor of Endothelial Dysfunction and Atherosclerotic Vascular Wall Damage. Antioxidants 2022, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef]
- Hui, D.Y.; Noel, J.G.; Harmony, J.A. Binding of plasma low density lipoproteins to erythrocytes. Biochim. Biophys. Acta 1981, 664, 513–526. [Google Scholar] [CrossRef]
- Durrington, P.N.; MacIver, J.E.; Holdsworth, G.; Galton, D.J. Severe hypertriglyceridemia associated with pancytopenia and lipoprotein lipase deficiency. Ann. Intern. Med. 1981, 94, 211–212. [Google Scholar] [CrossRef]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes Res. 2010, 2, 14. [Google Scholar] [CrossRef]
- Michel, C.C.; Nanjee, M.N.; Olszewski, W.L.; Miller, N.E. LDL and HDL transfer rates across peripheral microvascular endothelium agree with those predicted for passive ultrafiltration in humans. J. Lipid Res. 2015, 56, 122–128. [Google Scholar] [CrossRef]
- Mackness, M.I.; Mackness, B.; Arrol, S.; Wood, G.; Bhatnagar, D.; Durrington, P.N. Presence of paraoxonase in human interstitial fluid. FEBS Lett. 1997, 416, 377–380. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Cabodevilla, A.G.; Younis, W. In the Beginning, Lipoproteins Cross the Endothelial Barrier. J. Atheroscler. Thromb. 2024, 31, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Semorine, K.; Watts, G.F.; Mamo, J. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin. Chem. Lab. Med. 2003, 41, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Nakajima, K.; Niimi, M.; Fujita, M.Q.; Nakajima, Y.; Takeichi, S.; Kinoshita, M.; Matsushima, T.; Teramoto, T.; Tanaka, A. Detection of apolipoproteins B-48 and B-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin. Chim. Acta 2008, 390, 38–43. [Google Scholar] [CrossRef]
- Rapp, J.H.; Harris, H.W.; Hamilton, R.L.; Krupski, W.C.; Reilly, L.M.; Ehrenfeld, W.K.; Stoney, R.J.; Goldstone, J.; Kane, J.P. Particle size distribution of lipoproteins from human atherosclerotic plaque: A preliminary report. J. Vasc. Surg. 1989, 9, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wissler, R.W.; Vesselinovitch, D.; Getz, G.S. Abnormalities of the arterial wall and its metabolism in atherogenesis. Prog. Cardiovasc. Dis. 1976, 18, 341–369. [Google Scholar] [CrossRef]
- Schwartz, C.J.; Valente, A.J.; Sprague, E.A.; Kelley, J.L.; Nerem, R.M. The pathogenesis of atherosclerosis: An overview. Clin. Cardiol. 1991, 14 (Suppl. S1), 1–16. [Google Scholar] [CrossRef]
- Weinberg, P.D. Haemodynamic Wall Shear Stress, Endothelial Permeability and Atherosclerosis-A Triad of Controversy. Front. Bioeng. Biotechnol. 2022, 10, 836680. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- Medrano-Bosch, M.; Simón-Codina, B.; Jiménez, W.; Edelman, E.R.; Melgar-Lesmes, P. Monocyte-endothelial cell interactions in vascular and tissue remodeling. Front. Immunol. 2023, 14, 1196033. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Khotina, V.; Ivanova, E.A.; Orekhov, A.N. NADPH Oxidases and Their Role in Atherosclerosis. Biomedicines 2020, 8, 206. [Google Scholar] [CrossRef]
