In Vitro Effects of Lipopolysaccharide on Rabbit Sperm: Toll-like Receptor 4 Expression, Motility, and Oxidative Status
Abstract
1. Introduction
2. Materials and Methods
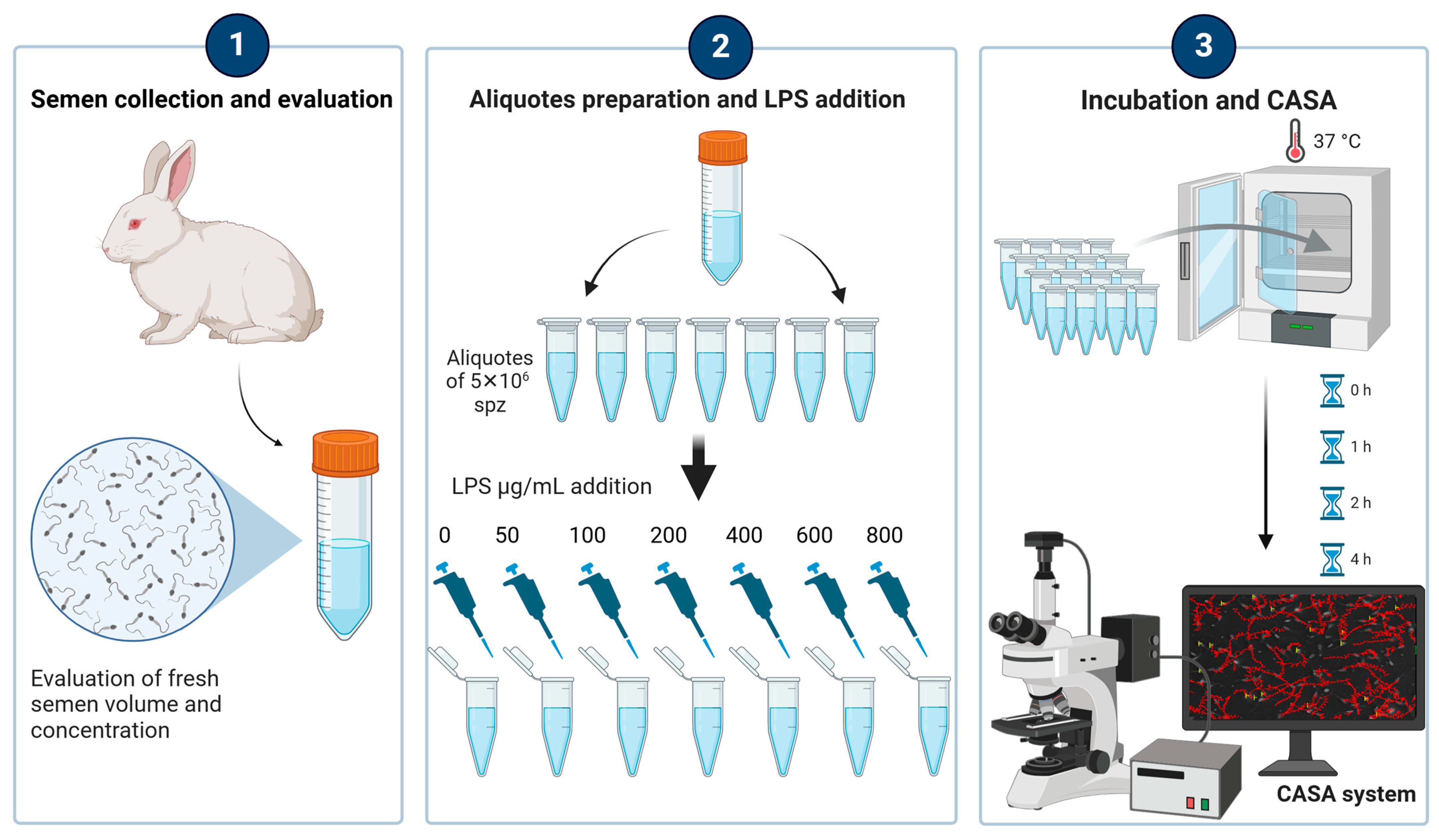
2.1. Animals and Experimental Design
2.2. Semen Collection and Analysis
2.3. Measurement of Lipid Peroxidation: Malondialdehyde (MDA) Assay
2.4. Expression of Toll-like Receptor-4 in Rabbit Sperm with Immunofluorescence Staining
2.5. Statistical Analysis
3. Results
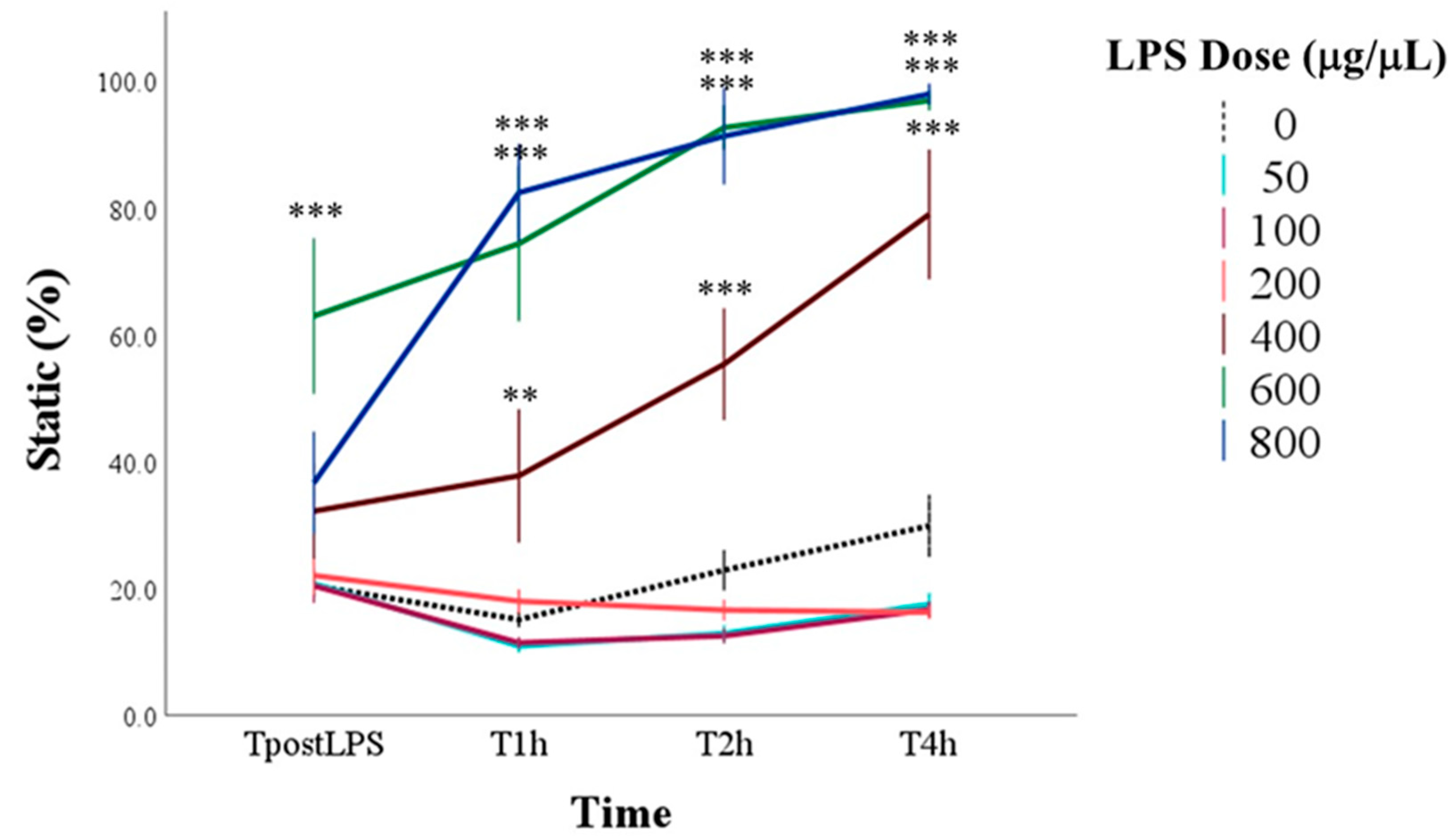
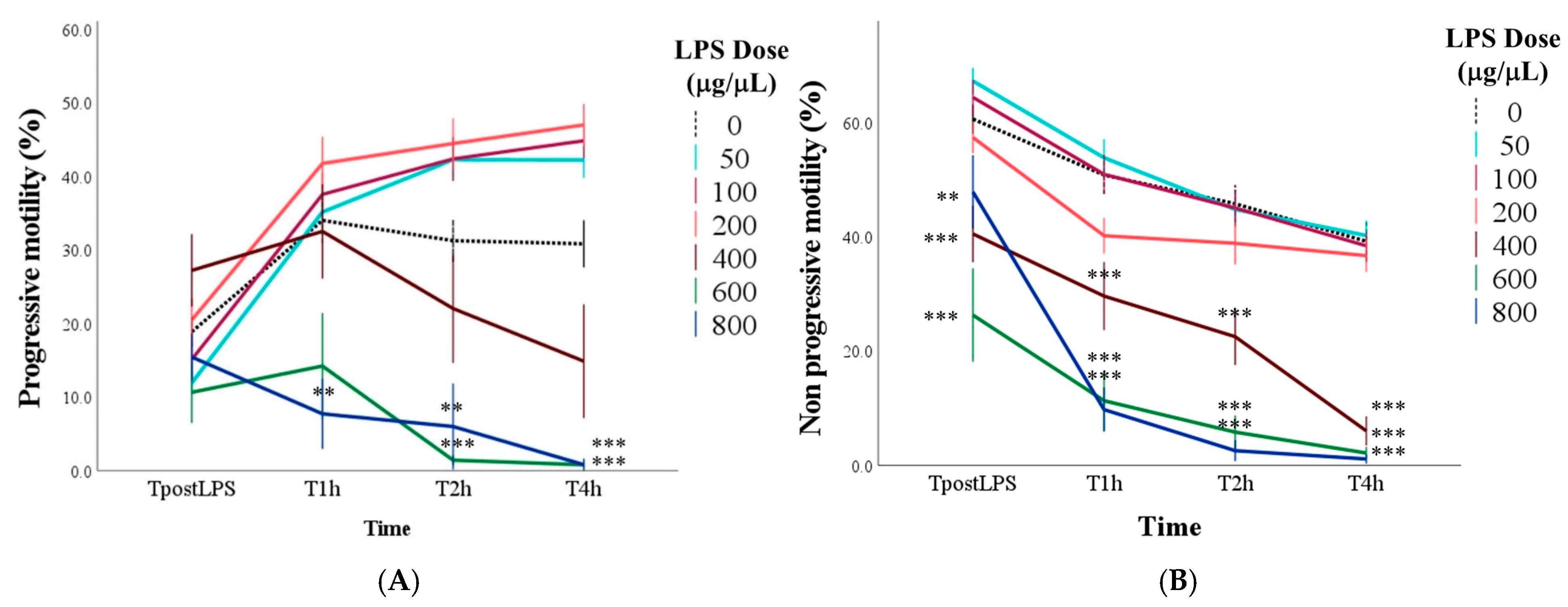
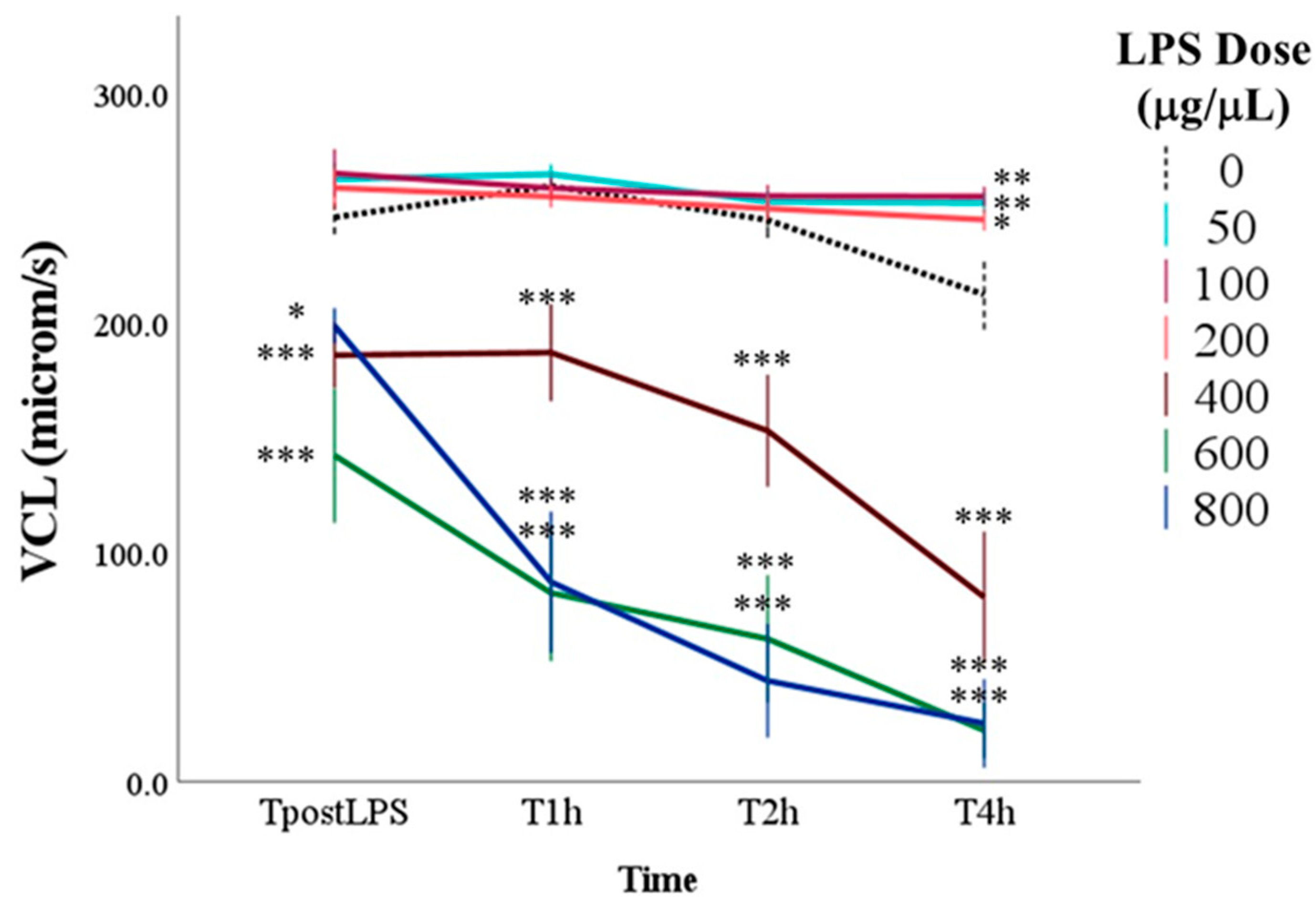
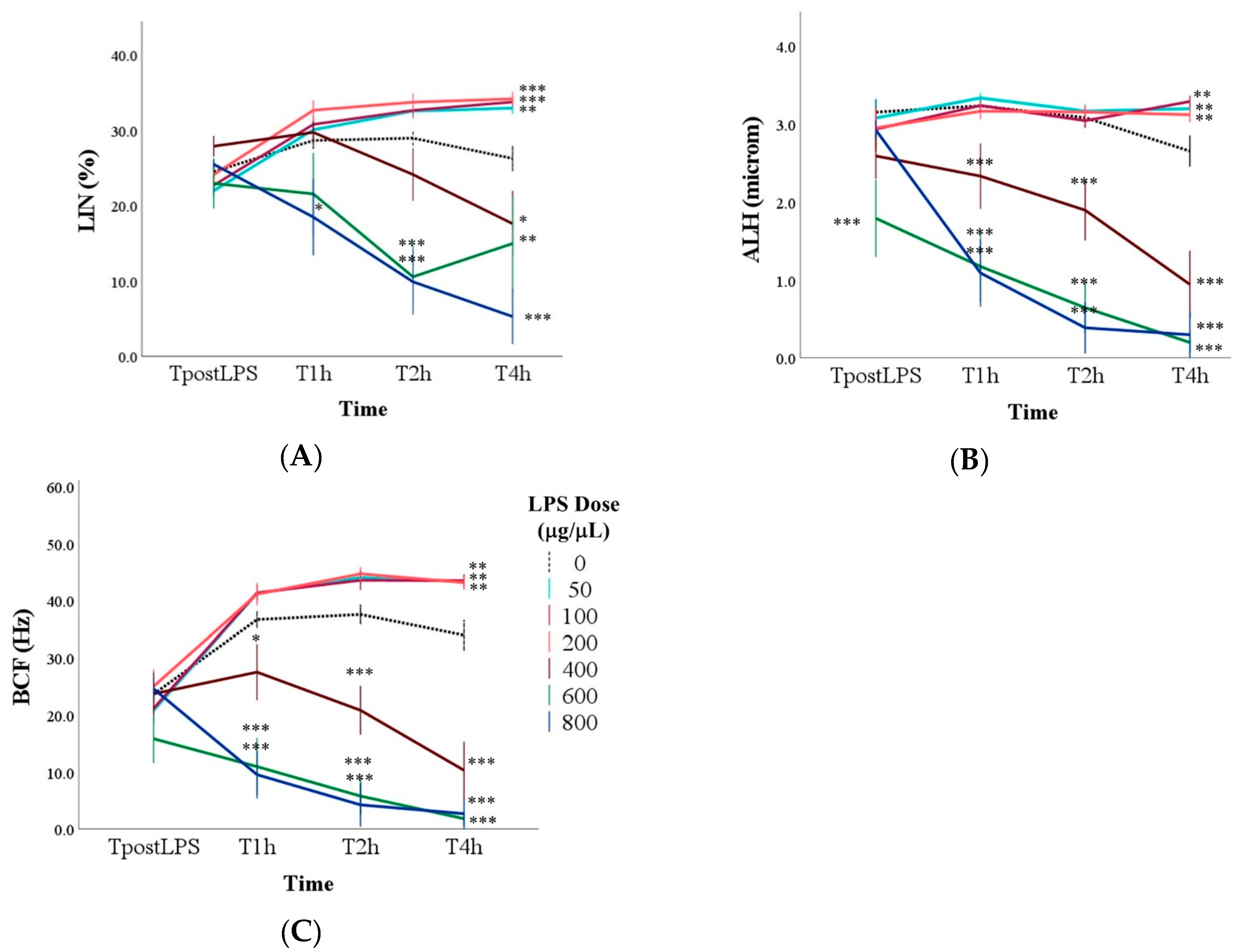
3.1. Effect of LPS on Sperm Motility
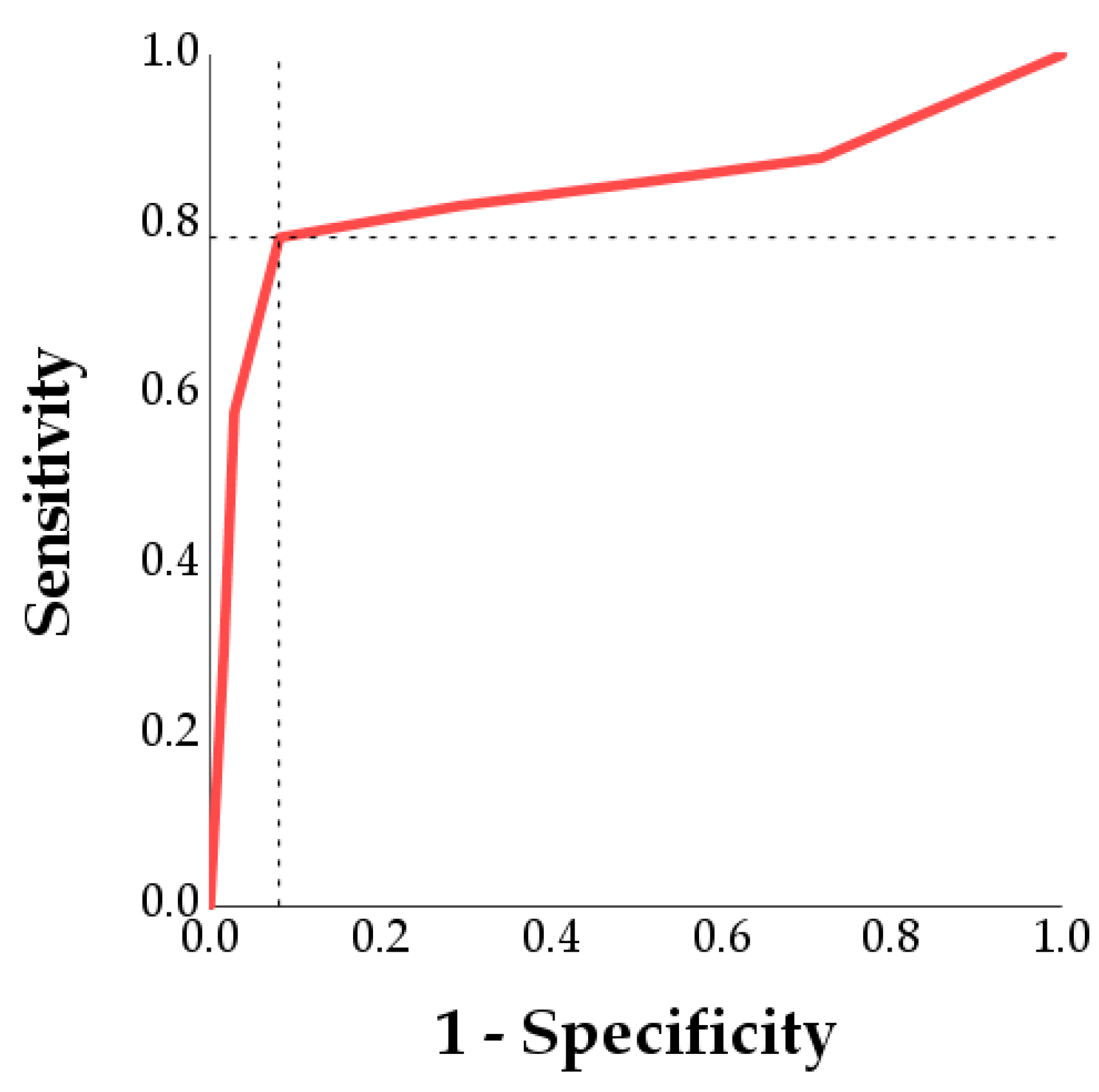
3.2. Determination of the Optimal LPS Dose to Induce Spermatic Damage
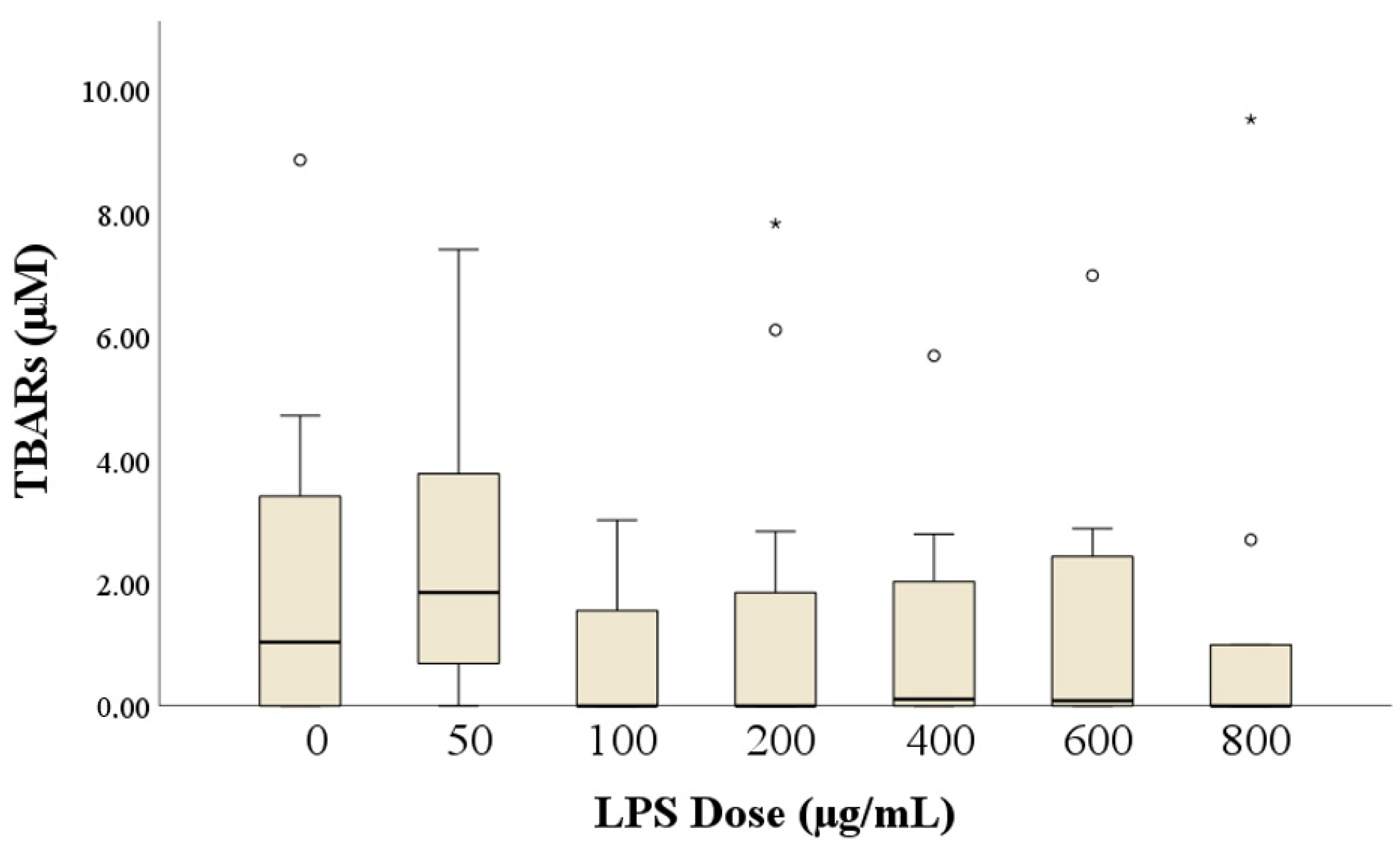
3.3. Assessment of Lipid Peroxidation: Malondialdehyde (MDA) Levels
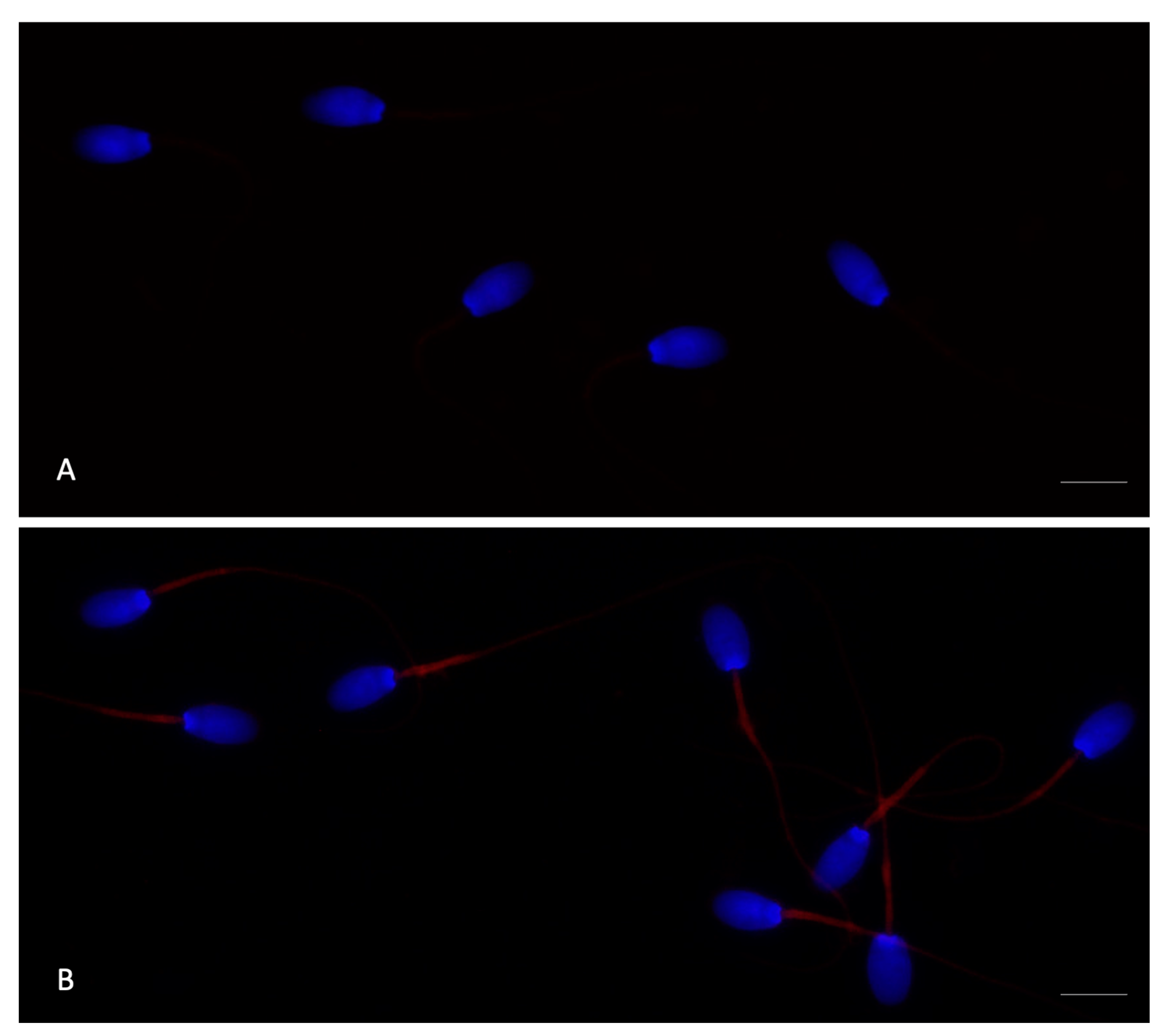
3.4. Expression of TLR4 in Rabbit Sperm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidner, W.; Pilatz, A.; Diemer, T.; Schuppe, H.C.; Rusz, A.; Wagenlehner, F. Male Urogenital Infections: Impact of Infection and Inflammation on Ejaculate Parameters. World J. Urol. 2013, 31, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, H.-C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic Orchitis: A Neglected Cause of Male Infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Brecchia, G.; Kuželová, L.; Arruda, J.; Mourvaki, E.; Castellini, C. Cytokines Release and Oxidative Status in Semen Samples from Rabbits Treated with Bacterial Lipopolysaccharide. Theriogenology 2015, 83, 1233–1240. [Google Scholar] [CrossRef]
