HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome
Abstract
1. The Metabolic Syndrome: Epidemiology and Introduction
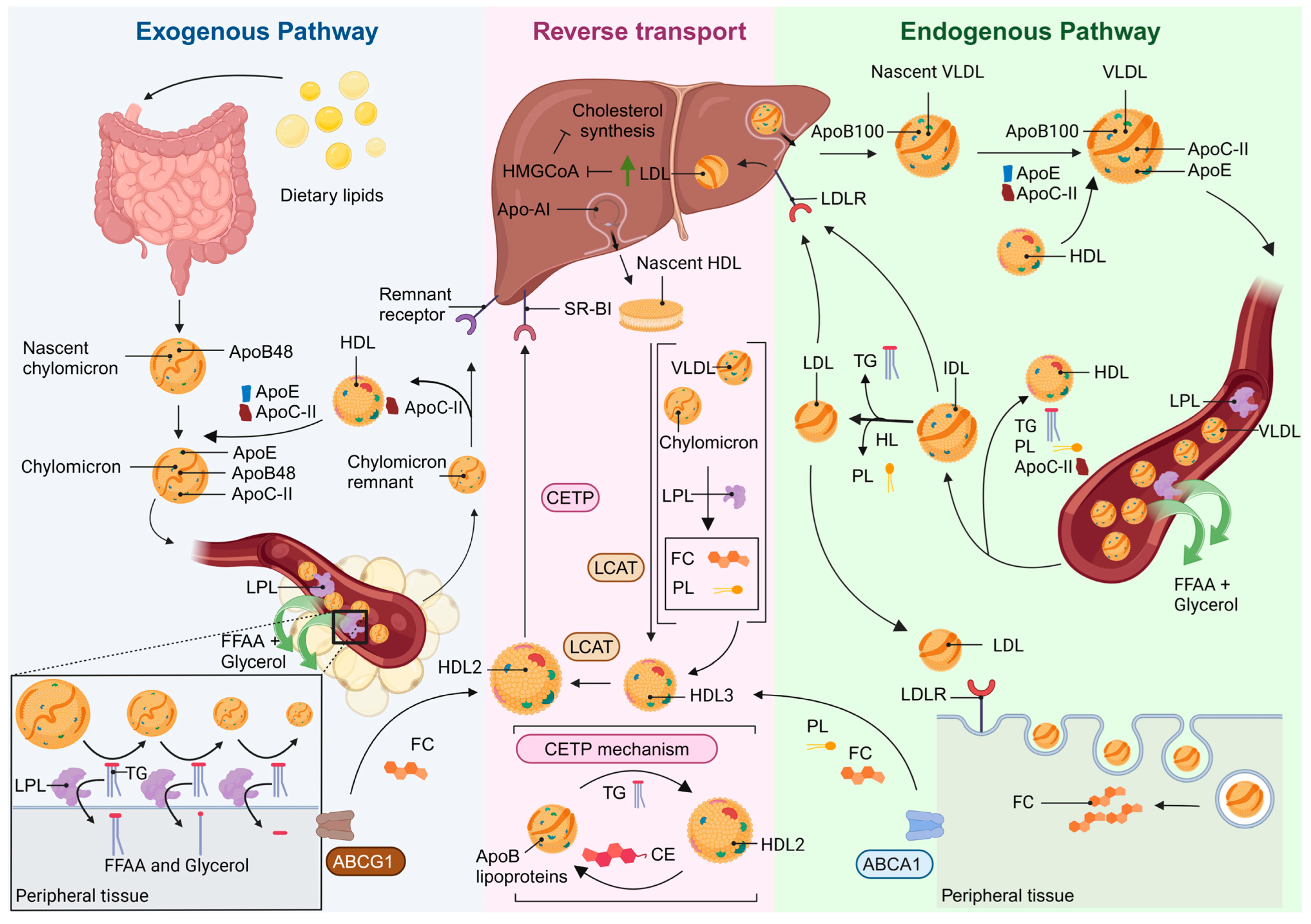
2. TG and HDL Biogenesis
3. Understanding HDL: Insights into Functional and Dysfunctional Particles
3.1. HDL Particles: Structure and Composition
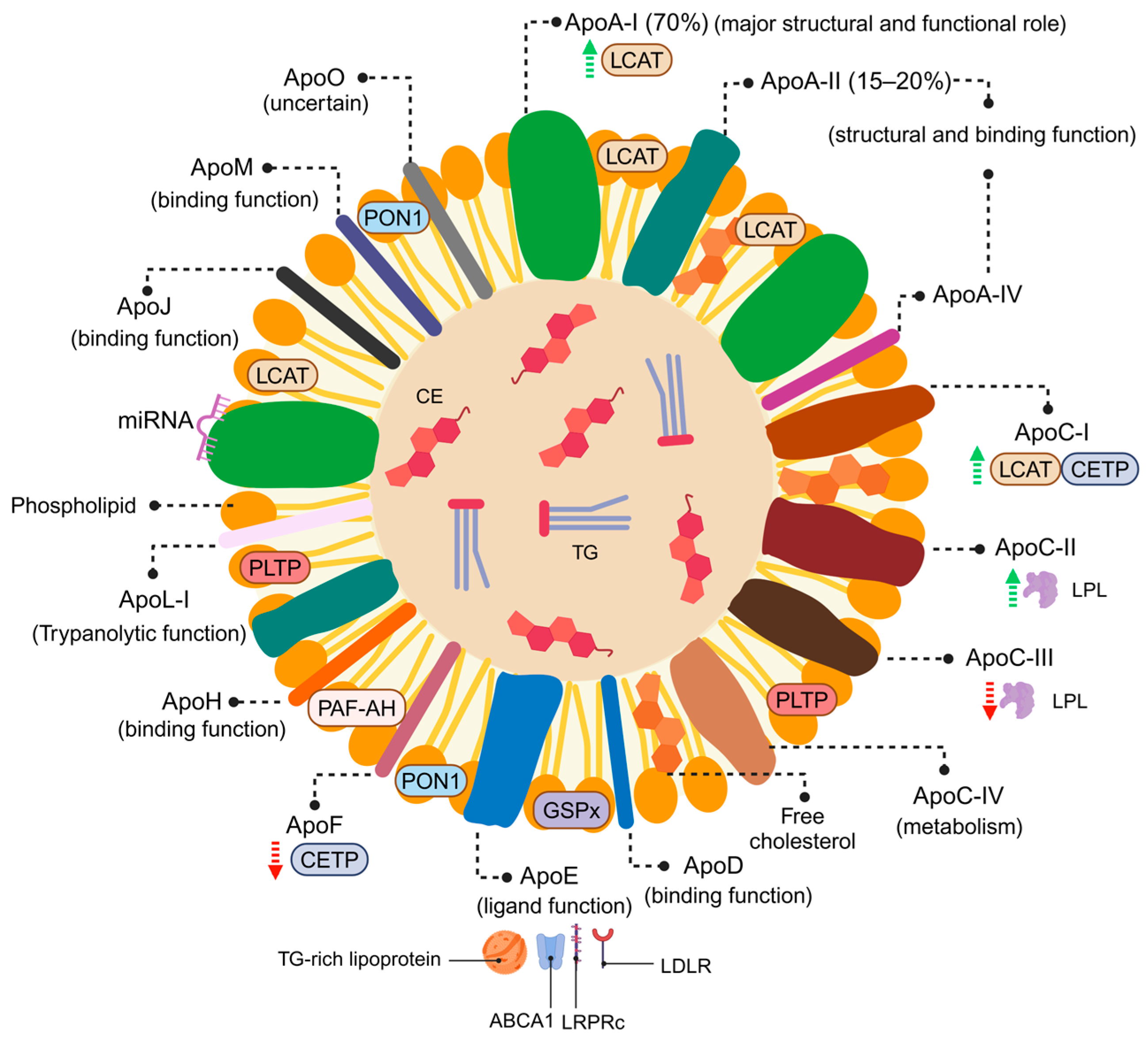
3.2. Functional HDL Particles
3.2.1. Antioxidant Properties
3.2.2. Vasodilatory and Antithrombotic Properties
3.2.3. Glucose Metabolism
3.2.4. Others
3.3. HDL Adverse Remodelling: The Impact of Cardiovascular Risk Factors and Comorbid Conditions in HDL Functionality
4. The Role of TG and FFAA in Metabolic and Cardiovascular Risk
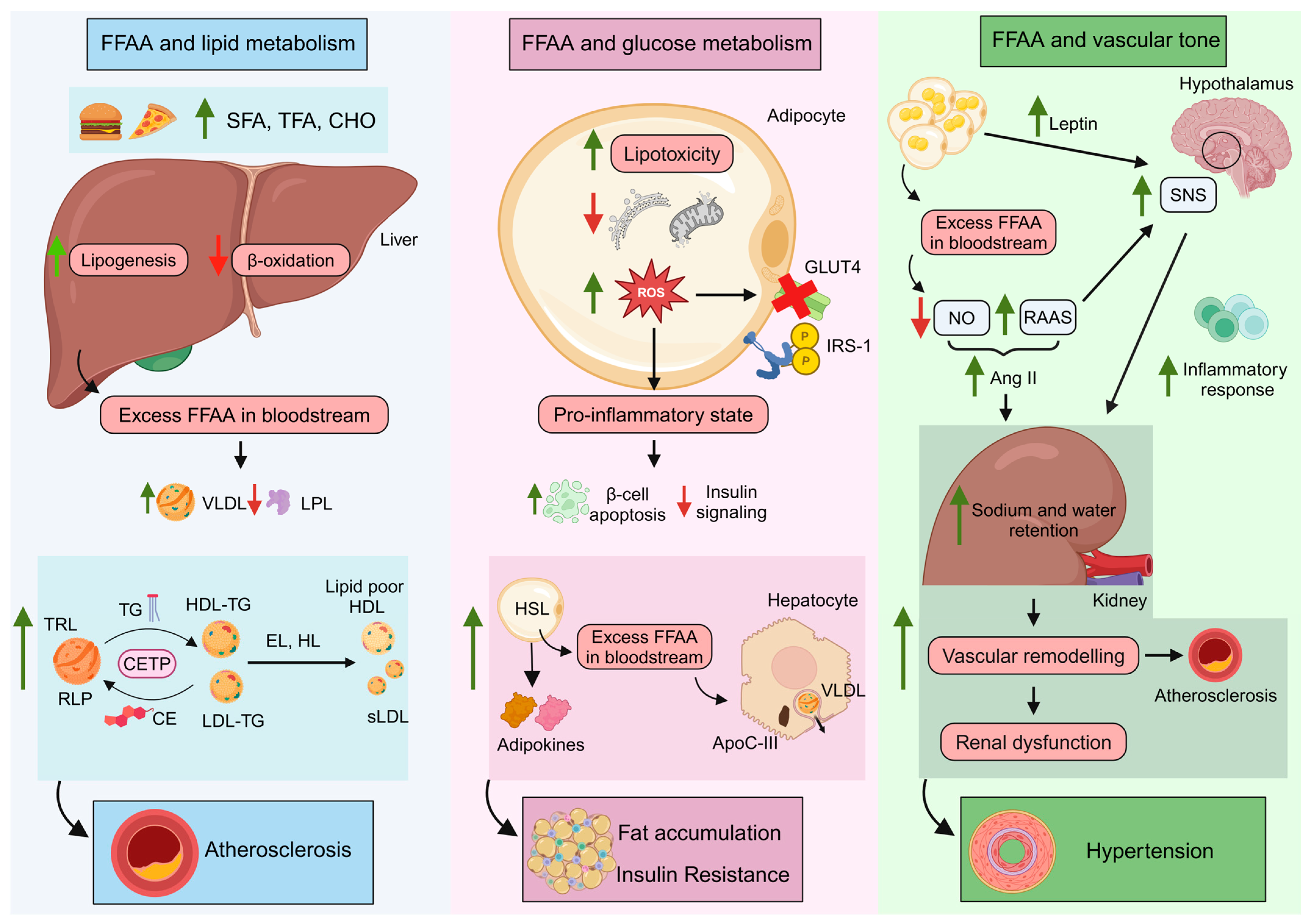
4.1. FFAA and Lipid Metabolism: TG-Rich Lipoproteins and Remnant Lipoprotein Particles
4.2. FFAA and Glucose Control
4.3. FFAA Levels and the Vascular Tone
5. The TG/HDL-C Ratio
6. Therapeutic Management to Target TG and HDL-C Levels
6.1. Lifestyle Changes
6.2. Drug Therapy
6.2.1. Effort to Improve Primary Cardiovascular Endpoints by Increasing HDL-C Levels or HDL-Mimetics
6.2.2. Others Therapeutic Strategies
7. Future Perspectives and Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette protein A1 |
| ABCG1 | ATP-binding cassette protein G1 |
| Apo | apolipoprotein |
| CAD | coronary artery disease |
| CE | cholesterol esters |
| CETP | cholesteryl ester transfer protein |
| CVD | cardiovascular disease |
| EVOO | extra virgin olive oil |
| FFAA | free fatty acids |
| FoxO1 | forkhead box protein O1 |
| HDL | high-density lipoproteins |
| IDL | intermediate-density lipoproteins |
| IR | insulin resistance |
| LCAT | lecithin–cholesterol acyltransferase |
| LDL | low-density lipoproteins |
| LDLR | LDL receptor |
| MetS | metabolic syndrome |
| MUFA | mono-saturated fatty acids |
| NO | nitric oxide |
| PL | phospholipids |
| RAAS | renin–angiotensin–aldosterone system |
| RCT | reverse cholesterol transport |
| RISK | reperfusion injury signaling kinase |
| RLP | remnant lipoprotein particles |
| ROS | reactive oxygen species |
| SAFE | survivor activating factor enhancement |
| S1P | sphingosine-1-phosphate |
| SR-BI | scavenger receptor class B type I |
| TG | triglycerides |
| TRL | TG-rich lipoprotein |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VLDL | very low-density lipoproteins |
References
- Enzi, G.; Busetto, L.; Inelmen, E.M.; Coin, A.; Sergi, G. Historical Perspective: Visceral Obesity and Related Comorbidity in Joannes Baptista Morgagni’s “De Sedibus et Causis Morborum per Anatomen Indagata”. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The Metabolic Syndrome—What Is It and How Should It Be Managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Martínez-Larrad, M.T.; Corbatón-Anchuelo, A.; Fernández-Pérez, C.; Lazcano-Redondo, Y.; Escobar-Jiménez, F.; Serrano-Ríos, M. Metabolic Syndrome, Glucose Tolerance Categories and the Cardiovascular Risk in Spanish Population. Diabetes Res. Clin. Pract. 2016, 114, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Fwu, C.-W.; Schulman, I.H.; Lawrence, J.M.; Kimmel, P.L.; Eggers, P.; Norton, J.; Chan, K.; Mendley, S.R.; Barthold, J.S. Association of Obesity, Metabolic Syndrome, and Diabetes With Urinary Incontinence and Chronic Kidney Disease: Analysis of the National Health and Nutrition Examination Survey, 2003–2020. J. Urol. 2024, 211, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.K.B. Epidemiology of Metabolic Syndrome: Global Scenario; Elsevier Inc.: Amsterdam, The Netherlands, 2024; ISBN 9780323857321. [Google Scholar]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Elabbassi, W.N.; Haddad, H.A. The Epidemic of the Metabolic Syndrome. Saudi Med. J. 2005, 26, 373–375. [Google Scholar]
- Badimon, L.; Chiva-Blanch, G. Lipid Metabolism in Dyslipidemia and Familial Hypercholesterolemia. In The Molecular Nutrition of Fats; Academic Press: Cambridge, MA, USA, 2019; pp. 307–322. ISBN 9780128112977. [Google Scholar]
- D’Aquila, T.; Hung, Y.H.; Carreiro, A.; Buhman, K.K. Recent Discoveries on Absorption of Dietary Fat: Presence, Synthesis, and Metabolism of Cytoplasmic Lipid Droplets within Enterocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 730–747. [Google Scholar]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. North. Am. 2022, 51, 437–458. [Google Scholar]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Orešič, M.; Vidal-Puig, A. A Systems Biology Approach to Study Metabolic Syndrome; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; ISBN 9783319010076. [Google Scholar]
- Moon, J.H.; Kim, K.; Choi, S.H. Lipoprotein Lipase: Is It a Magic Target for the Treatment of Hypertriglyceridemia. Endocrinol. Metab. 2022, 37, 575–586. [Google Scholar] [CrossRef]
- Borén, J.; Taskinen, M.R.; Björnson, E.; Packard, C.J. Metabolism of Triglyceride-Rich Lipoproteins in Health and Dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [PubMed]
- Bahiru, E.; Hsiao, R.; Phillipson, D.; Watson, K.E. Mechanisms and Treatment of Dyslipidemia in Diabetes. Curr. Cardiol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef]
- Karam, I. Hyperlipidemia Background and Progress. SM Atheroscler. J. 2017, 1, 1003. [Google Scholar]
- Feingold, K.R. Introduction to Lipids and Lipoproteins; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Rohrer, L.; Ohnsorg, P.M.; Lehner, M.; Landolt, F.; Rinninger, F.; von Eckardstein, A. High-Density Lipoprotein Transport through Aortic Endothelial Cells Involves Scavenger Receptor BI and ATP-Binding Cassette Transporter G1. Circ. Res. 2009, 104, 1142–1150. [Google Scholar] [CrossRef]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 224, pp. 3–51. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Nofer, J.R.; Assmann, G. High Density Lipoproteins and Arteriosclerosis. Role of Cholesterol Efflux and Reverse Cholesterol Transport. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 13–27. [Google Scholar] [CrossRef]
- Miller, G.J.; Miller, N.E. Plasma-High-Density-Lipoprotein Concentration and Development of Ischaemic Heart-Disease. Lancet 1975, 305, 16–19. [Google Scholar] [CrossRef]
- Schoch, L.; Alcover, S.; Padró, T.; Ben-Aicha, S.; Mendieta, G.; Badimon, L.; Vilahur, G. Update of HDL in Atherosclerotic Cardiovascular Disease. Clin. Investig. Arterioscler. 2023, 35, 297–314. [Google Scholar]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef]
- Vilahur, G. High-Density Lipoprotein Benefits beyond the Cardiovascular System: A Potential Key Role for Modulating Acquired Immunity through Cholesterol Efflux. Cardiovasc. Res. 2017, 113, e51–e53. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Han, G.; Liu, X.; Chen, B.; Peng, K.; Shi, Y.; Zhang, M.; Yang, Y.; Cui, J.; Song, L.; et al. Association of High-Density Lipoprotein Cholesterol with All-Cause and Cause-Specific Mortality in a Chinese Population of 3.3 Million Adults: A Prospective Cohort Study. Lancet Reg. Health West. Pac. 2024, 42, 100874. [Google Scholar] [CrossRef] [PubMed]