- Harrison, D.G.; Widder, J.; Grumbach, I.; Chen, W.; Weber, M.; Searles, C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J. Intern. Med. 2006, 259, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Gisterå, A.; Ketelhuth, D.F.J.; Malin, S.G.; Hansson, G.K. Animal Models of Atherosclerosis-Supportive Notes and Tricks of the Trade. Circ. Res. 2022, 130, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Klimov, A.N.; Nagornev, V.A. Current concepts of the pathogenesis of atherosclerosis in light of the development of N.N. Anichkov’s theory (on the centenary of his birth). Arkhiv Patol. 1985, 47, 12–23. [Google Scholar]
- Dai, G.; Kaazempur-Mofrad, M.R.; Natarajan, S.; Zhang, Y.; Vaughn, S.; Blackman, B.R.; Kamm, R.D.; García-Cardeña, G.; Gimbrone, M.A., Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. USA 2004, 101, 14871–14876. [Google Scholar] [CrossRef] [PubMed]
- Cancel, L.M.; Fitting, A.; Tarbell, J.M. In vitro study of LDL transport under pressurized (convective) conditions. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H126–H132. [Google Scholar] [CrossRef]
- Dabagh, M.; Jalali, P.; Tarbell, J.M. The transport of LDL across the deformable arterial wall: The effect of endothelial cell turnover and intimal deformation under hypertension. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H983–H996. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.P.; Zieske, A.W.; Malcom, G.T. Lipoproteins and atherosclerosis in children: An early marriage? Nutr. Metab. Cardiovasc. Dis. 2001, 11 (Suppl. S5), 16–22. [Google Scholar]
- Chen, Z.; Peto, R.; Collins, R.; MacMahon, S.; Lu, J.; Li, W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ 1991, 303, 276–282. [Google Scholar] [CrossRef]
- Lorey, M.B.; Öörni, K.; Kovanen, P.T. Modified Lipoproteins Induce Arterial Wall Inflammation During Atherogenesis. Front. Cardiovasc. Med. 2022, 9, 841545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sessa, W.C.; Fernández-Hernando, C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 130. [Google Scholar] [CrossRef]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fernández-Hernando, C. Transport of LDLs into the arterial wall: Impact in atherosclerosis. Curr. Opin. Lipidol. 2020, 31, 279–285. [Google Scholar] [CrossRef]
- Ghaffari, S.; Jang, E.; Naderinabi, F.; Sanwal, R.; Khosraviani, N.; Wang, C.; Steinberg, B.E.; Goldenberg, N.M.; Ikeda, J.; Lee, W.L. Endothelial HMGB1 Is a Critical Regulator of LDL Transcytosis via an SREBP2-SR-BI Axis. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Robert, J.; Rohrer, L.; von Eckardstein, A.; Lee, W.L. Transendothelial transport of lipoproteins. Atherosclerosis 2020, 315, 111–125. [Google Scholar] [CrossRef]
- Ho, T.W.W.; Henry, A.; Lee, W.L. LDL Transcytosis by the Arterial Endothelium-Atherosclerosis by a Thousand Cuts? Curr. Atheroscler. Rep. 2023, 25, 457–465. [Google Scholar] [CrossRef]
- Ma, B.; Jia, J.; Wang, X.; Zhang, R.; Niu, S.; Ni, L.; Di, X.; Liu, C. Differential roles of Scavenger receptor class B type I: A protective molecule and a facilitator of atherosclerosis (Review). Mol. Med. Rep. 2020, 22, 2599–2604. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef]
- Luchetti, F.; Crinelli, R.; Nasoni, M.G.; Benedetti, S.; Palma, F.; Fraternale, A.; Iuliano, L. LDL receptors, caveolae and cholesterol in endothelial dysfunction: oxLDLs accomplices or victims? Br. J. Pharmacol. 2021, 178, 3104–3114. [Google Scholar] [CrossRef]