- Brecchia, G.; Menchetti, L.; Cardinali, R.; Castellini, C.; Polisca, A.; Zerani, M.; Maranesi, M.; Boiti, C. Effects of a Bacterial Lipopolysaccharide on the Reproductive Functions of Rabbit Does. Anim. Reprod. Sci. 2014, 147, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Menchetti, L.; Barbato, O.; Filipescu, I.E.; Traina, G.; Leonardi, L.; Polisca, A.; Troisi, A.; Guelfi, G.; Piro, F.; Brecchia, G. Effects of Local Lipopolysaccharide Administration on the Expression of Toll-like Receptor 4 and pro-Inflammatory Cytokines in Uterus and Oviduct of Rabbit Does. Theriogenology 2018, 107, 162–174. [Google Scholar] [CrossRef]
- Brecchia, G.; Cardinali, R.; Mourvaki, E.; Collodel, G.; Moretti, E.; Dal Bosco, A.; Castellini, C. Short- and Long-Term Effects of Lipopolysaccharide-Induced Inflammation on Rabbit Sperm Quality. Anim. Reprod. Sci. 2010, 118, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.R.; Ribeiro, C.M.; Mirim, A.F.M.; Silva, A.A.S.; Romano, R.M.; Hallak, J.; Avellar, M.C.W. Lipopolysaccharide and Lipotheicoic Acid Differentially Modulate Epididymal Cytokine and Chemokine Profiles and Sperm Parameters in Experimental Acute Epididymitis. Sci. Rep. 2018, 8, 103. [Google Scholar] [CrossRef]
- Marchiani, S.; Baccani, I.; Tamburrino, L.; Mattiuz, G.; Nicolò, S.; Bonaiuto, C.; Panico, C.; Vignozzi, L.; Antonelli, A.; Rossolini, G.M.; et al. Effects of Common Gram-Negative Pathogens Causing Male Genitourinary-Tract Infections on Human Sperm Functions. Sci. Rep. 2021, 11, 19177. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, D.; Li, X.; Gong, W.; Wu, F.; Guo, X.; Xiao, H.; Liu, L.; Zhou, H. TLR Signalling Affects Sperm Mitochondrial Function and Motility via Phosphatidylinositol 3-Kinase and Glycogen Synthase Kinase-3α. Cell Signal. 2016, 28, 148–156. [Google Scholar] [CrossRef]
- Urata, K.; Narahara, H.; Tanaka, Y.; Egashira, T.; Takayama, F.; Miyakawa, I. Effect of Endotoxin-Induced Reactive Oxygen Species on Sperm Motility. Fertil. Steril. 2001, 76, 163–166. [Google Scholar] [CrossRef]