- Mørland, J.G.; Magnus, P.; Vollset, S.E.; Leon, D.A.; Selmer, R.; Tverdal, A. Associations between Serum High-Density Lipoprotein Cholesterol Levels and Cause-Specific Mortality in a General Population of 345 000 Men and Women Aged 20–79 Years. Int. J. Epidemiol. 2023, 52, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Li, Y.; Zhang, Y.-B.; Wang, Y.; Zhou, Y.-F.; Geng, T.; Liu, G.; Pan, A.; Liao, Y.-F. Nonlinear Relationship between High-Density Lipoprotein Cholesterol and Cardiovascular Disease: An Observational and Mendelian Randomization Analysis. Metabolism 2024, 154, 155817. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. HDL Particles—More Complex than We Thought. Thromb. Haemost. 2014, 112, 857. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Silva, R.A.G.D.; Huang, R.; Morris, J.; Fang, J.; Gracheva, E.O.; Ren, G.; Kontush, A.; Jerome, W.G.; Rye, K.A.; Davidson, W.S. Structure of Apolipoprotein A-I in Spherical High Density Lipoproteins of Different Sizes. Proc. Natl. Acad. Sci. USA 2008, 105, 12176–12181. [Google Scholar] [CrossRef]
- Rayner, K.J.; Moore, K.J. MicroRNA Control of High-Density Lipoprotein Metabolism and Function. Circ. Res. 2014, 114, 183–192. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Escate, R.; Casani, L.; Padro, T.; Pena, E.; Arderiu, G.; Mendieta, G.; Badimon, L.; Vilahur, G. High-Density Lipoprotein Remodelled in Hypercholesterolaemic Blood Induce Epigenetically Driven down-Regulation of Endothelial HIF-1a Expression in a Preclinical Animal Model. Cardiovasc. Res. 2021, 116, 1288–1299. [Google Scholar] [CrossRef]
- Wu, Q.; Sheng, Q.; Michell, D.; Ramirez-Solano, M.; Posey, O.; Phothisane, A.; Shaik, S.; Vickers, K.C.; Ormseth, M.J. Anti-Inflammatory Effect of High-Density Lipoprotein Blunted by Delivery of Altered MicroRNA Cargo in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2024, 76, 684–695. [Google Scholar] [CrossRef]
- Graham, A. Modulation of the Cellular MicroRNA Landscape: Contribution to the Protective Effects of High-Density Lipoproteins (HDL). Biolology 2023, 12, 1232. [Google Scholar] [CrossRef]
- Rossi-Herring, G.; Belmonte, T.; Rivas-Urbina, A.; Benítez, S.; Rotllan, N.; Crespo, J.; Llorente-Cortés, V.; Sánchez-Quesada, J.L.; de Gonzalo-Calvo, D. Circulating Lipoprotein-Carried MiRNome Analysis Reveals Novel VLDL-Enriched MicroRNAs That Strongly Correlate with the HDL-MicroRNA Profile. Biomed. Pharmacother. 2023, 162, 114623. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic Function of HDL Particle Subpopulations: Focus on Antioxidative Activities. Curr. Opin. Lipidol. 2010, 21, 312–318. [Google Scholar] [CrossRef]
- Oravec, S.; Demuth, K.; Myara, I.; Hornych, A. The Effect of High Density Lipoprotein Subfractions on Endothelial Eicosanoid Secretion. Thromb. Res. 1998, 92, 65–71. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2018, 26, 1644–1664. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M. Antiatherogenic Small, Dense HDL—Guardian Angel of the Arterial Wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Prats-Uribe, A.; Sayols-Baixeras, S.; Fernández-Sanlés, A.; Subirana, I.; Carreras-Torres, R.; Vilahur, G.; Civeira, F.; Marrugat, J.; Fitó, M.; Hernáez, Á.; et al. High-Density Lipoprotein Characteristics and Coronary Artery Disease: A Mendelian Randomization Study. Metabolism 2020, 112, 154351. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the Complexities of the HDL Lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Zerrad-Saadi, A.; Therond, P.; Chantepie, S.; Couturier, M.; Rye, K.-A.; Chapman, M.J.; Kontush, A. HDL3-Mediated Inactivation of LDL-Associated Phospholipid Hydroperoxides Is Determined by the Redox Status of Apolipoprotein A-I and HDL Particle Surface Lipid Rigidity: Relevance to Inflammation and Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2169–2175. [Google Scholar] [CrossRef]
- de Souza, J.A.; Vindis, C.; Nègre-Salvayre, A.; Rye, K.-A.; Couturier, M.; Therond, P.; Chantepie, S.; Salvayre, R.; Chapman, M.J.; Kontush, A. Small, Dense HDL 3 Particles Attenuate Apoptosis in Endothelial Cells: Pivotal Role of Apolipoprotein A-I. J. Cell Mol. Med. 2010, 14, 608–620. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, Dense HDL Particles Exert Potent Protection of Atherogenic LDL against Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef]
- Khoo, J.C.; Miller, E.; McLoughlin, P.; Steinberg, D. Prevention of Low Density Lipoprotein Aggregation by High Density Lipoprotein or Apolipoprotein A-I. J. Lipid Res. 1990, 31, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and Atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef]
- Jakubowski, H. The Molecular Bases of Anti-Oxidative and Anti-Inflammatory Properties of Paraoxonase 1. Antioxidants 2024, 13, 1292. [Google Scholar] [CrossRef]
- Rye, K.-A.; Barter, P.J. Cardioprotective Functions of HDLs1. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Panzenböck, U.; Stocker, R. Formation of Methionine Sulfoxide-Containing Specific Forms of Oxidized High-Density Lipoproteins. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1703, 171–181. [Google Scholar] [CrossRef]
- Brock, J.W.C.; Jenkins, A.J.; Lyons, T.J.; Klein, R.L.; Yim, E.; Lopes-Virella, M.; Carter, R.E.; Thorpe, S.R.; Baynes, J.W. Increased Methionine Sulfoxide Content of ApoA-I in Type 1 Diabetes. J. Lipid Res. 2008, 49, 847–855. [Google Scholar] [CrossRef]
- Hine, D.; MacKness, B.; MacKness, M. Cholesteryl-Ester Transfer Protein Enhances the Ability of High-Density Lipoprotein to Inhibit Low-Density Lipoprotein Oxidation. IUBMB Life 2011, 63, 772–774. [Google Scholar] [CrossRef]
- Elsøe, S.; Ahnström, J.; Christoffersen, C.; Hoofnagle, A.N.; Plomgaard, P.; Heinecke, J.W.; Binder, C.J.; Björkbacka, H.; Dahlbäck, B.; Nielsen, L.B. Apolipoprotein M Binds Oxidized Phospholipids and Increases the Antioxidant Effect of HDL. Atherosclerosis 2012, 221, 91–97. [Google Scholar] [CrossRef]
- Choi, B.G.; Vilahur, G.; Viles-Gonzalez, J.F.; Badimon, J.J. The Role of High-Density Lipoprotein Cholesterol in Atherothrombosis. Mt. Sinai J. Med. 2006, 73, 690–701. [Google Scholar]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.E.; Kingwell, B.A. High-Density Lipoprotein and Apolipoprotein AI Increase Endothelial NO Synthase Activity by Protein Association and Multisite Phosphorylation. Proc. Natl. Acad. Sci. USA 2004, 101, 6999–7004. [Google Scholar] [CrossRef]