- Tian, K.; Ogura, S.; Little, P.J.; Xu, S.W.; Sawamura, T. Targeting LOX-1 in atherosclerosis and vasculopathy: Current knowledge and future perspectives. Ann. N. Y. Acad. Sci. 2019, 1443, 34–53. [Google Scholar] [CrossRef]
- Akhmedov, A.; Sawamura, T.; Chen, C.H.; Kraler, S.; Vdovenko, D.; Lüscher, T.F. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic cardiovascular disease. Eur. Heart J. 2021, 42, 1797–1807. [Google Scholar] [CrossRef]
- Barreto, J.; Karathanasis, S.K.; Remaley, A.; Sposito, A.C. Role of LOX-1 (Lectin-Like Oxidized Low-Density Lipoprotein Receptor 1) as a Cardiovascular Risk Predictor: Mechanistic Insight and Potential Clinical Use. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Shu, M.; Kuang, L.; Bai, X.; Jia, X.; Zhao, Y.; Lu, Y.; Zhu, L.; Wang, L.; Zhu, Y.; et al. Scavenger receptor a mediates glycated LDL transcytosis across endothelial cells to promote atherosclerosis. Int. J. Biol. Macromol. 2023, 235, 123836. [Google Scholar] [CrossRef]
- Shu, M.; Cheng, W.; Jia, X.; Bai, X.; Zhao, Y.; Lu, Y.; Zhu, L.; Zhu, Y.; Wang, L.; Shu, Y.; et al. AGEs promote atherosclerosis by increasing LDL transcytosis across endothelial cells via RAGE/NF-κB/Caveolin-1 pathway. Mol. Med. 2023, 29, 113. [Google Scholar] [CrossRef]
- Goldberg, D.; Khatib, S. Atherogenesis, Transcytosis, and the Transmural Cholesterol Flux: A Critical Review. Oxid. Med. Cell. Longev. 2022, 2022, 2253478. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Ho, T.W.W.; Xu, Z.; Neculai, D.; Beauchemin, C.A.; Lee, W.L.; Fairn, G.D. Apolipoprotein A1 and high-density lipoprotein limit low-density lipoprotein transcytosis by binding SR-B1. J. Lipid Res. 2024, 65, 100530. [Google Scholar] [CrossRef]
- Miller, N.E.; Weinstein, D.B.; Carew, T.E.; Koschinsky, T.; Steinberg, D. Interaction between high density and low density lipoproteins uptake and degradation by cultured human fibroblasts. J. Clin. Investig. 1977, 60, 78–88. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of foam cell formation in atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Cuthbert, G.A.; Shaik, F.; Harrison, M.A.; Ponnambalam, S.; Homer-Vanniasinkam, S. Scavenger Receptors as Biomarkers and Therapeutic Targets in Cardiovascular Disease. Cells 2020, 9, 2453. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Apoprotein E and Reverse Cholesterol Transport. Int. J. Mol. Sci. 2018, 19, 3479. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Vaya, J.; Shih, D.; Aviram, M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis 2005, 179, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Deakin, S.P.; Bioletto, S.; Bochaton-Piallat, M.L.; James, R.W. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic. Biol. Med. 2011, 50, 102–109. [Google Scholar] [CrossRef]
- Evans, C.E.; Mylchreest, S.; Charlton-Menys, V.; Durrington, P. The role of hydrostatic pressure in foam cell formation upon exposure of macrophages to LDL and oxidized LDL. Atherosclerosis 2008, 197, 596–601. [Google Scholar] [CrossRef]
- Durrington, P.; Soran, H. Paraoxonase 1: Evolution of the enzyme and of its role in protecting against atherosclerosis. Curr. Opin. Lipidol. 2024, 35, 171–178. [Google Scholar] [CrossRef]
- Aldridge, W.N. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem. J. 1953, 53, 117–124. [Google Scholar] [CrossRef]