- Reddy, M.M.; Mahipal, S.V.K.; Subhashini, J.; Reddy, M.C.; Roy, K.R.; Reddy, G.V.; Reddy, P.R.K.; Reddanna, P. Bacterial Lipopolysaccharide-Induced Oxidative Stress in the Impairment of Steroidogenesis and Spermatogenesis in Rats. Reprod. Toxicol. 2006, 22, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Sibley, K.; Miller, A.N.; Lane, E.A.; Fishwick, J.; Nash, D.M.; Herath, S.; England, G.C.W.; Dobson, H.; Sheldon, I.M. Original Article: The Effect of Escherichia coli Lipopolysaccharide and Tumour Necrosis Factor Alpha on Ovarian Function. Am. J. Rep. Immunol. 2008, 60, 462–473. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Roberts, M.H. Toll-Like Receptor 4 Mediates the Response of Epithelial and Stromal Cells to Lipopolysaccharide in the Endometrium. PLoS ONE 2010, 5, e12906. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Schlatt, S.; Phillips, D.J.; De Kretser, D.M.; Hedger, M.P. Bacterial Lipopolysaccharide-Induced Inflammation Compromises Testicular Function at Multiple Levels In Vivo 1. Endocrinology 2000, 141, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wu, K.-H.; Wu, H.-P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef]
- Collodel, G.; Castellini, C.; Del Vecchio, M.; Cardinali, R.; Geminiani, M.; Rossi, B.; Spreafico, A.; Moretti, E. Effect of a Bacterial Lipopolysaccharide Treatment on Rabbit Testis and Ejaculated Sperm. Reprod. Domest. Anim. 2012, 47, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, H.; Rastgar Rezaei, Y.; Nikanfar, S.; Zarezadeh, R.; Sadegi, M.; Latifi, Z.; Nouri, M.; Fattahi, A.; Ahmadi, Y.; Bleisinger, N. Effects of Bacteria on Male Fertility: Spermatogenesis and Sperm Function. Life Sci. 2020, 256, 117891. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; He, Y.; Ding, Z.; Mao, F.; Luo, T.; Zhang, X. Lipopolysaccharide Compromises Human Sperm Function by Reducing Intracellular cAMP. Tohoku J. Exp. Med. 2016, 238, 105–112. [Google Scholar] [CrossRef]
- He, B.; Guo, H.; Gong, Y.; Zhao, R. Lipopolysaccharide-Induced Mitochondrial Dysfunction in Boar Sperm Is Mediated by Activation of Oxidative Phosphorylation. Theriogenology 2017, 87, 1–8. [Google Scholar] [CrossRef]