- Beitz, J.; Förster, W. Influence of Human Low Density and High Density Lipoprotein Cholesterol on the in Vitro Prostaglandin I2 Synthetase Activity. Biochim. Biophys. Acta 1980, 620, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-Density Lipoprotein Binding to Scavenger Receptor-BI Activates Endothelial Nitric Oxide Synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, N.; Yu, S.; Yvan-Charvet, L.; Wang, N.; Mzhavia, N.; Langlois, R.; Pagler, T.; Li, R.; Welch, C.L.; Goldberg, I.J.; et al. ABCG1 and HDL Protect against Endothelial Dysfunction in Mice Fed a High-Cholesterol Diet. J. Clin. Investig. 2008, 118, 3701–3713. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-Density Lipoprotein Protects Macrophages from Oxidized Low-Density Lipoprotein-Induced Apoptosis by Promoting Efflux of 7-Ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Catapano, A.L. HDLs, Immunity, and Atherosclerosis. Curr. Opin. Lipidol. 2011, 22, 410–416. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging Role of High Density Lipoproteins as a Player in the Immune System. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef]
- Kratzer, A.; Giral, H.; Landmesser, U. High-Density Lipoproteins as Modulators of Endothelial Cell Functions: Alterations in Patients with Coronary Artery Disease. Cardiovasc. Res. 2014, 103, 350–361. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G. LDL-Cholesterol versus HDL-Cholesterol in the Atherosclerotic Plaque: Inflammatory Resolution versus Thrombotic Chaos. Ann. N. Y. Acad. Sci. 2012, 1254, 18–32. [Google Scholar] [CrossRef]
- Calkin, A.C.; Drew, B.G.; Ono, A.; Duffy, S.J.; Gordon, M.V.; Schoenwaelder, S.M.; Sviridov, D.; Cooper, M.E.; Kingwell, B.A.; Jackson, S.P. Reconstituted High-Density Lipoprotein Attenuates Platelet Function in Individuals with Type 2 Diabetes Mellitus by Promoting Cholesterol Efflux. Circulation 2009, 120, 2095–2104. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, J.L. Inhibitory Effect of High-Density Lipoprotein on Platelet Function Is Mediated by Increase in Nitric Oxide Synthase Activity in Platelets. Life Sci. 1994, 55, 1815–1821. [Google Scholar] [CrossRef]
- Viswambharan, H.; Ming, X.-F.; Zhu, S.; Hubsch, A.; Lerch, P.; Vergères, G.; Rusconi, S.; Yang, Z. Reconstituted High-Density Lipoprotein Inhibits Thrombin-Induced Endothelial Tissue Factor Expression through Inhibition of RhoA and Stimulation of Phosphatidylinositol 3-Kinase but Not Akt/Endothelial Nitric Oxide Synthase. Circ. Res. 2004, 94, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Tian, R.; Lu, N. Nitric Oxide Protected against NADPH Oxidase-Derived Superoxide Generation in Vascular Endothelium: Critical Role for Heme Oxygenase-1. Int. J. Biol. Macromol. 2019, 126, 549–554. [Google Scholar] [CrossRef]
- Han, R.; Lai, R.; Ding, Q.; Wang, Z.; Luo, X.; Zhang, Y.; Cui, G.; He, J.; Liu, W.; Chen, Y. Apolipoprotein A-I Stimulates AMP-Activated Protein Kinase and Improves Glucose Metabolism. Diabetologia 2007, 50, 1960–1968. [Google Scholar] [CrossRef]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; de Courten, B.; Forbes, J.M.; et al. High-Density Lipoprotein Modulates Glucose Metabolism in Patients with Type 2 Diabetes Mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Tabet, F.; Cochran, B.J.; Cuesta Torres, L.F.; Wu, B.J.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I Enhances Insulin-Dependent and Insulin-Independent Glucose Uptake by Skeletal Muscle. Sci. Rep. 2019, 9, 1350. [Google Scholar] [CrossRef]
- Dugani, C.B.; Klip, A. Glucose Transporter 4: Cycling, Compartments and Controversies. EMBO Rep. 2005, 6, 1137–1142. [Google Scholar] [CrossRef]
- Karlsson, H.K.R.; Chibalin, A.V.; Koistinen, H.A.; Yang, J.; Koumanov, F.; Wallberg-Henriksson, H.; Zierath, J.R.; Holman, G.D. Kinetics of GLUT4 Trafficking in Rat and Human Skeletal Muscle. Diabetes 2009, 58, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Rye, K.A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The Emerging Role of HDL in Glucose Metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 Maintains β Cell Identity and Function by Repressing an α Cell Program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef]
- Cochran, B.J.; Bisoendial, R.J.; Hou, L.; Glaros, E.N.; Rossy, J.; Thomas, S.R.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I Increases Insulin Secretion and Production from Pancreatic β-Cells via a G-Protein-CAMPPKA-FoxO1-Dependent Mechanism. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2261–2267. [Google Scholar] [CrossRef]
- Nilsson, O.; Del Giudice, R.; Nagao, M.; Grönberg, C.; Eliasson, L.; Lagerstedt, J.O. Apolipoprotein A-I Primes Beta Cells to Increase Glucose Stimulated Insulin Secretion. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165613. [Google Scholar] [CrossRef]
- Mancuso, E.; Mannino, G.C.; Fuoco, A.; Leo, A.; Citraro, R.; Averta, C.; Spiga, R.; Russo, E.; De Sarro, G.; Andreozzi, F.; et al. HDL (High-Density Lipoprotein) and ApoA-1 (Apolipoprotein A-1) Potentially Modulate Pancreatic α-Cell Glucagon Secretion. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2941–2952. [Google Scholar] [CrossRef]
- Theilmeier, G.; Schmidt, C.; Herrmann, J.; Keul, P.; Schäfers, M.; Herrgott, I.; Mersmann, J.; Larmann, J.; Hermann, S.; Stypmann, J.; et al. High-Density Lipoproteins and Their Constituent, Sphingosine-1-Phosphate, Directly Protect the Heart against Ischemia/Reperfusion Injury in Vivo via the S1P3 Lysophospholipid Receptor. Circulation 2006, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Gutiérrez, M.; Casaní, L.; Cubedo, J.; Capdevila, A.; Pons-Llado, G.; Carreras, F.; Hidalgo, A.; Badimon, L. Hypercholesterolemia Abolishes High-Density Lipoprotein-Related Cardioprotective Effects in the Setting of Myocardial Infarction. J. Am. Coll. Cardiol. 2015, 66, 2469–2470. [Google Scholar]
- Sposito, A.C.; De Lima-Junior, J.C.; Moura, F.A.; Barreto, J.; Bonilha, I.; Santana, M.; Virginio, V.W.; Sun, L.; Carvalho, L.S.F.; Soares, A.A.S.; et al. Reciprocal Multifaceted Interaction between HDL (High-Density Lipoprotein) and Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1550–1564. [Google Scholar]
- Pajkrt, D.; Doran, J.E.; Koster, F.; Lerch, P.G.; Arnet, B.; van der Poll, T.; ten Cate, J.W.; van Deventer, S.J. Antiinflammatory Effects of Reconstituted High-Density Lipoprotein during Human Endotoxemia. J. Exp. Med. 1996, 184, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Hajduk, S.L.; Moore, D.R.; Vasudevacharya, J.; Siqueira, H.; Torri, A.F.; Tytler, E.M.; Esko, J.D. Lysis of Trypanosoma Brucei by a Toxic Subspecies of Human High Density Lipoprotein. J. Biol. Chem. 1989, 264, 5210–5217. [Google Scholar] [CrossRef]
- Cho, K.-H. Structural and Functional Impairments of Reconstituted High-Density Lipoprotein by Incorporation of Recombinant β-Amyloid42. Molecules 2021, 26, 4317. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Hsiao, J.-H.T.; Halliday, G.M.; Kim, W.S. α-Synuclein Regulates Neuronal Cholesterol Efflux. Molecules 2017, 22, 1769. [Google Scholar] [CrossRef]
- Stukas, S.; Robert, J.; Wellington, C.L. High-Density Lipoproteins and Cerebrovascular Integrity in Alzheimer’s Disease. Cell Metab. 2014, 19, 574–591. [Google Scholar] [CrossRef]