- Augustinsson, K.B. Arylesterases. J. Histochem. Cytochem. 1964, 12, 744–747. [Google Scholar] [CrossRef]
- Brogen, C.-H.; Bøg-Hansen, T.C. Enzyme Characterization in Quantitative Immunoelectrophoresis. Scand. J. Immunol. 1975, 4, 37–51. [Google Scholar] [CrossRef]
- Mackness, M.I.; Hallam, S.D.; Peard, T.; Warner, S.; Walker, C.H. The separation of sheep and human serum “A”-esterase activity into the lipoprotein fraction by ultracentrifugation. Comp. Biochem. Physiol. B 1985, 82, 675–677. [Google Scholar] [CrossRef]
- Furlong, C.E.; Costa, L.G.; Hassett, C.; Richter, R.J.; Sundstrom, J.A.; Adler, D.A.; Disteche, C.M.; Omiecinski, C.J.; Chapline, C.; Crabb, J.W.; et al. Human and rabbit paraoxonases: Purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxification. Chem. Biol. Interact. 1993, 87, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Draganov, D.I.; La Du, B.N. Pharmacogenetics of paraoxonases: A brief review. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 78–88. [Google Scholar] [CrossRef]
- Mackness, B.; Hunt, R.; Durrington, P.N.; Mackness, M.I. Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1233–1238. [Google Scholar] [CrossRef]
- McElveen, J.; Mackness, M.I.; Colley, C.M.; Peard, T.; Warner, S.; Walker, C.H. Distribution of paraoxon hydrolytic activity in the serum of patients after myocardial infarction. Clin. Chem. 1986, 32, 671–673. [Google Scholar] [PubMed]
- Ayub, A.; Mackness, M.I.; Arrol, S.; Mackness, B.; Patel, J.; Durrington, P.N. Serum paraoxonase after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 330–335. [Google Scholar] [CrossRef]
- Mackness, B.; Davies, G.K.; Turkie, W.; Lee, E.; Roberts, D.H.; Hill, E.; Roberts, C.; Durrington, P.N.; Mackness, M.I. Paraoxonase status in coronary heart disease: Are activity and concentration more important than genotype? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Durrington, P.; McElduff, P.; Yarnell, J.; Azam, N.; Watt, M.; Mackness, M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation 2003, 107, 2775–2779. [Google Scholar] [CrossRef]
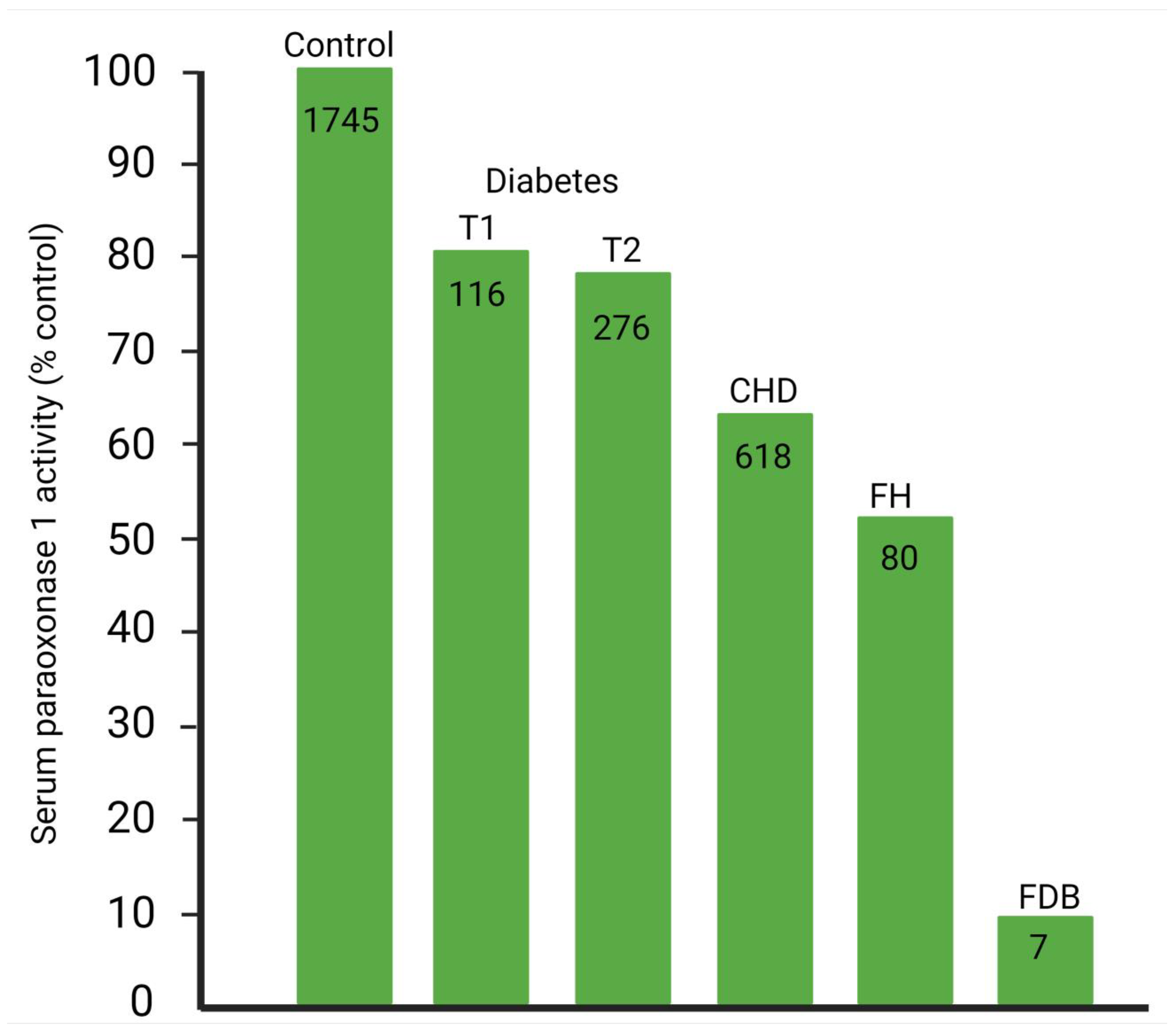
- Mackness, M.I.; Harty, D.; Bhatnagar, D.; Winocour, P.H.; Arrol, S.; Ishola, M.; Durrington, P.N. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis 1991, 86, 193–199. [Google Scholar] [CrossRef]
- Abbott, C.A.; Mackness, M.I.; Kumar, S.; Boulton, A.J.; Durrington, P.N. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1812–1818. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.N.; Boulton, A.J.M.; Hine, D.; Mackness, M.I. Serum paraoxonase activity in patients with type 1 diabetes compared to healthy controls. Eur. J. Clin. Investig. 2002, 32, 259–264. [Google Scholar] [CrossRef]
- Liu, Y. Clinical Study on Apolipoprotein E Distribution, Metabolism and Glycation. Ph.D. Thesis, University of Manchester, Manchester, UK, 2015. [Google Scholar]
- Garin, M.C.B.; Kalix, B.; Morabia, A.; James, R.W. Small, dense lipoprotein particles and reduced paraoxonase-1 in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Sentí, M.; Tomás, M.; Fitó, M.; Weinbrenner, T.; Covas, M.I.; Sala, J.; Masiá, R.; Marrugat, J. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 5422–5426. [Google Scholar] [CrossRef] [PubMed]