- Makvandi, A.; Kowsar, R.; Hajian, M.; Mahdavi, A.H.; Tanhaei Vash, N.; Nasr-Esfahani, M.H. Alpha Lipoic Acid Reverses the Negative Effect of LPS on Mouse Spermatozoa and Developmental Competence of Resultant Embryos in Vitro. Andrology 2019, 7, 350–356. [Google Scholar] [CrossRef]
- Aitken, R. Free Radicals, Lipid Peroxidation and Sperm Function. Reprod. Fertil. Dev. 1995, 7, 659. [Google Scholar] [CrossRef] [PubMed]
- Oborna, I.; Wojewodka, G.; De Sanctis, J.B.; Fingerova, H.; Svobodova, M.; Brezinova, J.; Hajduch, M.; Novotny, J.; Radova, L.; Radzioch, D. Increased Lipid Peroxidation and Abnormal Fatty Acid Profiles in Seminal and Blood Plasma of Normozoospermic Males from Infertile Couples. Hum. Reprod. 2010, 25, 308–316. [Google Scholar] [CrossRef]
- Blas, C.; Wiseman, J. Nutrition of the Rabbit, 2nd ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-669-3. [Google Scholar]
- Boiti, C.; Castellini, C.; Besenfelder, U.; Theau-Clément, M.; Liguori, L. Guidelines for the Handling of Rabbit Bucks and Semen. World Rabbit Sci. 2005, 13, 71–91. [Google Scholar] [CrossRef][Green Version]
- Castellini, C.; Dal Bosco, A.; Ruggeri, S.; Collodel, G. What Is the Best Frame Rate for Evaluation of Sperm Motility in Different Species by Computer-Assisted Sperm Analysis? Fertil. Steril. 2011, 96, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Bonet, S.; Rodríguez-Gil, J.E.; Del Álamo, M.M.R. Evaluation of Sperm Motility with CASA-Mot: Which Factors May Influence Our Measurements? Reprod. Fertil. Dev. 2018, 30, 789. [Google Scholar] [CrossRef]
- Montgomery, D.C. Introduction to Statistical Quality Control, 7th ed.; J. Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-14681-1. [Google Scholar]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and Practical Application of the Receiver-Operating Characteristic Analysis for Diagnostic Tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef]