- Shih, Y.-H.; Tsai, K.-J.; Lee, C.-W.; Shiesh, S.-C.; Chen, W.-T.; Pai, M.-C.; Kuo, Y.-M. Apolipoprotein C-III Is an Amyloid-β-Binding Protein and an Early Marker for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 41, 855–865. [Google Scholar] [CrossRef]
- Rader, D.J.; Hovingh, G.K. HDL and Cardiovascular Disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.T.; Lackner, S.; Mörkl, S.; Trakaki, A.; Scharnagl, H.; Borenich, A.; Wonisch, W.; Mangge, H.; Zelzer, S.; Meier-Allard, N.; et al. Obesity Affects HDL Metabolism, Composition and Subclass Distribution. Biomedicines 2021, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver Disease Alters High-Density Lipoprotein Composition, Metabolism and Function. Biochim. Biophys. Acta 2016, 1861, 630–638. [Google Scholar] [CrossRef]
- Srivastava, R.A.K. Dysfunctional HDL in Diabetes Mellitus and Its Role in the Pathogenesis of Cardiovascular Disease. Mol. Cell Biochem. 2018, 440, 167–187. [Google Scholar] [CrossRef]
- Ahmed, T.; Bowden, R.G. Assessing High-Density Lipoprotein: Shifting Focus from Quantity to Quality in Cardiovascular Disease Risk Assessment. Int. J. Transl. Med. 2024, 4, 369–380. [Google Scholar] [CrossRef]
- Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Casaní, L.; Muñoz-García, N.; Joan-Babot, O.; Peña, E.; Aržanauskaitė, M.; Gutierrez, M.; Mendieta, G.; Padró, T.; Badimon, L.; et al. HDL (High-Density Lipoprotein) Remodeling and Magnetic Resonance Imaging-Assessed Atherosclerotic Plaque Burden: Study in a Preclinical Experimental Model. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2481–2493. [Google Scholar] [CrossRef]
- Padró, T.; Cubedo, J.; Camino, S.; Béjar, M.T.; Ben-Aicha, S.; Mendieta, G.; Escolà-Gil, J.C.; Escate, R.; Gutiérrez, M.; Casani, L.; et al. Detrimental Effect of Hypercholesterolemia on High-Density Lipoprotein Particle Remodeling in Pigs. J. Am. Coll. Cardiol. 2017, 70, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Schoch, L.; Sutelman, P.; Suades, R.; Casani, L.; Padro, T.; Badimon, L.; Vilahur, G. Hypercholesterolemia-Induced HDL Dysfunction Can Be Reversed: The Impact of Diet and Statin Treatment in a Preclinical Animal Model. Int. J. Mol. Sci. 2022, 23, 8596. [Google Scholar] [CrossRef]
- Nobecourt, E.; Davies, M.J.; Brown, B.E.; Curtiss, L.K.; Bonnet, D.J.; Charlton, F.; Januszewski, A.S.; Jenkins, A.J.; Barter, P.J.; Rye, K.-A. The Impact of Glycation on Apolipoprotein A-I Structure and Its Ability to Activate Lecithin:Cholesterol Acyltransferase. Diabetologia 2007, 50, 643–653. [Google Scholar] [CrossRef]
- Hoang, A.; Murphy, A.J.; Coughlan, M.T.; Thomas, M.C.; Forbes, J.M.; O’Brien, R.; Cooper, M.E.; Chin-Dusting, J.P.F.; Sviridov, D. Advanced Glycation of Apolipoprotein A-I Impairs Its Anti-Atherogenic Properties. Diabetologia 2007, 50, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.R.; Osme, A.; Ilchenko, S.; Golizeh, M.; Lee, K.; Wang, S.; Bena, J.; Previs, S.F.; Smith, J.D.; Kasumov, T. Glycation Reduces the Stability of ApoAI and Increases HDL Dysfunction in Diet-Controlled Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 388–396. [Google Scholar] [CrossRef]
- Matsunaga, T.; Nakajima, T.; Miyazaki, T.; Koyama, I.; Hokari, S.; Inoue, I.; Kawai, S.; Shimomura, H.; Katayama, S.; Hara, A.; et al. Glycated High-Density Lipoprotein Regulates Reactive Oxygen Species and Reactive Nitrogen Species in Endothelial Cells. Metabolism 2003, 52, 42–49. [Google Scholar] [CrossRef]
- Skeggs, J.W.; Morton, R.E. LDL and HDL Enriched in Triglyceride Promote Abnormal Cholesterol Transport. J. Lipid Res. 2002, 43, 1264–1274. [Google Scholar] [CrossRef]
- Kontush, A.; De Faria, E.C.; Chantepie, S.; Chapman, M.J. A Normotriglyceridemic, Low HDL-Cholesterol Phenotype Is Characterised by Elevated Oxidative Stress and HDL Particles with Attenuated Antioxidative Activity. Atherosclerosis 2005, 182, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lagrost, L. Regulation of Cholesteryl Ester Transfer Protein (CETP) Activity: Review of in Vitro and in Vivo Studies. Biochim. Biophys. Acta 1994, 1215, 209–236. [Google Scholar] [CrossRef]
- Denimal, D.; Nguyen, A.; Pais de Barros, J.P.; Bouillet, B.; Petit, J.M.; Vergès, B.; Duvillard, L. Major Changes in the Sphingophospholipidome of HDL in Non-Diabetic Patients with Metabolic Syndrome. Atherosclerosis 2016, 246, 106–114. [Google Scholar] [CrossRef]
- Denimal, D.; Monier, S.; Brindisi, M.C.; Petit, J.M.; Bouillet, B.; Nguyen, A.; Demizieux, L.; Simoneau, I.; Pais De Barros, J.P.; Vergès, B.; et al. Impairment of the Ability of Hdl from Patients with Metabolic Syndrome but without Diabetes Mellitus to Activate ENOS: Correction by S1P Enrichment. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 804–811. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Fernandez-Garcia, D.; Gomez-Huelgas, R.; Tinahones, F.J.; Cardona, F. Adipose Tissue Gene Expression of Factors Related to Lipid Processing in Obesity. PLoS ONE 2011, 6, e24783. [Google Scholar] [CrossRef]
- Verwer, B.J.; Scheffer, P.G.; Vermue, R.P.; Pouwels, P.J.; Diamant, M.; Tushuizen, M.E. NAFLD Is Related to Post-Prandial Triglyceride-Enrichment of HDL Particles in Association with Endothelial and HDL Dysfunction. Liver Int. 2020, 40, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; Tietge, U.J.F. Role of Hepatic Lipase and Endothelial Lipase in High-Density Lipoprotein-Mediated Reverse Cholesterol Transport. Curr. Atheroscler. Rep. 2011, 13, 257–265. [Google Scholar] [CrossRef]
- Brites, F.D.; Bonavita, C.D.; De Geitere, C.; Cloës, M.; Delfly, B.; Yael, M.J.; Fruchart, J.; Wikinski, R.W.; Castro, G.R. Alterations in the Main Steps of Reverse Cholesterol Transport in Male Patients with Primary Hypertriglyceridemia and Low HDL-Cholesterol Levels. Atherosclerosis 2000, 152, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S. HDL Function and Atherosclerosis: Reactive Dicarbonyls as Promising Targets of Therapy. Circ. Res. 2023, 132, 1521–1545. [Google Scholar] [CrossRef]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Singh, P.; Anantharamaiah, G.M.; Chait, A.; Brunzell, J.; et al. The Myeloperoxidase Product Hypochlorous Acid Oxidizes HDL in the Human Artery Wall and Impairs ABCA1-Dependent Cholesterol Transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef]
- Lu, N.; Xie, S.; Li, J.; Tian, R.; Peng, Y.-Y. Myeloperoxidase-Mediated Oxidation Targets Serum Apolipoprotein A-I in Diabetic Patients and Represents a Potential Mechanism Leading to Impaired Anti-Apoptotic Activity of High Density Lipoprotein. Clin. Chim. Acta 2015, 441, 163–170. [Google Scholar] [CrossRef]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Brame, C.J.; Boutaud, O.; Davies, S.S.; Yang, T.; Oates, J.A.; Roden, D.; Roberts, L.J. Modification of Proteins by Isoketal-Containing Oxidized Phospholipids*. J. Biol. Chem. 2004, 279, 13447–13451. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Catapano, A.L. Modified HDL: Biological and Physiopathological Consequences. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, T.H.; Frikke-Schmidt, R.; Schou, J.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetic Inhibition of CETP, Ischemic Vascular Disease and Mortality, and Possible Adverse Effects. J. Am. Coll. Cardiol. 2012, 60, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Chantepie, S.; Bochem, A.E.; Chapman, M.J.; Hovingh, G.K.; Kontush, A. High-Density Lipoprotein (HDL) Particle Subpopulations in Heterozygous Cholesteryl Ester Transfer Protein (CETP) Deficiency: Maintenance of Antioxidative Activity. PLoS ONE 2012, 7, e49336. [Google Scholar]