- Mackness, B.; Quarck, R.; Verreth, W.; Mackness, M.; Holvoet, P. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Adhe-Rojekar, A.; Mogarekar, M.R.; Rojekar, M.V. Paraoxonase activity in metabolic syndrome in children and adolescents. Casp. J. Intern. Med. 2018, 9, 116–120. [Google Scholar] [CrossRef]
- Ferré, N.; Feliu, A.; García-Heredia, A.; Marsillach, J.; París, N.; Zaragoza-Jordana, M.; Mackness, B.; Mackness, M.; Escribano, J.; Closa-Monasterolo, R.; et al. Impaired paraoxonase-1 status in obese children. Relationships with insulin resistance and metabolic syndrome. Clin. Biochem. 2013, 46, 1830–1836. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Bijzet, J.; Hazenberg, B.P.; Dullaart, R.P.F. Lower serum paraoxonase-1 activity is related to higher serum amyloid a levels in metabolic syndrome. Arch. Med. Res. 2011, 42, 219–225. [Google Scholar] [CrossRef]
- Adam, S.; Ho, J.H.; Liu, Y.; Siahmansur, T.; Siddals, K.; Iqbal, Z.; Azmi, S.; Senapati, S.; New, J.; Jeziorska, M.; et al. Bariatric Surgery-induced High-density Lipoprotein Functionality Enhancement Is Associated With Reduced Inflammation. J. Clin. Endocrinol. Metab. 2022, 107, 2182–2194. [Google Scholar] [CrossRef]
- Jiang, X.; Ding, C.; Wang, C.; Yu, J.; Hua, Z.; Chen, X.; Ma, J.; Chen, W. Association of Paraoxonase 1 Gene Polymorphisms and Polycystic Ovarian Syndrome Susceptibility: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2020, 85, 167–177. [Google Scholar] [CrossRef]
- Samouilidou, E.C.; Liaouri, A.; Kostopoulos, V.; Nikas, D.; Grapsa, E. The importance of paraoxonase 1 activity in chronic kidney disease. Ren. Fail. 2024, 46, 2376930. [Google Scholar] [CrossRef]
- Mackness, B.; Mackness, M.I.; Arrol, S.; Turkie, W.; Durrington, P.N. Effect of the human serum paraoxonase 55 and 192 genetic polymorphisms on the protection by high density lipoprotein against low density lipoprotein oxidative modification. FEBS Lett. 1998, 423, 57–60. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, J. A meta-analysis on relationship between paraoxonase 1 polymorphisms and atherosclerotic cardiovascular diseases. Life Sci. 2019, 232, 116646. [Google Scholar] [CrossRef]
- Ashiq, S.; Ashiq, K. The Role of Paraoxonase 1 (PON1) Gene Polymorphisms in Coronary Artery Disease: A Systematic Review and Meta-Analysis. Biochem. Genet. 2021, 59, 919–939. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Trentini, A.; Marsillach, J.; D’Amuri, A.; Bosi, C.; Roncon, L.; Passaro, A.; Zuliani, G.; Mackness, M.; Cervellati, C. Paraoxonase-1 (PON-1) Arylesterase Activity Levels in Patients with Coronary Artery Disease: A Meta-Analysis. Dis. Markers 2022, 2022, 4264314. [Google Scholar] [CrossRef]
- Mahrooz, A. Pleiotropic functions and clinical importance of circulating HDL-PON1 complex. Adv. Clin. Chem. 2024, 121, 132–171. [Google Scholar] [CrossRef] [PubMed]
- James, R.W.; Garin, M.C.B.; Calabresi, L.; Miccoli, R.; von Eckardstein, A.; Tilly-Kiesi, M.; Taskinen, M.R.; Assmann, G.; Franceschini, G. Modulated serum activities and concentrations of paraoxonase in high density lipoprotein deficiency states. Atherosclerosis 1998, 139, 77–82. [Google Scholar] [CrossRef]