- Shibahara, H.; Obara, H.; Ayustawati; Hirano, Y.; Suzuki, T.; Ohno, A.; Takamizawa, S.; Suzuki, M. Prediction of Pregnancy by Intrauterine Insemination Using CASA Estimates and Strict Criteria in Patients with Male Factor Infertility. Int. J. Androl. 2004, 27, 63–68. [Google Scholar] [CrossRef]
- Di Iorio, M.; Lauriola, F.; Rusco, G.; Antenucci, E.; Schiavitto, M.; Iaffaldano, N. Cryopreserving Rabbit Semen: Impact of Varying Sperm Concentrations on Quality and the Standardization of Protocol. Vet. Sci. 2023, 11, 9. [Google Scholar] [CrossRef]
- Hagen, D.R.; Gilkey, A.L.; Foote, R.H. Spermatozoal velocity and motilityand its relationship to fertility in the rabbit inseminated with low sperm numbers. World Rabbit Sci. 2010, 10, 135–140. [Google Scholar] [CrossRef][Green Version]
- Lavara, R.; Mocé, E.; Lavara, F.; Viudes De Castro, M.P.; Vicente, J.S. Do Parameters of Seminal Quality Correlate with the Results of On-Farm Inseminations in Rabbits? Theriogenology 2005, 64, 1130–1141. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Türk, G.; Cantürk, F.; Yay, A.; Eken, A.; Akçay, A. The Effect of Bovine Serum Albumin and Fetal Calf Serum on Sperm Quality, DNA Fragmentation and Lipid Peroxidation of the Liquid Stored Rabbit Semen. Cryobiology 2013, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.-M.; Theau-Clément, M.; Bolet, G. The Relationship between Rabbit Semen Characteristics and Reproductive Performance after Artificial Insemination. Anim. Reprod. Sci. 2002, 70, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Mirshokraei, P.; Hassanpour, H.; Ragh, M.J.; Tajik, P.; Arab, H.A. In Vitro Effects of Bacterial Lipopolysaccharide (LPS) on Motion Parameters of Ram Epididymal Sperm. Comp. Clin. Pathol. 2010, 19, 329–334. [Google Scholar] [CrossRef]