- Gomaraschi, M.; Ossoli, A.; Pozzi, S.; Nilsson, P.; Cefalù, A.B.; Averna, M.; Kuivenhoven, J.A.; Hovingh, G.K.; Veglia, F.; Franceschini, G.; et al. ENOS Activation by HDL Is Impaired in Genetic CETP Deficiency. PLoS ONE 2014, 9, e95925. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Arranz-Martínez, E.; López-Uriarte, B.; Rivera-Teijido, M.; Palacios-Martínez, D.; Dávila-Blázquez, G.M.; Rosillo-González, A.; González-Posada Delgado, J.A.; Mariño-Suárez, J.E.; Revilla-Pascual, E.; et al. Prevalence of Hypertriglyceridemia in Adults and Related Cardiometabolic Factors. SIMETAP-HTG Study. Clin. Investig. Arterioscler. 2020, 32, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Philip, S.; Granowitz, C.; Toth, P.P.; Wong, N.D. Prevalence of US Adults with Triglycerides ≥ 150 Mg/Dl: NHANES 2007–2014. Cardiol. Ther. 2020, 9, 207–213. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a Cardiovascular Risk Factor: Current Status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef]
- Ildefonso Arocha Rodulfo, J.; Ponte Negretti, C.I.; Candia, F.A. Triglicéridos En Ayunas y Posprandiales, y Su Contribución al Estudio Del Riesgo Cardiometabólico. Clínica Investig. Arterioscler. 2009, 21, 290–297. [Google Scholar] [CrossRef]
- Koutsari, C.; Mundi, M.S.; Ali, A.H.; Patterson, B.W.; Jensen, M.D. Systemic Free Fatty Acid Disposal into Very Low-Density Lipoprotein Triglycerides. Diabetes 2013, 62, 2386–2395. [Google Scholar] [CrossRef]
- Capell, W.H.; Zambon, A.; Austin, M.A.; Brunzell, J.D.; Hokanson, J.E. Compositional Differences of LDL Particles in Normal Subjects with LDL Subclass Phenotype A and LDL Subclass Phenotype B. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patané, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged Exposure to Free Fatty Acids Has Cytostatic and Pro-Apoptotic Effects on Human Pancreatic Islets: Evidence That β-Cell Death Is Caspase Mediated, Partially Dependent on Ceramide Pathway, and Bcl-2 Regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Kambham, N.; Markowitz, G.S.; Valeri, A.M.; Lin, J.; D’Agati, V.D. Obesity-Related Glomerulopathy: An Emerging Epidemic. Kidney Int. 2001, 59, 1498–1509. [Google Scholar] [CrossRef]
- Simha, V. Management of Hypertriglyceridemia. BMJ 2020, 371, m3109. [Google Scholar] [CrossRef] [PubMed]
- Parafati, M.; Lascala, A.; Morittu, V.M.; Trimboli, F.; Rizzuto, A.; Brunelli, E.; Coscarelli, F.; Costa, N.; Britti, D.; Ehrlich, J.; et al. Bergamot Polyphenol Fraction Prevents Nonalcoholic Fatty Liver Disease via Stimulation of Lipophagy in Cafeteria Diet-Induced Rat Model of Metabolic Syndrome. J. Nutr. Biochem. 2015, 26, 938–948. [Google Scholar] [CrossRef]
- Dietschy, J.M. Dietary Fatty Acids and the Regulation of Plasma Low Density Lipoprotein Cholesterol Concentrations. J. Nutr. 1998, 128, 444S–448S. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Lichtenstein, A.H. Dietary Trans Fatty Acids and Cardiovascular Disease Risk: Past and Present. Curr. Atheroscler. Rep. 2014, 16, 433. [Google Scholar] [CrossRef]
- Matthan, N.R.; Welty, F.K.; Barrett, P.H.R.; Harausz, C.; Dolnikowski, G.G.; Parks, J.S.; Eckel, R.H.; Schaefer, E.J.; Lichtenstein, A.H. Dietary Hydrogenated Fat Increases High-Density Lipoprotein ApoA-I Catabolism and Decreases Low-Density Lipoprotein ApoB-100 Catabolism in Hypercholesterolemic Women. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1092–1097. [Google Scholar] [CrossRef]
- van Tol, A.; Zock, P.L.; van Gent, T.; Scheek, L.M.; Katan, M.B. Dietary Trans Fatty Acids Increase Serum Cholesterylester Transfer Protein Activity in Man. Atherosclerosis 1995, 115, 129–134. [Google Scholar] [CrossRef]
- Feingold, K.R. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Endotext: South Dartmouth, MA, USA, 2024. [Google Scholar]
- Softic, S.; Meyer, J.G.; Wang, G.-X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.P.M.M.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-Translational Modifications of Mitochondrial Proteins. Cell Metab. 2019, 30, 735–753.e4. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; López-Otín, C.; Madeo, F.; de Cabo, R. Carbotoxicity—Noxious Effects of Carbohydrates. Cell 2018, 175, 605–614. [Google Scholar] [CrossRef]
- Brunzell, J.D.; Hazzard, W.R.; Porte, D.J.; Bierman, E.L. Evidence for a Common, Saturable, Triglyceride Removal Mechanism for Chylomicrons and Very Low Density Lipoproteins in Man. J. Clin. Investig. 1973, 52, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Karpe, F.; Olivecrona, T.; Walldius, G.; Hamsten, A. Lipoprotein Lipase in Plasma after an Oral Fat Load: Relation to Free Fatty Acids. J. Lipid Res. 1992, 33, 975–984. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-Garcia, N.; Badimon, L. The Role of Triglycerides in the Origin and Progression of Atherosclerosis. Clin. Investig. Arterioscler. 2021, 33, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Nordestgaard, B.G. Remnant Cholesterol and Triglyceride-Rich Lipoproteins in Atherosclerosis Progression and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2133–2135. [Google Scholar]
- Nakajima, K.; Nakano, T.; Tanaka, A. The Oxidative Modification Hypothesis of Atherosclerosis: The Comparison of Atherogenic Effects on Oxidized LDL and Remnant Lipoproteins in Plasma. Clin. Chim. Acta 2006, 367, 36–47. [Google Scholar] [CrossRef]
- Meijssen, S.; Castro Cabezas, M.; Ballieux, C.G.M.; Derksen, R.J.; Bilecen, S.; Erkelens, D.W. Insulin Mediated Inhibition of Hormone Sensitive Lipase Activity in Vivo in Relation to Endogenous Catecholamines in Healthy Subjects. J. Clin. Endocrinol. Metab. 2001, 86, 4193–4197. [Google Scholar] [CrossRef]
- Sun, X.J.; Miralpeix, M.; Myers, M.G.; Glasheen, E.M.; Backer, J.M.; Kahn, C.R.; White, M.F. Expression and Function of IRS-1 in Insulin Signal Transmission. J. Biol. Chem. 1992, 267, 22662–22672. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Yu, C.; Chen, Y.; Cline, G.W.; Zhang, D.; Zong, H.; Wang, Y.; Bergeron, R.; Kim, J.K.; Cushman, S.W.; Cooney, G.J.; et al. Mechanism by Which Fatty Acids Inhibit Insulin Activation of Insulin Receptor Substrate-1 (IRS-1)-Associated Phosphatidylinositol 3-Kinase Activity in Muscle. J. Biol. Chem. 2002, 277, 50230–50236. [Google Scholar] [CrossRef] [PubMed]