- Bar-Rogovsky, H.; Hugenmatter, A.; Tawfik, D.S. The evolutionary origins of detoxifying enzymes: The mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases. J. Biol. Chem. 2013, 288, 23914–23927. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Nicholls, S.J.; Topol, E.J.; Zhang, R.; Yang, X.; Schmitt, D.; Fu, X.; Shao, M.; Brennan, D.M.; Ellis, S.G.; et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008, 299, 1265–1276. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Sparks, C.E.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein. J. Clin. Med. 2019, 8, 1357. [Google Scholar] [CrossRef]
- Soran, H.; Younis, N.N.; Charlton-Menys, V.; Durrington, P. Variation in paraoxonase-1 activity and atherosclerosis. Curr. Opin. Lipidol. 2009, 20, 265–274. [Google Scholar] [CrossRef]
- Mahrooz, A.; Mackness, M.; Bagheri, A.; Ghaffari-Cherati, M.; Masoumi, P. The epigenetic regulation of paraoxonase 1 (PON1) as an important enzyme in HDL function: The missing link between environmental and genetic regulation. Clin. Biochem. 2019, 73, 1–10. [Google Scholar] [CrossRef]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Lack of protection against oxidative modification of LDL by avian HDL. Biochem. Biophys. Res. Commun. 1998, 247, 443–446. [Google Scholar] [CrossRef]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Xia, Y.R.; Wang, X.P.; Miller, E.; Castellani, L.W.; Subbanagounder, G.; Cheroutre, H.; Faull, K.F.; Berliner, J.A.; Witztum, J.L.; et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000, 275, 17527–17535. [Google Scholar] [CrossRef]
- Tward, A.; Xia, Y.R.; Wang, X.P.; Shi, Y.S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Guns, P.J.; Van Assche, T.; Verreth, W.; Fransen, P.; Mackness, B.; Mackness, M.; Holvoet, P.; Bult, H. Paraoxonase 1 gene transfer lowers vascular oxidative stress and improves vasomotor function in apolipoprotein E-deficient mice with pre-existing atherosclerosis. Br. J. Pharmacol. 2008, 153, 508–516. [Google Scholar] [CrossRef] [PubMed]
- She, Z.G.; Zheng, W.; Wei, Y.S.; Chen, H.Z.; Wang, A.B.; Li, H.L.; Liu, G.; Zhang, R.; Liu, J.J. Human paraoxonase gene cluster transgenic overexpression represses atherogenesis and promotes atherosclerotic plaque stability in ApoE-null mice. Circ. Res. 2009, 104, 1160–1168. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, W.; Wang, M.; Zhu, J.; Zang, Y.; Shi, W.; Zhang, J.; Qin, J. Studies on protective effects of human paraoxonases 1 and 3 on atherosclerosis in apolipoprotein E knockout mice. Gene Ther. 2010, 17, 626–633. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer Jr, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Kriska, T.; Marathe, G.K.; Schmidt, J.C.; McIntyre, T.M.; Girotti, A.W. Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. J. Biol. Chem. 2007, 282, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Teiber, J.F.; Draganov, D.I.; La Du, B.N. Purified human serum PON1 does not protect LDL against oxidation in the in vitro assays initiated with copper or AAPH. J. Lipid Res. 2004, 45, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Khattib, A.; Musa, S.; Halabi, M.; Hayek, T.; Khatib, S. Lyso-DGTS Lipid Derivatives Enhance PON1 Activities and Prevent Oxidation of LDL: A Structure-Activity Relationship Study. Antioxidants 2022, 11, 2058. [Google Scholar] [CrossRef]