- Foote, R.H. Fertility of Rabbit Sperm Exposed in Vitro to Cadmium and Lead. Reprod. Toxicol. 1999, 13, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Mihara, T.; Okazaki, T.; Shitanaka, M.; Kushino, R.; Ikeda, C.; Negishi, H.; Liu, Z.; Richards, J.S.; Shimada, M. Toll-like Receptors (TLR) 2 and 4 on Human Sperm Recognize Bacterial Endotoxins and Mediate Apoptosis. Hum. Reprod. 2011, 26, 2799–2806. [Google Scholar] [CrossRef]
- Song, T.; Shi, Y.; Wang, Y.; Qazi, I.H.; Angel, C.; Zhang, M. Implication of Polyhistidine, a Novel Apoptosis Inhibitor, in Inhibiting Lipopolysaccharide-Induced Apoptosis in Boar Sperm. Animals 2019, 9, 719. [Google Scholar] [CrossRef]
- O’Doherty, A.M.; Di Fenza, M.; Kölle, S. Lipopolysaccharide (LPS) Disrupts Particle Transport, Cilia Function and Sperm Motility in an Ex Vivo Oviduct Model. Sci. Rep. 2016, 6, 24583. [Google Scholar] [CrossRef]
- Lasko, J.; Schlingmann, K.; Klocke, A.; Mengel, G.A.; Turner, R. Calcium/Calmodulin and cAMP/Protein Kinase-A Pathways Regulate Sperm Motility in the Stallion. Anim. Reprod. Sci. 2012, 132, 169–177. [Google Scholar] [CrossRef]
- Jansen, V.; Alvarez, L.; Balbach, M.; Strünker, T.; Hegemann, P.; Kaupp, U.B.; Wachten, D. Controlling Fertilization and cAMP Signaling in Sperm by Optogenetics. eLife 2015, 4, e05161. [Google Scholar] [CrossRef]
- Sahnoun, S.; Sellami, A.; Chakroun, N.; Mseddi, M.; Attia, H.; Rebai, T.; Lassoued, S. Human Sperm Toll-like Receptor 4 (TLR4) Mediates Acrosome Reaction, Oxidative Stress Markers, and Sperm Parameters in Response to Bacterial Lipopolysaccharide in Infertile Men. J. Assist. Reprod. Genet. 2017, 34, 1067–1077. [Google Scholar] [CrossRef]
- Metukuri, M.R.; Reddy, C.M.T.; Reddy, P.R.K.; Reddanna, P. Bacterial LPS Mediated Acute Inflammation-Induced Spermatogenic Failure in Rats: Role of Stress Response Proteins and Mitochondrial Dysfunction. Inflammation 2010, 33, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Vaure, C.; Liu, Y. A Comparative