- Kruszynska, Y.T.; Worrall, D.S.; Ofrecio, J.; Frias, J.P.; Macaraeg, G.; Olefsky, J.M. Fatty Acid-Induced Insulin Resistance: Decreased Muscle PI3K Activation but Unchanged Akt Phosphorylation. J. Clin. Endocrinol. Metab. 2002, 87, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhang, T.; Dong, H.H. Effect of Hepatic Insulin Expression on Lipid Metabolism in Diabetic Mice. J. Diabetes 2016, 8, 314–323. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Emerging Evidence That ApoC-III Inhibitors Provide Novel Options to Reduce the Residual CVD. Curr. Atheroscler. Rep. 2019, 21, 27. [Google Scholar] [CrossRef]
- Goyal, S.; Tanigawa, Y.; Zhang, W.; Chai, J.-F.; Almeida, M.; Sim, X.; Lerner, M.; Chainakul, J.; Ramiu, J.G.; Seraphin, C.; et al. APOC3 Genetic Variation, Serum Triglycerides, and Risk of Coronary Artery Disease in Asian Indians, Europeans, and Other Ethnic Groups. Lipids Health Dis. 2021, 20, 113. [Google Scholar] [CrossRef]
- Caron, S.; Verrijken, A.; Mertens, I.; Samanez, C.H.; Mautino, G.; Haas, J.T.; Duran-Sandoval, D.; Prawitt, J.; Francque, S.; Vallez, E.; et al. Transcriptional Activation of Apolipoprotein CIII Expression by Glucose May Contribute to Diabetic Dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, J.; Lü, J.-M.; Chai, H.; Wang, X.; Lin, P.H.; Yao, Q. Resistin Decreases Expression of Endothelial Nitric Oxide Synthase through Oxidative Stress in Human Coronary Artery Endothelial Cells. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H193–H201. [Google Scholar] [CrossRef]
- Tomita, Y.; Sakata, S.; Arima, H.; Yamato, I.; Ibaraki, A.; Ohtsubo, T.; Matsumura, K.; Fukuhara, M.; Goto, K.; Kitazono, T. Relationship between Casual Serum Triglyceride Levels and the Development of Hypertension in Japanese. J. Hypertens. 2021, 39, 677–682. [Google Scholar] [CrossRef]
- Oishi, K.; Zheng, B.; Kuo, J.F. Inhibition of Na,K-ATPase and Sodium Pump by Protein Kinase C Regulators Sphingosine, Lysophosphatidylcholine, and Oleic Acid. J. Biol. Chem. 1990, 265, 70–75. [Google Scholar] [CrossRef]
- Norlander, A.E.; Madhur, M.S.; Harrison, D.G. The Immunology of Hypertension. J. Exp. Med. 2018, 215, 21–33, Erratum in J. Exp. Med. 2018, 215, 719. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Fontana, V.; de Faria, A.P.C.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC Isoketal-Modified Proteins Activate T Cells and Promote Hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Kirabo, A.; Wu, J.; Saleh, M.A.; Zhu, L.; Wang, F.; Takahashi, T.; Loperena, R.; Foss, J.D.; Mernaugh, R.L.; et al. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II–Induced Hypertension. Circ. Res. 2015, 117, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Folkow, B. Physiological Aspects of Primary Hypertension. Physiol. Rev. 1982, 62, 347–504. [Google Scholar] [CrossRef]
- Bobik, A. The Structural Basis of Hypertension: Vascular Remodelling, Rarefaction and Angiogenesis/Arteriogenesis. J. Hypertens. 2005, 23, 1473–1475. [Google Scholar] [CrossRef][Green Version]
- Norlander, A.E.; Madhur, M.S. Inflammatory Cytokines Regulate Renal Sodium Transporters: How, Where, and Why? Am. J. Physiol.-Ren. Physiol. 2017, 313, F141–F144. [Google Scholar] [CrossRef]
- Criqui, M.H.; Heiss, G.; Cohn, R.; Cowan, L.D.; Suchindran, C.M.; Bangdiwala, S.; Kritchevsky, S.; Jacobs, D.R.J.; O’Grady, H.K.; Davis, C.E. Plasma Triglyceride Level and Mortality from Coronary Heart Disease. N. Engl. J. Med. 1993, 328, 1220–1225. [Google Scholar] [CrossRef]
- Gaziano, J.M.; Hennekens, C.H.; O’Donnell, C.J.; Breslow, J.L.; Buring, J.E. Fasting Triglycerides, High-Density Lipoprotein, and Risk of Myocardial Infarction. Circulation 1997, 96, 2520–2525. [Google Scholar] [CrossRef]
- Sultani, R.; Tong, D.C.; Peverelle, M.; Lee, Y.S.; Baradi, A.; Wilson, A.M. Elevated Triglycerides to High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio Predicts Long-Term Mortality in High-Risk Patients. Heart Lung Circ. 2020, 29, 414–421. [Google Scholar] [CrossRef]
- Bittner, V.; Johnson, B.D.; Zineh, I.; Rogers, W.J.; Vido, D.; Marroquin, O.C.; Bairey-Merz, C.N.; Sopko, G. The Triglyceride/High-Density Lipoprotein Cholesterol Ratio Predicts All-Cause Mortality in Women with Suspected Myocardial Ischemia: A Report from the Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2009, 157, 548–555. [Google Scholar] [CrossRef]
- Caselli, C.; De Caterina, R.; Smit, J.M.; Campolo, J.; El Mahdiui, M.; Ragusa, R.; Clemente, A.; Sampietro, T.; Clerico, A.; Liga, R.; et al. Triglycerides and Low HDL Cholesterol Predict Coronary Heart Disease Risk in Patients with Stable Angina. Sci. Rep. 2021, 11, 20714. [Google Scholar] [CrossRef]
- Rezapour, M.; Shahesmaeili, A.; Hossinzadeh, A.; Zahedi, R.; Najafipour, H.; Gozashti, M.H. Comparison of Lipid Ratios to Identify Metabolic Syndrome. Arch. Iran. Med. 2018, 21, 572–577. [Google Scholar] [PubMed]
- Shin, H.-G.; Kim, Y.-K.; Kim, Y.-H.; Jung, Y.-H.; Kang, H.-C. The Relationship between the Triglyceride to High-Density Lipoprotein Cholesterol Ratio and Metabolic Syndrome. Korean J. Fam. Med. 2017, 38, 352–357. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Daimon, T. Comparison of Discrimination for Cardio-Metabolic Risk by Different Cut-off Values of the Ratio of Triglycerides to HDL Cholesterol. Lipids Health Dis. 2019, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Gleeson, M.; Jeukendrup, A.E. Determination of the Exercise Intensity That Elicits Maximal Fat Oxidation. Med. Sci. Sports Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of Endogenous Fat and Carbohydrate Metabolism in Relation to Exercise Intensity and Duration. Am. J. Physiol. Endocrinol. Metab. 1993, 265, 380–391. [Google Scholar] [CrossRef]
- Fikenzer, K.; Fikenzer, S.; Laufs, U.; Werner, C. Effects of Endurance Training on Serum Lipids. Vasc. Pharmacol. 2018, 101, 9–20. [Google Scholar] [CrossRef]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of Exercise on HDL Functionality. Curr. Opin. Lipidol. 2019, 30, 16–23. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Ruiz-Ramie, J.J.; Barber, J.L.; Slentz, C.A.; Apolzan, J.W.; McGarrah, R.W.; Harris, M.N.; Church, T.S.; Borja, M.S.; He, Y.; et al. Effects of Increasing Exercise Intensity and Dose on Multiple Measures of HDL (High-Density Lipoprotein) Function. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 943–952. [Google Scholar] [CrossRef]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Vasankari, T.J. Strenuous Physical Exercise Accelerates the Lipid Peroxide Clearing Transport by HDL. Eur. J. Appl. Physiol. 2016, 116, 1683–1691. [Google Scholar] [CrossRef]