- Mishra, V.K.; Palgunachari, M.N.; McPherson, D.T.; Anantharamaiah, G.M. Lipid complex of apolipoprotein A-I mimetic peptide 4F is a novel platform for paraoxonase-1 binding and enhancing its activity and stability. Biochem. Biophys. Res. Commun. 2013, 430, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- James, R.W.; Deakin, S.P. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic. Biol. Med. 2004, 37, 1986–1994. [Google Scholar] [CrossRef]
- Rochu, D.; Renault, F.; Cléry-Barraud, C.; Chabrière, E.; Masson, P. Stability of highly purified human paraoxonase (PON1): Association with human phosphate binding protein (HPBP) is essential for preserving its active conformation(s). Biochim. Biophys. Acta 2007, 1774, 874–883. [Google Scholar] [CrossRef]
- Tavori, H.; Aviram, M.; Khatib, S.; Musa, R.; Nitecki, S.; Hoffman, A.; Vaya, J. Human carotid atherosclerotic plaque increases oxidative state of macrophages and low-density lipoproteins, whereas paraoxonase 1 (PON1) decreases such atherogenic effects. Free Radic. Biol. Med. 2009, 46, 607–615. [Google Scholar] [CrossRef]
- Aharoni, A.; Gaidukov, L.; Yagur, S.; Toker, L.; Silman, I.; Tawfik, D.S. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc. Natl. Acad. Sci. USA 2004, 101, 482–487. [Google Scholar] [CrossRef]
- Graham, A.; Hassall, D.G.; Rafique, S.; Owen, J.S. Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis 1997, 135, 193–204. [Google Scholar] [CrossRef]
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisgaier, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; La Du, B. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: Selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.G.; Keavney, B.D.; Watkins, H.; Collins, R.; Danesh, J. Four paraoxonase gene polymorphisms in 11212 cases of coronary heart disease and 12786 controls: Meta-analysis of 43 studies. Lancet 2004, 363, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.S. A parlous state of storm and stress. The life and times of James B. Herrick. Circulation 1983, 67, 955–959. [Google Scholar] [CrossRef]
- Perła-Kaján, J.; Borowczyk, K.; Głowacki, R.; Nygård, O.; Jakubowski, H. Paraoxonase 1 Q192R genotype and activity affect homocysteine thiolactone levels in humans. FASEB J. 2018, 32, 6019–6024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durrington, P.N.; Bashir, B.; Soran, H. How Does HDL Participate in Atherogenesis? Antioxidant Activity Versus Role in Reverse Cholesterol Transport. Antioxidants 2025, 14, 430. https://doi.org/10.3390/antiox14040430
Durrington PN, Bashir B, Soran H. How Does HDL Participate in Atherogenesis? Antioxidant Activity Versus Role in Reverse Cholesterol Transport. Antioxidants. 2025; 14(4):430. https://doi.org/10.3390/antiox14040430
Chicago/Turabian StyleDurrington, Paul N., Bilal Bashir, and Handrean Soran. 2025. "How Does HDL Participate in Atherogenesis? Antioxidant Activity Versus Role in Reverse Cholesterol Transport" Antioxidants 14, no. 4: 430. https://doi.org/10.3390/antiox14040430
APA StyleDurrington, P. N., Bashir, B., & Soran, H. (2025). How Does HDL Participate in Atherogenesis? Antioxidant Activity Versus Role in Reverse Cholesterol Transport. Antioxidants, 14(4), 430. https://doi.org/10.3390/antiox14040430