Review of Toll-Like Receptor 4 Expression and Functionality in Different Animal Species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z.H. Oxidative Stress Induced Sperm DNA Damage, a Possible Reason for Male Infertility. Iran. J. Reprod. Med. 2015, 13, 525–532. [Google Scholar]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen Characteristics and Malondialdehyde Levels in Men with Different Reproductive Problems. Andrology 2015, 3, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zribi, N.; Chakroun, N.; Elleuch, H.; Abdallah, F.; Ben Hamida, A.; Gargouri, J.; Fakhfakh, F.; Keskes, L. Sperm DNA Fragmentation and Oxidation Are Independent of Malondialdheyde. Reprod. Biol. Endocrinol. 2011, 9, 47. [Google Scholar] [CrossRef]
- Kuželová, L.; Svoradová, A.; Baláži, A.; Vašíček, J.; Langraf, V.; Kolesárová, A.; Sláma, P.; Chrenek, P. Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine. Antioxidants 2024, 13, 1360. [Google Scholar] [CrossRef]
| Parameter | Description | Definition | Units |
|---|---|---|---|
| Static | Immotile spermatozoa | % | |
| Progressive motility | Progressively motile spermatozoa | % | |
| Non-progressive motility | Spermatozoa that are moving but not progressing forward | % | |
| VCL | Curvilinear velocity | Velocity of the sperm head along its actual curvilinear path | μm/s |
| LIN | Linearity | Linearity of the curvilinear path (VS.L/VCL ratio) | % |
| ALH | Amplitude of lateral head displacement | The average value of the extreme side-to-side movement of the sperm head in each beat cycle | μm |
| BCF | Beat-cross frequency | Frequency of the head crossing the average path trajectory | Hz |
| Parameter | Control | Acceptability Limit | Optimal Minimal LPS Dosage to Overcome the Acceptability Limit | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | |||||
| Static | 22.1% | 19.8% | 41.9% | 300 μg/mL | 78.5% | 91.9% |
| Non-progressive motility | 49.1% | 21.2% | 27.97% | 300 μg/mL | 50.6% | 91.9% |