- Tiainen, S.; Luoto, R.; Ahotupa, M.; Raitanen, J.; Vasankari, T. 6-Mo Aerobic Exercise Intervention Enhances the Lipid Peroxide Transport Function of HDL. Free Radic. Res. 2016, 50, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Casella-Filho, A.; Chagas, A.C.P.; Maranhão, R.C.; Trombetta, I.C.; Cesena, F.H.Y.; Silva, V.M.; Tanus-Santos, J.E.; Negrão, C.E.; da Luz, P.L. Effect of Exercise Training on Plasma Levels and Functional Properties of High-Density Lipoprotein Cholesterol in the Metabolic Syndrome. Am. J. Cardiol. 2011, 107, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Yao, S.; Zhang, L.; Li, X.; Yang, N.; Zhao, J.; Zhao, L.; Si, Y.; Zhang, Y.; Lv, X.; et al. Walk-Run Training Improves the Anti-Inflammation Properties of High-Density Lipoprotein in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2015, 100, 870–879. [Google Scholar] [CrossRef]
- Roberts, C.K.; Ng, C.; Hama, S.; Eliseo, A.J.; Barnard, R.J. Effect of a Short-Term Diet and Exercise Intervention on Inflammatory/Anti-Inflammatory Properties of HDL in Overweight/Obese Men with Cardiovascular Risk Factors. J. Appl. Physiol. (1985) 2006, 101, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Garritson, J.D.; Boudina, S. The Effects of Exercise on White and Brown Adipose Tissue Cellularity, Metabolic Activity and Remodeling. Front. Physiol. 2021, 12, 772894. [Google Scholar]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin Are Type II Muscle Specific Myokines Protecting Pancreatic Beta-Cells against Proinflammatory Cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef]
- Wasserman, D.H.; Lacy, D.B.; Colburn, C.A.; Bracy, D.; Cherrington, A.D. Efficiency of Compensation for Absence of Fall in Insulin during Exercise. Am. J. Physiol. 1991, 261, E587–E597. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The Endocrine Coupling of Skeletal Muscle and Bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar] [CrossRef]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a Secreted Muscle Protein, Promotes Endothelial Cell Function and Revascularization in Ischemic Tissue through a Nitric-Oxide Synthase-Dependent Mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [PubMed]
- Badimon, L.; Vilahur, G.; Padro, T. Nutraceuticals and Atherosclerosis: Human Trials. Cardiovasc. Ther. 2010, 28, 202–215. [Google Scholar] [PubMed]
- Vilahur, G.; Cubedo, J.; Padró, T.; Casaní, L.; Mendieta, G.; González, A.; Badimon, L. Intake of Cooked Tomato Sauce Preserves Coronary Endothelial Function and Improves Apolipoprotein A-I and Apolipoprotein J Protein Profile in High-Density Lipoproteins. Transl. Res. 2015, 166, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Padró, T.; Casaní, L.; Mendieta, G.; López, J.A.; Streitenberger, S.; Badimon, L. Polyphenol-Enriched Diet Prevents Coronary Endothelial Dysfunction by Activating the Akt/ENOS Pathway. Rev. Española Cardiol. (Engl. Ed.) 2015, 68, 216–225. [Google Scholar] [CrossRef]
- Vilahur, G.; Badimon, L. Antiplatelet Properties of Natural Products. Vascul Pharmacol. 2013, 59, 67–75. [Google Scholar]
- López-Yerena, A.; Padro, T.; de Santisteban Villaplana, V.; Muñoz-García, N.; Pérez, A.; Vilahur, G.; Badimon, L. Vascular and Platelet Effects of Tomato Soffritto Intake in Overweight and Obese Subjects. Nutrients 2023, 15, 5084. [Google Scholar] [CrossRef]
- Padro, T.; Muñoz-García, N.; Vilahur, G.; Chagas, P.; Deyà, A.; Antonijoan, R.M.; Badimon, L. Moderate Beer Intake and Cardiovascular Health in Overweight Individuals. Nutrients 2018, 10, 1237. [Google Scholar] [CrossRef]
- Vilahur, G.; Casaní, L.; Peña, E.; Crespo, J.; Juan-Babot, O.; Ben-Aicha, S.; Mendieta, G.; Béjar, M.T.; Borrell, M.; Badimon, L. Silybum Marianum Provides Cardioprotection and Limits Adverse Remodeling Post-Myocardial Infarction by Mitigating Oxidative Stress and Reactive Fibrosis. Int. J. Cardiol. 2018, 270, 28–35. [Google Scholar] [CrossRef]
- Vilahur, G.; Sutelman, P.; Mendieta, G.; Ben-Aicha, S.; Borrell-Pages, M.; Peña, E.; Crespo, J.; Casaní, L.; Badimon, L. Triglyceride-Induced Cardiac Lipotoxicity Is Mitigated by Silybum Marianum. Atherosclerosis 2021, 324, 91–101. [Google Scholar] [CrossRef]
- Vilahur, G.; Casani, L.; Guerra, J.M.; Badimon, L. Intake of Fermented Beverages Protect against Acute Myocardial Injury: Target Organ Cardiac Effects and Vasculoprotective Effects. Basic. Res. Cardiol. 2012, 107, 291. [Google Scholar] [CrossRef] [PubMed]
- Ramón, E.; Emilio, R.; Jordi, S.-S.; Maria-Isabel, C.; Dolores, C.; Fernando, A.; Enrique, G.-G.; Valentina, R.-G.; Miquel, F.; José, L.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Wang, K.; Subbaiah, P.V. Importance of the Free Sulfhydryl Groups of Lecithin-Cholesterol Acyltransferase for Its Sensitivity to Oxidative Inactivation1The Results of These Studies Were Partly Presented at the American Heart Association Meeting in Dallas, TX, November 1998.1. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2000, 1488, 268–277. [Google Scholar] [CrossRef]
- Hernáez, A.; Farràs, M.; Fitó, M. Olive Oil Phenolic Compounds and High-Density Lipoprotein Function. Curr. Opin. Lipidol. 2016, 27, 47–53. [Google Scholar] [CrossRef]
- Hernáez, Á.; Fernández-Castillejo, S.; Farràs, M.; Catalán, Ú.; Subirana, I.; Montes, R.; Solà, R.; Muñoz-Aguayo, D.; Gelabert-Gorgues, A.; Díaz-Gil, Ó.; et al. Olive Oil Polyphenols Enhance High-Density Lipoprotein Function in Humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2115–2119. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, E673–E691. [Google Scholar] [PubMed]
- Tanaka, N.; Ishida, T.; Nagao, M.; Mori, T.; Monguchi, T.; Sasaki, M.; Mori, K.; Kondo, K.; Nakajima, H.; Honjo, T.; et al. Administration of High Dose Eicosapentaenoic Acid Enhances Anti-Inflammatory Properties of High-Density Lipoprotein in Japanese Patients with Dyslipidemia. Atherosclerosis 2014, 237, 577–583. [Google Scholar] [CrossRef]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene Intervention Reduces Inflammation and Improves HDL Functionality in Moderately Overweight Middle-Aged Individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.-Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute Consumption of Walnuts and Walnut Components Differentially Affect Postprandial Lipemia, Endothelial Function, Oxidative Stress, and Cholesterol Efflux in Humans with Mild Hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef]
- Wang, L.; Bordi, P.L.; Fleming, J.A.; Hill, A.M.; Kris-Etherton, P.M. Effect of a Moderate Fat Diet With and Without Avocados on Lipoprotein Particle Number, Size and Subclasses in Overweight and Obese Adults: A Randomized, Controlled Trial. J. Am. Heart Assoc. 2024, 4, e001355. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Sierksma, A.; van Tol, A.; Fournier, N.; van Gent, T.; Paul, J.-L.; Hendriks, H.F.J. Moderate Alcohol Consumption Increases Cholesterol Efflux Mediated by ABCA1. J. Lipid Res. 2004, 45, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, M.S.; van Tol, A.; Vermunt, S.H.F.; Scheek, L.M.; Schaafsma, G.; Hendriks, H.F.J. Alcohol Consumption Stimulates Early Steps in Reverse Cholesterol Transport. J. Lipid Res. 2001, 42, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, S.M.; Jauhiainen, M.; Ala-Korpela, M.; Metso, J.; Lehto, T.M.; Savolainen, M.J.; Hannuksela, M.L. HDL2 of Heavy Alcohol Drinkers Enhances Cholesterol Efflux from Raw Macrophages via Phospholipid-Rich HDL2b Particles. Alcohol. Clin. Exp. Res. 2008, 32, 991–1000. [Google Scholar] [CrossRef]
- Casani, L.; Segales, E.; Vilahur, G.; Bayes De Luna, A.; Badimon, L. Moderate Daily Intake of Red Wine Inhibits Mural Thrombosis and Monocyte Tissue Factor Expression in an Experimental Porcine Model. Circulation 2004, 110, 460–465. [Google Scholar] [CrossRef]
- Giacco, R.; Costabile, G.; Della Pepa, G.; Anniballi, G.; Griffo, E.; Mangione, A.; Cipriano, P.; Viscovo, D.; Clemente, G.; Landberg, R.; et al. A Whole-Grain Cereal-Based Diet Lowers Postprandial Plasma Insulin and Triglyceride Levels in Individuals with Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 837–844. [Google Scholar] [CrossRef]
- Bozzetto, L.; Annuzzi, G.; Costabile, G.; Costagliola, L.; Giorgini, M.; Alderisio, A.; Strazzullo, A.; Patti, L.; Cipriano, P.; Mangione, A.; et al. A CHO/Fibre Diet Reduces and a MUFA Diet Increases Postprandial Lipaemia in Type 2 Diabetes: No Supplementary Effects of Low-Volume Physical Training. Acta Diabetol. 2014, 51, 385–393. [Google Scholar] [CrossRef]
- Lambert, C.; Cubedo, J.; Padró, T.; Vilahur, G.; López-Bernal, S.; Rocha, M.; Hernández-Mijares, A.; Badimon, L. Effects of a Carob-Pod-Derived Sweetener on Glucose Metabolism. Nutrients 2018, 10, 271. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Gibson, C.M.; Duffy, D.; Korjian, S.; Bahit, M.C.; Chi, G.; Alexander, J.H.; Lincoff, A.M.; Heise, M.; Tricoci, P.; Deckelbaum, L.I.; et al. Apolipoprotein A1 Infusions and Cardiovascular Outcomes after Acute Myocardial Infarction. N. Engl. J. Med. 2024, 390, 1560–1571. [Google Scholar] [CrossRef]
- Guyton, J.R.; Slee, A.E.; Anderson, T.; Fleg, J.L.; Goldberg, R.B.; Kashyap, M.L.; Marcovina, S.M.; Nash, S.D.; O’Brien, K.D.; Weintraub, W.S.; et al. Relationship of Lipoproteins to Cardiovascular Events: The AIM-HIGH Trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 2013, 62, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.G.; Zhao, X.Q.; Chait, A.; Fisher, L.D.; Cheung, M.C.; Morse, J.S.; Dowdy, A.A.; Marino, E.K.; Bolson, E.L.; Alaupovic, P.; et al. Simvastatin and Niacin, Antioxidant Vitamins, or the Combination for the Prevention of Coronary Disease. N. Engl. J. Med. 2001, 345, 1583–1592. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, C.; Wu, G. Dietary Niacin Intake and Its Association with All-Cause and Cardiovascular Mortality Rates in Individuals with Metabolic Syndrome. Nutr. J. 2024, 23, 90. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Metzinger, M.P.; Saldanha, S.; Gulati, J.; Patel, K.V.; El-Ghazali, A.; Deodhar, S.; Joshi, P.H.; Ayers, C.; Rohatgi, A. Effect of Anacetrapib on Cholesterol Efflux Capacity: A Substudy of the DEFINE Trial. J. Am. Heart Assoc. 2020, 9, e018136. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.J.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of Long-Term Fenofibrate Therapy on Cardiovascular Events in 9795 People with Type 2 Diabetes Mellitus (the FIELD Study): Randomised Controlled Trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; et al. Gemfibrozil for the Secondary Prevention of Coronary Heart Disease in Men with Low Levels of High-Density Lipoprotein Cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 1999, 341, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; Probstfield, J.; et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary Prevention of Macrovascular Events in Patients with Type 2 Diabetes in the PROactive Study (PROspective PioglitAzone Clinical Trial In MacroVascular Events): A Randomised Controlled Trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.J.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Zheng, R.; Lind, L. A Combined Observational and Mendelian Randomization Investigation Reveals NMR-Measured Analytes to Be Risk Factors of Major Cardiovascular Diseases. Sci. Rep. 2024, 14, 10645. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Miao, Z.; Zhang, N.R.; Hennessy, S.; Small, D.S.; Rader, D.J. A Mendelian Randomization Study of the Role of Lipoprotein Subfractions in Coronary Artery Disease. eLife 2021, 10, e58361. [Google Scholar] [CrossRef]
| Drug Class | Example(s) | Doses (Oral) | Clinical Indications (Type of Patients) | Positive Outcomes | Clinical Outcomes | Reference |
|---|---|---|---|---|---|---|
| Vitamin B3 | Niacin | 500–2000 mg/d | CVD, MetS | ↑HDL by ~15–30%, ↓TG by ~20–50% | No effect or ↓CV events | [217,218,219] |
| CETP Inhibitors | Dalcetrapib; | 600 mg/d | Recent acute coronary syndrome; | ↑HDL by ~31–40%; no significant ↓TG; | No significant ↓CV events; | [220] |
| Evacetrapib; | 130 mg/d | Acute coronary syndrome, cerebrovascular disease, T2DM with CVD; | ↑HDL by ~130%, ↓TG by ~6%; | No significant ↓CV events; | [221] | |
| Torceratrip | 60 mg/d | High CV risk; | ↑HDL by ~72%, ↓TG by ~10%; | ↑CV events, ↑death; | [222] | |
| Anacetrapib | 100 mg/d | High CVD risk, low HDL levels | ↑HDL by ~145%, ↓TG by ~9% | ↓CV events (men) | [223] | |
| Omega-3 Fatty Acids | IPE | 4 g/d | High CV risk or T2DM | ↑HDL by ~3%, ↓TG by ~20% | ↓CV events | [224] |
| EPA + DHA | 4 g/d | High risk of CVD with low HDL, elevated TG | ↑HDL by ~5%, ↓TG by ~19% | No significant ↓MACE | [225] | |
| Fibrates | Fenofibrate | 200 mg/d | T2DM | ↑HDL by ~5%, ↓TG by ~29% | No significant ↓primary outcome | [226] |
| Gemfibrozil | 1200 mg/d | CVD, low HDL, elevated TG | ↑HDL by ~6%, ↓TG by ~31% | ↓MACE | [227] | |
| Statin + Fibrate | Simvastatin + Fenofibrate | 40 + 160 mg/d | Low HDL, high TG | ↑HDL by ~8%, ↓TG by ~26% | No significant ↓primary outcome | [228] |
| Thiazolidinediones | Pioglitazone | 15–45 mg/d, | T2DM with CVD | ↑HDL by ~19%, ↓TG by ~11% | ↓CV events, ↓death | [229] |
| Statins | Rosuvastatin | 20 mg/d | High CV risk | No significant ↑HDL, ↓TG by ~19% | ↓MACE | [230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcover, S.; Ramos-Regalado, L.; Girón, G.; Muñoz-García, N.; Vilahur, G. HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome. Antioxidants 2025, 14, 434. https://doi.org/10.3390/antiox14040434
Alcover S, Ramos-Regalado L, Girón G, Muñoz-García N, Vilahur G. HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome. Antioxidants. 2025; 14(4):434. https://doi.org/10.3390/antiox14040434
Chicago/Turabian StyleAlcover, Sebastià, Lisaidy Ramos-Regalado, Gabriela Girón, Natàlia Muñoz-García, and Gemma Vilahur. 2025. "HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome" Antioxidants 14, no. 4: 434. https://doi.org/10.3390/antiox14040434
APA StyleAlcover, S., Ramos-Regalado, L., Girón, G., Muñoz-García, N., & Vilahur, G. (2025). HDL-Cholesterol and Triglycerides Dynamics: Essential Players in Metabolic Syndrome. Antioxidants, 14(4), 434. https://doi.org/10.3390/antiox14040434