| Progressive motility | 28.8% | 18.3% | 10.50% | 300 μg/mL | 90.0% | 54.1% |
| VCL 1 | 240.36 | 62.61 | 177.75 | 300 μg/mL | 90.6% | 78.6% |
| LIN 2 | 27.10 | 7.62 | 19.48 | 300 μg/mL | 85.1% | 55.2% |
| ALH 3 | 3.03 | 0.94 | 2.09 | 300 μg/mL | 89.6% | 64.3% |
| BCF 4 | 32.97 | 13.02 | 19.96 | 300 μg/mL | 89.4% | 57.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quattrone, A.; Fehri, N.E.; Agradi, S.; Menchetti, L.; Barbato, O.; Castrica, M.; Sulçe, M.; Castellini, C.; Muça, G.; Mattioli, S.; et al. In Vitro Effects of Lipopolysaccharide on Rabbit Sperm: Toll-like Receptor 4 Expression, Motility, and Oxidative Status. Antioxidants 2025, 14, 431. https://doi.org/10.3390/antiox14040431
Quattrone A, Fehri NE, Agradi S, Menchetti L, Barbato O, Castrica M, Sulçe M, Castellini C, Muça G, Mattioli S, et al. In Vitro Effects of Lipopolysaccharide on Rabbit Sperm: Toll-like Receptor 4 Expression, Motility, and Oxidative Status. Antioxidants. 2025; 14(4):431. https://doi.org/10.3390/antiox14040431
Chicago/Turabian StyleQuattrone, Alda, Nour Elhouda Fehri, Stella Agradi, Laura Menchetti, Olimpia Barbato, Marta Castrica, Majlind Sulçe, Cesare Castellini, Gerald Muça, Simona Mattioli, and et al. 2025. "In Vitro Effects of Lipopolysaccharide on Rabbit Sperm: Toll-like Receptor 4 Expression, Motility, and Oxidative Status" Antioxidants 14, no. 4: 431. https://doi.org/10.3390/antiox14040431
APA StyleQuattrone, A., Fehri, N. E., Agradi, S., Menchetti, L., Barbato, O., Castrica, M., Sulçe, M., Castellini, C., Muça, G., Mattioli, S., Vigo, D., Migni, G., Nompleggio, L., Belabbas, R., Gualazzi, F., Ricci, G., Postoli, R., Di Federico, F., Moretti, E., ... Curone, G. (2025). In Vitro Effects of Lipopolysaccharide on Rabbit Sperm: Toll-like Receptor 4 Expression, Motility, and Oxidative Status. Antioxidants, 14(4), 431. https://doi.org/10.3390/antiox14040431















